Abstract
Background and Objectives
In this study, we examined the role of inflammatory parameters in an apical mural thrombus with a reduced ejection fraction due to large anterior myocardial infarction (MI).
Subjects and Methods
A total of 103 patients who had suffered from heart failure, 45 of whom had left ventricular apical thrombus (AT) after a large anterior MI, were enrolled in the study. A detailed clinical history was taken of each participant, biochemical inflammatory markers, which were obtained during admission, were analyzed and an echocardiographical and angiographical evaluation of specific parameters were performed.
Results
There were no statistically significant differences in terms of age, gender, and history of hypertension, diabetes mellitus, and atrial fibrillation between both groups (p>0.05). Similarly there were no statistically significant differences in terms of biochemical and echocardiographic parameters (p>0.05). However, there were significant differences in terms of neutrophil lymphocyte ratio (p=0.032). After a multivariate regression analysis, neutrophil lymphocyte ratio (NLR) was an independent predictor of thrombus formation (β: 0.296, p=0.024). The NLR >2.74 had a 78% sensivity and 61% specifity in predicting thrombus in patients with a low left ventricular ejection fraction.
Conclusion
In this study, neutrophil lymphocyte ratios were significantly higher in patients with apical thrombus.
Keywords: Inflammation, Thrombus, Myocardial infarction
Introduction
Left ventricular apical thrombus (AT) is one of the major complications after a myocardial infarction (MI).1) The incidence of AT is reported to be 30-40% as in postmortem.1) In most of the cases, left ventricular AT accompanies wall motion disorders such as ischemic and nonischemic heart diseases.1) Hypercoagulable or inflammatory states might rarely predispose to thrombus formation.2) It usually occurs with a large anterior MI, particularly in the presence of a left ventricular aneurysm and apical akinesia.1) The AMI represents a good model for studying thrombus formation since all pre-requisites for thrombus formation are present in this particular setting (i.e. endothelial injury, hypercoagulable state, and blood stasis). Reduced left ventricular ejection fraction (LVEF) after acute myocardial infarction (AMI) is known to be correlated strongly with AT and most often occurs as a result of anterior and apical AMI.3),4),5),6)
Neutrophil lymphocyte ratio (NLR) has been suggested as an important and cheap prognostic factor in patients with coronary heart disease (CHD).7),8) In addition, neutrophilia and relative lymphocytopenia were shown to be independent predictors of mortality in patients with heart failure9),10) as neutrophils play an important role in tissue destruction. In recent years, several studies have show the crucial role of neutrophils in thrombosis.9),10),11) Therefore, neutrophils might enhance or trigger thrombotic events via production and expression of tissue factor (TF).12)
Hence, the aim of this study was to determine the role of neutrophil to lymphocyte ratios (NLR) in an apical mural thrombus among post large anterior MI patients with reduced ejection fraction.
Subjects and Methods
In this study, 45 patients aged 58 with a left ventricular AT after a large anterior MI and ejection fraction, and sex-matched patients without a left ventricular AT after a large anterior MI were used as a control group and enrolled retrospectively. The exclusion criteria were the presence of infection, nonischemic cardiomyopathy, current therapy with corticosteroid, non steroidal anti-inflamatory drugs, warfarin and heparin derivatives, hematological disorders, and connective tissue diseases.
Blood samples were drawn from a large antecubital vein for determination of biochemical and hemostatic parameters (Symex K-1000, Kobe, Japan) on admission. All routine biochemical tests were performed using an auto-analyzer (Roche Diagnostic Modular Systems, Tokyo, Japan). The NLR was defined as the absolute neutrophil count in peripheral blood divided by total lymphocyte count, and the platelet to lymphocyte ratio (PLR) was defined as the absolute platelet count in the peripheral blood divided by the total lymphocyte count
The echocardiographic features of patients which had been performed were evaluated after 6 weeks of MI. A two-dimensional echocardiography was performed with a 3.5 MHz transducer (IE33, Philips Medical Systems, Andover, MA, USA). The left atrial diameter was assessed in a parasternal short axis view. Simpson's method was used to asses the left ventricular ejection fraction in a two-dimensional echocardiographic apical four chamber view, as recommended by the American society of echocardiography guidelines.13) All images had been archived and were evaluated by independent cardiologists who were blinded to each patient's data.
Statistical analysis
After assessing data compatibility with normal distribution using the Kolmogorov-Smirnov test, a Student's t-test was used to compare normally distributed data between groups, and the Mann-Whitney U test was used for nonnormally distributed data. The chi-square test was used in the investigation of categoric variables. A multiple linear regression analysis was performed for parameters affecting the presence of thrombus. Normally distributed data are expressed as mean±standard deviations and non-normally distributed data are expressed as a percentage. Receiver operating characteristic (ROC) curve analysis was performed to detect the cut-off value of the NLR ratio in predicting thrombus. All statistical analyses were performed with SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA). The p<0.05 was accepted as significant.
Results
The mean age of the patients was 62.52±13.56 years, while that of the control group was 61.49±7.88 years. Demographical, echocardiographical and angiographical parameters of patients are shown in Table 1. Echocardiographic parameters (left ventricular ejection fraction, left atrium diameter) did not demonstrate significant differences between the two groups as shown in Table 1. Embolic events were higher in patients with apical thrombus (p=0.048). There were no differences in terms of electrocardiography (ECG) changes for aneurysmal formation and zone of infarct relater artery (p=0.260 and p>0.005, respectively). There were no statistically significant differences between the two groups, except for smoking and embolic events (p=0.037 and p=0.048, respectively). The complete blood count parameters and biochemical parameters are given in Table 2. There were no significant differences between the two groups, except for NLR (3.84±1.6 in group 1, 2.85±1.6 in group 2, p=0.009).
Table 1. Demographical, echocardiographical and angiographical characteristics of study subjects and controls.
| Variables | Thrombus (+) patients (n=45) | Thrombus (−) patients (n=58) | p |
|---|---|---|---|
| Gender (female) (n, %) | 8 (17) | 12 (20.6) | 0.734 |
| Age (years±SD) | 62.52±13.56 | 61.49±7.88 | 0.803 |
| Diabetes mellitus (n, %) | 15 (33) | 10 (17) | 0.342 |
| Hypertension (n, %) | 34 (75) | 21 (36) | 0.125 |
| Coronary heart disease (n, %) | 41 (91) | 58 (100) | 0.089 |
| PCI (n, %) | 26 (57) | 32 (55) | 0.812 |
| CABG (n, %) | 14 (31) | 23 (39) | 0.351 |
| Atrial fibrillation (n, %) | 2 (0.4) | 1 (0.1) | 0.341 |
| Heart failure (n, %) | 45 (100) | 58 (100) | 1 |
| Smoking (n, %) | 34 (75) | 28 (48) | 0.029 |
| ACE blokers (n, %) | 45 (100) | 58 (100) | 1 |
| Aldosterone ant. (n, %) | 45 (100) | 58 (100) | 1 |
| Beta bloker (n, %) | 45 (100) | 58 (100) | 1 |
| Antiplatelet agents (n, %) | 45 (100) | 58 (100) | 1 |
| Anticoagulant (n, %) | 2 (0.2) | 1 (0.1) | 0.560 |
| Diuretics (loop diuretic) (n, %) | 45 (100) | 58 (100) | 1 |
| LV end diastolic diameter (cm±SD) | 5.59±0.89 | 5.57±0.88 | 0.540 |
| LV end systolic diameter (cm±SD) | 4.47±1.04 | 4.41±1.12 | 0.982 |
| LV ejection fraction (%±SD) | 28.63±11.69 | 29.19±12.16 | 0.822 |
| Left atrial diameter (cm±SD) | 4.49±0.78 | 4.28±0.47 | 0.220 |
| ECG changes for aneurysmal formation | 37 (82) | 45 (77) | 0.260 |
| LAD proksimal | 30 (66) | 25 (43) | 0.101 |
| LAD mid | 8 (17) | 16 (27) | 0.208 |
| LAD distal | 7 (15) | 17 (29) | 0.352 |
| Embolic events (stroke or TIA) | 8 (17) | 3 (0.5) | 0.048 |
Values are presented as mean±SD or number (%). PCI: percutaneous coronary intervention, CABG: coronary artery bypass graft, ACE: angiotensine converting enzyme, LV: left ventricle, ECG: electrocardiography, LAD: left anterior descending artery, TIA: transient ischemic attack
Table 2. Biochemical and hematological characteristics of study subjects and controls.
| Variables | Thrombus (+) patients (n=45) | Thrombus (−) patients (n=58) | p |
|---|---|---|---|
| Glucose (mg/dL±SD) | 141.36±73.53 | 139.22±48.43 | 0.837 |
| Creatinine (mg/dL±SD) | 1.31±1.49 | 1.09±0.67 | 0.210 |
| Sodium (mmol/dL±SD) | 138.47±3.37 | 138.64±1.29 | 0.480 |
| Potasium (mmol/dL±SD) | 4.43±0.63 | 4.52±0.53 | 0.464 |
| Total cholesterol (mg/dL±SD) | 171.53±44.82 | 186.41±53.44 | 0.622 |
| LDL cholesterol (mg/dL±SD) | 102.09±37.51 | 121.03±37.21 | 0.510 |
| HDL cholesterol (mg/dL±SD) | 38.09±10.80 | 54.77±25.67 | 0.180 |
| Triglyseride (mg/dL±SD) | 145.39±58.41 | 149.40±72.41 | 0.341 |
| Hemoglobine (g/L±SD) | 14.17±2.11 | 14.28±2.12 | 0.510 |
| Hematocrite (%±SD) | 42.34±4.92 | 42.52±3.32 | 0.349 |
| White blood cell (103/µL±SD) | 9.33±4.05 | 10.90±9.40 | 0.330 |
| Platelet (103/mm3±SD) | 230.04±55.59 | 254.15±52.46 | 0.724 |
| Glomeruler filtration rate (ml/min/1.73 m2±SD) | 49.5±4.5 | 58.6±2.3 | 0.124 |
| Neutrophil lymphocyte ratio (%±SD) | 3.84±1.6 | 2.85±1.6 | 0.003 |
| Platelet lymphocyte ratio (%±SD) | 131±64 | 130±62 | 0.508 |
Values are presented as mean±SD. LDL: low density lipoprotein cholesterol, HDL: high density lipoprotein cholesterol, SD: standard deviation
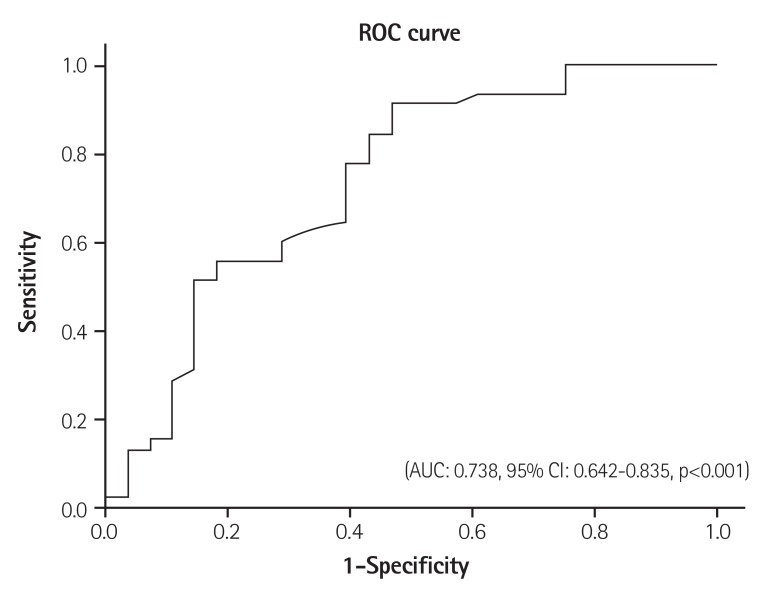
As shown in Table 3, after a multivariate linear analysis, NLR, the left anterior descending artery (LAD) proximal lesion, ECG changes for aneurysmal formation and smoking were independent predictors of apical thrombus (β: 0.296, p=0.024; β: 0.286, p=0.013; β: 0.482, p<0.001; β: 0.305, p=0.012, respectively). The NLR >2.74 (area under curve [AUC]: 0.738, 95% CI: 0.642-0.835, p<0.001) had a 78% sensivity and 61% specifity in predicting thrombus in patients with low left ventricular ejection fraction (Fig. 1).
Table 3. Multivariate regression analysis of confounding variables to predict apical thrombus.
| Multivariate regression | ||
|---|---|---|
| β | p | |
| NLR | 0.296 | 0.024 |
| PLR | 0.092 | 0.530 |
| LVEF | 0.102 | 0.103 |
| LAD proximal lesion | 0.286 | 0.013 |
| ECG changes | 0.482 | <0.001 |
| Smoking | 0.305 | 0.012 |
| Age | 0.106 | 0.277 |
| DM | 0.217 | 0.076 |
| HT | 0.248 | 0.067 |
| HDL cholesterol | −0.086 | 0.847 |
| LDL cholesterol | 0.180 | 0.112 |
NLR: neutrophil lymphocyte ratio, PLR: platelet lymphocyte ratio, LVEF: left ventricle ejection fraction, LAD: left anterior descending artery, ECG: electrocardiography, DM: diabetes mellitus, HT: hypertension, LVEF: left ventricular ejection fraction
Fig. 1. Sensitivity and specificity of neutrophil lymphocyte ratios for apical thrombus. ROC: receiver operating characteristic, AUC: area under curve, CI: confidence interval.
Discussion
In this study, we showed that NLR was significantly higher in patients with apical thrombus after a large anterior myocardial infarction than without apical thrombus. NLR was statistically significant independent predictors of left ventricular AT. The NLR >2.74 had a 78% sensivity and 61% specifity in predicting thrombus. In our study, we also showed that the zone of infarct related lesion at an LAD artery and ECG changes for aneurysmal formation are important for apical thrombus formation. There variables were independent predictor of thrombus formation.
Intracardiac AT forms as a result of low flow and inflammation states, including AMI and severe cardiomyopathy.1) AT is also shown in patients with severe congestive heart failure.14),15) Bakalli et al.15) demonstrated that 13.3% of patients with dilated cardiomyopathy with sinus rhythm has AT and they also identified a significant correlation between thrombus formation and reduced LVEF.
Beside the low LVEF, inflammatory states play an important role in the thrombotic process. Erythrocyte sedimation rate (ESR), c-reactive protein (CRP) and NLR, as indicators of systemic inflammation, have been studied in a great number of epidemiologic studies.16),17) After an MI, neutrophilia may be associated with the acute inflammatory response to tissue injury.18),19) Ommen et al.20) showed a reduced number of circulating lymphocytes during MI and Horne et al.21) described that the NLR has the highest predictive value for death/MI in patients with coronary artery disease.
Because of the routine use and inexpensive nature of complete blood count, various studies suggested NLR as a valuable tool in a wide spectrum of disorders. Doğan et al.22) demonstrated that NLR is independently associated with saphenous venous graft disease.
Tokgoz et al.23) showed the relationship between NLR and short term mortality in acute stroke. Yılmaz et al.24) reported that NLR was significantly increased in patients with coronary thrombus formation in non-ST segment elevated acute coronary syndrome. Gazi et al.25) showed that a high NLR is a strong and independent predictor of inpatient mortality among patients with ST-segment elevated MI. Yayla et al.26) demonstrated that PLR is independently associated with saphenous venous graft disease. In our study, we found that NLR levels are significantly different between two groups despite a similar ejection fraction, but we couldn't find any relation between PLR and thrombus formation.
This study had some limitations. It was a single center, and retrospective study with a small sample size. One major limitation was the use of a single sample of blood parameter. It might be conclusive, because acute coronary syndrome leads to activation of various blood parameters. The lack of examination of CRP, which is a highly sensitive marker, might be more predictable for thrombus formation, because its value in patients with cardiovascular disease is higher as shown in previous studies.
In this study, NLR was significantly greater in the AT (+) group than control (-) group and these inflammatory marker were independently associated with AT risk among our patients. These data suggested that the inflammatory process (as expressed by NLR) might have an important role in AT after ischemic heart disease. Therefore, our results should be consolidated further with large-scale prospective trials in order to extrapolate our findings for similar patient groups.
In conclusion, this simple blood count analysis, which is a cheap and approachable test, might indicate ischemic heart disease patients who are at greater risk for apical thrombus formation and may help to determine usage and timing of an anticoagulation.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Sharma ND, McCullough PA, Philbin EF, et al. Left ventricular thrombus and subsequent thromboembolism in patients with severe systolic dysfunction. Chest. 2000;117:314–320. doi: 10.1378/chest.117.2.314. [DOI] [PubMed] [Google Scholar]
- 2.Schneider C, Bahlmann E, Heuser C, et al. Images in cardiovascular medicine. Unusual biventricular thrombus formation in acute myeloid leukemia and factor V Leiden mutation. Circulation. 2003;107:e114–e116. doi: 10.1161/01.CIR.0000061023.00510.BF. [DOI] [PubMed] [Google Scholar]
- 3.Thomson SP, Gibbons RJ, Smars PA, et al. Incremental value of the leukocyte differential and the rapid creatine kinase-MB isoenzyme for the early diagnosis of myocardial infarction. Ann Intern Med. 1995;122:335–341. doi: 10.7326/0003-4819-122-5-199503010-00003. [DOI] [PubMed] [Google Scholar]
- 4.Delewi R, Zijlstra F, Piek JJ. Left ventricular thrombus formation after acute myocardial infarction. Heart. 2012;98:1743–1749. doi: 10.1136/heartjnl-2012-301962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johannessen KA, Nordrehaug JE, von der Lippe G. Increased occurrence of left ventricular thrombi during early treatment with timolol in patients with acute myocardial infarction. Circulation. 1987;75:151–155. doi: 10.1161/01.cir.75.1.151. [DOI] [PubMed] [Google Scholar]
- 6.Asinger RW, Mikell FL, Elsperger J, Hodges M. Incidence of leftventricular thrombosis after acute transmural myocardial infarction. Serial evaluation by two-dimensional echocardiography. N Engl J Med. 1981;305:297–302. doi: 10.1056/NEJM198108063050601. [DOI] [PubMed] [Google Scholar]
- 7.Visser CA, Kan G, Lie KI, Durrer D. Left ventricular thrombus following acute myocardial infarction: a prospective serial echocardiographic study of 96 patients. Eur Heart J. 1983;4:333–337. doi: 10.1093/oxfordjournals.eurheartj.a061470. [DOI] [PubMed] [Google Scholar]
- 8.Han YC, Yang TH, Kim DI, et al. Neutrophil to Lymphocyte ratio predicts long-term clinical outcomes in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Korean Circ J. 2013;43:93–99. doi: 10.4070/kcj.2013.43.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arruda-Olson AM, Reeder GS, Bell MR, Weston SA, Roger VL. Neutrophilia predicts death and heart failure after myocardial infarction: a community-based study. Circ Cardiovasc Qual Outcomes. 2009;2:656–662. doi: 10.1161/CIRCOUTCOMES.108.831024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudiger A, Burckhardt OA, Harpes P, Müller SA, Follath F. The relative lymphocyte count on hospital admission is a risk factor for longterm mortality in patients with acute heart failure. Am J Emerg Med. 2006;24:451–454. doi: 10.1016/j.ajem.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Looney MR, Su X, Van Ziffle JA, Lowell CA, Matthay MA. Neutrophils and their Fc gamma receptors are essential in a mouse model of transfusion-related acute lung injury. J Clin Invest. 2006;116:1615–1623. doi: 10.1172/JCI27238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rapaport SI, Rao LV. The tissue factor pathway: how it has become a "prima ballerina". Thromb Haemost. 1995;74:7–17. [PubMed] [Google Scholar]
- 13.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Roberts WC, Siegel RJ, McManus BM. Idiopathic dilated cardiomyopathy: analysis of 152 necropsy patients. Am J Cardiol. 1987;60:1340–1355. doi: 10.1016/0002-9149(87)90618-7. [DOI] [PubMed] [Google Scholar]
- 15.Bakalli A, Georgievska-Ismail L, Koçinaj D, Musliu N, Krasniqi A, Pllana E. Prevalence of left chamber cardiac thrombi in patients with dilated left ventricle at sinus rhythm: the role of transesophageal echocardiography. J Clin Ultrasound. 2013;41:38–45. doi: 10.1002/jcu.21953. [DOI] [PubMed] [Google Scholar]
- 16.Papa A, Emdin M, Passino C, Michelassi C, Battaglia D, Cocci F. Predictive value of elevated neutrophil-lymphocyte ratio on cardiac mortality in patients with stable coronary artery disease. Clin Chim Acta. 2008;395:27–31. doi: 10.1016/j.cca.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Momiyama Y, Kawaguchi A, Kajiwara I, et al. Prognostic value of plasma high-sensitivity C-reactive protein levels in Japanese patients with stable coronary artery disease: the Japan NCVC-Collaborative Inflammation Cohort (JNIC) Study. Atherosclerosis. 2009;207:272–276. doi: 10.1016/j.atherosclerosis.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Doğdu O, Akpek M, Yarlıoğlueş M, et al. Relationship between hematologic parameters and left ventricular systolic dysfunction in stable patients with multi-vessel coronary artery disease. Turk Kardiyol Dern Ars. 2012;40:706–713. doi: 10.5543/tkda.2012.82429. [DOI] [PubMed] [Google Scholar]
- 19.Jala VR, Haribabu B. Leukotrienes and atherosclerosis: new roles for old mediators. Trends Immunol. 2004;25:315–322. doi: 10.1016/j.it.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Ommen SR, Hodge DO, Rodeheffer RJ, McGregor CG, Thomson SP, Gibbons RJ. Predictive power of the relative lymphocyte concentration in patients with advanced heart failure. Circulation. 1998;97:19–22. doi: 10.1161/01.cir.97.1.19. [DOI] [PubMed] [Google Scholar]
- 21.Horne BD, Anderson JL, John JM, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45:1638–1643. doi: 10.1016/j.jacc.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 22.Doğan M, Akyel A, Cimen T, et al. Relationship between neutrophilto-lymphocyte ratio and saphenous vein graft disease in patients with coronary bypass. Clin Appl Thromb Hemost. 2015;21:25–29. doi: 10.1177/1076029613488935. [DOI] [PubMed] [Google Scholar]
- 23.Tokgoz S, Kayrak M, Akpinar Z, et al. Neutrophil lymphocyte ratio as a predictor of stroke. J Stroke Cerebrovasc Dis. 2013;22:1169–1174. doi: 10.1016/j.jstrokecerebrovasdis.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Yilmaz M, Tenekecioglu E, Arslan B, et al. White blood cell subtypes and neutrophil-lymphocyte ratio in prediction of coronary thrombus formation in non-ST-segment elevated acute coronary syndrome. Clin Appl Thromb Hemost. 2015;21:446–452. doi: 10.1177/1076029613507337. [DOI] [PubMed] [Google Scholar]
- 25.Gazi E, Bayram B, Gazi S, et al. Prognostic Value of the Neutrophil-Lymphocyte Ratio in Patients With ST-Elevated Acute Myocardial Infarction. Clin Appl Thromb Hemost. 2015;21:155–159. doi: 10.1177/1076029613492011. [DOI] [PubMed] [Google Scholar]
- 26.Yayla Ç, Canpolat U, Akyel A, et al. Association Between Platelet to Lymphocyte Ratio and Saphenous Vein Graft Disease. Angiology. 2016;67:133–138. doi: 10.1177/0003319715578258. [DOI] [PubMed] [Google Scholar]