Abstract
Background
Endometrial carcinoma comprises a group of tumors with distinct histologic and molecular features, and clinical behavior. Here we sought to define the biological processes that govern the clinical behavior of endometrial cancers.
Methods
Sixteen prototype genes representative of different biological processes that would likely play a role in endometrial and other hormone-driven cancers were defined. RNA-sequencing gene expression data from 323 endometrial cancers from The Cancer Genome Atlas were used to determine the transcription module of each prototype gene. The expression of prototype genes and modules and their association with outcome was assessed in univariate and multivariate survival analyses. The association of MSH6 expression with outcome was validated in an independent cohort of 243 primary endometrial cancers using immunohistochemistry.
Results
We observed that the clinical behavior of endometrial carcinomas as a group was associated with hormone receptor signaling, PI3K pathway signaling and DNA mismatch repair processes. When analyzed separately, in endometrioid carcinomas, hormone receptor, PI3K and DNA mismatch repair modules were significantly associated with outcome in univariate analysis, whereas the clinical behavior of serous cancers was likely governed by apoptosis and Wnt signaling. Multivariate survival analysis revealed that MSH6 expression was associated with outcome of endometrial cancer patients independently from traditional prognostic clinicopathologic parameters, which was confirmed in an independent cohort at the protein level.
Conclusion
Endometrioid and serous endometrial cancers are underpinned by distinct molecular pathways. MSH6 expression levels may be associated with outcome in endometrial cancers as a group.
Keywords: endometrial cancer, gene expression, biological process, outcome
1. INTRODUCTION
Endometrial cancer (EC), the most common gynecologic malignancy in the USA, comprises a heterogeneous group of tumors with distinct histologic features, biological behavior and treatment response. Endometrioid and serous carcinomas account for the majority of ECs. Treatment decisions for patients with EC are primarily determined by surgical stage at presentation, histologic type and grade[1].
There is evidence to suggest that some molecular alterations are preferentially found in endometrioid endometrial carcinomas (EECs), including mutations in PTEN and CTNNB1, whereas others are more prevalent in serous endometrial carcinomas (SECs), such as TP53 mutations[2,3]. These observations have been corroborated and expanded by the transcriptomic and genomic analyses of a large set of EECs and SECs carried out by The Cancer Genome Atlas (TCGA)[4]. The analyses performed by TCGA have led to an integrated genomic classification of EECs and SECs and the identification of the POLE (ultramutated), microsatellite instability (MSI) (hypermutated), copy-number low (endometrioid) and copy-number high (serous-like) subtypes, which have been shown to be underpinned by distinct combinations of genomic and epigenetic alterations[4].
Based on the molecular heterogeneity observed in ECs, we posited that the biological processes and pathways associated with outcome may be distinct between ECs of different histologic types, grades or integrated genomic subtypes, and that additional markers predictive of clinical behavior may be present in different subsets of the disease.
The aims of this study were i) to determine genes or gene expression modules representative of biological processes known to play a role in EC or in other hormone-driven cancers, and ii) to define the association of these genes and/or gene expression modules with outcome in ECs as a group, and in subgroups stratified according to histologic type, grade or integrated genomic types using RNA-sequencing data from the TCGA study[4].
2. METHODS
2.1. Transcription modules and univariate analysis
RNA-sequencing gene expression and clinicopathologic data including outcome from 323 ECs were retrieved from the TCGA data portal (https://tcga-data.nci.nih.gov/docs/publications/ucec_2013/; files ‘RNASeq’ and ‘Key Clinical Data’; accessed December 2015)[4](Supplementary Table 1). The RSEM normalized gene-level expression data were obtained. As described by TCGA[4], genes lacking HGNC annotation or with small expression values in at least one-fourth of the samples were removed. The expression values of the final set of 20,502 genes were log2 transformed. We selected 16 ‘prototype’ genes representative of biological processes that have been shown to play a role in EC or other hormone-driven cancers. The details of the gene selection are described in the Supplementary Methods[3-5]. The transcription modules of each prototype gene, which comprise the genes specifically co-expressed with each prototype gene (Supplementary Tables 2 and 3), were defined essentially as described by Desmedt et al.[5] and are described in the Supplementary Methods. The R script for the univariate models, transcription module development and module score calculations is available in the Supplement.
2.2. Gene ontology enrichment analysis
Gene ontology enrichment analyses of the 16 transcription modules were performed using Cytoscape v.2.8.3 with the BinGO plugin (v.2.44)[6], and a hypergeometric test, with the False Discovery Rate controlled using the Benjamini and Hochberg procedure. Biological processes with a corrected P<0.05 were deemed significant.
2.3. Tissue microarrays and immunohistochemistry
Tissue microarrays (TMAs) from the University Hospital Basel, Basel, Switzerland and University Hospital Virgen del Rocío, Seville, Spain containing two replicate 0.6 mm and 1.0 mm cores, respectively, from 276 ECs were constructed as previously described[7] and the expression levels of MSH6 protein were assessed by immunohistochemistry (Supplementary Methods). For the purpose of this study, only ECs of endometrioid (n=228) and serous (n=15) types based on review of the diagnostic histologic slides were included.
2.4. Statistics
A detailed description of the statistical methods employed is available in the Supplementary Methods. For the analysis of the RNA-sequencing gene expression data, univariate and multivariate analyses for overall survival were performed using the Cox proportional hazards regression model, with the survival data censored at 5 years (Supplementary Methods). Forest plots were generated using ggplot2 (http://ggplot2.org/) in R (http://www.r-project.org/; v 3.0.1). For the identification of the optimal cut-offs for survival analysis, the X-tile software[8] was employed, dividing the cohort into training and validation subsets (split sample approach; see Supplementary Methods). For the MSH6 protein expression analysis, disease-free survival was expressed as the number of months from diagnosis to the occurrence of distant or local relapse, as overall survival was not available for this cohort, using Kaplan–Meier method and log-rank test (Supplementary Methods).
3. RESULTS
3.1 Biological processes associated with outcome in EC
We selected 16 ‘prototype genes’ that are either recurrently (over-) expressed and/or amplified or targeted by mutations in ECs, or have been shown to be associated with outcome in breast cancer, which akin to EC, is a hormone-dependent disease (Supplementary Methods). Transcription modules representative of biological processes of these 16 prototype genes were defined (Supplementary Tables 2 and 3), and a module score[5] was determined for each of the transcription modules and used for univariate and multivariate survival analyses.
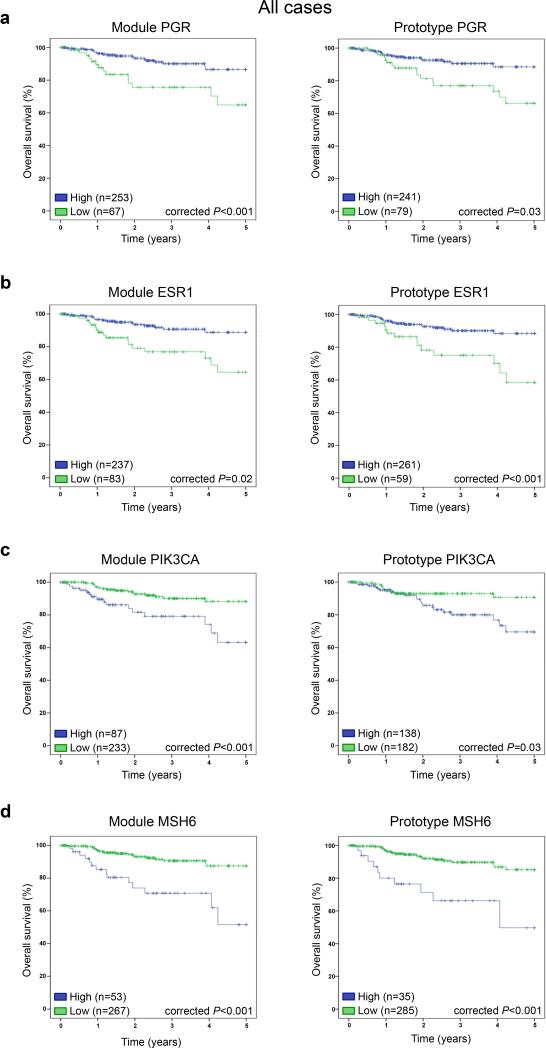
On univariate analysis, the clinicopathologic parameters International Federation of Gynecology and Obstetrics (FIGO) stage, age, histologic type and grade were significantly associated with progression-free and overall survival in this set of 323 ECs (Table 1; Supplementary Table 4). For the remaining analyses of this study, we focused on overall survival as the end point. In this cohort, in addition to the clinicopathologic parameters, the expression of both the prototype genes CTNNB1, PIK3CA, ERBB2, ESR1, PGR, and MSH6 and their respective Wnt signaling, PI3K pathway, HER2 signaling, estrogen receptor (ER) signaling, progesterone (PR) signaling and MSH6 DNA mismatch repair modules were associated significantly with outcome in univariate analysis, as was the chromatin organization ARID1A module (P<0.05, Table 1, Supplementary Fig. 1). Prototype genes and transcription modules whose continuous values were significantly associated with overall survival were subsequently converted into categorical variables using X-tile. These survival analyses revealed that in ECs not only the prototype genes ESR1, PGR, PIK3CA and MSH6 were significantly associated with overall survival (P<0.05), but also the hormone receptor modules ESR1 and PGR, whose expression is highly correlated (Supplementary Table 5), and the PI3K pathway (as defined by the PIK3CA module) and DNA mismatch repair (MSH6) modules (Fig. 1). Furthermore, the ERBB2 prototype gene and the ARID1A module were found to be significantly associated with outcome in this EC dataset using a categorical analysis (Supplementary Fig. 2a). These data provide evidence to suggest that ECs with high gene expression levels of ESR1 or PGR, and low gene expression levels of PIK3CA or MSH6 have a more favorable outcome. Multivariate survival analysis including the clinicopathologic parameters revealed that the DNA MSH6 prototype gene remained an independent predictor of overall survival in EC (Table 2). The MSH6 module was of borderline significance (P=0.05; Table 2); however, in a model omitting the PIK3CA module, which showed a strong correlation with the MSH6 module, a statistically significant association between the MSH6 DNA mismatch repair module and overall survival was observed (P=0.031; Supplementary Tables 5 and 6).
Table 1.
Univariate Cox's regression analysis of 5-year overall survival in endometrial cancer patients (TCGA) including standard clinicopathologic parameters, prototype genes and module scores.
| All cases (n=323) | Endometrioid cancers (n=271) | Serous cancers (n=52) | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age | 1.047 (1.011-1.083) | 0.009 | 1.041 (1.001-1.082) | 0.044 | 1.034 (0.94-1.137) | 0.493 |
| Grade | 2.935 (1.586-5.433) | 0.001 | 2.866 (1.496-5.493) | 0.002 | ||
| Histology | 2.191 (1.042-4.606) | 0.039 | ||||
| FIGO stage | 2.072 (1.553-2.763) | <0.001 | 1.966 (1.401-2.759) | <0.001 | 2.324 (1.117-4.837) | 0.024 |
| Prototype ESR1 | 0.830 (0.729-0.945) | 0.005 | 0.788 (0.664-0.935) | 0.006 | 1.056 (0.743-1.5) | 0.763 |
| Prototype PGR | 0.888 (0.804-0.982) | 0.021 | 0.836 (0.733-0.955) | 0.008 | 1.223 (0.93-1.608) | 0.150 |
| Prototype PTEN | 0.722 (0.501-1.039) | 0.079 | 0.662 (0.446-0.984) | 0.041 | 0.968 (0.293-3.194) | 0.958 |
| Prototype PIK3CA | 1.477 (1.04-2.097) | 0.029 | 2.015 (1.089-3.729) | 0.026 | 0.787 (0.348-1.78) | 0.565 |
| Prototype CTNNB1 | 1.942 (1.206-3.128) | 0.006 | 1.748 (0.991-3.084) | 0.054 | 2.632 (1.048-6.612) | 0.039 |
| Prototype ARID1A | 1.433 (0.886-2.319) | 0.143 | 1.329 (0.744-2.374) | 0.336 | 1.356 (0.553-3.323) | 0.506 |
| Prototype MLH1 | 1.061 (0.845-1.332) | 0.611 | 0.982 (0.771-1.25) | 0.881 | 1.581 (0.431-5.803) | 0.490 |
| Prototype MSH6 | 2.119 (1.297-3.461) | 0.003 | 2.072 (1.113-3.859) | 0.022 | 1.731 (0.64-4.678) | 0.279 |
| Prototype POLE | 1.178 (0.741-1.874) | 0.488 | 1.114 (0.646-1.921) | 0.699 | 1.311 (0.481-3.576) | 0.597 |
| Prototype TP53 | 1.041 (0.685-1.583) | 0.851 | 0.774 (0.441-1.358) | 0.371 | 1.506 (0.819-2.769) | 0.187 |
| Prototype ERBB2 | 1.283 (1.024-1.608) | 0.030 | 1.262 (0.741-2.147) | 0.392 | 1.146 (0.832-1.577) | 0.404 |
| Prototype AURKA | 1.286 (0.902-1.833) | 0.165 | 1.332 (0.859-2.066) | 0.200 | 0.550 (0.209-1.447) | 0.226 |
| Prototype PLAU | 0.809 (0.598-1.095) | 0.170 | 0.804 (0.563-1.149) | 0.231 | 0.805 (0.445-1.455) | 0.473 |
| Prototype STAT1 | 1.058 (0.781-1.432) | 0.718 | 0.987 (0.653-1.493) | 0.952 | 0.765 (0.448-1.307) | 0.328 |
| Prototype VEGF | 1.143 (0.832-1.571) | 0.410 | 1.198 (0.814-1.764) | 0.359 | 0.971 (0.554-1.703) | 0.919 |
| Prototype CASP3 | 0.728 (0.359-1.476) | 0.379 | 1.536 (0.61-3.87) | 0.362 | 0.282 (0.096-0.825) | 0.021 |
| Module ESR1 | 0.991 (0.985-0.998) | 0.007 | 0.989 (0.981-0.997) | 0.006 | 1.019 (0.995-1.044) | 0.118 |
| Module PGR | 0.993 (0.988-0.998) | 0.003 | 0.991 (0.984-0.997) | 0.004 | 1.016 (0.995-1.038) | 0.134 |
| Module PTEN | 0.840 (0.703-1.003) | 0.054 | 0.806 (0.664-0.978) | 0.029 | 0.918 (0.518-1.626) | 0.769 |
| Module PIK3CA | 1.010 (1.004-1.017) | 0.002 | 1.011 (1.003-1.02) | 0.011 | 1.005 (0.992-1.018) | 0.469 |
| Module CTNNB1 | 1.006 (1.001-1.011) | 0.015 | 1.004 (0.999-1.01) | 0.136 | 1.013 (1-1.026) | 0.046 |
| Module ARID1A | 1.024 (1.003-1.045) | 0.024 | 1.016 (0.992-1.04) | 0.191 | 1.050 (0.996-1.107) | 0.069 |
| Module MLH1 | 1.017 (0.942-1.097) | 0.675 | 0.976 (0.899-1.059) | 0.557 | 1.429 (0.795-2.566) | 0.232 |
| Module MSH6 | 1.117 (1.041-1.198) | 0.002 | 1.103 (1.015-1.2) | 0.021 | 1.115 (0.928-1.338) | 0.245 |
| Module POLE | 1.005 (0.998-1.012) | 0.156 | 1.005 (0.997-1.013) | 0.249 | 0.996 (0.977-1.015) | 0.688 |
| Module TP53 | 1.041 (0.685-1.583) | 0.851 | 0.774 (0.441-1.358) | 0.371 | 1.506 (0.819-2.769) | 0.187 |
| Module ERBB2 | 1.113 (1.031-1.201) | 0.006 | 1.175 (0.98-1.409) | 0.081 | 1.052 (0.937-1.182) | 0.390 |
| Module AURKA | 1.002 (1-1.004) | 0.058 | 1.002 (1-1.005) | 0.109 | 0.999 (0.991-1.006) | 0.709 |
| Module PLAU | 0.945 (0.815-1.097) | 0.461 | 0.914 (0.765-1.092) | 0.322 | 0.973 (0.727-1.301) | 0.852 |
| Module STAT1 | 0.999 (0.991-1.007) | 0.835 | 0.999 (0.988-1.01) | 0.812 | 0.990 (0.976-1.004) | 0.144 |
| Module VEGF | 1.040 (0.904-1.196) | 0.581 | 1.062 (0.895-1.259) | 0.490 | 1.014 (0.802-1.282) | 0.909 |
| Module CASP3 | 0.851 (0.623-1.164) | 0.313 | 1.062 (0.684-1.648) | 0.789 | 0.738 (0.475-1.147) | 0.177 |
All module scores and prototype genes are considered as continuous variables. Statistically significant P-values are highlighted in bold. CI, confidence interval; HR, hazard ratio; TCGA, The Cancer Genome Atlas.
Fig. 1. Overall survival curves of module scores and prototype genes in endometrial carcinomas.
The optimal cut-point for stratification was determined using X-tile [8] for module scores and prototype genes of a, PGR, b, ESR1, c, PIK3CA, and d, MSH6. Monte Carlo-corrected P-values of the log-rank test are shown. Categorical values obtained with the split-sample approach of X-tile (i.e. low- and high-levels) of prototype genes and module scores were employed in the Kaplan-Meier curves.
Table 2.
Multivariate Cox's regression analysis of 5-year overall survival in endometrial cancer patients (TCGA) including standard clinicopathologic parameters, prototype genes and module scores.
| All cases (n=323) – Prototype genes | All cases (n=323) - Modules | ||||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age | 1.070 (1.027-1.114) | 0.001 | Age | 1.059 (1.019-1.101) | 0.004 |
| Grade | 1.787 (0.886-3.602) | 0.105 | Grade | 1.904 (0.939-3.863) | 0.074 |
| Histology | 0.366 (0.146-0.920) | 0.032 | Histology | 0.346 (0.126-0.954) | 0.040 |
| FIGO stage | 1.951 (1.383-2.751) | <0.001 | FIGO stage | 1.918 (1.366-2.693) | <0.001 |
| Prototype ESR1 | 0.629 (0.231-1.714) | 0.364 | Module ESR1 | 1.864 (0.452-7.689) | 0.389 |
| Prototype PGR | 1.291 (0.479-3.478) | 0.614 | Module PGR | 0.447 (0.112-1.786) | 0.254 |
| Prototype PIK3CA | 1.030 (0.443-2.393) | 0.945 | Module PIK3CA | 1.076 (0.455-2.544) | 0.868 |
| Prototype MSH6 | 3.542 (1.504-8.339) | 0.004 | Module MSH6 | 2.406 (0.999-5.794) | 0.050 |
| Endometrioid cancers (n=271) – Prototype genes | Endometrioid cancers (n=271) - Modules | ||||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age | 1.074 (1.029-1.120) | 0.001 | Age | 1.073 (1.027-1.121) | 0.002 |
| Grade | 1.612 (0.772-3.365) | 0.203 | Grade | 1.891 (0.919-3.891) | 0.084 |
| FIGO stage | 2.167 (1.411-3.328) | <0.001 | FIGO stage | 2.104 (1.365-3.242) | 0.001 |
| Prototype ESR1 | 0.842 (0.281-2.525) | 0.759 | Module ESR1 | 1.547 (0.250-9.558) | 0.639 |
| Prototype PGR | 1.1164 (0.398-3.406) | 0.782 | Module PGR | 0.528 (0.085-3.282) | 0.493 |
| Prototype PTEN | 0.370 (0.148-0.923) | 0.033 | Module PTEN | 0.240 (0.095-0.605) | 0.002 |
| Prototype MSH6 | 3.705 (1.265-10.854) | 0.017 | Module MSH6 | 1.689 (0.637-4.480) | 0.293 |
All module scores and prototype genes are considered as categorical variables. Statistically significant P-values are highlighted in bold. CI, confidence interval; HR, hazard ratio; TCGA, The Cancer Genome Atlas.
3.2. Biological processes associated with outcome are distinct between EECs and SECs
Next we sought to define whether outcome of EECs (n=271) and SECs (n=52) would be governed by distinct biological processes. While in EECs the prototype genes ESR1, PGR, PIK3CA, and PTEN, the hormone receptor modules (i.e. ESR1 and PGR) and the PI3K pathway signaling modules (i.e. PIK3CA and PTEN), and the prototype gene MSH6 and the DNA mismatch repair module were associated significantly with outcome in univariate analysis, in SECs only the apoptosis-related CASP3 and CTNNB1 prototype genes and the Wnt signaling module were significantly associated with overall survival (P<0.05; Table 1, Supplementary Fig. 1). Survival analysis using the X-tile threshold values of the prototype genes and transcription modules revealed that in EECs the prototype genes ESR1 and PGR and their respective hormone receptor modules, as well as the prototype genes PTEN and MSH6, and the DNA mismatch repair module were significantly associated with overall survival (P<0.05; Fig. 2). None of the biological processes or prototype genes identified to be governing clinical behavior of SECs in univariate analysis were found to be significant when used as categorical variables as defined by the X-tile software (data not shown). Multivariate analysis correcting for age, grade and stage revealed that the prototype gene and PI3K pathway module PTEN and the MSH6 prototype gene were independent predictors of outcome in EECs (Table 2).
Fig. 2. Overall survival curves of module scores and prototype genes in endometrial cancers of endometrioid histology.
The optimal cut-point for stratification was determined using X-tile [8] for module scores and prototype genes of a, PGR, b, ESR1, c, PTEN, and d, MSH6. Monte Carlo-corrected P-values of the log-rank test are shown. Categorical values obtained with the split-sample approach of X-tile (i.e. low- and high-levels) of prototype genes and module scores were employed in the Kaplan-Meier curves.
3.3. Biological processes associated with outcome in ECs of distinct grades and genomic subtypes
The stratification of all ECs according to grade revealed that, in univariate survival analysis, the tumor invasion and metastasis module and the prototype gene PLAU were significantly associated with outcome in grade 3 ECs, the prototype gene and module ESR1 and the prototype gene CTNNB1 were significantly associated with outcome in grade 2 ECs, however no prototype genes or transcription modules governing overall survival in grade 1 ECs were identified (Supplementary Table 7, Supplementary Fig. 2b). Likewise, stratification into the genomic subtypes as described by TCGA[4] showed that the chromatin organization module and prototype gene ARID1A and the Wnt signaling module CTNNB1 were significantly associated with the overall survival in ECs of copy-number high (serous-like) subtype (Supplementary Table 7, Supplementary Fig. 2c) but no associations with other genomics subtypes were found. It should be noted, however, that with the exception of grade in the copy-number low cancers none of the clinicopathologic parameters were significantly associated with overall survival in the EC genomic subgroups (Supplementary Table 7).
3.4. MSH6 protein expression is associated with outcome in EC
To independently validate our RNA-sequencing-based findings, we selected the only prototype gene found to be an independent predictor of outcome in ECs in multivariate analysis, namely the prototype gene MSH6. To assess whether high levels of MSH6 protein expression were also associated with outcome in EC, we performed MSH6 immunohistochemical analysis in an independent series of ECs (n=228 EECs, n=15 SECs; Supplementary Table 8). Akin to the associations with outcome observed in the TCGA series, the clinicopathologic parameters FIGO stage, age, histologic type and grade were significantly associated with disease-free survival in this set of 243 ECs (Supplementary Fig. 3). We further observed that high MSH6 protein expression levels were significantly associated with disease-free survival in univariate and multivariate analyses in ECs (Fig. 3; Table 3), confirming our observations made based on MSH6 mRNA analysis. Finally, MSH6 protein levels were found to be significantly associated with disease-free survival in the 228 EECs of the TMA cohort on univariate analysis, but not on multivariate analysis (Supplementary Fig. 4, Supplementary Table 9).
Fig. 3. MSH6 protein expression and association with outcome in endometrial cancers.
a, Representative micrographs of endometrial cancers with low/absent levels of MSH6 expression and high levels of MSH6 expression as assessed by immunohistochemistry on tissue microarrays. b, Disease-free survival curves of MSH6 protein expression in an independent series of 243 endometrial cancers. P-value of the log-rank test is shown. Categorical values for MSH6 (i.e. MSH6 high expression (Allred score ≥6) and MSH6 low expression (Allred score of <6)) were employed in the Kaplan-Meier curves.
Table 3.
Multivariate Cox's regression analysis of disease-free survival in endometrial cancer patients of the validation series including standard clinicopathologic parameters and MSH6 protein expression assessed by immunohistochemical analysis.
| All endometrial cancers validation series (n=243) | ||
|---|---|---|
| HR (95% CI) | P | |
| Age | 2.552 (1.359-4.793) | 0.004 |
| Grade | 1.834 (1.225-2.747) | 0.003 |
| Histology | 1.092 (0.445-2.680) | 0.848 |
| FIGO stage | 2.025 (1.387-2.956) | <0.001 |
| MSH6 protein expression (high vs low) | 2.150 (1.104-4.190) | 0.024 |
Statistically significant P-values are highlighted in bold. CI, confidence interval; HR, hazard ratio.
4. DISCUSSION
Here we demonstrate through the analysis of prototype genes and gene expression modules that low levels of ESR1 and PGR gene expression and their respective gene expression modules are associated with less favorable outcome in ECs in general as well as in EECs. Our findings corroborate and expand on observations demonstrating that PR-negativity as determined by immunohistochemistry is an independent prognostic factor for disease-free survival in patients with EEC[9,10]. Prospective assessment of ER and PR expression using pre-determined cut-offs would be required, however, to ascertain their value as prognostic biomarkers[11].
Our findings expand on the observations that EECs and SECs are distinct. Here we observed that PI3K signaling and DNA mismatch repair processes may play a role in the clinical behavior of EECs, as opposed to Wnt signaling (CTNNB1) and apoptosis (CASP3)-related processes in SECs. In a subset of ER-negative breast cancers, increased expression of the Wnt/β-catenin signaling transcriptome has been observed[12], although activation of the canonical Wnt pathway through mutations seems to be rare in breast cancer. A similar phenomenon may be observed in SECs as these rarely display β-catenin nuclear expression (<3%) and only up to 3% harbor CTNNB1 mutations[4,13-15]. Of note, high levels of caspase-3 protein expression as determined by immunohistochemistry have been shown to be associated with favorable prognosis in high-grade serous ovarian cancer[16,17].
Here we provide transcriptomic evidence to demonstrate that the PIK3CA prototype gene and pathway module are associated with overall survival in EC. While the PTEN prototype gene and module expression were found to be independent prognostic factors of overall survival in patients with EEC, neither PTEN nor PIK3CA prototype genes or modules were found to be associated with the outcome of SECs, despite the presence of PIK3CA mutations in approximately 40% of cases[4,18]. The prognostic value of loss of PTEN protein expression in EC has proven controversial and conflicting results have been reported[19], which may in part be due to the challenges posed by PTEN immunohistochemical analysis[20] and the lack of standardized thresholds for its interpretation. It should be noted that expression levels of the PIK3CA prototype gene and module were found not to correlate with the PIK3CA gene mutation status (Supplementary Fig. 5), which is in agreement with previous reports based on the analysis of breast cancers[21]. Our results support previous reports suggesting that co-occurring mutations and epigenetic aberrations affecting different components of the PI3K pathway may converge on regulation of PTEN expression levels and function in ECs[22].
The gene expression module and prototype gene MSH6 were found to be an independent predictor of outcome in this large dataset of ECs, where cases with low MSH6 expression have a more favorable clinical behavior. This observation was confirmed at the protein level in an independent cohort of ECs. Whilst germline mutations in MSH6 are associated with increased risk for Lynch syndrome-associated EC[23], the role of MSH6 in sporadic EC is less well understood. MSH6 promoter hypermethylation seems to be rare in EC[24] and MSH6 somatic mutations were found in 7% of cases included in the TCGA study (cBioPortal, http://www.cbioportal.org/, accessed May 2015)[4,25], however these did not correlate with MSH6 prototype or module expression (Supplementary Fig. 5). Of note, the MSH6 expression module included MCM3 and MCM6, and MCM2, MCM3 and MCM7 have been suggested to act as proliferation markers in the endometrium and in EC[26,27]. This is of interest given that, in contrast to ER-positive breast cancer where the expression of proliferation-related genes is an important determinant of outcome[5], no significant association of the proliferation module and survival in ECs was found. These data provide evidence to suggest that the impact of proliferation on the outcome of these two hormone-driven cancers is likely distinct. In addition, further studies are warranted to determine the biological basis of the high expression of the MSH6 prototype gene in sporadic ECs with a poor outcome.
The limitations of our study include the analysis of a retrospective cohort, of which a subset received adjuvant chemotherapy with or without radiation therapy; however the therapies could not be included in the survival model as the adjuvant treatment information is not available for a large subset of patients in the TCGA study[4]. In addition, our analysis was performed assessing mRNA expression levels, which do not always translate into protein expression changes; however, similar analyses of breast cancers have yielded important insights for the identification of biological processes and transcriptional modules that define the outcome of hormone receptor disease[5] and the observations related to the MSH6 prototype gene expression were validated at the protein level. Regrettably, validation of the transcriptomic results derived from the analysis of the TCGA dataset could not be performed, given the lack of sufficiently-sized publicly-available independent EC gene expression datasets with follow-up information. Moreover, the composition of ECs in terms of grade was different between our validation cohort and the TCGA dataset, as the latter was enriched for grade 3 ECs[4]. Finally, our study was limited to EECs and SECs; additional studies investigating the biological processes that govern the outcome of clear cell carcinomas and carcinosarcomas are warranted.
Despite these limitations, here we demonstrate that the expression of the DNA mismatch repair MSH6 gene and its expression module are independent predictors of outcome in EC, and that the clinical behavior of EECs and SECs is governed by distinct processes. Given the molecular diversity of ECs, the development of prognostic models taking into account molecular features in addition to the current anatomical prognostic factors is warranted, as is the stratification of ECs not only according to histologic type, but also molecular characteristics in studies aiming to define novel markers of prognosis and therapy response in EC.
Supplementary Material
HIGHLIGHTS.
Endometrioid and serous carcinomas are underpinned by distinct molecular pathways.
PI3K and DNA mismatch repair modules govern the outcome of endometrioid cancers.
The clinical behavior of serous cancers is defined by apoptosis and Wnt signaling.
MSH6 expression is associated with outcome of patients with endometrial carcinomas.
Acknowledgements/ Funding
AM Schultheis is supported by a stipend from the German Cancer Aid (Dr. Mildred Scheel Stiftung), S Piscuoglio in part by a Susan G Komen Postdoctoral Fellowship Grant (PDF14298348), C Marchiò by grants from AIRC (MFAG13310), and JC Palacios by grants from ISCIII (RD12/0036/0064 and PI13/02477 co-financed by the European Development Regional Fund, ‘A way to achieve Europe’ EDRF). Research reported in this publication was supported in part by a Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute (Grant No. P30CA008748). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Role of the funding source
Funding bodies had no role in the design of the study, collection, analysis and interpretation of the data, or the writing of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Appendix A. Supplementary data
REFERENCES
- 1.Abu-Rustum NR, Zhou Q, Gomez JD, Alektiar KM, Hensley ML, Soslow RA, et al. A nomogram for predicting overall survival of women with endometrial cancer following primary therapy: toward improving individualized cancer care. Gynecol Oncol. 2010;116(3):399–403. doi: 10.1016/j.ygyno.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matias-Guiu X, Prat J. Molecular pathology of endometrial carcinoma. Histopathology. 2013;62(1):111–23. doi: 10.1111/his.12053. [DOI] [PubMed] [Google Scholar]
- 3.Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Lancet Oncol. 2014;15(7):e268–78. doi: 10.1016/S1470-2045(13)70591-6. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Research Network. Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desmedt C, Haibe-Kains B, Wirapati P, Buyse M, Larsimont D, Bontempi G, et al. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res. 2008;14(16):5158–65. doi: 10.1158/1078-0432.CCR-07-4756. [DOI] [PubMed] [Google Scholar]
- 6.Smoot ME, Ono K, Ruscheinski J, Wang PL, Ideker T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics. 2011;27(3):431–2. doi: 10.1093/bioinformatics/btq675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biscuola M, Van de Vijver K, Castilla MA, Romero-Perez L, Lopez-Garcia MA, Diaz-Martin J, et al. Oncogene alterations in endometrial carcinosarcomas. Hum Pathol. 2013;44(5):852–9. doi: 10.1016/j.humpath.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 8.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–9. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 9.Huvila J, Talve L, Carpen O, Edqvist PH, Ponten F, Grenman S, et al. Progesterone receptor negativity is an independent risk factor for relapse in patients with early stage endometrioid endometrial adenocarcinoma. Gynecol Oncol. 2013;130(3):463–9. doi: 10.1016/j.ygyno.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Jongen V, Briet J, de Jong R, ten Hoor K, Boezen M, van der Zee A, et al. Expression of estrogen receptor-alpha and -beta and progesterone receptor-A and -B in a large cohort of patients with endometrioid endometrial cancer. Gynecol Oncol. 2009;112(3):537–42. doi: 10.1016/j.ygyno.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 11.Salvesen HB, Haldorsen IS, Trovik J. Markers for individualised therapy in endometrial carcinoma. Lancet Oncol. 2012;13(8):e353–61. doi: 10.1016/S1470-2045(12)70213-9. [DOI] [PubMed] [Google Scholar]
- 12.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–67. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McConechy MK, Ding J, Cheang MC, Wiegand KC, Senz J, Tone AA, et al. Use of mutation profiles to refine the classification of endometrial carcinomas. J Pathol. 2012;228(1):20–30. doi: 10.1002/path.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreno-Bueno G, Hardisson D, Sanchez C, Sarrio D, Cassia R, Garcia-Rostan G, et al. Abnormalities of the APC/beta-catenin pathway in endometrial cancer. Oncogene. 2002;21(52):7981–90. doi: 10.1038/sj.onc.1205924. [DOI] [PubMed] [Google Scholar]
- 15.Dedes KJ, Wetterskog D, Ashworth A, Kaye SB, Reis-Filho JS. Emerging therapeutic targets in endometrial cancer. Nat Rev Clin Oncol. 2011;8(5):261–71. doi: 10.1038/nrclinonc.2010.216. [DOI] [PubMed] [Google Scholar]
- 16.Materna V, Surowiak P, Markwitz E, Spaczynski M, Drag-Zalesinska M, Zabel M, et al. Expression of factors involved in regulation of DNA mismatch repair- and apoptosis pathways in ovarian cancer patients. Oncol Rep. 2007;17(3):505–16. [PubMed] [Google Scholar]
- 17.Espinosa I, Catasus L, Canet B, D'Angelo E, Munoz J, Prat J. Gene expression analysis identifies two groups of ovarian high-grade serous carcinomas with different prognosis. Mod Pathol. 2011;24(6):846–54. doi: 10.1038/modpathol.2011.12. [DOI] [PubMed] [Google Scholar]
- 18.Urick ME, Rudd ML, Godwin AK, Sgroi D, Merino M, Bell DW. PIK3R1 (p85alpha) is somatically mutated at high frequency in primary endometrial cancer. Cancer Res. 2011;71(12):4061–7. doi: 10.1158/0008-5472.CAN-11-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackay HJ, Gallinger S, Tsao MS, McLachlin CM, Tu D, Keiser K, et al. Prognostic value of microsatellite instability (MSI) and PTEN expression in women with endometrial cancer: results from studies of the NCIC Clinical Trials Group (NCIC CTG). Eur J Cancer. 2010;46(8):1365–73. doi: 10.1016/j.ejca.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 20.Pallares J, Bussaglia E, Martinez-Guitarte JL, Dolcet X, Llobet D, Rue M, et al. Immunohistochemical analysis of PTEN in endometrial carcinoma: a tissue microarray study with a comparison of four commercial antibodies in correlation with molecular abnormalities. Mod Pathol. 2005;18(5):719–27. doi: 10.1038/modpathol.3800347. [DOI] [PubMed] [Google Scholar]
- 21.Palimaru I, Brugmann A, Wium-Andersen MK, Nexo E, Sorensen BS. Expression of PIK3CA, PTEN mRNA and PIK3CA mutations in primary breast cancer: association with lymph node metastases. Springerplus. 2013;2:464. doi: 10.1186/2193-1801-2-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheung LW, Hennessy BT, Li J, Yu S, Myers AP, Djordjevic B, et al. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer Discov. 2011;1(2):170–85. doi: 10.1158/2159-8290.CD-11-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baglietto L, Lindor NM, Dowty JG, White DM, Wagner A, Gomez Garcia EB, et al. Risks of Lynch syndrome cancers for MSH6 mutation carriers. J Natl Cancer Inst. 2010;102(3):193–201. doi: 10.1093/jnci/djp473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodfellow PJ, Buttin BM, Herzog TJ, Rader JS, Gibb RK, Swisher E, et al. Prevalence of defective DNA mismatch repair and MSH6 mutation in an unselected series of endometrial cancers. Proc Natl Acad Sci U S A. 2003;100(10):5908–13. doi: 10.1073/pnas.1030231100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato K, Toki T, Shimizu M, Shiozawa T, Fujii S, Nikaido T, et al. Expression of replication-licensing factors MCM2 and MCM3 in normal, hyperplastic, and carcinomatous endometrium: correlation with expression of Ki-67 and estrogen and progesterone receptors. Int J Gynecol Pathol. 2003;22(4):334–40. doi: 10.1097/01.pgp.0000092129.10100.5e. [DOI] [PubMed] [Google Scholar]
- 27.Li SS, Xue WC, Khoo US, Ngan HY, Chan KY, Tam IY, et al. Replicative MCM7 protein as a proliferation marker in endometrial carcinoma: a tissue microarray and clinicopathological analysis. Histopathology. 2005;46(3):307–13. doi: 10.1111/j.1365-2559.2005.02069.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.