Abstract
Cisplatin-induced ototoxicity remains a primary dose-limiting adverse effect of this highly effective anticancer drug. The clinical utility of cisplatin could be enhanced if the signaling pathways that regulate the toxic side-effects are delineated. In previous studies, we reported cisplatin-induced nitration of cochlear proteins and provided the first evidence for nitration and downregulation of cochlear LIM domain only 4 (LMO4) in cisplatin ototoxicity. Here, we extend these findings to define the critical role of nitrative stress in cisplatin-induced downregulation of LMO4 and its consequent ototoxic effects in UBOC1 cell cultures derived from sensory epithelial cells of the inner ear and in CBA/J mice. Cisplatin treatment increased the levels of nitrotyrosine and active caspase 3 in UBOC1 cells, which was detected by immunocytochemical and flow cytometry analysis, respectively. The cisplatin-induced nitrative stress and apoptosis were attenuated by co-treatment with SRI110, a peroxynitrite decomposition catalyst (PNDC), which also attenuated the cisplatin-induced downregulation of LMO4 in a dose-dependent manner. Furthermore, transient overexpression of LMO4 in UBOC1 cells prevented cisplatin-induced cytotoxicity while repression of LMO4 exacerbated cisplatin-induced cell death, indicating a direct link between LMO4 protein levels and cisplatin ototoxicity. Finally, auditory brainstem responses (ABR) recorded from CBA/J mice indicated that co-treatment with SRI110 mitigated cisplatin-induced hearing loss. Together, these results suggest that cisplatin-induced nitrative stress leads to a decrease in the levels of LMO4, downregulation of LMO4 is a critical determinant in cisplatin-induced ototoxicity, and targeting peroxynitrite could be a promising strategy for mitigating cisplatin-induced hearing loss.
Abbreviations: LMO4, Lim domain only 4; PNDC, peroxynitrite decomposition catalyst; ABR, auditory brainstem responses; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
Keywords: Cisplatin, Ototoxicity, Protein nitration, LMO4, Cochlea, Hearing loss
Graphical abstract
Schematic representation of the role of nitrative stress and LMO4 downregulation in cisplatin-induced ototoxicity. Cisplatin is known to induce cochlear oxidative stress by activating NOX3. The cisplatin-induced generation of reactive oxygen species eventually leads to the nitration of LMO4 and decreases its level in the cochlea. Generally, LMO4 acts as a scaffold for protein complexes and regulates cellular apoptosis. The cisplatin-induced decrease in LMO4 probably compromises the LMO4 associated anti-apoptotic machinery and thereby facilitates cochlear apoptosis and ototoxicity.

Highlights
-
•
Cisplatin-induced nitrative stress leads to a decrease in the levels of LMO4.
-
•
Downregulation of LMO4 is a critical factor in cisplatin-induced ototoxicity.
-
•
SRI110 appears to be a promising candidate for preventing cisplatin ototoxicity.
1. Introduction
Cisplatin, a first-generation platinum-based anti-neoplastic drug, is used to treat cancers of the bladder, cervix, lung (non-small cell), ovary, head & neck (squamous cell), testicle, and malignant mesothelioma. According to the National Cancer Institute, 10–20% of all cancer patients are prescribed cisplatin or its analogues. Cisplatin arrests cell division to prevent tumor growth and induces programmed cell death to reduce tumor size. However, cisplatin-induced apoptosis is not restricted to tumor cells but extends to susceptible cells, such as the outer hair cells in the cochlea. Therefore, ototoxicity is a major side-effect of cisplatin, which significantly affects the quality of life in cancer survivors and has devastating consequences in children because it affects their speech and language development, education, and social integration [1], [2]. Although progress has been made in delineating the mechanisms underlying cisplatin-induced ototoxicity [3], [4], [5], [6], [7], the signaling pathways that facilitate cochlear apoptosis are yet to be fully characterized. Thus, we do not yet have a comprehensive solution to this important problem.
Among the cochlear cell death mechanisms reported so far, oxidative stress is considered to play a causal role in cisplatin ototoxicity [3], [8] as cisplatin activates the enzyme NOX3, which increases the production of superoxide radicals in the inner ear [9]. In addition, the activation of iNOS pathway and the generation of nitric oxide have been detected in cisplatin ototoxicity [10], [11]. Although antioxidants are employed to prevent cisplatin ototoxicity they do not provide a comprehensive solution as many of them interfere with the anticancer activity of cisplatin. Hence, there is a need to identify alternative downstream targets for intervention. We reported cisplatin-induced increase in the nitration of proteins in the cochlear sensory epithelium [12] and detected a strong correlation between cochlear protein nitration and cisplatin-induced hearing loss. Nitrative stress is emerging as an important factor in acquired hearing loss as increased levels of nitrotyrosine have been detected in several inner ear pathologies and associated with pathways related to the cochlear stress response [13], [14], [15], [16], [17], [18]. Targeting nitrative stress appears to be a plausible strategy to prevent cisplatin-induced ototoxicity because co-treatment with a PNDC did not interfere with the anticancer activity of paclitaxel [19].
Our studies identified the nitration of cochlear LMO4 in cisplatin ototoxicity [4]. As a molecular adaptor for protein-protein interactions, LMO4 forms transcriptional complexes and thereby regulates cell survival and cell death [20], [21], [22], [23]. LMO4 plays an important role in the development of the inner ear [24] and its downstream target STAT3 is also implicated in cisplatin-ototoxicity [25], [26], [27]. We co-localized LMO4 and nitrotyrosine in the outer hair cells, which are primary targets of cisplatin-induced ototoxicity [4]. Protein nitration can modulate phosphorylation cascades, alter protein function, and facilitate proteolytic degradation of nitrated proteins [28], [29], [30], [31], [32], [33], [34]. Consistent with these reports, cisplatin-induced nitration of LMO4 was associated with a significant decrease in its protein levels, not only in the auditory cells, but in the renal and neuronal cells, which are also susceptible to the toxic side-effects of cisplatin [35]. Repression of LMO4 has been reported to facilitate cellular apoptosis in other models [36], [37].
Our objective, in this study, is to validate and extend the previous correlational observations by clarifying the critical link between cisplatin-induced nitrative stress, downregulation of LMO4, and ototoxicity. Based on our studies and other reports, we hypothesized that downregulation of LMO4 by cisplatin-induced nitrative stress compromises the anti-apoptotic machinery to facilitate ototoxicity. We objectively tested our hypothesis by employing two approaches: 1) using a PNDC to selectively inhibit nitrative stress and 2) genetically manipulating the expression of LMO4 in sensory epithelial cells of the inner ear.
2. Materials and methods
2.1. Animals
Six-week-old male CBA/J mice were purchased from Jackson Laboratories (Jackson Laboratories, Bar Harbor, ME). All animals were housed at the Laboratory Animal Facility of Wayne State University and maintained in a temperature-controlled room with a 12-h light/dark cycle and allowed free access to food and water. Every effort was made to minimize pain and discomfort and all animals were handled and treated according to guidelines established by the National Institutes of Health and the Institutional Animal Care and Use Committee (# A 04-07-14).
2.2. Cell culture
UBOC1 cells derived from the mouse organ of Corti were provided by Dr. Mathew C Holley (University of Sheffield, UK). The cells were cultured in minimum essential medium (GlutaMAX, catalog no. 41090-036, Thermo Fisher Scientific, Rockford, IL) with 10% fetal bovine serum (Gibco BRL, Gaithersburg, MD) and incubated in a humidified atmosphere containing 10% CO2. For propagation the cells were initially cultured in a medium containing 50 U/ml γ-interferon (catalog no. 315-05, PeproTech, Rocky Hill, NJ, USA) and to facilitate differentiation the cells were cultured for a week at 37 °C without γ-interferon [38], [39]. Fully differentiated cells were used for genetic manipulation, drug treatment, and biochemical analyses. The expression of myosin VIIa was tested to verify differentiation.
2.3. Drug treatment
All chemicals and reagents including cisplatin (P4394) were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless noted otherwise. UBOC1 cells were treated with 5 or 10 µm of cisplatin mixed with culture medium for 24 h. SRI110, a manganese(III) bis(hydroxyphenyl)dipyrromethene catalyst, was synthesized as reported previously [19]. SRI110 (50 µm) was mixed with the culture medium, and the cells were exposed to the drug 1 h prior to cisplatin treatment. In mice, veterinary-grade cisplatin (Pharmachemie B.V., Haarlem, Netherlands) was administered at 3 mg/kg body weight for 5 days [41] by slow intraperitoneal infusion at the concentration of 1 mg/ml in sterile saline (0.9%). Control animals were infused with an equal volume of saline. SRI110 (10 mg/kg) was mixed with Nutella and administered orally 1 h prior to cisplatin treatment on all 5 days. All animals were hydrated daily with 10 ml/kg subcutaneous injection of saline until they were sacrificed.
2.4. Protein extraction
The cells were homogenized in radio-immunoprecipitation assay buffer supplemented with 5 mM EDTA, phosphatase, and protease inhibitors (Thermo Fisher Scientific, Rockford, IL). The homogenate was extracted on ice for 45 min and centrifuged at 14,000×g for 10 min. Protein concentration of the supernatant was determined by Bradford assay [40].
2.5. Immunoblotting
Protein extracts were separated on 4–20% Mini-Protean TGX gel (456-1093, Bio-Rad Laboratories, Inc., Hercules, CA), transferred to polyvinylidene difluoride membranes, blocked with 5% fat-free milk in tris-buffered saline containing 0.05% Tween 20 (Sigma-Aldrich) and probed with antibodies using chemiluminescence detection (34076, Thermo Fisher Scientific, Rockford, IL). The FluorChem E imaging system (ProteinSimple, Santa Clara, CA) was used to visualize bands, which were quantified using NIH ImageJ software. Background corrected bands were normalized against actin [4].
2.6. Immunocytochemistry
UBOC1 Cells were plated on two-well chamber slides (Nunc Lab-Tek II Chamber Slide system, 154461, Fisher Scientific, Pittsburgh, PA, USA) and treated with 10 µm cisplatin for 24 h. The cells were fixed, permeabilized, and blocked as described previously [35]. Then the cells were incubated with anti-nitrotyrosine, anti-myosin VIIa (catalog no. sc-32757, sc-74516, Santa Cruz Biotechnology Inc., Santa Cruz, CA) or anti-LMO4 (catalog no. ab39383, Abcam, Cambridge, MA) followed by incubation with Alexa Fluor 568 donkey anti-mouse or Alexa Fluor 647 goat anti-rabbit secondary antibody (catalog no. A10037 or A21244, Life Technologies, Carlsbad, CA) and fluorescein phalloidin (catalog no. F432, Life Technologies). ProLong Gold antifade reagent containing DAPI (catalog no. P36935, Life Technologies) was used for mounting the cells and Carl Zeiss Laser Scanning Systems (Zeiss LSM 780, Jena, Germany) was used to capture the images of the stained cells.
2.7. Silencing of LMO4
UBOC1 cells were transfected with a combination of four siRNAs (Qiagen, Valencia, CA): Hs_LMO4_8 (catalog no. SI04270966), CGGCACGTCCTGTTACACCAA; Hs_LMO4_9 (catalog no. SI04312973), CCGCCTCTCGCAATATTGCAA; HsLMO4_6 (catalog no. SI03185777), CCCGGGAGATCGGTTTCACTA; Hs_LMO4_7 (catalog no. SI04151231), AGGAAACGTGTTTCAATCAAA in Opti-MEM reduced serum medium (Invitrogen, catalog no. 31985) using Oligofectamine (Invitrogen, catalog no. 12252-011). AllStars Negative Control siRNA (Qiagen, catalog no. 1027280), CAGGGTATCGACGATTACAAA, was used as a negative control. The cells were incubated for 24 h for silencing the gene and then treated with 5 µm cisplatin treatment for another 24 h [4]. Repression of LMO4 was verified by immunoblotting with anti-LMO4.
2.8. Transient overexpression of LMO4
Mammalian expression vector pRK5 (catalog no. 22964, Addgene, Cambridge, MA) was used for the overexpression of LMO4, following the manufacturer's protocol. UBOC1 cells were transfected with HA-tagged LMO4 using lipofectamine reagent (Invitrogen, Carlsbad, CA) at 50–60% confluence and cultured for 48 h. Transfection of the plasmid DNA was verified by immunoblotting with HA-Tag (6E2) mouse antibody (Cell Signaling, Danvers, MA) and overexpression of LMO4 was verified by immunoblotting with anti-LMO4 [35].
2.9. Cell viability count
The viability of the cells was determined by counting the number of cells that were not stained with trypan blue (live cell count) relative to the total number of cells (total cell count), using a hemocytometer.
2.10. MTT assay
UBOC1 cells were treated with 10 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (5 mg/ml in PBS) and incubated at 37 °C in 5% CO2 for 4 h, following the manufacturer's protocol (catalog no. CT02, EMD Millipore Corporation, Temecula, CA). The formazan crystals, formed by the reduction of MTT by active mitochondria present in the viable cells, were dissolved by adding 100 μl of 0.04 N HCl in isopropanol. The absorbance was measured at 570 nm using a microplate reader, with a reference wavelength of 630 nm.
2.11. Caspase 3 fluorescence assay
Activation of caspase 3 was assayed as a biomarker of apoptosis, using a Fluorescein Active Caspase 3 Staining Kit (catalog no. ab39383, Abcam, Cambridge, MA). UBOC1 cells were plated in six-well culture plates and treated with cisplatin or SRI110 or both for 24 h. The cells were re-suspended and treated with FITC-DEVD-FMK reagent following the manufacturer's protocol. Fluorescence generated by the reaction between a caspase 3 substrate (FITC-DEVD-FMK) and active caspase 3, upon cleavage, was analyzed by flow cytometry. Z-VAD-FMK reagent, a caspase inhibitor, was used for the negative controls.
2.12. Auditory brainstem response
Hearing thresholds were measured after anesthetizing the animals with isoflurane (4% induction, 1.5% maintenance with 1 L/min O2). ABR was recorded using subcutaneous differential active needle electrodes with sound stimuli of 1-ms tone bursts (4, 8, 16, 24, or 32 kHz) or 25-µs clicks, generated using Tucker-Davis Technologies BioSigRZ software and TDT System3 hardware (TDT, Alachua, FL). The stimuli were presented to the external auditory meatus and two hundred stimulus presentations, delivered at 21/s, was averaged to obtain the waveform of the brainstem response. The lowest intensity of stimulation at which a waveform with an identifiable peak was detected was considered as the hearing threshold [12].
2.13. Data analysis
All data were statistically analyzed using two-tailed unpaired t-test and P value of <0.05 was considered significant. GraphPad Prism 6 software (GraphPad, La Jolla, CA) was used for statistical analysis and the results are expressed as mean±standard deviation.
3. Results
3.1. Cisplatin-induced protein nitration was inhibited by treatment with PNDC
We reported that cisplatin treatment increased the nitration of proteins in rat cochlea as well as UBOC1 cell cultures [4], [35]. In order to determine whether treatment with PNDC could prevent cisplatin-induced protein nitration UBOC1 cells were treated with 50 µm of SRI110 1 h before 10 µm cisplatin treatment. After 24 h, the cells were probed with anti-nitrotyrosine and the immunoreactivity with nitrotyrosine was visualized by confocal microscopy. Consistent with our previous studies cisplatin significantly increased the levels of nitrotyrosine and induced apoptosis, which was evident from the condensed nuclei. Co-treatment with SRI110 attenuated the cisplatin-induced increase in nitrotyrosine as well as the apoptotic changes observed in the nuclei (Fig. 1). This suggested that PNDCs inhibit the cisplatin-induced nitrative stress in the inner ear cells.
Fig. 1.
Cisplatin-induced increase in nitrotyrosine is attenuated by SRI110. A) Increased levels of nitrotyrosine as well as condensed nuclei (white arrows) were detected in UBOC1 cells treated with 10 µm cisplatin. Co-treatment with 50 µm SRI110 attenuated the cisplatin-induced increase in nitrotyrosine. Red staining indicates immunoreactivity to anti-nitrotyrosine, blue indicates nuclear staining with DAPI, while green indicates actin staining with phalloidin. The images are representative of four biological replicates. Scale bar, 20 µm. (B) The mean pixel intensities for nitrotyrosine immunostaining was significantly higher in cisplatin treated cells (****P<0.0001), which was attenuated by SRI110 co-treatment (***P<0.001). The results are expressed as mean±standard deviation, n=4. (C) Immunostaining (violet) with anti-myosin VIIa indicated the differentiated state of UBOC1 cells. The image is representative of three biological replicates. Scale bar, 20 µm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Inhibition of nitrative stress attenuated cisplatin-induced downregulation of LMO4 in a dose-dependent manner
Our previous studies indicated that in addition to increased nitration of cochlear proteins, cisplatin treatment decreased the levels of LMO4 in the cochlea. We hypothesized that cisplatin-induced nitrative stress leads to a decrease in LMO4 protein levels because nitrated proteins are potential candidates for proteasomal degradation [28], [31]. To test whether scavenging of peroxynitrite could attenuate the cisplatin-induced downregulation of LMO4, we treated UBOC1 cells with two different doses of SRI110 (25 or 50 µm), 1 h before 10 µm cisplatin treatment. After 24 h, the proteins were extracted from the cells, separated on a gel, transferred to a polyvinylidene fluoride membrane, and probed with anti-LMO4. The detected LMO4 protein bands were quantified by normalizing with that of actin. Cisplatin treatment decreased the levels of LMO4, which was at 58%, relative to controls, while the levels of LMO4 after co-treatment with 25 or 50 µm of SRI110 was at 70% and 88%, respectively (Fig. 2). The observations were supported by scatter plots generated through immunocytochemical analysis, which indicated that SRI110 co-treatment (50 µm) attenuated not only the cisplatin-induced increase in nitrotyrosine levels but also the decrease in LMO4 levels. These findings indicated that scavenging of peroxynitrite attenuates cisplatin-induced downregulation of LMO4 in a dose-dependent manner and suggested that cisplatin-induced nitrative stress is partly responsible for the decrease in the levels of LMO4.
Fig. 2.
SRI110 attenuates cisplatin-induced downregulation of LMO4 in a dose-dependent manner. A) Immunoblots with anti-LMO4 indicated that cisplatin treatment significantly decreased the protein levels of LMO4. Co-treatment with 25 µm of SRI110 did not alter the cisplatin-induced decrease in LMO4 levels. However, co-treatment with 50 µm of SRI110 significantly attenuated the cisplatin-induced decrease in LMO4, suggesting a dose-dependent response to SRI110 treatment. The results are expressed as mean±standard deviation, n=4. ** P<0.01, relative to control. B) Scatter plots indicated that in control cells LMO4 (orange) is heavily expressed while cisplatin treatment induced an increase in nitrotyrosine (red) levels. SRI110 co-treatment attenuated the cisplatin-induced decrease in LMO4 levels. The plots are representative of biological replicates. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Downregulation of LMO4 exacerbated cisplatin-induced cytotoxicity
To determine whether the downregulation of LMO4 could promote the ototoxic effects of cisplatin, we silenced LMO4 expression in UBOC1 cells using SiRNAs and assessed the cisplatin-induced cytotoxic effects using cell counts. UBOC1 cells were transfected with 4 different LMO4 SiRNAs and the decrease in LMO4 protein levels were analyzed by immunoblotting with anti-LMO4. Actin was used to normalize the expression of LMO4. Repression of LMO4 with SiRNAs resulted in a 40% decrease in the protein levels of LMO4. Treatment of wild-type cells with cisplatin induced only 18% decrease in cell viability. However, treatment of the LMO4 deficient cells with 5 µm cisplatin exacerbated the cisplatin-induced cytotoxicity, which was indicated by 46% decrease in the cell counts (Fig. 3). This was also supported by the increase in the number of condensed nuclei in LMO4 deficient cells relative to wild-type cells treated with cisplatin. These findings suggested that in the absence of LMO4 the susceptibility of the inner ear cells to cisplatin-induced toxicity is significantly enhanced and is consistent with other reports, which indicate repression of LMO4 promotes apoptosis [37]. The viability of UBOC1 cells treated with scrambled RNA was similar to that of the control cells.
Fig. 3.
Silencing of LMO4 exacerbates cisplatin-induced cell death. (A) LMO4 was repressed in UBOC1 cells by using siRNAs. Treatment of LMO4 deficient cells with 5 µm cisplatin significantly decreased the number of viable cells when compared to cisplatin-treated wild-type UBOC1 cells. The results are expressed as mean±standard deviation, n=3. ****P<0.0001. (B) Repression of LMO4 by siRNAs was quantified by immunoblotting with anti-LMO4 and normalized with that of actin. Treatment with SiRNAs induced a 40% decrease in the levels of LMO4 (n=3, *P<0.05). (C) Nuclear staining with DAPI indicated that the number of condensed nuclei (white arrows) is much higher in the LMO4 deficient cells than in the wild-type cells, after cisplatin treatment. The images are representative of four biological replicates. Scale bar, 20 µm.
3.4. Overexpression of LMO4 attenuated cisplatin-induced cytotoxicity
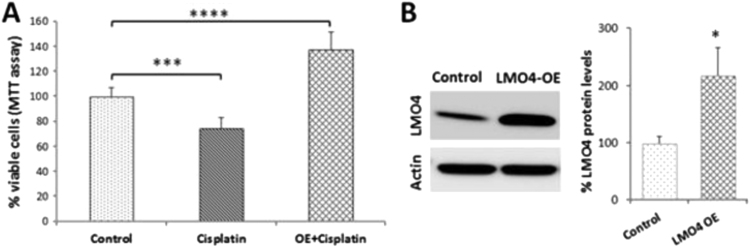
Because LMO4 regulates cellular apoptosis and downregulation of LMO4 facilitated cisplatin-induced cytotoxicity we hypothesized that overexpression of LMO4 will prevent the cytotoxic effects induced by cisplatin. To test this hypothesis, we transiently overexpressed LMO4 in UBOC1 cells and evaluated the cytotoxic effects of cisplatin using MTT assay. Cisplatin treatment (5 µm) significantly decreased the cell viability by 25% in wild-type UBOC1 cells while overexpression of LMO4 not only prevented the cisplatin-induced cell death but significantly increased the cell viability by 38% (Fig. 4). This suggested that at normal or higher levels LMO4 plays a protective role and prevents cisplatin-induced cell death. Overexpression of LMO4 was verified by immunoblotting with anti-LMO4, which was normalized by immunoblotting with anti-actin.
Fig. 4.
Overexpression of LMO4 prevents cisplatin-induced decrease in cell viability. (A) MTT assay indicated that cisplatin treatment decreased the viability of UBOC1 cells. Overexpression of LMO4 attenuated the cisplatin-induced decrease in cell viability. The results are expressed as mean±standard deviation, n=3. ***P<0.001, ****P<0.0001. (B) The efficiency of transfection was indicated by immunoblots, which showed a 117% increase in the expression of LMO4 in overexpressed cells, but not in control cells. LMO4 expression was normalized with that of actin (n=3, *P<0.05).
3.5. Inhibition of nitrative stress attenuated cisplatin-induced apoptosis
Our results indicated that the expression levels of LMO4 are a critical determinant of cisplatin-induced cytotoxicity and inhibition of nitrative stress attenuated cisplatin-induced downregulation of LMO4. Therefore, scavenging of peroxynitrite is also expected to attenuate cisplatin-induced cytotoxicity, which primarily occurs through apoptosis. To test this hypothesis we treated UBOC1 cells with 50 µm of SRI110 and assessed the expression of active caspase 3, a biomarker of apoptosis, by flow cytometry. Cisplatin treatment (10 µm) induced a significant increase in active caspase 3 levels in UBOC1 cells (p>0.001), when compared to the controls. Co-treatment with SRI110 attenuated the cisplatin-induced increase in caspase levels (p>0.001). In the cells that were treated with only SRI110 the caspase activity was similar to that of the controls (Fig. 5). This suggested that inhibition of nitrative stress prevents cisplatin-induced apoptosis in the inner ear cells.
Fig. 5.
Inhibition of nitrative stress attenuates cisplatin-induced apoptosis. The illustrations indicate the changes in the expression of active caspase 3 in UBOC1 cells, detected by flow cytometry analysis. Cisplatin treatment induced an increase in active caspase 3 levels while SRI110 co-treatment attenuated it. Treatment with SRI110 alone did not alter the active caspase 3 levels. The images are representative of three biological replicates. The results are expressed as mean±standard deviation, n=3. ***P<0.001.
3.6. Inhibition of nitrative stress mitigated cisplatin-induced hearing loss
Though the cytotoxic effects of cisplatin in this inner ear cell culture model were mitigated by treatment with PNDC, this interventional approach may not be relevant to cisplatin-induced ototoxicity unless the efficacy is verified in a murine model. Therefore, we used CBAJ mice, which are good models for studying cisplatin ototoxicity [41], and treated them with 3 mg/kg dose of cisplatin daily for five consecutive days. Hearing was assessed on the eighth day by testing ABRs (Fig. 6A and B). Cisplatin treatment induced a 10–15 dB shift in the hearing thresholds while co-treatment of SRI110 at 10 mg/kg dose daily, by oral administration for five consecutive days, attenuated the cisplatin-induced hearing loss (p>0.05, at 8 kHz). In addition, SRI110 co-treatment significantly attenuated the cisplatin-induced weight loss (Fig. 6C). This suggested that PNDCs could emerge as a promising interventional approach to prevent cisplatin ototoxicity.
Fig. 6.
Inhibition of nitrative stress attenuates cisplatin-induced hearing loss. (A) CBAJ mice treated with 3 mg/kg dose of cisplatin for 5 days induced a 10–20 dB shift in hearing threshold on day 8. Co-treatment with SRI110 attenuated the cisplatin-induced hearing loss (P<0.05, at 8 kHz). ABR recorded from the left ear of 4 mice are illustrated. The results are expressed as mean±standard deviation. (B) Representative ABR waveform in response to click stimuli is illustrated. (C) SRI110 co-treatment prevented the cisplatin-induced weight loss. The results are expressed as mean±standard deviation, n=5.
4. Discussion
Nitrated proteins play an important role in mediating cell death responses, and their signaling has been effectively targeted to inhibit cell death in several experimental models [42], [43], [44]. However, the nitrative stress mechanism underlying cisplatin-induced ototoxicity remains only partially understood. In previous studies, we reported that cisplatin treatment leads to the nitration and downregulation of cochlear LMO4 [4], [35]. Here, we provide evidence that selective inhibition of nitrative stress by SRI110 attenuates cisplatin-induced downregulation of LMO4 and apoptosis in cell cultures of the auditory sensory epithelium. In addition, co-treatment of SRI110 mitigated cisplatin-induced hearing loss in CBA/J mice indicating the functional significance of nitrative stress in cisplatin ototoxicity. Furthermore, transient overexpression of LMO4 in cell cultures prevented cisplatin-induced cytotoxicity while repression of LMO4 worsened the toxic effects of cisplatin. Together, these findings suggest that cisplatin-induced nitrative stress and downregulation of LMO4 plays a critical role in facilitating cisplatin-induced ototoxicity.
In this study, UBOC1 cell cultures and CBA/J mice were used to determine the contribution of LMO4 and nitrative stress to cisplatin ototoxicity. These are good experimental models for studying ototoxicity and have been employed by other researchers to investigate the molecular mechanisms underlying cisplatin-induced ototoxicity [5], [41]. We used fully differentiated UBOC1 cells in all our experiments, which were verified by testing the expression of myosin VIIa, a biomarker of hair cells. In animal studies, only male mice were used in order to avoid potential interference in LMO4 signaling from any changes in the sex hormones, particularly, estrogen because LMO4 is known to bind with estrogen receptors and repress its transactivation activities [45]. The CBA/J mice were administered cisplatin by following a 5-day treatment regimen, in order to mimic the clinical use of cisplatin, and their hearing loss was assessed by recording auditory brainstem responses before and after cisplatin treatment. Pharmaceutical grade cisplatin was used in the in-vivo studies while SRI110 was synthesized in-house [19]. The effect of the drug treatment was analyzed by immunoblotting and immunocytochemistry and the specificity of the immunoreaction with nitrotyrosine and LMO4 antibodies were verified as described in previous publications [4], [35]. The efficiency of transient overexpression as well as repression of LMO4 in UBOC1 cell cultures was verified by immunoblots with anti-LMO4.
The ototoxic side-effects of cisplatin are predominantly mediated by oxidative stress [3], [9] and protein nitration is an important sequela of oxidative stress. In agreement, cisplatin treatment increased the levels of nitrotyrosine in UBOC1 cells. In addition, it also increased the expression of active caspase 3, indicating cisplatin-induced apoptosis in these cell cultures. Because protein nitration can cause vital changes in biological function depending on the functional characteristics of the nitrated protein, identification of the nitrated protein(s) is essential to understand its functional significance. We previously identified cisplatin-induced nitration of cochlear LMO4, in Wistar rats, by immunoprecipitation followed by Matrix Assisted Laser Desorption/Ionization–Time Of Flight mass spectrometry [4], and verified the co-localization of nitrotyrosine and LMO4 in UBOC1 cells [35]. In addition to nitration of LMO4, cisplatin treatment decreased the levels of LMO4, which is consistent with reports that indicate nitrated proteins are potential targets for proteasomal degradation [31], [43], [46]. However, the functional implications of decreased levels of cochlear LMO4 in cisplatin-induced ototoxicity are not fully known.
LMO4 has the potential to mediate cytotoxicity, as it controls pathways regulating cell survival and cell death by modulating the formation of transcriptional complexes that repress or promote transcription of pertinent genes [20], [22], [23], [45], [47]. Our recent studies indicated that cisplatin-induced nitration and downregulation of LMO4 occurs not only in the auditory cells (UBOC1), but also in the renal (HK2), and neuronal (SH-SY5Y) cells [35]. To clarify the functional significance of downregulation of LMO4 in cisplatin-induced cytotoxicity we overexpressed LMO4 in UBOC1 cells using pRK5 vectors. The transient overexpression of LMO4 not only prevented cisplatin-induced cytotoxicity at this dose but also significantly increased cell viability indicating a potential anti-apoptotic role of LMO4. Moreover, silencing of LMO4 with siRNAs exacerbated the cisplatin-induced cytotoxicity in UBOC1 cells, which is consistent with other reports that indicated repression of LMO4 promotes apoptosis [37]. Collectively, these results suggested a direct link between LMO4 protein levels and cellular apoptosis in cisplatin-mediated cytotoxicity.
In order to decipher the contribution of nitrative stress to cisplatin-induced decrease in LMO4 levels and thus to ototoxicity we used SRI110 to scavenge cisplatin-induced generation of peroxynitrite. SRI110 is a manganese (III) bishydroxyphenyldipyrromethene-based peroxynitrite decomposition catalyst that was designed to selectively target peroxynitrite [19]. Targeting the nitrative stress pathway that facilitates the toxic side-effects of cisplatin appears to be an attractive strategy for intervention because it minimizes potential interference with the anti-cancer activity of cisplatin, which is primarily mediated by the formation of DNA adducts. Related metalloporphyrins have been reported to prevent cisplatin-induced protein nitration and nephrotoxicity [48]. SRI110 has several advantages as a potential otoprotective compound because of the following reasons. First, it did not interfere with the anticancer activities of paclitaxel. Second, it spares the superoxide radical, which has other signaling roles particularly in learning and memory. Third, its oral bioavailability enables it to be easily and continuously used throughout cancer treatment. In this study, inhibition of nitrative stress by SRI110 decreased the cisplatin-induced increase in nitrotyrosine and caspase 3 levels, attenuated the cisplatin-induced downregulation of LMO4 in UBOC1 cells in a dose dependent manner, and mitigated cisplatin-induced hearing loss and weight loss in CBA/J mice.
5. Conclusions
Our results provide compelling evidence to suggest that PNDCs could be effectively employed to mitigate nitrative stress-induced otopathology and they could be administered orally to selectively target peroxynitrite, which is not known to have any beneficial roles. These findings are significant and may have broader implications because nitrative stress is emerging as an important factor not only in cisplatin-induced hearing loss, but also in other types of acquired hearing loss such as noise-induced and age-related hearing loss [13], [17]. Furthermore, by defining the critical link between cisplatin-induced hearing loss, nitrative stress, and downregulation of LMO4, which was also detected in cisplatin-induced damage to renal and neuronal cells [35], this study provides a strong foundation for the identification and development of novel targets for therapeutic intervention. These outcomes, in turn, will increase the clinical utility of this crucial life-saving drug and improve the quality of life of the cancer survivors treated with cisplatin.
Funding
This research was supported by start-up funds to SJ from Wayne State University and P30 grant (P30 ES020957) to Center for Urban Responses to Environmental Stressors (CURES) from NIEHS. The Microscopy, Imaging and Cytometry Resources Core is supported, in part, by NIH Center grant P30 CA022453.
References
- 1.Brock P.R., Knight K.R., Freyer D.R., Campbell K.C., Steyger P.S., Blakley B.W., Rassekh S.R., Chang K.W., Fligor B.J., Rajput K., Sullivan M., Neuwelt E.A. Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J. Clin. Oncol. 2012;30(19):2408–2417. doi: 10.1200/JCO.2011.39.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langer T., am Zehnhoff-Dinnesen A., Radtke S., Meitert J., Zolk O. Understanding platinum-induced ototoxicity. Trends Pharm. Sci. 2013;34(8):458–469. doi: 10.1016/j.tips.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Rybak L.P., Whitworth C.A., Mukherjea D., Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear. Res. 2007;226(1–2):157–167. doi: 10.1016/j.heares.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Jamesdaniel S., Coling D., Hinduja S., Ding D., Li J., Cassidy L., Seigel G.M., Qu J., Salvi R. Cisplatin-induced ototoxicity is mediated by nitroxidative modification of cochlear proteins characterized by nitration of Lmo4. J. Biol. Chem. 2012;287(22):18674–18686. doi: 10.1074/jbc.M111.297960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaur T., Borse V., Sheth S., Sheehan K., Ghosh S., Tupal S., Jajoo S., Mukherjea D., Rybak L.P., Ramkumar V. Adenosine A1 receptor protects against cisplatin ototoxicity by suppressing the NOX3/STAT1 inflammatory pathway in the cochlea. J. Neurosci. 2016;36(14):3962–3977. doi: 10.1523/JNEUROSCI.3111-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.More S.S., Akil O., Ianculescu A.G., Geier E.G., Lustig L.R., Giacomini K.M. Role of the copper transporter, CTR1, in platinum-induced ototoxicity. J. Neurosci. 2010;30(28):9500–9509. doi: 10.1523/JNEUROSCI.1544-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas A.J., Hailey D.W., Stawicki T.M., Wu P., Coffin A.B., Rubel E.W., Raible D.W., Simon J.A., Ou H.C. Functional mechanotransduction is required for cisplatin-induced hair cell death in the zebrafish lateral line. J. Neurosci. 2013;33(10):4405–4414. doi: 10.1523/JNEUROSCI.3940-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berndtsson M., Hagg M., Panaretakis T., Havelka A.M., Shoshan M.C., Linder S. Acute apoptosis by cisplatin requires induction of reactive oxygen species but is not associated with damage to nuclear DNA. Int. J. Cancer. 2007;120(1):175–180. doi: 10.1002/ijc.22132. [DOI] [PubMed] [Google Scholar]
- 9.Banfi B., Malgrange B., Knisz J., Steger K., Dubois-Dauphin M., Krause K.H. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J. Biol. Chem. 2004;279(44):46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- 10.Li G., Liu W., Frenz D. Cisplatin ototoxicity to the rat inner ear: a role for HMG1 and iNOS. Neurotoxicology. 2006;27(1):22–30. doi: 10.1016/j.neuro.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe K., Inai S., Jinnouchi K., Bada S., Hess A., Michel O., Yagi T. Nuclear-factor kappa B (NF-kappa B)-inducible nitric oxide synthase (iNOS/NOS II) pathway damages the stria vascularis in cisplatin-treated mice. Anticancer Res. 2002;22(6C):4081–4085. [PubMed] [Google Scholar]
- 12.Jamesdaniel S., Ding D., Kermany M.H., Davidson B.A., Knight P.R., 3rd, Salvi R., Coling D.E. Proteomic analysis of the balance between survival and cell death responses in cisplatin-mediated ototoxicity. J. Proteome Res. 2008;7(8):3516–3524. doi: 10.1021/pr8002479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang H., Talaska A.E., Schacht J., Sha S.H. Oxidative imbalance in the aging inner ear. Neurobiol. Aging. 2007;28(10):1605–1612. doi: 10.1016/j.neurobiolaging.2006.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labbe D., Teranishi M.A., Hess A., Bloch W., Michel O. Activation of caspase-3 is associated with oxidative stress in the hydropic guinea pig cochlea. Hear. Res. 2005;202(1–2):21–27. doi: 10.1016/j.heares.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Lee J.E., Nakagawa T., Kim T.S., Endo T., Shiga A., Iguchi F., Lee S.H., Ito J. Role of reactive radicals in degeneration of the auditory system of mice following cisplatin treatment. Acta Otolaryngol. 2004;124(10):1131–1135. doi: 10.1080/00016480410017521. [DOI] [PubMed] [Google Scholar]
- 16.Lopez I.A., Acuna D., Beltran-Parrazal L., Espinosa-Jeffrey A., Edmond J. Oxidative stress and the deleterious consequences to the rat cochlea after prenatal chronic mild exposure to carbon monoxide in air. Neuroscience. 2008;151(3):854–867. doi: 10.1016/j.neuroscience.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 17.Shi X., Han W., Yamamoto H., Omelchenko I., Nuttall A. Nitric oxide and mitochondrial status in noise-induced hearing loss. Free Radic. Res. 2007;41(12):1313–1325. doi: 10.1080/10715760701687117. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita D., Jiang H.Y., Le Prell C.G., Schacht J., Miller J.M. Post-exposure treatment attenuates noise-induced hearing loss. Neuroscience. 2005;134(2):633–642. doi: 10.1016/j.neuroscience.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Doyle T., Chen Z., Muscoli C., Bryant L., Esposito E., Cuzzocrea S., Dagostino C., Ryerse J., Rausaria S., Kamadulski A., Neumann W.L., Salvemini D. Targeting the overproduction of peroxynitrite for the prevention and reversal of paclitaxel-induced neuropathic pain. J. Neurosci. 2012;32(18):6149–6160. doi: 10.1523/JNEUROSCI.6343-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novotny-Diermayr V., Lin B., Gu L., Cao X. Modulation of the interleukin-6 receptor subunit glycoprotein 130 complex and its signaling by LMO4 interaction. J. Biol. Chem. 2005;280(13):12747–12757. doi: 10.1074/jbc.M500175200. [DOI] [PubMed] [Google Scholar]
- 21.Schock S.C., Xu J., Duquette P.M., Qin Z., Lewandowski A.J., Rai P.S., Thompson C.S., Seifert E.L., Harper M.E., Chen H.H. Rescue of neurons from ischemic injury by peroxisome proliferator-activated receptor-gamma requires a novel essential cofactor LMO4. J. Neurosci. 2008;28(47):12433–12444. doi: 10.1523/JNEUROSCI.2897-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Setogawa T., Shinozaki-Yabana S., Masuda T., Matsuura K., Akiyama T. The tumor suppressor LKB1 induces p21 expression in collaboration with LMO4, GATA-6, and Ldb1. Biochem. Biophys. Res. Commun. 2006;343(4):1186–1190. doi: 10.1016/j.bbrc.2006.03.077. [DOI] [PubMed] [Google Scholar]
- 23.Wang N., Lin K.K., Lu Z., Lam K.S., Newton R., Xu X., Yu Z., Gill G.N., Andersen B. The LIM-only factor LMO4 regulates expression of the BMP7 gene through an HDAC2-dependent mechanism, and controls cell proliferation and apoptosis of mammary epithelial cells. Oncogene. 2007;26(44):6431–6441. doi: 10.1038/sj.onc.1210465. [DOI] [PubMed] [Google Scholar]
- 24.Deng M., Luo X.J., Pan L., Yang H., Xie X., Liang G., Huang L., Hu F., Kiernan A.E., Gan L. LMO4 functions as a negative regulator of sensory organ formation in the mammalian cochlea. J. Neurosci. 2014;34(30):10072–10077. doi: 10.1523/JNEUROSCI.0352-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jamesdaniel S. Downstream targets of Lmo4 are modulated by cisplatin in the inner ear of Wistar rats. PLoS One. 2014;9(12):e115263. doi: 10.1371/journal.pone.0115263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levano S., Bodmer D. Loss of STAT1 protects hair cells from ototoxicity through modulation of STAT3, c-Jun, Akt, and autophagy factors. Cell Death Dis. 2015;6:e2019. doi: 10.1038/cddis.2015.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fetoni A.R., Paciello F., Mezzogori D., Rolesi R., Eramo S.L., Paludetti G., Troiani D. Molecular targets for anticancer redox chemotherapy and cisplatin-induced ototoxicity: the role of curcumin on pSTAT3 and Nrf-2 signalling. Br. J. Cancer. 2015;113(10):1434–1444. doi: 10.1038/bjc.2015.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curry-McCoy T.V., Osna N.A., Donohue T.M., Jr. Modulation of lysozyme function and degradation after nitration with peroxynitrite. Biochim Biophys. Acta. 2009;1790(8):778–786. doi: 10.1016/j.bbagen.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franco M.C., Ye Y., Refakis C.A., Feldman J.L., Stokes A.L., Basso M., Melero Fernandez de Mera R.M., Sparrow N.A., Calingasan N.Y., Kiaei M., Rhoads T.W., Ma T.C., Grumet M., Barnes S., Beal M.F., Beckman J.S., Mehl R., Estevez A.G. Nitration of Hsp90 induces cell death. Proc. Natl. Acad. Sci. USA. 2013;110(12):E1102–E1111. doi: 10.1073/pnas.1215177110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joshi M.S., Mihm M.J., Cook A.C., Schanbacher B.L., Bauer J.A. Alterations in connexin 43 during diabetic cardiomyopathy: competition of tyrosine nitration versus phosphorylation. J. Diabetes. 2015;7(2):250–259. doi: 10.1111/1753-0407.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Souza J.M., Choi I., Chen Q., Weisse M., Daikhin E., Yudkoff M., Obin M., Ara J., Horwitz J., Ischiropoulos H. Proteolytic degradation of tyrosine nitrated proteins. Arch. Biochem. Biophys. 2000;380(2):360–366. doi: 10.1006/abbi.2000.1940. [DOI] [PubMed] [Google Scholar]
- 32.Venkatesan A., Uzasci L., Chen Z., Rajbhandari L., Anderson C., Lee M.H., Bianchet M.A., Cotter R., Song H., Nath A. Impairment of adult hippocampal neural progenitor proliferation by methamphetamine: role for nitrotyrosination. Mol. Brain. 2011;4:28. doi: 10.1186/1756-6606-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Low I.C., Loh T., Huang Y., Virshup D.M., Pervaiz S. Ser70 phosphorylation of Bcl-2 by selective tyrosine nitration of PP2A-B56delta stabilizes its antiapoptotic activity. Blood. 2014;124(14):2223–2234. doi: 10.1182/blood-2014-03-563296. [DOI] [PubMed] [Google Scholar]
- 34.Blanchard-Fillion B., Souza J.M., Friel T., Jiang G.C., Vrana K., Sharov V., Barron L., Schoneich C., Quijano C., Alvarez B., Radi R., Przedborski S., Fernando G.S., Horwitz J., Ischiropoulos H. Nitration and inactivation of tyrosine hydroxylase by peroxynitrite. J. Biol. Chem. 2001;276(49):46017–46023. doi: 10.1074/jbc.M105564200. [DOI] [PubMed] [Google Scholar]
- 35.Rathinam R., Ghosh S., Neumann W.L., Jamesdaniel S. Cisplatin-induced apoptosis in auditory, renal, and neuronal cells is associated with nitration and downregulation of LMO4. Cell Death Discov. 2015;1 doi: 10.1038/cddiscovery.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen H.H., Schock S.C., Xu J., Safarpour F., Thompson C.S., Stewart A.F. Extracellular ATP-dependent upregulation of the transcription cofactor LMO4 promotes neuron survival from hypoxia. Exp. Cell Res. 2007;313(14):3106–3116. doi: 10.1016/j.yexcr.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 37.Tian Y., Wang N., Lu Z. Repression of Lim only protein 4-activated transcription inhibits proliferation and induces apoptosis of normal mammary epithelial cells and breast cancer cells. Clin. Exp. Metastasis. 2010;27(7):455–463. doi: 10.1007/s10585-010-9332-1. [DOI] [PubMed] [Google Scholar]
- 38.Rivolta M.N., Grix N., Lawlor P., Ashmore J.F., Jagger D.J., Holley M.C. Auditory hair cell precursors immortalized from the mammalian inner ear. Proc. Biol. Sci. 1998;265(1406):1595–1603. doi: 10.1098/rspb.1998.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rivolta M.N., Halsall A., Johnson C.M., Tones M.A., Holley M.C. Transcript profiling of functionally related groups of genes during conditional differentiation of a mammalian cochlear hair cell line. Genome Res. 2002;12(7):1091–1099. doi: 10.1101/gr.225602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 41.Hughes A.L., Hussain N., Pafford R., Parham K. Dexamethasone otoprotection in a multidose cisplatin ototoxicity mouse model. Otolaryngol. Head Neck Surg. 2014;150(1):115–120. doi: 10.1177/0194599813511948. [DOI] [PubMed] [Google Scholar]
- 42.Abdelsaid M.A., Pillai B.A., Matragoon S., Prakash R., Al-Shabrawey M., El-Remessy A.B. Early intervention of tyrosine nitration prevents vaso-obliteration and neovascularization in ischemic retinopathy. J. Pharm. Exp. Ther. 2010;332(1):125–134. doi: 10.1124/jpet.109.157941. [DOI] [PubMed] [Google Scholar]
- 43.Kim J.Y., Song E.H., Lee H.J., Oh Y.K., Park Y.S., Park J.W., Kim B.J., Kim D.J., Lee I., Song J., Kim W.H. Chronic ethanol consumption-induced pancreatic {beta}-cell dysfunction and apoptosis through glucokinase nitration and its down-regulation. J. Biol. Chem. 2010;285(48):37251–37262. doi: 10.1074/jbc.M110.142315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou M.H., Li H., He C., Lin M., Lyons T.J., Xie Z. Tyrosine nitration of prostacyclin synthase is associated with enhanced retinal cell apoptosis in diabetes. Am. J. Pathol. 2011;179(6):2835–2844. doi: 10.1016/j.ajpath.2011.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh R.R., Barnes C.J., Talukder A.H., Fuqua S.A., Kumar R. Negative regulation of estrogen receptor alpha transactivation functions by LIM domain only 4 protein. Cancer Res. 2005;65(22):10594–10601. doi: 10.1158/0008-5472.CAN-05-2268. [DOI] [PubMed] [Google Scholar]
- 46.Abdelmegeed M.A., Moon K.H., Chen C., Gonzalez F.J., Song B.J. Role of cytochrome P450 2E1 in protein nitration and ubiquitin-mediated degradation during acetaminophen toxicity. Biochem Pharm. 2010;79(1):57–66. doi: 10.1016/j.bcp.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sum E.Y., Peng B., Yu X., Chen J., Byrne J., Lindeman G.J., Visvader J.E. The LIM domain protein LMO4 interacts with the cofactor CtIP and the tumor suppressor BRCA1 and inhibits BRCA1 activity. J. Biol. Chem. 2002;277(10):7849–7856. doi: 10.1074/jbc.M110603200. [DOI] [PubMed] [Google Scholar]
- 48.Pan H., Shen K., Wang X., Meng H., Wang C., Jin B. Protective effect of metalloporphyrins against cisplatin-induced kidney injury in mice. PLoS One. 2014;9(1):e86057. doi: 10.1371/journal.pone.0086057. [DOI] [PMC free article] [PubMed] [Google Scholar]