Abstract
Currently, percutaneous coronary intervention (PCI) with drug-eluting stents (DES) is the most commonly employed modality in the treatment of patients with coronary artery disease. PCI has come of age over the last four decades with enormous forays in the technology and drugs which have greatly enhanced its capability. Angioplasty and bare metal stents were plagued by high failure rates on account of restenosis leading to repeat revascularization procedures. Insights into pathophysiology of instent restenosis (ISR) and neointimal hyperplasia triggered the development of DES. The dreamlike remarkable reduction in ISR with DES was enthusiastically welcomed. Soon thereafter emerged the spectre of very late stent thrombosis (VLST) with DES. VLST was a new entity seen predominantly with DES and pathological insights as to the cause was instrumental in the development and efficacy of new generation DES. This review will highlight the evolution and present day DES for coronary interventions.
Keywords: Drug eluting stents, Evolution, Present day scenario
Introduction
Percutaneous coronary intervention (PCI) with stenting today represents the standard of care in the treatment of patients with coronary artery disease. The first path-breaking modality to treat coronary stenosis was percutaneous coronary angioplasty (PTCA) in 1977 with a catheter improvised in his kitchen by Gruntzig.1 PTCA was fraught with a 50% failures at one year which was on account of acute elastic recoil, plaque prolapse and constrictive remodelling on account of balloon mediated vascular injury. This balloon-mediated injury had an initial phase similar to wound healing with platelet adhesion, fibrin deposition and cellular infiltrate to be followed by a subsequent phase re-endothelisation, smooth muscle cell (SMC) migration and proliferation.
Bare metal stents (BMS) – the rise and fall
The quandary of restenosis with PTCA was addressed by the next innovation in the form of a metallic stent implant in the coronary in 1986 by Sigwart et al.2 The deployed stent promptly sealed disrupted plaques and splinted angioplasty induced dissection with resultant plaque stabilisation. The stanchion provided by the metal platform resulted in acute gain in vessel calibre and offset the vessel recoil and constrictive remodelling. This resulted in significantly reduced rates in restenosis to 20% (11–40%) with BMS. Stent implantation was quickly adopted as the default strategy in PCI but soon lost their sheen on account of two drawbacks in the form of stent thrombosis (ST) and instent restenosis (ISR).
-
(a)
Stent thrombosis (ST): ST is the acute and complete thrombotic occlusion of a coronary stent. ST occurred in up to 25% of the cases within the first 14 days in early experience.3 ST is a very serious clinical event typically resulting in ST-elevation myocardial infarction (MI) in 70–80% of cases4 with mortality rates that may be as high as 20–40%.5 Aggressive initial attempts to tackle ST with high dose heparin, dextran and urokinase infusions lead to considerable morbidity and mortality with major bleeding occurring in up to 9% of the cases. Two developments were salutary in reducing the incidence of ST to less than 1%: firstly the institution of dual anti-platelet therapy and secondly the use of high balloon pressures during stent deployment to maximise their apposition. The battle of early stent failures on account of ST was won and this unfolded the second worrisome area of ISR.
-
(b)
Instent restenosis (ISR): The delayed temporal response to the higher degree of vascular injury on account of implantation of stents in the first month initially comprises mild luminal thrombus formation on account of platelet aggregation and fibrin deposition to be followed by inflammatory cellular infiltrate, SMC migration and collagen deposition that constitutes neo-intimal hyperplasia (NIH). NIH with BMS peaked at 6 months and was the cause of ISR. The clinical presentation of ISR is usually angina but in a third may be an acute MI.
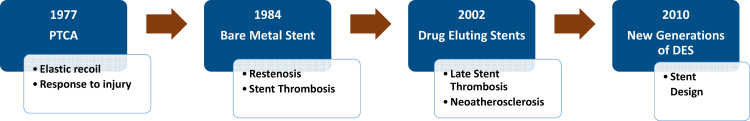
The next forays were targeted against NIH. The therapeutic option of controlling the cellular proliferation was intuitive and was accomplished and ensured by local delivery of anti-proliferative drugs. Concomitantly it was important to have the optimal and desired drug concentrations coinciding with the period when NIH occurred. This temporo-spatial profile of drug delivery was done by coating the metal platform with a polymer which in turn served as the drug delivery carrier. The evolution of PCI is given in Fig. 1.
Fig. 1.
The evolution of PCI.
First generation drug-eluting stents (DES) – the new kid in town!
The first DES to be launched were in 2002 and they were the sirolimus eluting stent (SES) – Cypher® stent and the paclitaxel eluting stent (PES) – Taxus® stent. Early DES versus BMS trials demonstrated DES superiority with significantly reduced rates of target lesion revascularisation (TLR) and angiographic restenosis to less than10% in the RAVEL trial,6 the SIRIUS trials,7 and the TAXUS trials.8 Consequently, both first-generation DES, the SES and the PES received approval by the regulatory bodies in Europe and the USA in 2002/2003. Following the success of initial clinical trials of first-generation DES there was a rapid and fervent expansion to their use in almost 90% of PCI over the next 3 years. Simultaneously these DES were also used for ‘off-label’ indications in complex lesions such as small vessels, long and tortuous lesions, chronic total occlusions and left main disease. The euphoria was tempered by the occurrence of ST after a year of implantation of these DES called very late stent thrombosis (VLST). Case reports and preliminary data from the large Swedish Coronary Angiography and Angioplasty Register (SCAAR), and other groups9 noted a significant increase in VLST. The 4-year rates of VLST were 1.2% in SES vs 0.6% in BMS (p = 0.20) and 1.3% in PES vs 0.9% in BMS (p = 0.30). Furthermore, the risk of VLST (0.4–0.6% per year) continued for another 5 years. The causes of VLST were ascribed to a distinct and different kind ISR seen with DES as compared to BMS as shown in Table 1. The DES ISR delayed healing, impaired endothelisation, allergic reactions, inflammation, vascular dysfunction and neo-atherosclerosis in the stented segment of the coronary artery. Among these neo-atherosclerosis was a new and a perilous phenomenon.
Table 1.
Instent restenosis – BMS vs DES.
| BMS | DES | |
|---|---|---|
| Smooth muscle cell (SMC) content | Rich | Hypocellular |
| Proteoglycan content | Moderate | High |
| Peri-stent inflammation | Occasional | Frequent |
| Complete endothelisation | 3–6 months | Up to 4years |
| Neo-atherosclerosis | Infrequent and late | Frequent and accelerated |
| Angiography | Diffuse pattern | Focal pattern |
| Late luminal loss | 6–8 months | Up to 5 years |
Neo-atherosclerosis
Neo-atherosclerosis is the development of an atherosclerotic plaque inside an implanted coronary stent. Histopathologically, the process is characterised by three main stages: (i) early foamy macrophage infiltration, (ii) manifest atherosclerotic plaque development, and (iii) necrotic core plaque formation with or without thin fibrous caps. Although neo-atherosclerosis is also observed after bare metal stenting, it occurs earlier and more frequently after stenting with DES (35% vs 10%). The primary cause is the delayed and variable endothelisation which results in anatomical and functional impairment allowing infiltration by circulating lipid particles that culminate in neo-atherosclerosis.
This looming spectre of VLST dwindled the initial enthusiasm and we witnessed a decline in the use of first generation DES by 2007. This triggered a rigorous introspection as to the causes of the consequences of the new pathobiology of DES ISR and remedial measures which could be undertaken. The putative culprit for the VLST were intuitively ascribed to the composition and design of the DES. There were successive refinements and iterations of DES over the next few years and we witnessed the new generations of DES.
DES – the new generations
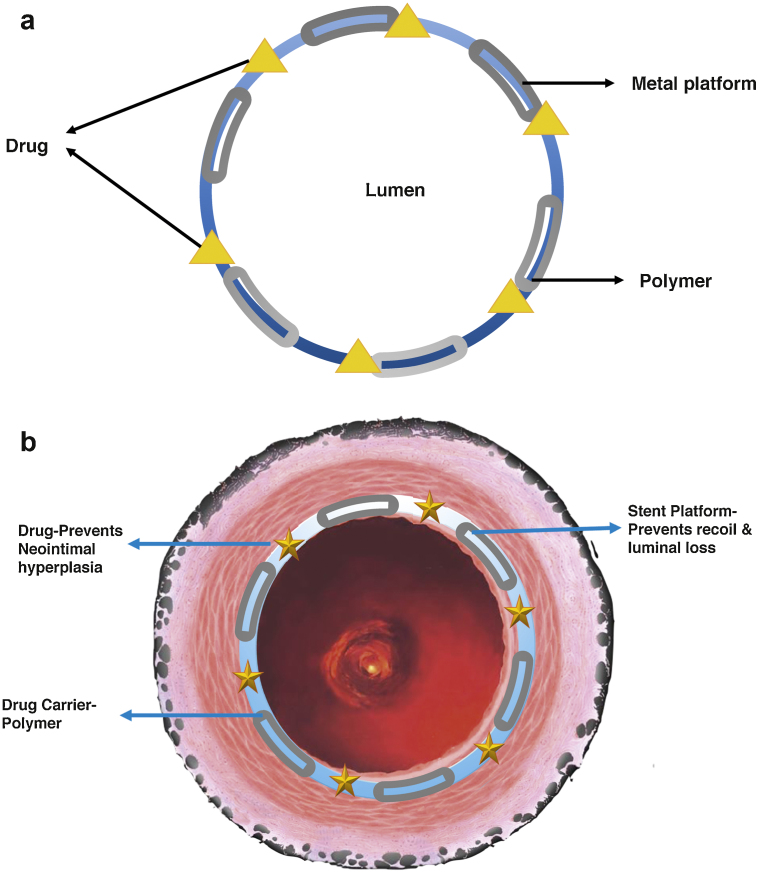
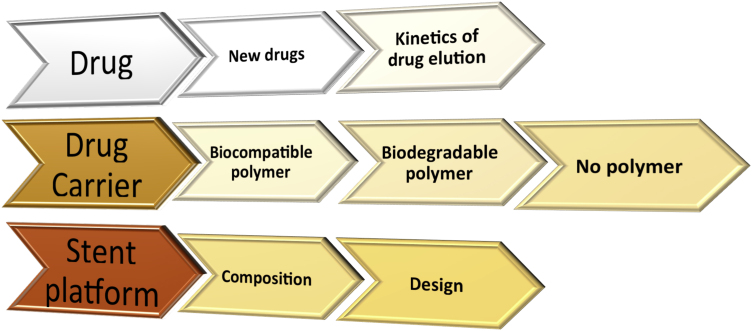
There are three components to a DES as illustrated in Fig. 2 are (i) the anti-proliferative drug, (ii) the vehicle for drug delivery which is usually a polymer coated to the stent and the (iii) drug metallic stent platform. Each of these components underwent modification and refinement to minimise the risk of VLST in a series of avatars in the first generations of DES. The categorisation of the generations of DES were based on the characteristic of the polymers and are first-generation DES with a durable polymer (DP), second-generation DES with a biocompatible polymer, third-generation DES with a biodegradable polymer, and fourth-generation DES with no polymer. The innovations in the polymer were concurrently associated with other modifications in the drug and the metallic platform as shown in Fig. 3. The nomenclature and the major changes undertaken are listed in Table 2.
Fig. 2.
(a) DES design and (b) DES function.
Fig. 3.
Modifications in the new generation DES.
Table 2.
The generation of DES based on the carrier platform.
| Metal platform | Strut thickness | Drug delivery vehicle/thickness | Drug/concentration | Drug elution kinetics | |
|---|---|---|---|---|---|
| First generation DES | Durable polymer | ||||
| CYPHER® | Stainless Steel | 140 μm | Durable polymer/12.6 μm | Sirolimus/140 μg/cm2 | Complete in 3 months |
| TAXUS® | Stainless Steel | 97 μm | Durable polymer/16.9 μm | Paclitaxel/100 μg/cm2 | 10% in 10 days. Rest not bioavailable and remains in polymer |
| Second generation DES | Biocompatible polymer | ||||
| Xience Family | Cobalt chromium | 81 μm | Biocompatible polymer/7.6 μm | Everolimus/100 μg/cm2 | Complete in six months |
| Resolute®, Integrity® | Cobalt chromium | 91 μm | Biocompatible polymer/4.1 μm | Zotarilimus/160 μg/cm2 | Complete in six months |
| Onyx® | Platinum-iridium | 81 μm | Biocompatible polymer/4.1 μm | Zotarilimus/160 μg/cm2 | Complete in six months |
| Promus element®, Promus Premier® | Platinum-chromium | 81 μm | Biocompatible polymer/6.0 μm | Everolimus/100 μg/cm2 | Complete in six months |
| Third generation DES | Biodegradable polymer | ||||
| Synergy® | Platinum-chromium | 71 μm | Biodegradable polymer/4.0 μm | Everolimus/100 μg/20 mm | Complete in four months |
| Fourth generation DES | No polymer | ||||
| Biofreedom™ | Stainless steel | 112 μm | No polymer | Biolimus/15.6 μg/mm | Complete in one month |
New generation DES – the transformation
The new generations of DES underwent concurrent changes in the three major components and proved their incremental safety and efficacy in the real world. The multitude of these changes were guided by the extant data and scientific insight. An overview of these changes which encompassed the drug, the delivery platform and the stent design are discussed below.
-
(a)
The drug: The ideal anti-proliferative drug had to meet three requirements (i) a retention time that would permit endothelium to be restored at the earliest and at the same time inhibit NIH (ii) a wide therapeutic window and (iii) lipophilic properties. The first generation DES had sirolimus (SES) and paclitaxel (PES) as the anti-proliferative drug. The polymer on SES eluted ∼80% of the drug in the first month, while the polymer on PES eluted ∼10% of the drug in the first 10 days and sequestered the rest indefinitely. Clinical data showed that SES were overall superior to in terms of rates of restenosis, VLST and need for revascularisation.10 This led to the singular preference of sirolimus and its analogues in the next generation of stents. As a consequence the new generation stents had modifications of the carbon 40 of the sirolimus molecule in the form of Everolimus, Zotarilimus and Biolimus A-9. Compared with PESs, newer everolimus-eluting stents (EES) have been shown to reduce composite of death or MI, ST and TLR.11 The EES also decreased the incidence of VLST and the need for repeat revascularisation as compared to SES in a meta-analysis of 11 trials.12
-
(b)
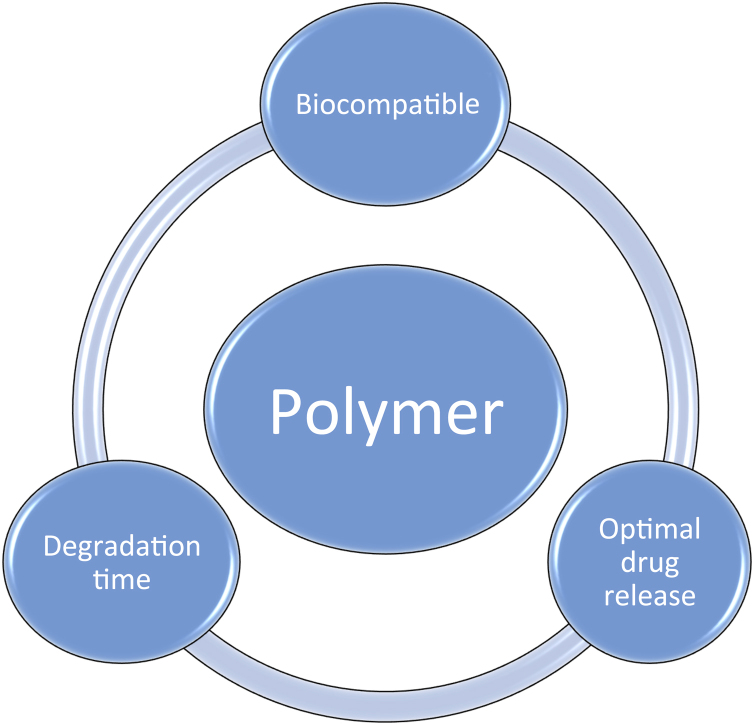
The drug delivery platform: The carrier for drug is a polymer which is coated on the stent. The polymers that were used in the SES drug delivery in the first generation SES and PES were already being used in bone cement and intraocular devices. They were DPs with the drawback was that once the drug had eluted the polymer had no role and was an inert entity contributing in the causation of VLST. The critical issues in selecting a polymer are given in Fig. 4 and they are biocompatibility, drug release kinetics and the inherent fate of the polymer.
Biocompatibility is related to the immune response generated by the polymer and its degradation products. The polymer must ensure the desired temporo-spatial drug release kinetics and ideally should degrade after accomplishing its role in drug elution. The second generation DES used biocompatible polymers in a reduced quantity as is given in Table 2. This resulted in significant reductions in VLST with second generation DES as compared to the first generation.
The next development was to have bio-absorbable polymers in the third generation of DES. These polymers are bio-resorbed in 6–9 months and thereafter this stent behaves like a BMS and dispenses with the persistent trigger for the chronic inflammatory response. Notwithstanding this, the flip side is that these bio-absorbable polymers evoke an intense inflammatory reaction on account of their breakdown products. The clinical evaluation of these third generation DES did not conclusively demonstrate superiority over the second generation DES in terms of reduction of VLST.
The final development was in the fourth generation DES which the polymer chrysalis was shed. The possible physical and inflammatory adverse effects of the polymer on account peeling and cracking were eliminated. The technological innovation in these DES is drug-filled stents and nanotechnology. In drug filled stents the drug is put in the hollow stent platform and the rate of elution is controlled through laser drilled holes on the abluminal surface of the stent. Nanotechnology uses a coating of a nano-matrix on the stent that consists of the anti-proliferative drug and nanoparticles. The drug release kinetics can be precisely controlled in these stents. However to date there is no convincing data regarding their superiority over second generation DES.13
-
(c)
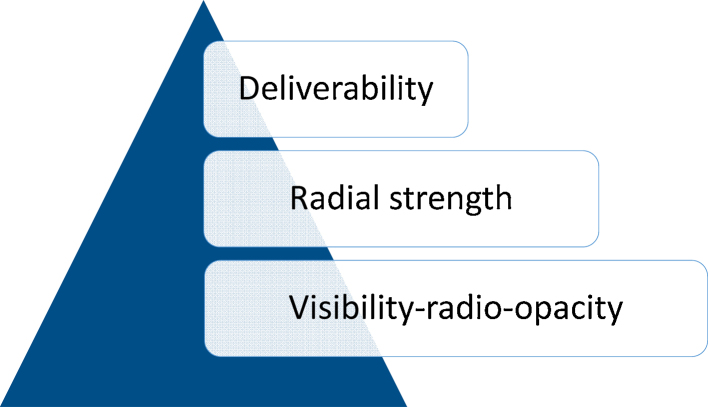
The stent platform: The coronary stent is a metallic trellis which (i) acutely seals the balloon mediated controlled dissection and prevent closure on account of recoil and (ii) continues to preserve the luminal gain by acting as a scaffold thereafter. The ideal stent should have ease of deliverability, good radial strength and should be visible as given in Fig. 5. The foremost characteristic of an ideal stent is ease of deliverability to the target lesion. The radial strength governs the mechanical performance and visibility is critical to ensure accurate deployment and subsequent assessments. All these are variables capable of revolving around the components of the metal platform, the strut thickness and the inherent design of the stent.
The metal platforms used in the first generation DES was stainless steel 316 L which was used in BMS. The 140 μm strut thickness coated with a polymer of 12.6 μm thickness limited the deliverability and was indicted for the VLST. The second generation DES used cobalt chromium alloy and reduced the polymer thickness by 50%. The thinner strut thickness with preserved radial strength increased the trackability and performance. Studies also confirmed the reduced rates of ISR and VLST when compared with the first generation DES.14, 15 Consequent to these salutary effects further refinements in the second generation DES took place. In the third generation DES the cobalt chromium alloy was replaced by platinum chromium which had greater visibility and radial strength. The platinum chromium alloy permitted a nearly 50% reduction in strut thickness to 71 μm as compared to the first generation DES. This coupled with the increased radio-opacity resulted in improved deliverability and accuracy during deployment.
Fig. 4.
Drug carrier.
Fig. 5.
The ideal stent platform.
Bioresorbable scaffolds (BRS) – the Prince of ‘Dissolve’
The evolution of DES was characterised by refinements in technology which focussed on optimising the drug kinetics, carrier and the metal platform. The pivotal concept was to minimise the consequences the side effects and reactions of a foreign implant vis-a-vis its putative role. The culmination was to ‘leave nothing behind’ – to dissolve everything completely and led to the creation of a Bioresorbable Scaffolds (BRS). The BRS highlights the temporary nature of the scaffold that provides transient vessel support through lactide polymers-poly-levo-lactic acid (PLLA), poly anhydrides and polycarbonates. The initial experience has been promising, with results comparable to the third generation DES however there are emerging concerns prior to their universal application. The technique of BRS implantation is more demanding in terms of the preparation of the bed, reduced radio-opacity and the need for frequent post deployment optimisation mandating a higher skill-set with the operator. There are also emerging concerns of an increased incidence of early ST which are being addressed through modifications of the scaffold.
Since BRS are not stents which by definition are characterised by a permanent metallic implant, they have been excluded in this review. Furthermore, we need to exercise restraint in the universal adoption of BRS, as this transforming technology, which is only a couple of years old, has not undergone the rigorous evaluation of DES, has considerable incremental cost limiting its usage and presently requires additional technical precision in implantation.
Conclusion
The introduction of DES heralded a new era in interventional cardiology. The first generation DES established their undisputed superiority in terms of reduction of ISR when compared to BMS and soon assumed the most favoured status in PCI. Their burgeoning acceptance was offset by the emergence of data with respect to VLST. The concurrent insights into the new pathobiology of ISR and VLST with DES prompted a comprehensive analysis and modifications culminating in a series of innovations. These encompassed improvements in stent platform, polymer and drug in the successive new generations of DES. The second generation DES made a salutary overall impact in terms ease of deliverability, reduction of clinical events and reduction in VLST. The risk of ST is no longer the limiting factor in the use these second generation DES and they are the gold standard for further comparisons. In contemporary practice the new generation DES have replaced most first-generation DES and are compatible with shorter duration of dual antiplatelet therapy. Cardiologists today have a variety of options and the stent choice should be tailored to the individual patient and progress indeed has been remarkable from the kitchen table to contemporary practice of PCI. We are at the interregnum where only time will tell whether we need to focus our efforts to further evolve the resolute DES or to adopt a – ‘dissolve all strategy’ which albeit conceptually transforming is nascent and evolving.
Conflicts of interest
The authors have none to declare.
References
- 1.Gruntzig A. Transluminal dilatation of coronary artery stenosis. Lancet. 1978;311:263. doi: 10.1016/s0140-6736(78)90500-7. [DOI] [PubMed] [Google Scholar]
- 2.Sigwart U., Puel J., Mirkovitch V., Joffre F., Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med. 1987;316:701–706. doi: 10.1056/NEJM198703193161201. [DOI] [PubMed] [Google Scholar]
- 3.Serruys P.W., Strauss B.H., Beatt K.J. Angiographic follow-up after placement of a self-expanding coronary-artery stent. N Engl J Med. 1991;324:13–17. doi: 10.1056/NEJM199101033240103. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong E.J., Feldman D.N., Wang T.Y. Clinical presentation, management, and outcomes of angiographically documented early, late, and very late stent thrombosis. JACC Cardiovasc Interv. 2012;5:131–140. doi: 10.1016/j.jcin.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Schulz S., Schuster T., Mehilli J. Stent thrombosis after drug-eluting stent implantation: incidence, timing, and relation to discontinuation of clopidogrel therapy over a 4-year period. Eur Heart J. 2009;30:2714–2721. doi: 10.1093/eurheartj/ehp275. [DOI] [PubMed] [Google Scholar]
- 6.Stone G.W., Moses J.W., Ellis S.G. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356(10):998–1008. doi: 10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- 7.Weisz G., Leon M.B., Holmes D.R., Jr. Five-year follow-up after sirolimus-eluting stent implantation results of the SIRIUS (Sirolimus-Eluting Stent in De-Novo Native Coronary Lesions) trial. J Am Coll Cardiol. 2009;53:1488–1497. doi: 10.1016/j.jacc.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 8.Grube E., Silber S., Hauptmann K.E. TAXUS I: six- and twelve-month results from a randomized, double-blind trial on a slow-release paclitaxel-eluting stent for de novo coronary lesions. Circulation. 2003;107:38–42. doi: 10.1161/01.cir.0000047700.58683.a1. [DOI] [PubMed] [Google Scholar]
- 9.Sarno G., Lagerqvist B., Frobert O. Lower risk of stent thrombosis and restenosis with unrestricted use of ‘new-generation’ drug-eluting stents: a report from the nationwide Swedish Coronary Angiography and Angioplasty Registry (SCAAR) Eur Heart J. 2012;33:606–613. doi: 10.1093/eurheartj/ehr479. [DOI] [PubMed] [Google Scholar]
- 10.Schomig A., Dibra A., Windecker S. A meta-analysis of 16 randomized trials of sirolimus-eluting stents versus paclitaxel-eluting stents in patients with coronary artery disease. J Am Coll Cardiol. 2007;50:1373–1380. doi: 10.1016/j.jacc.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 11.Kimura T., Morimoto T., Natsuaki M. Comparison of everolimus-eluting and sirolimus-eluting coronary stents: 1-year outcomes from the Randomized Evaluation of Sirolimus-Eluting versus Everolimus-Eluting Stent Trial (RESET) Circulation. 2012;126:1225–1236. doi: 10.1161/CIRCULATIONAHA.112.104059. [DOI] [PubMed] [Google Scholar]
- 12.Bangalore S., Kumar S., Fusaro M. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow- up from randomized trials. Circulation. 2012;125:2873–2891. doi: 10.1161/CIRCULATIONAHA.112.097014. [DOI] [PubMed] [Google Scholar]
- 13.Bangalore S., Toklu B., Amoroso N. Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: mixed treatment comparison meta-analysis. BMJ. 2013:347. doi: 10.1136/bmj.f6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cannon L. Two-year outcomes from the PERSEUS randomised trial of the paclitaxel-eluting platinum chromium stent versus paclitaxel-eluting stainless steel. Presented at 23rd Transcatheter Cardiovascular Therapeutics, 2011.
- 15.Stone G. Two-year results of the PLATINUM Randomized Trial Comparing Platinum Chromium PROMUS Element and Cobalt Chromium PROMUS/XIENCE V everolimus-eluting stents in de novo coronary artery lesions. Presented at the ACC Scientific Sessions, 2012.