Abstract
The overall decrease in proteolytic activity in aging can promote and accelerate protein accumulation and metabolic disturbances. To specifically analyze changes in macroautophagy (MA) we quantified different autophagy-related proteins (ATGs) in young, adult and old murine tissue as well as in young and senescent human fibroblasts. Thus, we revealed significantly reduced levels of ATG5-ATG12, LC3-II/LC3-I ratio, Beclin-1 and p62 in old brain tissue and senescent human fibroblasts. To investigate the role of mTOR, the protein itself and its target proteins p70S6 kinase and 4E-BP1 were quantified. Significant increased mTOR protein levels were determined in old tissue and cells. Determination of phosphorylated and basal amount of both proteins suggested higher mTOR activity in old murine tissue and senescent human fibroblasts. Besides the reduced levels of ATGs, mTOR can additionally reduce MA, promoting further acceleration of protein accumulation and metabolic disturbances during aging.
Abbreviations: ConA, Concanamycin A (lysosomal inhibitor); LC3, Microtubule-associated protein 1A/1B-light chain 3; mTOR, mammalian target of rapamycin; ATGs, Autophagy-related proteins; ALP, Autophagy-Lysosome pathway; MA, Macroautophagy
Keywords: Autophagy-lysosome pathway, Senescence, ATGs, Aging, Fibroblasts, MTOR
1. Introduction
Dysfunction of regular protein turnover in aging can promote the accumulation of oxidized, cross-linked and modified proteins, resulting in protein aggregates which are involved in many age-related diseases [1], [2], [3]. Besides the Ubiquitin-Proteasome-System (UPS), cells possess the Autophagy-Lysosome pathway (ALP) (reviewed in [4]), responsible for the degradation of long-lived proteins, cell organelles as well as specifically sequestered cytoplasmic cargo [5], [6]. To maintain the autophagic delivery of cell constituents into the lysosomes, three different types of autophagy are available: chaperone-mediated autophagy (CMA)[7], delivering soluble cytosolic proteins, microautophagy and macroautophagy (MA). The initiation of MA is given by the formation of a de novo- membrane which further maturates into a double-membrane vesicle, the autophagosome. During selective MA specifically ubiquitinated cargo (linked to K-63 polyubiquintin chains) is delivered to the autophagosomal membrane by proteins such as p62 [5]. The initiation of autophagosome formation is a very well-regulated conjunction, among others carried out by Beclin-1-VPS34 and mammalian target of rapamycin complex I (mTORC1) [8]. Particularly MA has been reported to be inhibited by mTORC1 (referred to as mTOR from here on) [9]. Besides the inhibition of autophagosome initiation, it has also been suggested that mTOR directly acts on ATGs by regulating phosphatase PP2A [10]. Thus, different ATG markers can be used for monitoring autophagy (reviewed in [11]). One important protein is Beclin-1, mainly responsible for autophagosome assembly and the recruitment of other ATGs [12]. For instance, ATG5-ATG12/ATG16 belongs to those ATGs which are necessary for the early autophagosome formation. Additionally, the complex is also important for LC3-II conversion [13], [14], one of the commonly used autophagy protein markers. To estimate the autophagic flux, the formation of the unbound LC3-I into the membrane-bound LC3-II can be determined. Another important protein to follow the protein delivery by autophagy is p62/SQSTM1 (p62). In general, since autophagy is a highly dynamic process; it has to be clearly distinguished between an impaired degradation and a decreased autophagic flux.
To monitor the changes in MA during aging, this study will compare MA by analyzing different ATGs in young, adult and old murine brain tissue as well as in senescent human fibroblasts. To verify the functionality of MA, quantification of ferritin H as a substrate for the ALP will be performed additionally. Finally, the role of mTOR on MA in both “aging models” will be examined, quantifying mTOR and analyzing its target proteins p70S6K (Thr389) and 4E-BP1 (Thr37/Thr46).
2. Materials and methods
2.1. Cell culture
Cell culture materials were received from Biochrom and all other chemicals were purchased from Sigma-Aldrich. Human dermal fibroblasts were obtained from human foreskin tissue of a 1-year old donor, kindly provided by Prof. Scharffetter-Kochanek from the University of Ulm, Germany. Cells were grown in DMEM (10% fetal bovine serum (FBS) and 1% L-glutamine) and were kept in 5% CO2 at 37 °C and 95% humidity. Cells were passaged once a week or when reaching 85% confluency. Fibroblasts with 60 population doublings (PD) were defined as “old, senescent” cells, up to 20 as “young cells”. Inhibition of lysosomal activity was performed using Concanamycin A (ConA, Sigma-Aldrich, C9705) at a final concentration of 250 nM.
2.2. Mice
C57/BL/6 J male mice were housed in polycarbonate cages, received standard diet and were kept under standard light-dark cycles. Mice were sacrificed by cervical dislocation at different stages of age: 8–10 weeks (young), 6 months (adult) and 18–25 months (old). Brains were immediately placed in liquid nitrogen and subsequently stored at −80 °C for further analysis. For immunoblot analysis brain tissue was homogenized by the Potter-Elvehjem homogenization method, using a 60 mM Imidazole/HCl lysis buffer pH 6.8. Protein concentrations were determined by the Bradford assay (BioRad, 5000006) and immunoblot analyses were performed as described below.
2.3. Immunoblot analysis
For protein quantification, controls and treated cells were harvested, lysed and protein concentration was determined by Lowry assay (BioRad, 5000111). Further, 20 µg protein lysate was added to Laemmli buffer (0.25 M Tris (pH 6.8), 40% glycerol, 8% SDS, 0.03% Orange G (Carl Roth, 0318.2) and loaded on a 12 or 15% acrylamide gel SDS-PAGE (BioRad, Munich, Germany). Afterwards, proteins were transferred to nitrocellulose (VWR, 10600002) and subsequently blocked in Odyssey© blocking buffer (LI-COR Biosciences, 927-40003) for 1 h at RT. Incubation with the primary antibodies was carried out for 1 h and the following antibodies were used: rabbit monoclonal ferritin H antibody (Cell signaling, 3998 S), rabbit and mouse monoclonal GAPDH antibody (Abcam, ab8245, ab37168), mouse monoclonal p62 antibody (Abcam, ab56416), rabbit monoclonal Beclin-1 antibody (Cell signaling, 3738 S), mouse monoclonal ATG5-7C6 antibody (specifically recognizes the ATG5-ATG12 protein complex at 55 kDa, NanoTools 0262-100) and LC3 antibody (specifically recognizes LC3-I at 18 kDa and LC3-II at 16 kDa (NanoTools, 0231-100). For the determination of the mTOR pathway we used a mTOR Substrate Antibody Sampler Kit (Cell signaling, #9862) and antibodies against the basal substrate proteins p70S6 Kinase (Cell signaling, 2708 P) and 4E-BP1 (Cell signaling, 5492 S). Secondary antibodies (all LI-COR Biosciences) were used according to the specifications of the manufacturer and membranes were quantified with the Odyssey Infrared Imaging System (LI-COR Biosciences). Blot quantifications were performed using the Image Studio™ Software (LI-COR Biosciences).
2.4. Immunohistochemical staining of ferritin H in young and old murine brain tissue
Paraffin-embedded brain sections (4 µm) were stained and analyzed for ferritin H, using rabbit monoclonal ferritin H antibody (Cell signaling, 3998 S) and the EnVision+ System-HRP (DAB) (Dako, K4010) as well as haematoxylin–eosin to evaluate histology. Representative images were generated with the MIRAX Digital Slide Scanner from Zeiss and pictured with the MIRAX Viewer 1.2.
3. Results
3.1. Investigation of MA in young, adult and old murine brain tissue
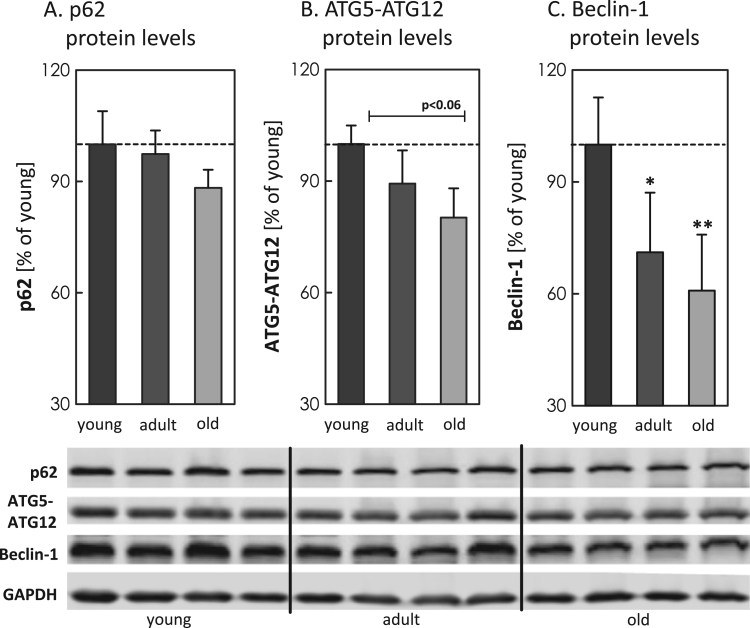
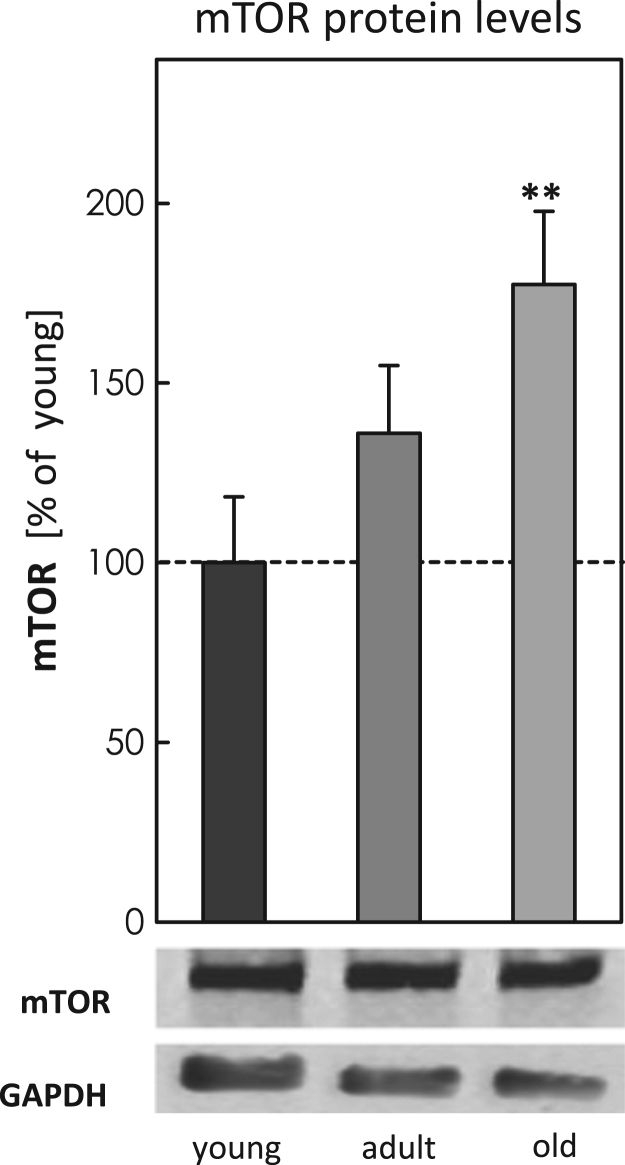
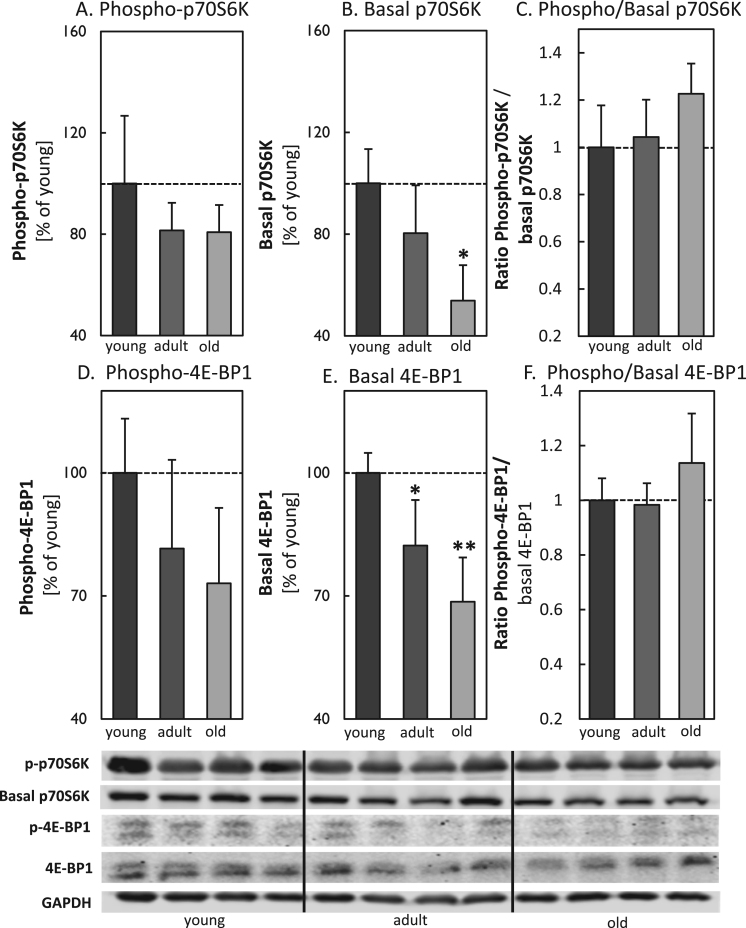
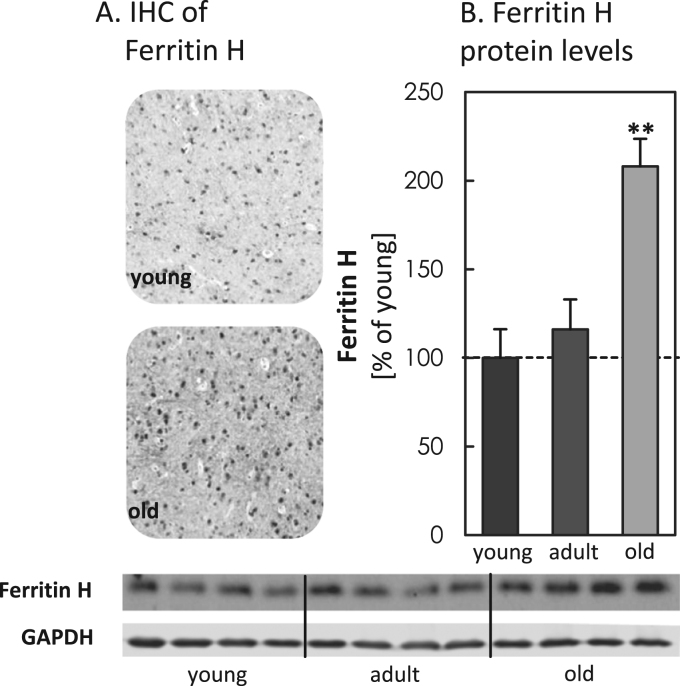
To analyze potential differences in MA during aging, we analyzed different ATGs, such as p62, ATG5-ATG12 and Beclin-1 in young, adult and old mice brain tissue (Fig. 1A-C). In total the quantification shows an overall decrease in all analyzed proteins with increasing age, particularly for ATG5-ATG12 and Beclin-1 the values were strongly reduced. To investigate a potential influence of mTOR, we also quantified basal mTOR levels, obtaining significantly increased protein levels in the old murine brain samples (Fig. 2). To estimate the activity of mTOR, we calculated the ratio of the phosphorylated to the basal form of p70S6K and 4E-BP1 (Fig. 3A-F), indicating a strong presence of mTOR activity in tissues of old mice. Additionally we also analyzed ferritin H protein levels in all three aging conditions, since degradation of this protein is largely dependent on functional MA. We performed immunohistological analyses (IHC) (Fig. 4A) as well as immunoblot analyses of the protein in young, adult and old murine brain tissue (Fig. 4B). As one can see, in both cases ferritin H values are significantly higher in the old murine brain samples.
Fig. 1.
Examination of autophagy-associated proteins in young, adult and old C57BL/6J male mice. Panels depict immunoblot analyses of (A) p62, (B) ATG5-ATG12 and (C) Beclin-1 in young, adult and old brain tissue from C57BL/6J male mice. Four lanes represent four individual mice per age group. All data were analyzed in relation to GAPDH and the young control was set to 100%. Statistical significant differences between young, adult and old tissue were calculated, using one-way ANOVA, followed by Tukey's post hoc test and are shown by *p<0.05, **p<0.01, all compared to young control.
Fig. 2.
Relative quantification of mTOR protein levels in brain tissue of young, adult and old C57BL/6J mice. To quantify mTOR protein levels samples were prepared as described above (see methods) and all data were analyzed in relation to GAPDH, setting the young control at 100%. Statistical significant differences between young, adult and old murine brain tissue samples were obtained, using one-way ANOVA, followed by Tukey's post hoc test and are shown by **p<0.01, all compared to young control.
Fig. 3.
Determination of mTOR activity by p70S6K and 4E-BP1 in brain tissue of young, adult and old C57BL/6J mice. Panel (A) shows the amount of p-p70S6K, while panel (B) demonstrates the basal amount of the protein in young, adult and old murine brain tissues from C57BL/6J male mice. The immunoblots are shown for four individual mice per age group. In panel (C) the ratio of p-p70S6K to basal p70S6K is given. Panels (D-F) show the analysis of 4E-BP1. Panel (D) demonstrates the amount of the p-4E-BP1, followed by panel (E), indicating the amount of the basal 4E-BP1 protein. The ratio of the p-4E-BP1 to 4E-BP1 is given in panel (F). All data were analyzed in relation to GAPDH and data are shown as percentages in relation to the young control. Statistical significant differences between young, adult and old murine brain samples were calculated, using one-way ANOVA, followed by Tukey's post hoc test and are indicated by *p<0.05 and **p<0.01, all compared to young control..
Fig. 4.
Steady state levels of ferritin H in brain tissue of young, adult and old C57BL/6J mice. In panel (A) representative immunohistochemistry of the distribution of ferritin H in young and old murine brain sections is shown. Panel (B) displays the protein expression of ferritin H in young, adult and old murine brain tissues from C57BL/6J male mice. The immunoblots are shown for four individual mice per age group. All data are protein levels in relation to GAPDH, setting the young control at 100%, statistical significant differences between young, adult and old murine brain tissue were calculated, using one-way ANOVA, followed by Tukey's post hoc test and are shown by **p<0.01, all compared to young control.
3.2. Protein expression of autophagy-related protein marker in young and old fibroblasts
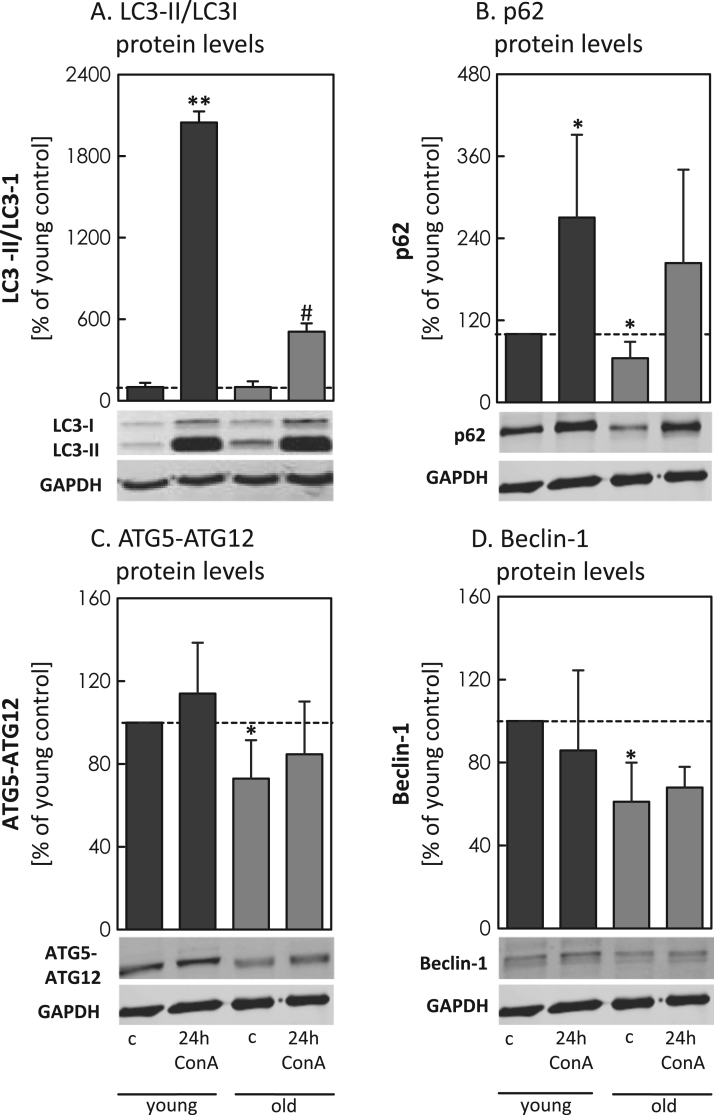
To monitor the age-related changes in MA in cells, we analyzed the relative protein expression of the ATGs p62, ATG5-ATG12 and Beclin-1 in fibroblasts (Fig. 5B-D). To further estimate the autophagic flux, we quantified the conversion of LC3-II by the ratio of LC3-II/LC3-I with and without the lysosomal inhibitor Concanamycin A (ConA) (Fig. 5A). We observed a statistical significant increase of the LC3-II/LC3-I ratio in the young controls (no ConA), compared to the young 24 h ConA treated, which was 4-fold higher than this ratio in old cells. To analyze the delivery of proteins to the lysosomes we quantified p62 (Fig. 5B), obtaining significant higher concentrations of the protein in young, compared to the old cells. Comparing control cell p62 to cells treated with ConA, higher values of p62, up to 25% in young cells compared to old, were detected. Additionally, quantification of ATG5-ATG12 (Fig. 5C) and Beclin-1 (Fig. 5D) showed significantly decreased levels of both proteins in old cells compared to the young. ConA incubation had no further effect on both protein levels.
Fig. 5.
Examination of autophagy-associated proteins in young and old cells. Relative quantification of ATGs were performed in young and old fibroblasts with and without 24 h incubation of Concanamycin A (24 h ConA). Analyses were performed by immunoblotting in cell lysates (see methods), always comparing untreated and 24 h ConA treated samples. Panels show: (A) quantification of LC3-II/LC3-I ratio, (B) the amount of p62, (C) protein levels of ATG5-ATG12, (D) the amount of Beclin-1. All data were analyzed in relation to GAPDH, setting the corresponding control young at 100%. Statistical significant differences between controls and treated cells is shown by #p<0.05, **p<0.01, compared to the corresponding control.
3.3. Quantification of mTOR and determination of its activity, using p70S6K and 4E-BP1 in young and senescent fibroblasts
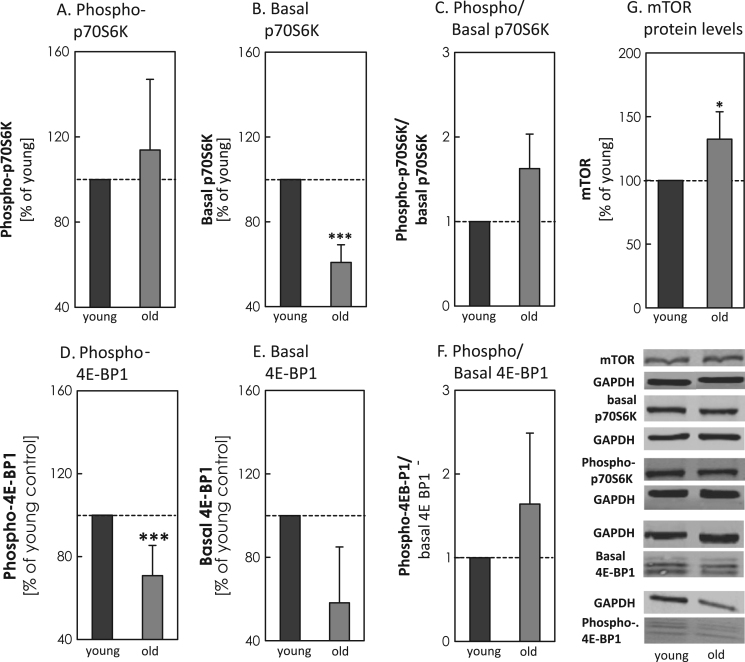
To clarify a potential involvement of mTOR in downregulation of MA during cellular senescence and to confirm the data in the old murine tissue, relative protein levels of mTOR, p70S6K and 4E-BP1 (Fig. 6A-G) were quantified in young and senescent fibroblasts. Immunoblot analyses of mTOR showed significantly higher protein levels in the old, compared to the young cells. By determining the phosphorylated (Fig. 6A/D) and the basal forms (Fig. 6B/E) of p70S6K and 4E-BP1, the ratios also suggested a higher activity of mTOR in the old cells.
Fig. 6.
Determination of mTOR amount and its activity by p70S6K and 4E-BP1 in young and old human fibroblasts. Panels show the levels of (A) phosphorylated p-p70S6K and (B) basal p70S6K in young and old human fibroblast. Panel (C) shows the calculated ratio of p-p70S6K to basal p70S6K. In panels (D-F), the analyses of the second target protein 4E-BP1 is shown, while panel (D) shows the amount of the p-4E-BP1, panel (E) demonstrates the amount of the basal 4E-BP1 protein. The calculated ratio of p-4E-BP1 to 4E-BP1 is given in panel (F). mTOR analysis is presented in panel G. All data were analyzed in relation to GAPDH and data are shown as percentages, setting the young cells at 100%. Statistical significant differences between control and treated cells were calculated, using Turkey's post hoc test, followed by Tukey's post hoc test and are shown by *** p<0.001, all compared to the young.
4. Discussion
To ensure cellular protein maintenance eukaryotic cells exert two proteolytic quality control systems: the UPS and the ALP. During aging, a progressive loss of cellular function and a decrease of turnover and repair systems leads to an increase in modified and cross-linked protein aggregates, such as lipofuscin [15], [16] or advanced glycation end products [17], [18], [19]. In cellular senescence, a decrease of 20S activity was already reported by our group [1], [20], [21] and others [22], [23] in different models. In order to obtain also more information about the changes in MA during the aging process, we analyzed different MA-related proteins in young, adult and old murine brain tissue (Fig. 1A-C). Particularly for complex ATG5-ATG12 and Beclin-1 the protein levels are highly reduced in old tissue. Both proteins are responsible for the early autophagosome formation. Previous studies have already shown that knock-out of ATG5 led to an inevitably reduced transfer of autophagy substrates into the lysosomes [16], [24]. Moreover, it has been previously described that ATG5 in complex with ATG12 is essential for the formation of autophagosomes [13], [25]. Due to the capability of ATG5 to bind to the autophagosomal membrane, it also transfers ATG12 close to the membrane. The tethering activity of ATG12 further enables LC3-I to get close to the phosphatidylethanolamine in the autophagic membrane, necessary to form the LC3-II [14]. The results for the ATG5-ATG12 complex showed a high decrease of protein levels in old, compared to young murine brain tissue (Fig. 1B). Due to the fact that ATG12, as well as Beclin-1, are assumed to be recycled by the proteasome, the lysosomal inhibitor ConA showed no further effect on their protein expression levels (as demonstrated for the fibroblasts in Fig. 5C/D). Thus, both proteins account as good MA markers in tissue samples without lysosomal inhibitor control [26], [27]. Beclin-1 is also a key component of the phosphatidylinositol 3-kinase class III (PtdIns3KC3) complex, which regulates the initiation of autophagosomes and enables the recruitment of other autophagy-related proteins [12], [28], [29]. By immunoblot analysis we were able to show a significant decrease of the steady state levels of Beclin-1 in the old tissue. To further validate the autophagic delivery we also analyzed p62, having a major part in this process (reviewed in [30], [31]). According to its capability to act as a linker between ubiquitinated proteins and LC3-I, it functions as an important “delivery” enzyme for specific autolysosomal degradation [32]. Analyzing p62 we obtained a higher formation rate of the protein in young, compared to old cells. Lower basal p62 level can be due to a reduced protein expression or increased autophagy flux, based on its incorporation into the complete autolysosomes. Thus, comparisons of control and ConA treated cells are necessary to judge the autophagic flux. Lower levels of p62 in old cells reduce the transfer of proteins to the autophagic membrane and thus slow down the degradation rate. Degradation of ferritin H by lysosomal activities has been reported in different studies. Besides ferritin H turnover by the proteasome [33], [34], the ALP seems to be mainly responsible for the degradation of the protein [35], [36], [37], assuming higher ferritin H levels when lysosomal activity is inhibited. Recent studies have shown, that particularly macroautophagy is involved in ferritin H removal [38], [39]. Besides decreased levels of ATGs, increased levels of ferritin H can further be indicative for a decline of MA in aged tissues. Due to high ferritin H levels in the brain and an already described increase in brain ferritin levels within aging [40], [41] we assumed that possibly impaired MA is responsible for elevated ferritin H levels in aging. To clearly demonstrate impairment in MA we additionally quantified ferritin H, known to be removed by MA [38], [39]. The analyzed ferritin H levels by IHC and immunoblot analyses clearly demonstrated a consistent rise of the protein levels with increasing age, resulting in significantly higher ferritin H levels in brain homogenates of old mice, compared to the young ones (Fig. 4). To confirm that our results in murine tissue are not the result of changes in brain cell composition or other factors then aging, we also repeated the experiments in young and senescent fibroblasts, a senescence model already described and published by Jung et al. [42]. The use of these fibroblasts also enables us to compare the MA-related proteins also to a lysosomal inhibitor control, providing better monitoring of the autophagic flux. For inhibition of the lysosomal activity, we used the lysosomal inhibitor ConA, which is able to block the acidification of the lysosomes by inhibiting the membrane-bound V-ATPases [16], [43]. Thus, we analyzed p62, ATG5-ATG12 and Beclin-1in the young and old fibroblast (Fig. 5B-D) and were able to confirm a significant decrease in all MA-related marker proteins in the old cells, compared to the respective ConA control. In addition we also examined the conversion of LC3-I into LC3-II. Regarding LC3 it is widely accepted that LC3-II conversion can be used as a reliable marker of autophagosome formation under the consideration of certain parameters (reviewed in [11], [27]). Since LC3-II can also be degraded by the ALP, a comparison to a sample treated with a lysosomal inhibitor, such as ConA, is required, explaining why we were unable to quantify the protein in murine tissue samples. Without comparison to a lysosomal inhibitor, conclusion whether the content of LC3-II results from an increased expression or an enhanced accumulation of the protein itself is not possible [44]. Old cells can also have higher basal LC3-II levels due to impairment in the further pathway. But our results clearly demonstrate a significant reduced formation rate of LC3-II in old cells, compared to young fibroblasts (Fig. 5A). To further investigate whether mTOR plays a role in the age-related reduction of MA, we additionally analyzed its protein and activity levels. Immunoblot analyses of mTOR clearly showed a significant increase in protein levels in both, old murine brain tissue (Fig. 2) and old fibroblasts (Fig. 6G). To demonstrate whether the enzyme is also more active, we additionally analyzed p70S6K and 4E-BP1, both well-known to be phosphorylated by the rapamycin affected mTORC1, particularly known to impair autophagy [45], [46]. The more phosphorylated these proteins are, the more active is mTOR. To correctly estimate the results, the ratio of the phosphorylated to the basal form of the respective proteins has to be calculated, since a decrease in target phosphorylation seems not to be accompanied by a decrease in mTOR activity. Possibly, the result can also be affected by lower basal levels of the proteins themselves. By determining the phosphorylated and the basal amounts of both substrates, we were able to show a higher phosphorylation rate (Fig. 3, Fig. 6), suggesting a still high mTOR activity in old tissue and old fibroblasts. In combination with the results for the mTOR quantity we concluded that mTOR might also play a decisive role, together with a distinct decrease of all analyzed ATGs, in age-related deregulation of autophagy, causing the higher ferritin H levels in the old tissue.
In summary, our results emphasize that MA is reduced in old murine brain tissue and in senescent human fibroblasts. Differences in ATGs between young and old tissue and cells, confirmed our assumption that the MA processes might be declined. An impairment of MA can also be assumed by the higher basal ferritin H levels in the old tissue, known to be mainly removed by the ALP. Furthermore, protein expression and activity analyses of mTOR revealed a still high activity of the enzyme, which can additionally promote the impairment of MA in old tissue and cells. Further elucidating the pathway of MA to show how mTOR influences autophagosome initiation or whether changes in transcription factors, such as STAT3, might be responsible for the lesser expression of ATGs in aging, would be interesting approaches for further studies on impaired proteolysis and protein accumulation in aging and age-related disease.
Competing interests
None of the authors have any actual or potential conflict of interest related to this manuscript.
Conflict of interest
None.
Funding
This work was funded by the German Research Foundation (DFG).
Acknowledgments
This study was supported by the German Research Foundation (DFG).
Contributor Information
Christiane Ott, Email: Christiane.Ott@dife.de.
Jeannette König, Email: Jeannette.Koenig@dife.de.
Annika Höhn, Email: Annika.Hoehn@dife.de.
Tobias Jung, Email: Tobias.Jung@dife.de.
Tilman Grune, Email: scientific.director@dife.de.
References
- 1.Hohn A., Konig J., Grune T. Protein oxidation in aging and the removal of oxidized proteins. J. Proteom. 2013;92:132–159. doi: 10.1016/j.jprot.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Friguet B. Oxidized protein degradation and repair in ageing and oxidative stress. FEBS Lett. 2006;580:2910–2916. doi: 10.1016/j.febslet.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 3.Ott C., Grune T. Protein oxidation and proteolytic signalling in aging. Curr. Pharm. Des. 2014;20:3040–3051. doi: 10.2174/13816128113196660709. [DOI] [PubMed] [Google Scholar]
- 4.Klionsky D.J., Abdelmohsen K., Abe A., Abedin M.J., Abeliovich H., Acevedo Arozena A., Zughaier S.M. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd ed.) Autophagy. 2016;12 doi: 10.1080/15548627.2015.1100356. (1-222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korolchuk V.I., Menzies F.M., Rubinsztein D.C. Mechanisms of cross-talk between the ubiquitin-proteasome and autophagy-lysosome systems. FEBS Lett. 2010;584:1393–1398. doi: 10.1016/j.febslet.2009.12.047. [DOI] [PubMed] [Google Scholar]
- 6.Hohn A., Grune T. Lipofuscin: formation, effects and role of macroautophagy. Redox Biol. 2013;1:140–144. doi: 10.1016/j.redox.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuervo A.M., Wong E. Chaperone-mediated autophagy: roles in disease and aging. Cell Res. 2014;24:92–104. doi: 10.1038/cr.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moller A.P., Chabi Y., Cuervo J.J., De Lope F., Kilpimaa J., Kose M., von Hirschheydt J. An analysis of continent-wide patterns of sexual selection in a passerine bird. Evolution. 2006;60:856–868. [PubMed] [Google Scholar]
- 9.Cao Y., Espinola J.A., Fossale E., Massey A.C., Cuervo A.M., MacDonald M.E., Cotman S.L. Autophagy is disrupted in a knock-in mouse model of juvenile neuronal ceroid lipofuscinosis. J. Biol. Chem. 2006;281:20483–20493. doi: 10.1074/jbc.M602180200. [DOI] [PubMed] [Google Scholar]
- 10.Reid C., Rushe M., Jarpe M., van Vlijmen H., Dolinski B., Qian F., Boriack-Sjodin P.A. Structure activity relationships of monocyte chemoattractant proteins in complex with a blocking antibody. Protein Eng. Des. Sel. 2006;19:317–324. doi: 10.1093/protein/gzl015. [DOI] [PubMed] [Google Scholar]
- 11.Massey A.C., Kaushik S., Sovak G., Kiffin R., Cuervo A.M. Consequences of the selective blockage of chaperone-mediated autophagy. Proc. Natl. Acad. Sci. USA. 2006;103:5805–5810. doi: 10.1073/pnas.0507436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuervo A.M. Autophagy in neurons: it is not all about food. Trends Mol. Med. 2006;12:461–464. doi: 10.1016/j.molmed.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Romanov J., Walczak M., Ibiricu I., Schuchner S., Ogris E., Kraft C., Martens S. Mechanism and functions of membrane binding by the Atg5-Atg12/Atg16 complex during autophagosome formation. EMBO J. 2012;31:4304–4317. doi: 10.1038/emboj.2012.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaushik S., Massey A.C., Cuervo A.M. Lysosome membrane lipid microdomains: novel regulators of chaperone-mediated autophagy. EMBO J. 2006;25:3921–3933. doi: 10.1038/sj.emboj.7601283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hohn A., Jung T., Grimm S., Grune T. Lipofuscin-bound iron is a major intracellular source of oxidants: role in senescent cells. Free Radic. Biol. Med. 2010;48:1100–1108. doi: 10.1016/j.freeradbiomed.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 16.Hohn A., Sittig A., Jung T., Grimm S., Grune T. Lipofuscin is formed independently of macroautophagy and lysosomal activity in stress-induced prematurely senescent human fibroblasts. Free Radic. Biol. Med. 2012;53:1760–1769. doi: 10.1016/j.freeradbiomed.2012.08.591. [DOI] [PubMed] [Google Scholar]
- 17.Grimm S., Ott C., Horlacher M., Weber D., Hohn A., Grune T. Advanced-glycation-end-product-induced formation of immunoproteasomes: involvement of RAGE and Jak2/STAT1. Biochem J. 2012;448:127–139. doi: 10.1042/BJ20120298. [DOI] [PubMed] [Google Scholar]
- 18.Ott C., Jacobs K., Haucke E., Navarrete Santos A., Grune T., Simm A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–429. doi: 10.1016/j.redox.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simm A., Casselmann C., Schubert A., Hofmann S., Reimann A., Silber R.E. Age associated changes of Age-receptor expression: rage upregulation is associated with human heart dysfunction. Exp. Gerontol. 2004;39:407–413. doi: 10.1016/j.exger.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Sitte N., Huber M., Grune T., Ladhoff A., Doecke W.D., Von Zglinicki T., Davies K.J. Proteasome inhibition by lipofuscin/ceroid during postmitotic aging of fibroblasts. FASEB J. 2000;14:1490–1498. doi: 10.1096/fj.14.11.1490. [DOI] [PubMed] [Google Scholar]
- 21.Grune T., Jung T., Merker K., Davies K.J. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and 'aggresomes' during oxidative stress, aging, and disease. Int J. Biochem Cell Biol. 2004;36:2519–2530. doi: 10.1016/j.biocel.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Chondrogianni N., Gonos E.S. Proteasome dysfunction in mammalian aging: steps and factors involved. Exp. Gerontol. 2005;40:931–938. doi: 10.1016/j.exger.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Petropoulos I., Conconi M., Wang X., Hoenel B., Bregegere F., Milner Y., Friguet B. Increase of oxidatively modified protein is associated with a decrease of proteasome activity and content in aging epidermal cells. J. Gerontol. A Biol. Sci. Med. Sci. 2000;55:B220–B227. doi: 10.1093/gerona/55.5.b220. [DOI] [PubMed] [Google Scholar]
- 24.Tashiro K., Shishido M., Fujimoto K., Hirota Y., Yo K., Gomi T., Tanaka Y. Age-related disruption of autophagy in dermal fibroblasts modulates extracellular matrix components. Biochem. Biophys. Res Commun. 2013;443:162–172. doi: 10.1016/j.bbrc.2013.11.066. [DOI] [PubMed] [Google Scholar]
- 25.Hanada T., Noda N.N., Satomi Y., Ichimura Y., Fujioka Y., Takao T., Ohsumi Y. The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J. Biol. Chem. 2007;282:37298–37302. doi: 10.1074/jbc.C700195200. [DOI] [PubMed] [Google Scholar]
- 26.Cortez M.R., Pinho A.P., Cuervo P., Alfaro F., Solano M., Xavier S.C., Jansen A.M. Trypanosoma cruzi (Kinetoplastida Trypanosomatidae): ecology of the transmission cycle in the wild environment of the Andean valley of Cochabamba, Bolivia. Exp. Parasitol. 2006;114:305–313. doi: 10.1016/j.exppara.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Klionsky D.J., Abdalla F.C., Abeliovich H., Abraham R.T., Acevedo-Arozena A., Adeli K., Zuckerbraun B. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massey A.C., Zhang C., Cuervo A.M. Chaperone-mediated autophagy in aging and disease. Curr. Top. Dev. Biol. 2006;73:205–235. doi: 10.1016/S0070-2153(05)73007-6. [DOI] [PubMed] [Google Scholar]
- 29.Ratcliff S.L., Brown A., Rosenberg L., Rosenberg M., Robert R.S., Cuervo L.J., Meyer W.J., 3rd The effectiveness of a pain and anxiety protocol to treat the acute pediatric burn patient. Burns. 2006;32:554–562. doi: 10.1016/j.burns.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Ichimura Y., Komatsu M. Selective degradation of p62 by autophagy. Semin Immunopathol. 2010;32:431–436. doi: 10.1007/s00281-010-0220-1. [DOI] [PubMed] [Google Scholar]
- 31.Klionsky D.J., Abdelmohsen K., Abe A., Abedin M.J., Abeliovich H., Acevedo Arozena A., Zughaier S.M. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12 doi: 10.1080/15548627.2015.1100356. (1-222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komatsu M., Ichimura Y. Physiological significance of selective degradation of p62 by autophagy. FEBS Lett. 2010;584:1374–1378. doi: 10.1016/j.febslet.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 33.De Domenico I., Vaughn M.B., Li L., Bagley D., Musci G., Ward D.M., Kaplan J. Ferroportin-mediated mobilization of ferritin iron precedes ferritin degradation by the proteasome. EMBO J. 2006;25:5396–5404. doi: 10.1038/sj.emboj.7601409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voss P., Horakova L., Jakstadt M., Kiekebusch D., Grune T. Ferritin oxidation and proteasomal degradation: protection by antioxidants. Free Radic. Res. 2006;40:673–683. doi: 10.1080/10715760500419357. [DOI] [PubMed] [Google Scholar]
- 35.Kidane T.Z., Sauble E., Linder M.C. Release of iron from ferritin requires lysosomal activity. Am. J. Physiol. Cell Physiol. 2006;291 doi: 10.1152/ajpcell.00505.2005. (C445-455) [DOI] [PubMed] [Google Scholar]
- 36.Asano T., Komatsu M., Yamaguchi-Iwai Y., Ishikawa F., Mizushima N., Iwai K. Distinct mechanisms of ferritin delivery to lysosomes in iron-depleted and iron-replete cells. Mol. Cell Biol. 2011;31:2040–2052. doi: 10.1128/MCB.01437-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radisky D.C., Kaplan J. Iron in cytosolic ferritin can be recycled through lysosomal degradation in human fibroblasts. Biochem J. 1998;336:201–205. doi: 10.1042/bj3360201. (Pt 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hou W., Xie Y., Song X., Sun X., Lotze M.T., Zeh H.J., 3rd, Tang D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425–1428. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mancias J.D., Wang X., Gygi S.P., Harper J.W., Kimmelman A.C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509:105–109. doi: 10.1038/nature13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Connor J.R., Menzies S.L., Martin S.M., St, Mufson E.J. Cellular distribution of transferrin, ferritin, and iron in normal and aged human brains. J. Neurosci. Res. 1990;27:595–611. doi: 10.1002/jnr.490270421. [DOI] [PubMed] [Google Scholar]
- 41.Connor J.R., Snyder B.S., Arosio P., Loeffler D.A., LeWitt P. A quantitative analysis of isoferritins in select regions of aged, parkinsonian, and Alzheimer's diseased brains. J. Neurochem. 1995;65:717–724. doi: 10.1046/j.1471-4159.1995.65020717.x. [DOI] [PubMed] [Google Scholar]
- 42.Jung T., Hohn A., Catalgol B., Grune T. Age-related differences in oxidative protein-damage in young and senescent fibroblasts. Arch. Biochem Biophys. 2009;483:127–135. doi: 10.1016/j.abb.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 43.Sobota J.A., Back N., Eipper B.A., Mains R.E. Inhibitors of the V0 subunit of the vacuolar H+-ATPase prevent segregation of lysosomal- and secretory-pathway proteins. J. Cell Sci. 2009;122:3542–3553. doi: 10.1242/jcs.034298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diaz J.A., Cuervo C., Valderrama A.M., Kohles J. Valdecoxib provides effective pain relief following acute ankle sprain. J. Int. Med. Res. 2006;34:456–467. doi: 10.1177/147323000603400502. [DOI] [PubMed] [Google Scholar]
- 45.Massey A.C., Kiffin R., Cuervo A.M. Autophagic defects in aging: looking for an "emergency exit"? Cell Cycle. 2006;5:1292–1296. doi: 10.4161/cc.5.12.2865. [DOI] [PubMed] [Google Scholar]
- 46.Giordano S., Darley-Usmar V., Zhang J. Autophagy as an essential cellular antioxidant pathway in neurodegenerative disease. Redox Biol. 2014;2:82–90. doi: 10.1016/j.redox.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]