Abstract
Background
To find association between prostate gland volume to components of the metabolic syndrome.
Methods
Cross-sectional, observational study in a tertiary care hospital of the Armed Forces of India. A total of 115 male patients aged 50–65 years attending the Urology OPD between Jan 2014 and July 2015 with lower urinary tract symptoms (LUTS) were included. Men with known malignant disease including carcinoma prostate, those on medical management for BPH and individuals with previous history of surgery related to urinary bladder/prostate were excluded. Blood Pressure (BP), weight in kgs, height, waist and hip circumference to nearest cm were recorded. Body Mass Index (BMI) and Waist/Hip ratio (WHR) were calculated. Fresh serum was analysed for lipid profile and glycaemic levels. The International Diabetes Federation (IDF) – 2005 guideline for metabolic syndrome was used for the diagnosis.
The total prostate volume and the severity of LUTS as per AUA Symptom index were considered as the primary and secondary outcome measure respectively. Statistical software SPSS version 20 was used for analysis. Mean prostate volume was compared with the components of MetS. An alpha level of 5% was considered significant.
Results
The study showed positive association between prostate volume with metabolic syndrome and its four components – raised blood pressure, fasting blood glucose and triglycerides and HDL ≤ 40 mg/dl. No correlation was found with waist circumference.
Conclusion
Our study indicates that metabolic syndrome and its individual components may predispose patients to a higher risk of prostatic enlargement/LUTS.
Keywords: Benign prostatic hyperplasia, Metabolic syndrome, Lower urinary tract symptoms, Prostate volume
Introduction
Amongst the elderly male, Benign prostatic hyperplasia (BPH) is one of the most common diseases. Besides other factors including inflammation, ageing and androgens play an important role in the aetiology of BPH. Although many studies have attributed obesity and increased Body Mass Index (BMI) with BPH, exact relationship is unclear.1 Metabolic Syndrome (MetS) has become a major public health problem worldwide today. Interest initially arose due to its association with cardiovascular diseases. However, of late, its secondary impact on lower urinary tract symptoms (LUTS) associated with BPH has attracted researchers’ attention. Besides being the diabetic capital of the world, India is having an ever increasing number of obese and hypertensive individuals. Due to genetic predisposition, Indians have high prevalence of MetS. Indian studies have shown their prevalence to be as high as 41% in Chennai.2 Although the relationship between BPH and MetS has been studied in western world since 1998, hardly any study existed highlighting the relationship in pan Indian population.3, 4, 5 The present study was initiated to find association between prostate gland volume to components of the MetS.
Materials and methods
This cross-sectional, observational study was performed on 94 male patients aged 50–65 years, who came to the Urology department between January 2014 and June 2015 with LUTS.
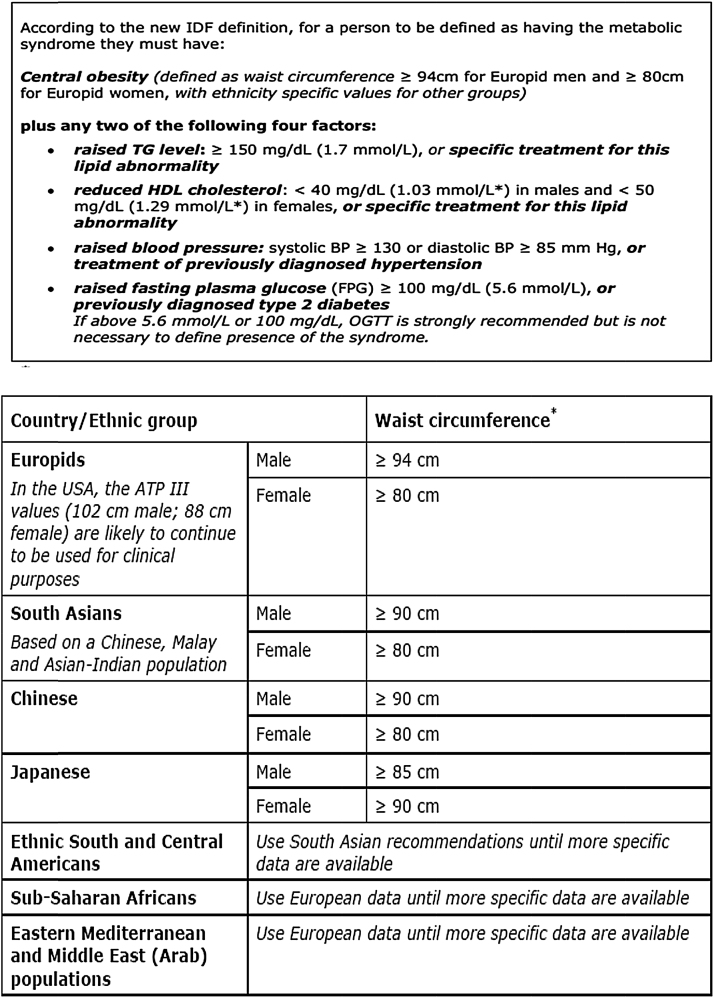
Data from a total of 115 men was collected during the study period. Those men with a history of any malignancy including prostate cancer (n = 04); those with incomplete data (n = 06); those taking 5∝Reductase Inhibitor (5ARI) medications that decrease prostate volume (n = 11) were excluded from the study. Individuals with previous history of surgery related to urinary bladder/prostate were excluded and any endocrinological disorders other than Type-2 Diabetes Mellitus were also excluded. This left 94 men for the study. Patients’ medical record, clinical findings and blood sugar levels provided the diagnosis of hypertension and diabetes mellitus. The following measurements were recorded: Blood Pressure (BP) to nearest mm Hg, weight in kgs, height to nearest 0.1 cm, waist and hip circumference to nearest cm. BMI and Waist/Hip ratio (WHR) were calculated. Fresh serum was analysed for lipid profile. International Diabetes Federation (IDF) – 2005 guideline for MetS was used for the diagnosis (Fig. 1). As per the IDF 2005 criteria for detection of MetS among Indian population, waist circumference more than or equal to 90 cm was considered as central obesity.
Fig. 1.
New International Diabetes Federation definition for metabolic syndrome along with ethnic variations.
The total prostate volume was considered the primary outcome measure. The gland was examined by digital rectal examination and volume was measured using ultrasound equipment applying Ellipsoid method.
International prostate Symptom score (IPSS) was used to determine the severity of LUTS which was considered the secondary outcome measure. With the total score running from 0 to 35 points, patients scoring 0 to 7, 8 to 19 and 20 to 35 points were classified as mild, moderate and severely symptomatic respectively.
Prostate biopsy was performed in men with a serum prostate-specific antigen (PSA) concentration more than 4.0 ng/ml as measured by ELISA and/or an abnormal digital rectal examination for the detection of prostate cancer. Statistical software SPSS version 20 was used for Statistical analysis. Mean Prostate Volume across age groups was compared.
Prostate Volume was expressed as Mean and Standard Deviation and compared across BMI, IPS score, waist circumference, BP, Fasting Blood Sugar, Triglyceride and HDL. An alpha level of 5% was taken, i.e. if any p value was less than 0.05, it was considered as significant.
Results
The age of the patients included in this study was between 50 and 65 years. They were stratified into three age groups 50–55, 56– 60 and 61– 65 with 23, 34 and 37 patients respectively. Of the 94 patients presented with LUTS, 31 had moderate and 63 severe symptoms. No one had IPS score <7 (Table 1).
Table 1.
Distribution of cases with respect to IPS scores.
| IPS score | No. of patients | Percentage (%) |
|---|---|---|
| Mild | 00 | 00.00 |
| Moderate | 31 | 32.98 |
| Severe | 63 | 67.02 |
| Total | 94 | 100 |
As per the IDF criteria, majority of the patients (85.11%) in our study had central obesity. Further analysis of the data revealed that there was no statistically significant difference in mean prostate volume between patients with central obesity and without central obesity (p value: 0.264).
In our study, 29 patients (30.85%) were found to have serum triglyceride ≥150 mg/dl. Mean prostate volume in patients having raised serum triglyceride was 49.72 ml, which was higher than their counterparts having normal serum triglycerides (mean prostate volume 35.55 ml). The result was statistically significant (p value: 0.000), that is, there is positive association between serum triglyceride and BPH (Table 2).
Table 2.
Prostate volume correlate and its significance with serum triglyceride, HDL cholesterol, fasting blood glucose, and metabolic syndrome.
| Prostate volume |
||||
|---|---|---|---|---|
| Mean ± Std deviation | P value | Significance | ||
| Serum triglyceride | <150 mg/dl | 35.55 ± 13.35 | 0.000 | Significant |
| >150 mg/dl | 49.72 ± 21.8 | |||
| HDL cholestrol | >40 mg/dl | 38.35 ± 19.15 | 0.047 | Significant |
| <40 mg/dl | 42.71 ± 14.24 | |||
| Fasting blood glucose | <100 mg/dl | 35.82 ± 18.55 | 0.001 | Significant |
| >100 mg/dl | 43.69 ± 15.9 | |||
| Metabolic syndrome | Absent | 32.78 ± 11.43 | <0.001 | Significant |
| Present | 50.00 ± 19.81 | |||
Serum HDL cholesterol < 40 mg/dl, which is another component of MetS, was found in 36% of cases in this study. And in this case also mean prostate volume was found to be higher among patients with low serum HDL than normal serum HDL level (Table 2).
High blood pressure or evidence of hypertension was found in 35 (37.23%, n = 94) patients. Among these hypertensive patients, mean prostate volume was found to be higher than the patients having normal blood pressure (p value 0.000).
In this study, 49 patients (52.13%, n = 94) had increased fasting blood glucose or positive history of diabetes mellitus. Patients having deranged serum fasting blood glucose had raised mean prostate volume compared to those with normal serum fasting blood glucose. There was positive correlation between raised serum fasting blood glucose and prostate volume as the result was statistically significant with p value <0.001 (Table 2). In our study, 39 patients were overweight (BMI: 25–29.9), 9 patients were obese (BMI: >30) and the rest were having normal BMI (18.8–24.9). No one had under-nutrition or morbid obesity. There was no statistically significant difference in prostate volume of patients with or without obesity (p value: 0.083).
MetS was found in 39 patients (41.49%) and mean prostate volume among these patients (50.00 ml) was significantly higher than patients without MetS (32.78 ml). Thus, there was a positive association between MetS and BPH as the result was statistically significant (p value: <0.001) (Table 2).
Compared with people without any MetS components (mean prostate volume 28.00 ml), the mean prostate volumes were 49.14 ml (p value: 0.057) for those with three components, 44.6 ml (p value: 0.0041) for those with four components and 53.8 ml (p value: 0.0013) for those with all five components (Table 3).
Table 3.
Comparisons of prostate volume according to metabolic syndrome and metabolic syndrome components.
| Components | Number of patients | Percentage (%) | Mean prostate volume | p value |
|---|---|---|---|---|
| No. of MetS components | ||||
| None | 04 | (04.25) | 28.00 ± 3.56 | |
| 1 | 20 | (21.27) | 29.95 ± 9.36 | |
| 2 | 31 | (32.98) | 34.81 ± 13.19 | |
| 3 | 21 | (22.34) | 49.14 ± 25.25 | 0.057 |
| 4 | 10 | (10.64) | 44.6 ± 10.05 | 0.004 |
| 5 | 10 | (10.64) | 53.8 ± 13.09 | 0.001 |
| Metabolic syndrome | ||||
| MetS | 39 | (41.49) | 50.00 ± 19.81 | 0.001 |
| Non MetS | 55 | (58.51) | 32.78 ± 11.43 | |
Out of 63 patients who had presented with severe LUTS, 58 patients had central obesity. And the result was statistically significant (p value: 0.0069) which suggested that central obesity in men may increase the frequency and severity of urinary symptoms. No positive relationship was found with LUTS and other components of MetS (hypertension, serum blood glucose, HDL cholesterol, serum triglycerides).
Discussion
BPH is most common neoplastic abnormality in elderly men and is also one of the most prevalent diseases in male more than 50 years of age. In United States, the prevalence among men above 60 years of age is 40% and above 80 years is 90%.6 Worldwide, the prevalence exceeds 50% in men more than 60 years of age.7
With increasing age, mean prostate volumes among the study population were observed to increase.
BPH often present with LUTS. In the U.S. Health Professionals Follow-up Study (HPFS), obese men over 40–75 years of age had severe urinary symptoms.8 Similarly men above 60 years of age had severe symptoms in the Third National Health and Nutrition Examination Survey.9 In BPH symptom, severity often does not correlate with prostate volume and LUTS are a non-specific surrogate.10
The relationship between the MetS and BPH was first evaluated by Hammarsten et al.11 Very few Indian studies exist evaluating the relationship and Indian data often differ from their western data due to genetic variations.12 The result of our study suggested that central obesity in men may increase the frequency and severity of urinary obstructive symptoms (p value: 0.0069) but no significant association was found between lower urinary tract symptom severity and prostate volume (p value: 0.917).
Obesity is often defined using various anthropometric measures such as waist circumference, WHR, and BMI. HPFS showed that BPH in obese patients with waist circumference (>109 cm) often required surgical treatment compared to those with waist circumference (<89 cm).8
Along with ageing, increased BMI and diabetes predisposes to greater prostatic enlargement. It is observed that men with BMI < 25 kg/m2 had smaller prostate compared to individuals with BMI≥35 kg/m2.13 It appears that BPH/LUTS are more often found in obese individuals.
In our study, we did not find any significant association between BPH and BMI/central obesity. This result may be due to small sample size and limited variation in BMI among study subjects. In the study by Tewari et al.,12 WHR and not BMI was associated with elevated prostate volume. WHR is considered to be more efficient predictor of obesity than BMI and more closely correlates with the various metabolic sequelae of obesity because Indians have more abdominal fat compared to the westerners who have more subcutaneous fat.
Furthermore, in calculating central obesity, IDF 2005 criteria were taken and accordingly majority of our patients (85.11%) had central obesity and this might be another reason for not finding any association between BPH and waist circumference in our study. With ageing peripherally circulating androgen decreases but intraprostatic levels of dihydrotestosterone (DHT) increase along with serum oestrogen. With ageing, fat starts accumulating and itaromatizes circulating testosterone into oestrogen and this altered testosterone and oestrogen levels in the prostate tissue are also considered as one of the contributory factors for BPH.12
Hypertensive patients have been found to have significant LUTS compared to those without hypertension.14 We also found positive association between hypertension and larger prostate volume (mean: 50.94 vs. 33.39 ml; p value: 0.000).
In our study, we found positive association between BPH and raised serum fasting blood glucose (mean prostate volume: 43.69 vs. 35.82 ml; p value: 0.001).
Similar to obesity, the relationship between BPH and dyslipidaemia has been documented in several studies.15, 16 Individuals with a low level of HDL cholesterol usually have a larger prostate volume and a higher annual BPH growth rate than individuals with a high level of HDL cholesterol. In the study conducted by Rohrmann et al. using data from NHANES III, they did not find any relationship between LUTS and lipid profile.14 A positive association between BPH and raised serum triglyceride was found in our study (mean prostate volume: 49.72 vs. 35.55 ml; p value: 0.000) but we could not find any correlation between BPH and serum HDL cholesterol (mean prostate volume: 42.71 vs. 38.35 ml; p value: 0.047).
MetS is considered as an independent predictor of moderate-to-severe LUTS.17 Men with MetS tend to have a larger prostate volume and a higher annual BPH growth rate compared to men without MetS.15
BPH as the name implies, is a benign proliferation of prostate gland promoted by oxidative stress and inflammatory mediators. MetS also has similar mediators. The relationship between BPH/LUTS and MetS or one of its components continues to be an enigma and the findings are contradictory and the Indian perspective is sparse. No relationship between BMI and the rate of surgery for BPH has been reported.18 Similarly, there was no correlation between obesity and blood pressure and subsequent clinical BPH development within a mean follow-up of 8.8 years.19 The IPSS showed no significant difference between men with and without MetS.20
In our study, we have found positive association between MetS and BPH. The mean prostate volumes in patients with MetS and without MetS were 50.00 ml and 32.78 ml respectively (p value: <0.001). Compared with people without any MetS components (mean prostate volume 28.00 ml), the mean prostate volumes were significantly more with those with three, four and with all five components of MetS (Table 3).
We have also found positive association between BPH and four components of MetS as per IDF 2006 criteria, the components being hypertension, raised fasting plasma glucose, serum triglyceride and serum HDL. No correlation was found with waist circumference level.
Finally, compared to the westerners, Asian Indian population have different genetic profiles, dietary habits, and environmental risk factors. The potential relationship between MetS and BPH may hence vary due to these factors.
Conclusion
Our study indicates that risk of prostatic enlargement is higher in individuals with components of MetS. Further studies with larger sample size are required to establish strong association between the presence of MetS and the development of BPH. These may provide newer insights into the cause of these entities and thereby leading to development of new treatment and prevention protocol. There is a need for awareness programmes to combat hypertension, obesity and diabetes and life style modifications to prevent those diseases for promoting healthy happy life.
Conflicts of interest
The authors have none to declare.
References
- 1.Gupta A., Gupta S., Pavuk M., Roehrborn C.G. Anthropometric and metabolic factors and risk of benign prostatic hyperplasia: a prospective cohort study for Air Force veterans. Urology. 2006;68:1198–1205. doi: 10.1016/j.urology.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 2.Gogia A., Agarwal P.K. Metabolic syndrome. Indian Med J Sci. 2006;60:72–81. [PubMed] [Google Scholar]
- 3.Madersbacher S., Alivizatos G., Nordling J., Sanz C.R., Emberton M., de la Rosette J.J. EAU 2004 guidelines on assessment, therapy and follow up of men with lower urinary tract symptoms suggestive of benign prostatic obstruction (BPH guidelines) Eur Urol. 2004;46:547–554. doi: 10.1016/j.eururo.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Athyros V.G., Bouloukos V.I., Pehlivanidis A.N. The prevalence of the metabolic syndrome in Greece: The MetS-Greece Multicentre Study. Diabetes Obes Metab. 2005;7:397–405. doi: 10.1111/j.1463-1326.2004.00409.x. [DOI] [PubMed] [Google Scholar]
- 5.Girman C.J., Jacobsen S.J., Tsukamoto T. Health-related quality of life associated with lower urinary tract symptoms in four countries. Urology. 1998;51:428–436. doi: 10.1016/s0090-4295(97)00717-6. [DOI] [PubMed] [Google Scholar]
- 6.Roehrborn C.G. Benign prostatic hyperplasia: etiology, pathophysiology, epidemiology, and natural history. In: Wein A.J., Kavoussi L.R., Novic A.C., Partin A.W., Peters C.A., editors. Campbell-Walsh Urology. 10th ed. Elsevier Saunders; 2012. pp. 2570–2610. [Google Scholar]
- 7.Marberger M., Harkaway R., de la Rosette J. Optimising the medical management of benign prostatic hyperplasia. Eur Urol. 2004;45:411–419. doi: 10.1016/j.eururo.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Giovannucci E., Rimm E.B., Chute C.G. Obesity and benign prostatic hyperplasia. Am J Epidemiol. 1994;140:989–1002. doi: 10.1093/oxfordjournals.aje.a117206. [DOI] [PubMed] [Google Scholar]
- 9.Rohrman S., Smit E., Giovannucci E. Associations of obesity with lower urinary tract symptoms and noncancer prostate surgery in the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2004;159:390–397. doi: 10.1093/aje/kwh060. [DOI] [PubMed] [Google Scholar]
- 10.AUA guideline on management of benign prostatic hyperplasia 2003: I. Diagnosis and treatment recommendations. J Urol. 2003;170:530–547. doi: 10.1097/01.ju.0000078083.38675.79. [DOI] [PubMed] [Google Scholar]
- 11.Hammarsten J., Hogstedt B., Holthuis N. Components of the metabolic syndrome – risk factors for the development of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 1998;1:157–162. doi: 10.1038/sj.pcan.4500221. [DOI] [PubMed] [Google Scholar]
- 12.Tewari R., Prabhat P., Natu S.M. Association of benign prostatic hyperplasia (BPH) with the metabolic syndrome (MS) and its components – ‘a growing dilemma’. JMH. 2011;8:66–71. [Google Scholar]
- 13.Parsons J.K. Modifiable risk factors for benign prostatic hyperplasia and lower urinary tract symptoms: new approaches to old problems. J Urol. 2007;178(2):395–401. doi: 10.1016/j.juro.2007.03.103. [DOI] [PubMed] [Google Scholar]
- 14.Rohrmann S., Smit E., Giovannuci E. Association between markers of metabolic syndrome and lower urinary tract symptoms in the Third National Health and Nutrition Examination Survey (NHANES III) Int J Obes (Lond) 2005;29:310–316. doi: 10.1038/sj.ijo.0802881. [DOI] [PubMed] [Google Scholar]
- 15.Hammarsten J., Högstedt B. Hyperinsulinaemia as a risk factor for developing benign prostatic hyperplasia. Eur Urol. 2001;39:151–158. doi: 10.1159/000052430. [DOI] [PubMed] [Google Scholar]
- 16.Nandeesha H., Koner B.C., Dorairajan L.N., Sen S.K. Hyperinsulinemia and dyslipidemia in non-diabetic benign prostatic hyperplasia. Clin Chim Acta. 2006;370:89–93. doi: 10.1016/j.cca.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 17.Kupelian V., Mcvary K.T., Kaplan S.A. Association of lower urinary tract symptoms and the metabolic syndrome: results from the Boston Area community health survey. J Urol. 2009;182:616–624. doi: 10.1016/j.juro.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seitter W.R., Barret-Connor E. Cigarette smoking, obesity, and benign prostatic hypertrophy: a prospective population based study. Am J Epidemiol. 1992;135:500–503. doi: 10.1093/oxfordjournals.aje.a116316. [DOI] [PubMed] [Google Scholar]
- 19.Meigs J.B., Mohr B., Barry M.J., Collins M.M., Mckinlay J.B. Risk factors for clinical benign prostatic hyperplasia in a community-based population of healthy aging men. J Clin Epidemiol. 2001;54:935–944. doi: 10.1016/s0895-4356(01)00351-1. [DOI] [PubMed] [Google Scholar]
- 20.Hong G.S., Shim B.S., Chung W.S., Yoon H. Correlation between metabolic syndrome and lower urinary tract symptoms of males and females in the aspect of gender specific medicine: a single institutional study. Korean J Urol. 2010;51:631–635. doi: 10.4111/kju.2010.51.9.631. [DOI] [PMC free article] [PubMed] [Google Scholar]