Abstract
Dengue virus infection affects the heart structurally and functionally. Clinical manifestations of cardiac complications secondary to dengue virus infection vary from self-limiting arrhythmias to severe myocardial infarction, leading to hypotension, pulmonary edema, and cardiogenic shock. However, we report a case of dengue hemorrhagic fever (DHF) complicated by a complete heart block. A female with DHF due to dengue virus serotype 2, presented to the emergency department with fever, headache, rash, and fatigue followed by an episode of syncope. She was found to have a third-degree atrioventricular block, with pulseless polymorphic ventricular tachycardia. Patient was resuscitated and a temporary trans-venous pacemaker was placed. She reverted back to normal sinus rhythm after 4 days of syncope and was subsequently discharged from the hospital after complete resolution of symptoms, without the need for a permanent pacemaker. Physicians are warranted to have high index of suspicion for dengue virus infection as an etiology in patients with acute cardiovascular compromise, especially in tropical areas.
Keywords: Dengue hemorrhagic fever, Heart block
Introduction
Dengue is the most prevalent viral disease transmitted by mosquitoes, producing clinical illness in 96 million cases annually.1) Most of the dengue virus infections are symptomatic and can present with a wide range of clinical manifestations, from mild febrile illness to life-threatening dengue shock syndrome.2) Aside from systemic manifestations, variable cardiac complications have been reported including atrioventricular conduction disorders, supraventricular arrhythmias, myocarditis, and cardiogenic shock. We report this unusual case of complete heart block secondary to dengue hemorrhagic fever (DHF) and discuss the implications of cardiac complications in dengue patients, understanding of which is essential in improving morbidity and mortality.
Case
A 31-year-old female presented to the Emergency Department of Mount Sinai St. Luke's and Mount Sinai Roosevelt Hospitals with low grade fever, headaches, arthralgia/myalgia, and fatigue one day after returning from a trip to Brazil. The patient denied any significant past medical problems, sick contacts, illicit drug abuse, or any family history of cardiovascular diseases. Upon arrival, she was febrile to 38.5℃, tachycardiac with the heart rate 110/min, respiratory rate 20/min, and blood pressure of 110/70 mmHg. Physical examination was notable for maculopapular rash on all extremities and a positive tourniquet test.
On laboratory evaluation, her white cell count was 10.7×109/L (4-11×109/L) with 72% polymorphonuclear cells, hemoglobin 14 g/dL (12.5-14.5 g/dL in females), hematocrit 41.7% (35-45%), platelet count 28.0×109/L (150-400×109/L), prothrombin time 10.2 s (control, 10.6 s), and activated partial thromboplastin time was 71.4 s (control, 30.2 s). A hepatic panel revealed elevated levels of aspartate aminotransferase to 1810 IU/L (<40 IU/L), alanine transaminase 1280 IU/L (<40 IU/L), alkaline phosphatase to 750 IU/L (<150 IU/L) and a decreased albumin level to 2.3 g/dL (>4 g/dL). On serology, dengue virus specific IgM antibodies were positive and the IgG titer during active infection in our subject was 1:1160. In our patient, electrocardiogram was normal at the time of initial presentation with no ST-T wave abnormalities (Fig. 1) and troponins were indeterminate. A chest radiograph showed a moderate right pleural effusion and normal cardiac silhouette. History and physical examination did not suggest congestive heart failure. Thereby, diagnosis of DHF was made, based on the findings of fever, thrombocytopenia, pleural effusion, and bleeding tendency (positive tourniquet sign).
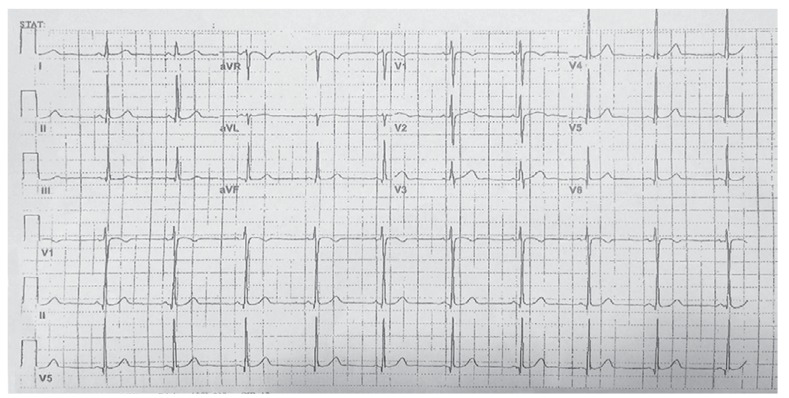
Fig. 1. Baseline normal sinus rhythm.
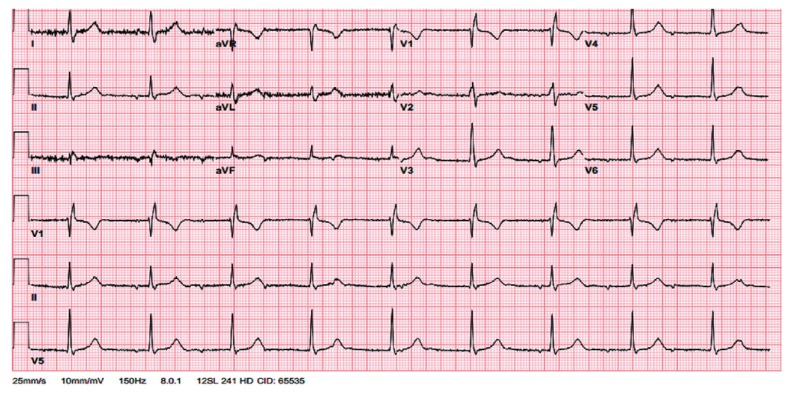
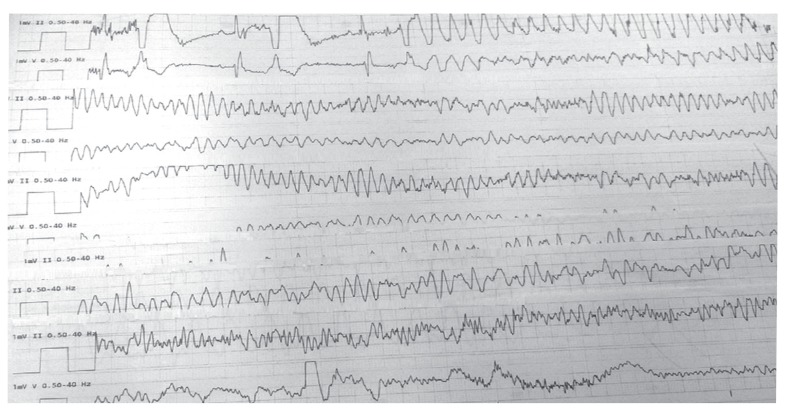
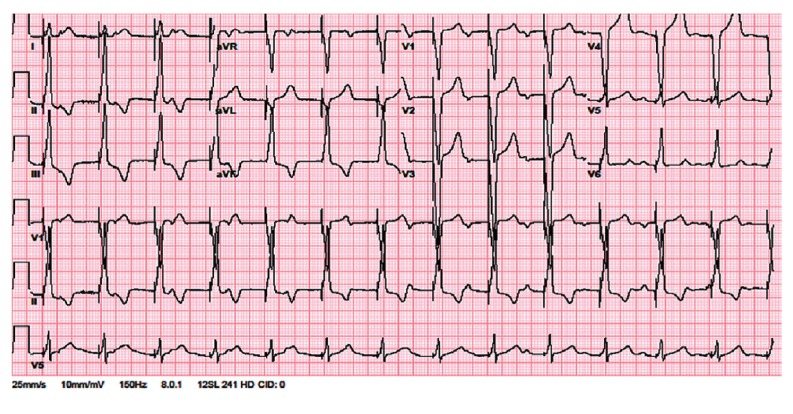
Subsequently, aggressive intravenous fluid resuscitation with isotonic saline was initiated. Follow-up chest X-ray was performed the next day, which did not show worsening of the pleural effusion. The patient remained hemodynamically stable and showed an increased platelet count to 66.2×109/L. On the third day of admission, she had a syncopal episode while walking. Initially, there was a concern of progression of DHF to dengue shock syndrome, but her vitals were stable. Physical examination was unchanged and no electrolyte abnormality was observed. The electrocardiogram revealed a third degree heart block (Fig. 2). She was transferred to the cardiac care unit for close monitoring. On the fifth day of hospital admission, the patient became unresponsive with no pulse, a code blue was called and cardiopulmonary resuscitation (CPR) was initiated. The cardiac monitor showed polymorphic ventricular tachycardia with a run of torsade (Fig. 3). During torsade de pointes, a laboratory evaluation revealed potassium 3.8 mmol/L (3.5-5.1 mmol/L), magnesium 1.9 mmol/L (1.6-2.3 mmol/L), and calcium 8.5 mg/dL (8.4-10.3 mg/dL). Furthermore, none of the QTc interval prolonging drugs were administered at torsade de pointes. A return of spontaneous circulation (ROSC) was achieved after three cycles of CPR including defibrillation, administration of epinephrine and intravenous (IV) magnesium. After ROSC, the patient was hypotensive to 70's/40's, so she was started on an IV infusion of dopamine, and a temporary, trans-venous, atrial sensed ventricular pacemaker was placed (Fig. 4). On day six of admission, the patient started recovering and was titrated off the dopamine drip. Transthoracic echocardiography demonstrated a normal ejection fraction with no wall motion or valvular abnormality. On day seven, she reverted back to normal sinus rhythm. After close monitoring for 24 hours, the trans-venous pacemaker was removed. The patient remained afebrile, her platelet count improved to >100000/L and the right sided pleural effusion started to resolve on follow-up chest X-rays. Thereafter, no further syncopal episodes were noted and her blood pressure was stable with good peripheral perfusion. She received a total of five liters of isotonic saline in this hospital admission.
Fig. 2. Complete/third-degree heart block following syncopal attack.
Fig. 3. Polymorphic ventricular tachycardia (V-tach) without pulse.
Fig. 4. After achieving return of spontaneous circulation and placing temporary trans-venous pacemaker.
Blood cultures were negative for bacterial growth. A reverse transcriptase-polymerase chain reaction was positive for dengue virus serotype 2 (DENV-2). Clinically, the patient improved significantly and refused further coronary angiographic investigations. Subsequently, she was discharged from the hospital with cardiology follow-up appointments.
Discussion
Like many viral diseases, dengue virus has a predilection to affect the cardiovascular system which may not always be benign and can increase morbidity and mortality. Cardiac involvement can be asymptomatic.3) or can present with bradycardia,4) atrial fibrillation,5) Mobitz type II second degree heart block,6) and transient ventricular arrhythmia7) or with acute pulmonary edema and cardiogenic shock due to severe myocardial cell damage with left ventricular failure.8),9) These symptoms can occur in the febrile phase of the disease or during the resolution phase. But, regardless of the phase, myocardial compromise is more severe in DHF and dengue shock syndrome (DSS) as compared to dengue fever.10)
Previously, DENV-2 and DENV-3 are reported to be the causative serotypes in patients with cardiac complications.3) Our patient had DENV-2 serotype as well. Agarwal et al.11) reported that only 1 out of 206 patients experienced cardiac symptoms after complete cardiovascular evaluation. Wali et al.12) demonstrated that 70% of 17 patients with DHF/DSS who underwent myocardial scintigraphic studies suffered diffuse left ventricular hypokinesis with a mean ejection fraction of 40%. A report from Sri Lanka showed that 62.5% of 120 adults with dengue fever had associated abnormalities in respective electrocardiograms.8) It can be safely assumed that cardiovascular complications of DF are under-diagnosed because they are mild and reversible in most of the cases without the need for further intervention.
Our case demonstrates one of the rare yet fatal complications, complete heart block secondary to DHF. Unlike previously reported cases in the literature, a complete heart block in our patient was not preceded by bradycardia or first degree heart block.13),14) Bradycardia is commonly observed in the convalescence phase due to predominant parasympathetic activity. It can potentially proceed to other arrhythmias including complete heart block. This rare finding in our case demonstrates that physicians, particularly in tropical and sub-tropical areas, should closely monitor patients with DHF for any cardiovascular compromise which can be an initial indicator of more severe problems ranging from arrhythmias to fulminant myocarditis.15)
From the pathophysiological standpoint, viral tropism towards cardiac muscle causes myocarditis. The resultant cytokine storm causes the inflammation which can damage the heart structurally and functionally, making it a substrate for arrhythmias.16) Furthermore, owing to low levels of platelets, there remains a bleeding tendency in or near the sinoatrial or atrioventricular node that can lead to conduction abnormalities and arrhythmias. Transient abnormalities can occur due to altered autonomic tone, adenosine metabolism, and electrolyte abnormalities. These electrolyte abnormalities include low serum levels of calcium, sodium, and potassium. Hypocalcaemia, as noted in our patient, has been commonly seen in DHF even after correction for low albumin levels due to leakage into the third space. It can lengthen the ST segment and prolong the QT interval posing a risk for atrial and ventricular arrhythmias including Torsade de pointes.17) Hyponatremia is also common in dengue virus infections due to salt deficiencies, renal loss, redistribution into cells due to sodium-potassium pump dysfunction, and excess of water due to reduced renal excretion.18) Hyponatremia associated conduction abnormalities include first degree, second degree, and even complete heart block.19) Hypokalemia is hypothesized to be secondary to the redistribution of potassium into cells, renal loss secondary to renal tubular injury and/or catecholamine surge seen in the viral infections.20) It causes an increase in resting membrane potential resulting in a prolonged duration of the action potential and refractory period, leading to the genesis of re-entrant arrhythmias. Increasing the threshold potential and automaticity is another mechanism by which hypokalemia may cause rhythm disturbances.20)
In conclusion, early diagnosis of dengue hemorrhagic fever related cardiac complication is essential. Physicians should focus on ruling out other reversible causes of cardiac dysfunction with assessment and replenishing the electrolyte abnormalities before interventions like cardiac pacing are considered. Unnecessary invasive procedures, like permanent pacemaker placement, can be avoided if the transient nature of these possible cardiovascular complications is kept in mind and supportive care provided. Therefore, the morbidity and mortality associated with the invasive measures can be significantly decreased. These possible cardiovascular complications is kept in mind and supportive care provided, thus decreasing the morbidity and mortality associated with invasive measures. However, definite recommendations and management protocols are warranted in order to help physicians diagnose, and manage these potential life-threatening complications associated with dengue hemorrhagic fever. Hence, this article recommends further studies to address this important aspect of care in the management of dengue hemorrhagic fever.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teixeira MG, Barreto ML. Diagnosis and management of dengue. BMJ. 2009;339:b4338. doi: 10.1136/bmj.b4338. [DOI] [PubMed] [Google Scholar]
- 3.Satarasinghe RL, Arultnithy K, Amerasena NL, Bulugahapitiya U, Sahayam DV. Asymptomatic myocardial involvement in acute dengue virus infection in a cohort of adult Sri Lankans admitted to a tertiary referral centre. Br J Cardiol. 2007;14:171–173. [Google Scholar]
- 4.Lateef A, Fisher DA, Tambyah PA. Dengue and relative bradycardia. Emerg Infect Dis. 2007;13:650–651. doi: 10.3201/eid1304.061212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horta Veloso H, Ferreira Júnior JA, Braga de Paiva JM, et al. Acute atrial fibrillation during dengue hemorrhagic fever. Braz J Infect Dis. 2003;7:418–422. doi: 10.1590/s1413-86702003000600010. [DOI] [PubMed] [Google Scholar]
- 6.Khongphatthallayothin A, Chotivitayatarakorn P, Somchit S, Mitprasart A, Sakolsattayadorn S, Thisyakorn C. Morbitz type I second degree AV block during recovery from dengue hemorrhagic fever. Southeast Asian J Trop Med Public Health. 2000;31:642–645. [PubMed] [Google Scholar]
- 7.Chuah SK. Transient ventricular arrhythmia as a cardiac manifestation in dengue haemorrhagic fever--a case report. Singapore Med J. 1987;28:569–572. [PubMed] [Google Scholar]
- 8.Kularatne SA, Pathirage MM, Kumarasiri PV, Gunasena S, Mahindawanse SI. Cardiac complications of a dengue fever outbreak in Sri Lanka, 2005. Trans R Soc Trop Med Hyg. 2007;101:804–808. doi: 10.1016/j.trstmh.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 9.Lee CH, Teo C, Low AF. Fulminant dengue myocarditis masquerading as acute myocardial infarction. Int J Cardiol. 2009;136:e69–e71. doi: 10.1016/j.ijcard.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 10.Khongphatthanayothin A, Lertsapcharoen P, Supachokchaiwattana P, et al. Myocardial depression in dengue hemorrhagic fever: prevalence and clinical description. Pediatr Crit Care Med. 2007;8:524–529. doi: 10.1097/01.PCC.0000288672.77782.D4. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal R, Kapoor S, Nagar R, et al. A clinical study of the patients with dengue hemorrhagic fever during the epidemic of 1996 at Lucknow, India. Southeast Asian J Trop Med Public Health. 1999;30:735–740. [PubMed] [Google Scholar]
- 12.Wali JP, Biswas A, Chandra S, et al. Cardiac involvement in dengue haemorrhagic fever. Int J Cardiol. 1998;64:31–36. doi: 10.1016/s0167-5273(98)00008-4. [DOI] [PubMed] [Google Scholar]
- 13.Lim SMS, Hoo FK, Sulaiman WAW. A case of dengue hemorrhagic fever with myocarditis and complete heart block. RMJ. 2014;39:104–106. [Google Scholar]
- 14.Martha JW. Total atrioventricular block due to Dengue myocarditis. J Cardiovasc Electrophysiol. 2009;20(S59):1045–3873. [Google Scholar]
- 15.La-Orkhun V, Supachokchaiwattana P, Lertsapcharoen P, Khongphatthanayothin A. Spectrum of cardiac rhythm abnormalities and heart rate variability during the convalescent stage of dengue virus infection: a Holter study. Ann Trop Paediatr. 2011;31:123–128. doi: 10.1179/1465328111Y.0000000008. [DOI] [PubMed] [Google Scholar]
- 16.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nijjer S, Ghosh AK, Dubrey SW. Hypocalcaemia, long QT interval and atrial arrhythmias. BMJ Case Rep. 2010;2010:bcr0820092216. doi: 10.1136/bcr.08.2009.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mekmullica J, Suwanphatra A, Thienpaitoon H, et al. Serum and urine sodium levels in dengue patients. Southeast Asian J Trop Med Public Health. 2005;36:197–199. [PubMed] [Google Scholar]
- 19.Mouallem M, Friedman E, Shemesh Y, Mayan H, Pauzner R, Farfel Z. Cardiac conduction defects associated with hyponatremia. Clin Cardiol. 1991;14:165–168. doi: 10.1002/clc.4960140214. [DOI] [PubMed] [Google Scholar]
- 20.Kayal AK, Goswami M, Das M, Jain R. Clinical and biochemical spectrum of hypokalemic paralysis in North: East India. Ann Indian Acad Neurol. 2013;16:211–217. doi: 10.4103/0972-2327.112469. [DOI] [PMC free article] [PubMed] [Google Scholar]