Abstract
Purpose
We investigated behavioral problems, attention problems, and cognitive function in children and adolescents born small for gestational age (SGA).
Methods
Forty-six SGA children born at term and 46 appropriate for gestational age (AGA) children born at term were compared. Psychiatric symptoms were examined with reference to the Korean-Child Behavior Checklist, Korean-Youth Self Report, and Attention Deficit Hyperactivity Disorder Rating Scale (ADHD-RS). Cognitive function was estimated using the Wechsler Intelligence Scale. Sociodemographic data were recorded from interviews.
Results
SGA children had high scores on delinquent behavior, aggressive behavior, and the externalizing scale, and they also showed a propensity for anxiety and depression. The SGA group had a higher mean ADHD-RS score than the AGA group (10.52±8.10 vs.9.93±7.23), but the difference was not significant. The SGA group had a significantly lower verbal intelligence quotient (IQ) than the AGA group, but the mean scores of both groups were within normal limits.
Conclusion
This study indicates marked behavioral problems, such as delinquency, aggressiveness, and anxiety and depression, as well as low verbal IQ in the SGA group than in the AGA group. Even in cases in which these symptoms are not severe, early detection and proper treatment can help these children adapt to society.
Keywords: Small for gestational age, Child behavior disorders, Cognition
Introduction
Small for gestational age (SGA) infants are those whose birth weight or length below the 10th percentile of all infants adjusted for gestational age1). SGA infants have greater morbidity and mortality than appropriate growth, gestational-age-matched infants. As neonatal intensive care has advanced, mortality of SGA infants has rapidly decreased. In the 2000s, there has been a paucity of data on differences between school-aged term SGA and appropriate for gestational age (AGA) children beyond the preschool years.
However, several previous studies have shown that term SGA infants are at increased risk for mild cognitive deficits in childhood and adolescenc2,3,4), as well as for learning difficulties and poorer performance in school2,5,6). Studies also demonstrated that they have problems related to behavior and mood control, in addition to psychological problems, such as attention deficit hyperactive disorder (ADHD)6).
Not all studies demonstrated this association; some found no significant difference in intelligence quotient (IQ) between term-born SGA and AGA adolescents7,8). O'Keeffe et al.6) reported that SGA status seemed to have only modest effects on learning, cognition and attention in adolescence. Another study concluded that term SGA infants may have relatively mild disorders that can easily be overlooked but later could have a major impact on quality of life in adulthood9). The aim of the present study was to investigate behavioral and attention problems, as well as cognitive function in children and adolescents born SGA at term.
Materials and methods
1. Materials
SGA was defined as a birth weight below the 10th percentile of all infants adjusted for gestational age, gender and parity10). Forty-six children and adolescents born SGA at term (gestational age≥37 weeks) between July 2003 and April 2009 at Hallym University Kangdong Sacred Heart Hospital in Korea were included. The mean birth weight was 2.30±0.28 kg. The control group comprised 46 children and adolescents born at term with birth weights above the 10th percentile for gestational age. The mean birth weight of the controls was 3.30±0.38 kg.
Patients who were born preterm or born with chromosomal or congenital abnormalities were excluded. Sociodemographic data were obtained by interview based on the socioeconomic status (SES) of subjects' parents.
2. Methods
1) Assessment of psychiatric symptoms
Psychiatric symptoms were evaluated using the Achenbach System of Empirically Based Assessment (ASEBA) with Korean-Child Behavior Checklist (K-CBCL) and the Korea-Youth Self Report (K-YSR).
The Child Behavior Checklist was developed in 1966 by Dr. Thomas Achenbach and later translated into Korean and standardized (K-CBCL)11). The K-CBCL is used to obtain reports from parents, other close relatives, or caregivers who reside with the 4- to 17-year-old children. The questionnaire consists of social competence scales and behavior problem scales. Similar questions are grouped into various syndromes. The social ability checklist has 3 subgroups: social competence, academic performance, and total competence. The behavior problem checklist is composed of eight subscales; withdrawal, somatization, anxiety/depression, social problem, thought problems, attention problems, aggressive behavior, and delinquent behavior. Syndrome scores are further summed to provide scores for internalizing and externalizing problem scales. The internalizing problem score is the sum of the scores from withdrawal, somatization, and anxiety/depression while the externalizing problem score is the sum of the scores from aggressive behavior and delinquent behavior. A total score from all questions is also derived for the total problem score. In this study, sex problems and emotional lability were not assessed in, 4- to 11-year-old children. Each item is appraised on a 3-point Likert scale; 0 (never), 1 (occasionally or the degree is not serious), or 2 (frequent or serious). The total possible number of points is 234. The social ability checklists are interpreted as normal at the 5th percentile and below 33 points on T-scores. The behavior problem checklists are interpreted as abnormal over the 98th percentile, above 70 points of T-scores. Internalizing, externalizing, and total problem measures are interpreted as abnormal over the 90th percentile and above 63 points on T-scores.
The Youth Self Report was developed in 1991 by Dr. Thomas Achenbach, then later translated into Korean and standardized in 1998 (K-YSR)12,13). The K-YSR is a self-report measure and was applied only to the 12- to17-year-old youths in our study. It consists of social ability scales and behavior problem scales. We analyzed only the subscales used in the K-CBCL. Social ability in the 5th percentile is interpreted as normal, below 33 points on raw scores. The problem behavior syndrome measure is interpreted as normal below 98th percentile, and 70 points on raw score.
ADHD was evaluated using the ADHD Rating Scale (ADHD-RS) form that was completed by parents. Jang et al.14) reported continuous normative data with T score and subdivided cutoff points for ADHD screening. According to the results of the study, the 80th and 90th percentiles could be used as a screening test, and the 93rd and 98th could be used for diagnosis. We determined the cutoff value of ADHD-RS to be 19 (the 93rd percentile), and children with raw scores greater than 19 were regarded as having ADHD15).
2) Cognitive function assessment
The Korea-Wechsler Intelligence Scale III (K-WISC-III) was used for IQ evaluation. This scale was developed in 1939 by David Wechsler, then later translated into Korean and standardized16). It contains a verbal and performance subscales. The verbal subscale is composed of information, similarities, arithmetic, vocabulary, comprehension, and digit span. The performance subscale is composed of picture completion, coding, picture arrangement, block design, object assembly, and symbol-searching.
3) Statistical analysis
Statistical analysis was performed using IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA). Student t test and Fisher exact tests were used to test the differences between the groups, with the significance level set at 0.05.
Results
1. Group characteristics
The birth weight of the SGA group was significantly lower than that of the AGA group (2.11±0.315 kg vs. 3.28±0.46 kg, respectively, P=0.000). There were no significant differences in children' age (12.14±1.71 years vs. 12.27±1.44 years, P=0.684), sex (P=0.093), and body mass index (19.62±5.09 kg/m2 vs. 18.56±4.69 kg/m2, respectively, P=0.304) between the 2 groups. SES did not significantly differ among the subjects (Table 1).
Table 1. Clinical characteristics of SGA and AGA groups.
| Characteristic | SGA (n=46) | AGA (n=46) | P value |
|---|---|---|---|
| Age (yr) | 12.14±1.71 | 12.27±1.44 | 0.684 |
| Sex, male:female | 16 30 | 25:21 | 0.093 |
| Birth weight (kg) | 2.11±0.315 | 3.28±0.46 | 0.000* |
| Height SDS | –0.54±1.19 | 0.20±1.17 | 0.003* |
| Weight SDS | –0.04±1.01 | –0.02±1.10 | 0.922 |
| BMI (kg/m2) | 19.62±5.09 | 18.56±4.69 | 0.304 |
| SES | |||
| Middle-high | 3 (6.5) | 8 (17.4) | |
| Middle-middle | 29 (63.0) | 32 (69.6) | 0.085 |
| Middle-low | 13 (28.3) | 5 (10.9) | |
| Lowest | 1 (2.2) | 1 (2.2) |
Values are presented as mean±standard deviation or number (%).
SGA, small for gestational age; AGA, appropriate for gestational age; SDS, standard deviation score; BMI, body mass index; SES, socioeconomic status.
*P<0.05, statistically significant difference between groups (SGA and AGA).
2. Psychiatric assessment
1) K-CBCL
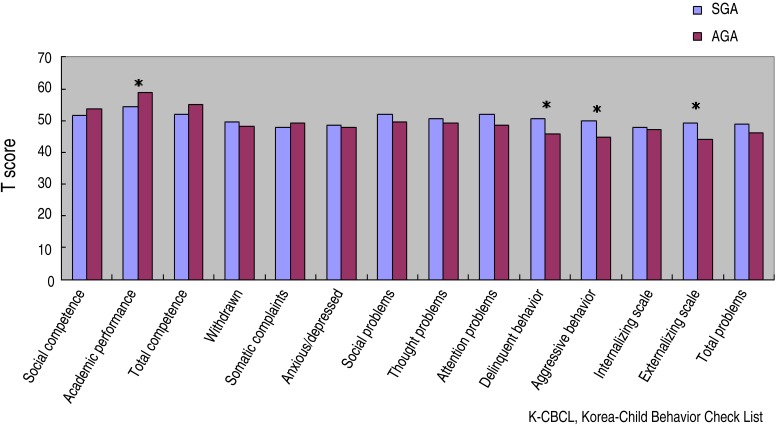
The results of the K-CBCL showed that the scores of SGA children were statistically significantly higher than those of the normal birth-weight children on the items relating to delinquent behavior (50.50±9.78 vs. 45.65±6.49, P=0.006), aggressive behavior (49.85±11.20 vs. 44.85±9.39, P=0.023), and the externalizing scale (49.39±10.99 vs. 44.22±9.10, P=0.016). However, academic performance scores were higher in the AGA group compared to SGA (54.39±11.53 vs. 58.91±9.47, P=0.043) (Fig. 1).
Fig. 1. Comparison of K-CBCL scores between SGA and AGA groups. K-CBCL, Korean-Child Behavior Checklist; SGA, small for gestational age; AGA, appropriate for gestational age. Asterisks showed that the scores of SGA children were statistically significantly higher than those of AGA.
2) K-YSR
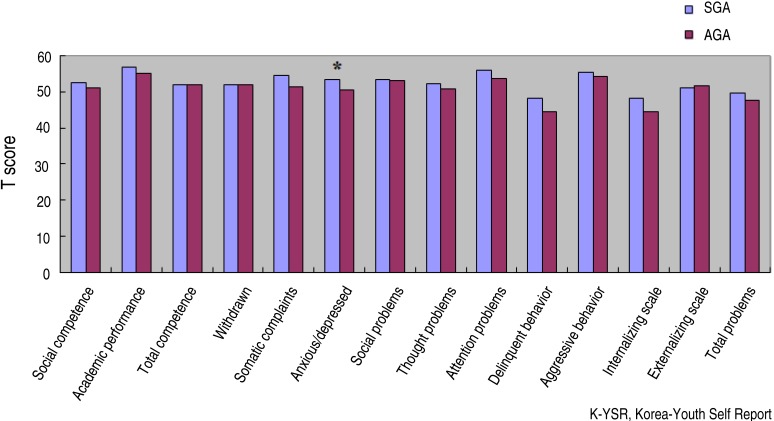
K-YSR was performed in 52 children. The scores of the SGA children on the K-YSR (53.42±6.62) were significantly higher for anxiety/depression than those of the AGA counterparts (50.43±1.06, P=0.022) (Fig. 2).
Fig. 2. K-YSR scores in SGA and AGA groups (*P<0.05 vs. AGA). K-YSR, Korean-Youth Self-Report; SGA, small for gestational age; AGA, appropriate for gestational age.
3) ADHD-RS
The parents of SGA children (50%) suspected and worried more about ADHD of their children before test than those of AGA children (23.9%; P=0.017). On the ADHD-RS, parents of SGA children reported higher score than those of AGA children (10.52±8.10 vs. 9.93±7.23), and 17.4% of SGA children had a total score greater than 19, whereas only 6.5% of AGA children did. However, the differences were not statistically significant (P=0.71 and P=0.197, respectively) (Table 2). Four of 8 patients (50%) in the SGA and one of 3 (33.3%) in the AGA who diagnosed with ADHD were on medication.
Table 2. ADHD-RS scores in SGA and AGA groups.
| Variable | SGA (n=46) | AGA (n=46) | P value |
|---|---|---|---|
| ADHD-RS | 10.52±8.10 | 9.93±7.23 | 0.714 |
| Score >19 | 8 (17.4) | 3 (6.5) | 0.197 |
| ADHD recognition by parents | 23 (50.0) | 11 (23.9) | 0.017* |
| ADHD treatment | 4/8 (50.0) | 1 /3 (33.3) | 1.000 |
Values are presented as mean±standard deviation or number (%).
ADHD-RS, attention deficit hyperactivity disorder rating scale; SGA, small for gestational age; AGA, appropriate for gestational age; ADHD, attention deficit hyperactivity disorder.
*P<0.05, statistically significant difference between groups (SGA and AGA).
3. Cognitive assessment
The SGA children had significantly lower scores in full-scale IQ (100.52±15.24 vs. 109.52±12.53, P=0.003) and verbal IQ (102.24±12.78 vs. 111.54±13.57, P=0.001) than the controls. When we separated and compared the verbal IQ and performance IQ, we found that the scores of both groups were within normal limits but the SGA group had significantly lower verbal IQ than the controls (102.24±12.78 vs. 111.54±13.57, P=0.001) (Table 3).
Table 3. Neurocognitive performance in SGA and AGA groups.
| Variable | SGA (n=46) | AGA (n=46) | P value |
|---|---|---|---|
| IQ score | |||
| Verbal subscale IQ score | 102.24±12.78 | 111.54±13.57 | 0.001* |
| Performance subscale IQ score | 98.39±17.80 | 104.17±13.27 | 0.081 |
| Full-scale IQ score | 100.52±15.24 | 109.52±12.53 | 0.003* |
| Verbal comprehension | 100.26±17.99 | 110.70±13.21 | 0.002* |
| Visuomotor perception | 98.48±16.79 | 104.33±13.56 | 0.069 |
| Attention | 100.93±15.61 | 111.13±11.96 | 0.001* |
| Processing speed | 98.54±16.30 | 104.09±14.54 | 0.089 |
Values are presented as mean±standard deviation.
ADHD-RS, attention deficit hyperactivity disorder rating scale; SGA, small for gestational age; AGA, appropriate for gestational age; IQ, intelligence quotient.
*P<0.05, statistically significant difference between groups (SGA and AGA).
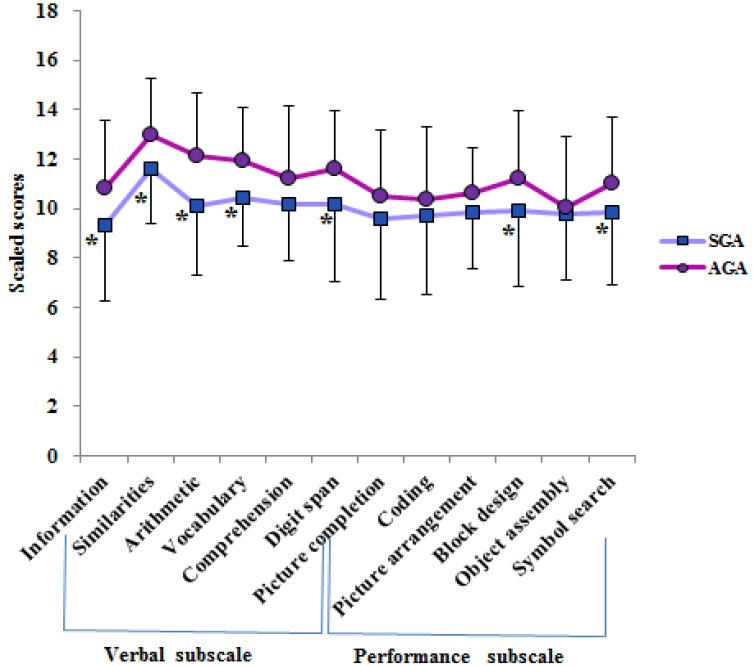
The subsets of K-WISC III are presented in Fig. 3. SGA children had significantly lower scores in subsets of information, similarities, arithmetic, vocabulary, digit span, block design and symbol-searching than the controls (P<0.05).
Fig. 3. K-WISC III scores in SGA and AGA groups (*P<0.05 vs. AGA). K-WISC III, Korean-Wechsler Intelligence Scale III; SGA, small for gestational age; AGA, appropriate for gestational age.
Discussion
Our study demonstrated that term SGA children had higher scores for delinquent behavior, aggressive behavior, and the externalizing scale on the K-CBCL. They also had higher scores for anxiety and depression on the K-YSR compared to the controls. There were no significant differences between term SGA children and their AGA counterparts in attention problems according to the scores of the ADHD-RS. Moreover, term SGA children had lower verbal IQs than AGA children, but the mean scores of both groups were within normal limits.
Birth weight can affect neurodevelopmental impairment. The prevalence of neurobehavioral symptoms in 11-year-olds was reported to be 40% for low-birth-weight children17). Developmental impairments have been known to be common in prematurity, especially in children born with very low birth weight. Botting et al.18) reported that very-low-birth-weight infants without cerebral palsy exhibit problems with sensory-motor function, visuospatial sensation, self-control, suppression, and making plans. Many previous studies have focused on problems associated with prematurity.
However, Hall and Wolke19) reported that a high incidence of emotional problems was significantly associated with very preterm, but not term, SGA births. In that study, consistent additional determinants of emotional problems included male gender and lower family SES. On the other hand, several studies previously reported that low-birth-weight children show reduced behavioral and emotion-control abilities compared to normal-birth-weight children.
The term SGA children in our study showed higher ASEBA scores for delinquent behavior, aggressiveness, anxiety, and depression compared to the controls. SGA children scored high for delinquent behavior, aggressive tendencies, and externalizing behavioral problems on the K-CBCL. The K-YSR, which is a self-reported module, showed tendencies toward mental instability demonstrated by high points for the anxiety and depression items. These results could support that the term SGA children had more behavioral problems compared to the AGA group.
According to previous reports, the prevalence of ADHD varies between 5% and 32%20,21,22,23). Another report examined the interrelationship between psychological symptoms and brain magnetic resonance imaging (MRI) of low-birth-weight infants, and frequently observed disorders such as periventricular lesions, dilated lateral ventricles, and thinning of the corpus callosum24). These observations point to a meaningful interrelationship between low birth-weight and the prevalence of ADHD. The present study did not include brain MRIs. However, the SGA group had a higher ADHD-RS score than the AGA group, although the difference was not statistically significant. The proportion of parents who recognized their children as having ADHD was also significantly higher in the SGA group than in the control group. The prevalence of ADHD in this study was 17.4% in the SGA group, whereas it was 6.5% in the control group.
According to a study by Matte et al.25) on the influence on IQ of variations in birth weight within normal range and among siblings at the age of 7 years, mean IQ increased with higher birth weight. Therefore, we can assume that fetal growth and brain development are closely related. Cohort studies showed that these effects are not limited to childhood or adolescence, but can influence cognitive ability in adulthood26,27). However, the results of other studies were inconclusive in this regard, and one suggested that the long-term effects of birth weight on cognition are probably negligible28,29). However, the IQ distribution in the SGA groups appears to be skewed toward the lower part of the scale30,31,32). Although the mean IQ of term SGA children in our study was within normal range and the effect was less pronounced for those born SGA at term than prematurely, we found that the overall intellectual performance of the term SGA group was significantly lower than that of the AGA group.
In particular, verbal IQ was lower in the term SGA children than in the AGA children. Hollo et al.5) showed three powerful independent predictors of academic achievement: a child's inattentive-passive behavior at school, verbal IQ score, and restless behavior during outpatient clinic visits. Although we did not measure learning difficulties, the low verbal IQ of the SGA children could affect academic achievement. In one recent study of term SGA children, not only verbal IQ but also performance IQ scores were significantly lower in the SGA group aged 19–20 years32). However, in our study, the overall scores for performance IQ were not significantly different between the two groups. Our results suggest that verbal IQ remains unresolved as an intelligence problem found in term SGA children.
Low SES is associated with poor developmental outcomes and emotional problems33,34). Socioeconomic variables, such as income, maternal age and education, ethnicity, and residence in a two-parent household have been found to influence the language outcomes of low-birth-weight children35). In the present study, SES was controlled between the SGA and AGA children, and therefore we could exclude the potential influence of economic variables on the behavioral problems and low IQ scores shown in the SGA group.
This study had several limitations. First, it had a small sample size and a retrospective design. Second, we did not include data on other possible covariates, such as perinatal risk factors, neonatal complications, and brain lesions. Additional research is needed to identify other causes of psychiatric and cognitive problems in SGA children and adolescents. Third, the results of verbal IQ in the term SGA group were within normal range, but an obvious difference between the 2 groups was present. We also found an association between term SGA status and psychiatric problems, and the relationship between term SGA status and a low verbal IQ could not be explained by SES alone. The strength of this study is that by excluding prematurity, SGA children born after at least 37 weeks of gestational age were compared to their AGA counterparts.
In conclusion, term SGA children had higher levels of delinquent and aggressive behaviors, anxiety, and depression. Although all IQ scores were within normal range, children and adolescents born at term with SGA had significantly lower verbal IQs than those born AGA. Under-recognition or inadequate treatment of these disorders may cause deterioration of not only the patients' quality of life, but also their ability to adapt to society.
Acknowledgments
This paper was supported by Wonkwang University in 2013.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Wollmann HA. Intrauterine growth restriction: definition and etiology. Horm Res. 1998;49(Suppl 2):1–6. [PubMed] [Google Scholar]
- 2.Paz I, Gale R, Laor A, Danon YL, Stevenson DK, Seidman DS. The cognitive outcome of full-term small for gestational age infants at late adolescence. Obstet Gynecol. 1995;85:452–456. doi: 10.1016/0029-7844(94)00430-l. [DOI] [PubMed] [Google Scholar]
- 3.Sommerfelt K, Andersson HW, Sonnander K, Ahlsten G, Ellertsen B, Markestad T, et al. Cognitive development of term small for gestational age children at five years of age. Arch Dis Child. 2000;83:25–30. doi: 10.1136/adc.83.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viggedal G, Lundalv E, Carlsson G, Kjellmer I. Neuropsychological follow-up into young adulthood of term infants born small for gestational age. Med Sci Monit. 2004;10:CR8–CR16. [PubMed] [Google Scholar]
- 5.Hollo O, Rautava P, Korhonen T, Helenius H, Kero P, Sillanpää M. Academic achievement of small-for-gestational-age children at age 10 years. Arch Pediatr Adolesc Med. 2002;156:179–187. doi: 10.1001/archpedi.156.2.179. [DOI] [PubMed] [Google Scholar]
- 6.O'Keeffe MJ, O'Callaghan M, Williams GM, Najman JM, Bor W. Learning, cognitive, and attentional problems in adolescents born small for gestational age. Pediatrics. 2003;112:301–307. doi: 10.1542/peds.112.2.301. [DOI] [PubMed] [Google Scholar]
- 7.Kulseng S, Jennekens-Schinkel A, Naess P, Romundstad P, Indredavik M, Vik T, et al. Very-low-birthweight and term small-for-gestational-age adolescents: attention revisited. Acta Paediatr. 2006;95:224–230. doi: 10.1080/08035250500421568. [DOI] [PubMed] [Google Scholar]
- 8.Theodore RF, Thompson JM, Waldie KE, Becroft DM, Robinson E, Wild CJ, et al. Determinants of cognitive ability at 7 years: a longitudinal case-control study of children born small-for-gestational age at term. Eur J Pediatr. 2009;168:1217–1224. doi: 10.1007/s00431-008-0913-9. [DOI] [PubMed] [Google Scholar]
- 9.Dinesen SJ, Greisen G. Quality of life in young adults with very low birth weight. Arch Dis Child Fetal Neonatal Ed. 2001;85:F165–F169. doi: 10.1136/fn.85.3.F165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin SM, Chang YP, Lee ES, Lee YA, Son DW, Kim MH, et al. Low birth weight, very low birth weight rates and gestational age-specific birth weight distribution of Korean newborn infants. J Korean Med Sci. 2005;20:182–187. doi: 10.3346/jkms.2005.20.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han MH, Yoo AJ. The validation of the child behavior checklist. Korean J Child Stud. 1995;16:5–21. [Google Scholar]
- 12.Lee HG, Shin HS, Lee KS. Development of a self-report form of the adolescent problem behavior rating scale: tests of its reliability and validity. Korean J Dev Psychol. 2004;17:147–170. [Google Scholar]
- 13.Ha EH. A validation study of the Korean Youth Self Report (K-YSR) SM J child study. 2005;18:83–104. [Google Scholar]
- 14.Jang SJ, Suh DS, Byun HJ. Normative study of the K-ARS (Korean ADHD Rating Scale) for parents. J Korean Acad Child Adolesc Psychiatry. 2007;18:38–48. [Google Scholar]
- 15.Kim YS, So YK, Noh JS, Choi NK, Kim SJ, Koh YJ. Normative data on the Korean ADHD Rating Scales (K-ARS) for parents and teacher. J Korean Neuropsychiatr Assoc. 2003;42:352–359. [Google Scholar]
- 16.Kwak KJ, Choi HP, Kim CT. A study for the standardization of K-WISC-III. Korean J Dev Psychol. 2002;15:19–33. [Google Scholar]
- 17.Elgen I, Sommerfelt K, Markestad T. Population based, controlled study of behavioural problems and psychiatric disorders in low birthweight children at 11 years of age. Arch Dis Child Fetal Neonatal Ed. 2002;87:F128–F132. doi: 10.1136/fn.87.2.F128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Botting N, Powls A, Cooke RW, Marlow N. Attention deficit hyperactivity disorders and other psychiatric outcomes in very low birthweight children at 12 years. J Child Psychol Psychiatry. 1997;38:931–941. doi: 10.1111/j.1469-7610.1997.tb01612.x. [DOI] [PubMed] [Google Scholar]
- 19.Hall J, Wolke D. A comparison of prematurity and small for gestational age as risk factors for age 6-13 year emotional problems. Early Hum Dev. 2012;88:797–804. doi: 10.1016/j.earlhumdev.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Strauss RS. Adult functional outcome of those born small for gestational age: twenty-six-year follow-up of the 1970 British Birth Cohort. JAMA. 2000;283:625–632. doi: 10.1001/jama.283.5.625. [DOI] [PubMed] [Google Scholar]
- 21.Stevenson CJ, Blackburn P, Pharoah PO. Longitudinal study of behaviour disorders in low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 1999;81:F5–F9. doi: 10.1136/fn.81.1.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rickards AL, Kelly EA, Doyle LW, Callanan C. Cognition, academic progress, behavior and self-concept at 14 years of very low birth weight children. J Dev Behav Pediatr. 2001;22:11–18. doi: 10.1097/00004703-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Stjernqvist K, Svenningsen NW. Ten-year follow-up of children born before 29 gestational weeks: health, cognitive development, behaviour and school achievement. Acta Paediatr. 1999;88:557–562. doi: 10.1080/08035259950169594. [DOI] [PubMed] [Google Scholar]
- 24.Odberg MD, Aukland SM, Rosendahl K, Elgen IB. Cerebral MRI and cognition in nonhandicapped, low birth weight adults. Pediatr Neurol. 2010;43:258–262. doi: 10.1016/j.pediatrneurol.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Matte TD, Bresnahan M, Begg MD, Susser E. Influence of variation in birth weight within normal range and within sibships on IQ at age 7 years: cohort study. BMJ. 2001;323:310–314. doi: 10.1136/bmj.323.7308.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richards M, Hardy R, Kuh D, Wadsworth ME. Birth weight and cognitive function in the British 1946 birth cohort: longitudinal population based study. BMJ. 2001;322:199–203. doi: 10.1136/bmj.322.7280.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sørensen HT, Sabroe S, Olsen J, Rothman KJ, Gillman MW, Fischer P. Birth weight and cognitive function in young adult life: historical cohort study. BMJ. 1997;315:401–403. doi: 10.1136/bmj.315.7105.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grantham-McGregor SM. Small for gestational age, term babies, in the first six years of life. Eur J Clin Nutr. 1998;52(Suppl 1):S59–S64. [PubMed] [Google Scholar]
- 29.Hack M. Effects of intrauterine growth retardation on mental performance and behavior, outcomes during adolescence and adulthood. Eur J Clin Nutr. 1998;52(Suppl 1):S65–S70. [PubMed] [Google Scholar]
- 30.Hack M, Flannery DJ, Schluchter M, Cartar L, Borawski E, Klein N. Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med. 2002;346:149–157. doi: 10.1056/NEJMoa010856. [DOI] [PubMed] [Google Scholar]
- 31.Lagrou K, Vanderfaeillie J, Froidecoeur C, Thomas M, Massa G, Tenoutasse S, et al. Effect of 2 years of high-dose growth hormone therapy on cognitive and psychosocial development in short children born small for gestational age. Eur J Endocrinol. 2007;156:195–201. doi: 10.1530/eje.1.02335. [DOI] [PubMed] [Google Scholar]
- 32.Løhaugen GC, Østgård HF, Andreassen S, Jacobsen GW, Vik T, Brubakk AM, et al. Small for gestational age and intrauterine growth restriction decreases cognitive function in young adults. J Pediatr. 2013;163:447–453. doi: 10.1016/j.jpeds.2013.01.060. [DOI] [PubMed] [Google Scholar]
- 33.Brooks-Gunn J, Klebanov PK, Duncan GJ. Ethnic differences in children's intelligence test scores: role of economic deprivation, home environment, and maternal characteristics. Child Dev. 1996;67:396–408. [PubMed] [Google Scholar]
- 34.Pungello EP, Iruka IU, Dotterer AM, Mills-Koonce R, Reznick JS. The effects of socioeconomic status, race, and parenting on language development in early childhood. Dev Psychol. 2009;45:544–557. doi: 10.1037/a0013917. [DOI] [PubMed] [Google Scholar]
- 35.Ment LR, Vohr B, Allan W, Katz KH, Schneider KC, Westerveld M, et al. Change in cognitive function over time in very low-birth-weight infants. JAMA. 2003;289:705–711. doi: 10.1001/jama.289.6.705. [DOI] [PubMed] [Google Scholar]