Abstract
Much excitement has been generated with the approval of idarucizumab, a humanized monoclonal antigen‐binding antibody fragment that is capable of reversing the anticoagulant activity of dabigatran. Here, we describe our initial experience of using tissue plasminogen activator (tPA) in an acute posterior circulation ischemic stroke after dabigatran reversal with idarucizumab. Both treatments were well tolerated and no hemorrhagic or procoagulatory complications were observed. We propose that the option of dabigatran reversal needs to be considered for contemporary treatment concepts of acute ischemic stroke.
Introduction
Dabigatran etexilate (Pradaxa®) is a specific and reversible thrombin inhibitor approved for stroke prevention in patients with nonvalvular atrial fibrillation (AF).1 Further indications include treatment and prevention of pulmonary embolism (PE) and deep vein thrombosis (DVT).2, 3, 4
Much excitement was generated with the approval of idarucizumab (Praxbind®) by the Food and Drug Administration in October 2015. This humanized monoclonal antibody fragment is capable of reversing anticoagulant activity of dabigatran within minutes and is thus recommended for patients who develop serious bleeding or require an urgent surgical procedure.5 However, whether the safety data can be translated to the setting of ischemic stroke and thrombolysis with intravenous tissue plasminogen activator (t‐PA) has not been evaluated so far.
Case
Here, we report the case of a 68‐year‐old man who presented with acute ischemic stroke in the posterior circulation. He provided informed written consent for the publication of the unique case in which systemic thrombolysis was performed after dabigatran was reversed with idarucizumab.
Because he had impaired kidney function, he was started on the lower dose of dabigatran, 110 mg twice a day, for paroxysmal AF and presumed cardioembolic stroke 20 months prior to this stroke. In addition, he was taking citalopram 10 mg. His family physician approved discontinuation of antihypertensive and cholesterol‐lowering medication in 2015. His BMI was 27 and he had stopped smoking at age 30.
On the day of self‐referral in May 2016, he was well when he woke up and took his morning dose of dabigatran. At 6:50 am, he noticed sudden onset of visual disturbances, dizziness, and slight headache. Neurological examination 25 min later revealed right‐sided homonymous hemianopsia and marginal evidence of nonfluent aphasia from his stroke 20 months earlier (National Institutes of Health Stroke Scale [NIHSS] of 3). We decided to neutralize the anticoagulant activity of dabigatran and perform thrombolysis with t‐PA considering hemianopsia as a functionally relevant deficit (modified Rankin Scale [mRS] 3), presence of a diffusion‐weighted imaging (DWI)/mismatch, and an early time window. In retrospect, the dabigatran concentration on admission, presumably around 45 min since the most recent intake, was 34.1 μg/L. Coagulation parameters and kidney function tests are shown in table 1.
Table 1.
Kidney function and coagulation parameters
| Value | Normal range | |
|---|---|---|
| Kidney function | ||
| Creatinine | 0.76 mg/dL | 0.67–1.17 |
| Glomerular filtration rate | 102 mL/min | >90 |
| Coagulation | ||
| aPTT | 34 sec | 23–42 |
| INR | <1.2 | |
| Prothrombin time | 90% | 70–130 |
| Fibrinogen | 547 mg/dL | 160–400 |
| Dabigatran | 34.1 μg/L |
2 × 150 mg: trough 31–225, peak: 64–443. Risk of hemorrhage with trough >200 μg/L. |
| Antithrombin III | 90% | 80–120 |
| Protein C (free) | 71% | 68–139 |
| LA‐PTT | 63 sec | 29–46 |
aPTT, activated partial thromboplastin time; INR, international normalized ratio; LA, lupus anticoagulant; PTT partial thromboplastin time. Abormal test results are marked in bold.
Two vials of 2.5 g idarucizumab each were given as rapid i.v. infusion. We started intravenous thrombolysis with t‐PA (0.9 mg/kg) after an additional interval of 10 min and total of 110 min from symptom onset. Transcranial doppler sonography shortly after completion of t‐PA infusion disclosed regular blood flow in both posterior cerebral arteries. Transthoracic echocardiography on day 2 did not uncover cardiac thrombi or structural heart disease. A low‐density lipoprotein (LDL)‐cholesterol of 102 mg/dL (range 50–150) on admission lead to reuptake of treatment with simvastatin 20 mg. He received weight‐adjusted low‐molecular‐weight heparin on day 2 and was discharged on day 3 with slightly improved hemianopsia (NIHSS 3, mRS 2) and raised dabigatran dosage (2 × 150 mg/day) as kidney function tests were normal.
Discussion
Patients who experience acute ischemic stroke should be considered for thrombolytic therapy to restore perfusion and function of the ischemic brain. In this regard, patients on direct anticoagulants (DOAC) presenting with acute ischemic stroke pose a significant management challenge as the risk and consequences of intracerebral hemorrhage after t‐PA therapy are assumed to be significant. While case series of accidental thrombolysis in DOAC‐treated patients implicate an acceptable safety profile, systemic and intracranial bleeding complications are likely to be higher even at subtherapeutic concentrations as seen with warfarin.6 Notably, t‐PA may be given if more than 12 h have passed since the last intake of dabigatran and coagulation assays are consistent with an absence or very low level of anticoagulant activity in the setting of normal renal function.7, 8 Some authorities even recommend that two to four half‐lives of the anticoagulant should have elapsed prior to thrombolysis (24 and 48 h, respectively).9 For patients with renal impairment, this period may be extended even further.
Measurement of dabigatran plasma concentration is currently not feasible outside of large stroke centers. To this end, Kate and coworkers devised a treatment protocol based on the relationship of dabigatran concentration and thrombin time (TT) and activated partial thromboplastin time (aPTT).10 Yet, limited use of TT hampers implementation and aPTT is prolonged at therapeutic levels but does not have a linear relationship with dabigatran. Notably, more than 50% of U.S. board‐certified vascular neurologists would not give t‐PA in ischemic stroke on dabigatran, regardless of the aPTT results.11
In the majority of patients, however, dabigatran levels or coagulation parameters are detected or expected in a range where t‐PA is contraindicated. Idarucizumab quickly reverses the anticoagulant effects and could expand the group of candidates for thrombolysis.5 The dabigatran concentration measured in our patient was in the lower range of the trough of 31–225 μg/L. The most potent anticoagulant effect and subsequently risk for hemorrhage is expected at peak plasma concentration 2–3 h after ingestion and an overlap with the time of thrombolysis was anticipated (Fig. 1A). General toxicologic therapies may apply in recent ingestions.12 In this regard, activated charcoal to adsorb dabigatran could be considered within 1–2 h after intake of the drug.
Figure 1.
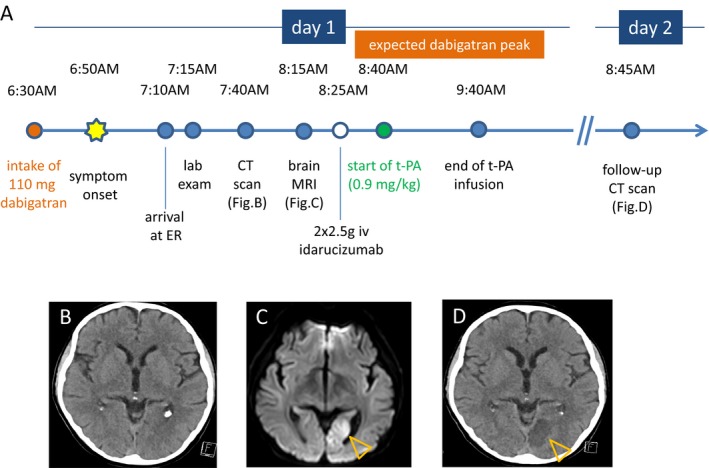
Time course of events in a patient who developed acute ischemic stroke on treatment with dabigatran and received t‐PA after reversal of anticoagulant activity with idarucizumab (A). Noncontrast CT 50 min from symptom onset did not visualize signs of acute ischemic stroke (B). Brain MRI revealed a diffusion‐weighted imaging (DWI)/fluid‐attenuated inversion recovery (FLAIR) mismatch in the medial part of the left occipital lobe. The axial DWI image is shown in image (C), the lesion is marked with orange arrowheads. Follow‐up CT on day 2 (D) showed demarcation of an embolic infarction in the territory supplied be the left posterior artery (orange arrowheads).
Insufficient anticoagulant activity with only 110 mg twice a day is most likely the reason for stroke recurrence in our patient as kidney function had recovered over time. Kate argues that even subtherapeutic plasma concentrations of dabigatran and other antithrombotic agents exert a protective effect by reducing clot burden and/or increasing the likelihood of spontaneous recanalization.10 Pfeilschifter et al. reported that all cases of stroke under dabigatran which received t‐PA so far did not show relevant alterations of the aPTT.13 While noncompliance may be an issue for DOACs, our patient plausibly denied irregular intake. Switching‐off anticoagulation in patients with an embolic source such as AF bears the risk of thrombosis and embolism, as observed in the pivotal trial. Thus, we bridged with heparin on day 2 and reinstituted dabigatran with the adequate dosage on day 3 to prevent additional ischemic events. When to reinstitute treatment with DOACs after an ischemic event is another challenge in clinical practice. In the lack of clinical trials, the 1–3–6–12‐day rule has been proposed in 2013 for patients with nonvalvular AF and has been followed in many centers worldwide. The most recent update of this recommendation suggests reinstitution of anticoagulation in patients with a transient ischemic attack (TIA) after 1 day, with minor stroke (NIHSS <8) after 3 days, a moderate stroke (NIHSS 8–16) after 6 days, and severe stroke (NIHSS >16) after 12 days.14 In the latter two situations, hemorrhagic transformation needs to be excluded prior to treatment initiation.
This is the third case of t‐PA treatment after dabigatran reversal in the literature.15, 16 While the two previous cases corroborate that dabigatran reversal is well tolerated and no hemorrhagic or procoagulatory complications occurred, prospective studies are mandatory to confirm the safety profile of idarucizumab for this purpose.
Author contributions
J. S. Mutzenbach, F. Otto, U. Halwachs, and F. Weymayr revised the manuscript and carried out acquisition of data, accepted responsibility for conduct of research, and approved the final manuscript. S. Pikija drafted/revised the manuscript, accepted responsibility for conduct of research, and approved the final manuscript. J. Sellner designed, conceptualized, and drafted/revised the manuscript, carried out acquisition of data, accepted responsibility for conduct of research, and approved the final manuscript.
Conflict of Interest
J. S. Mutzenbach has received speaker's honoraria from Bayer, Boehringer Ingelheim, Genzyme, and EVER NeuroPharma. S. Pikija has received speaker's honoraria from Boehringer Ingelheim and Teva‐Ratiopharm. F. Otto has received speaker's honoraria from Teva‐Rationpharm and Genzyme. U. Halwachs reports no disclosures. F. Weymayr reports no disclosures. J. Sellner has received speaker's honoraria from Biogen, EVER NeuroPharma, Genzyme, Teva‐Ratiopharm, and Novartis.
Acknowledgments
The authors thank the emergency room and stroke unit team at Christian Doppler Medical Center.
References
- 1. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. New England J Med 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 2. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;361:2342–2352. [DOI] [PubMed] [Google Scholar]
- 3. Goldhaber SZ, Schellong S, Kakkar A, et al. Treatment of acute pulmonary embolism with dabigatran versus warfarin. A pooled analysis of data from RE‐COVER and RE‐COVER II. Thromb Haemost 2016;116: [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 4. Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE‐MODEL randomized trial. J Thromb Haemost 2007;5:2178–2185. [DOI] [PubMed] [Google Scholar]
- 5. Pollack CV Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. New England J Med 2015;373:511–520. [DOI] [PubMed] [Google Scholar]
- 6. Shahjouei S, Tsivgoulis G, Bavarsad Shahripour R, et al. Safety of intravenous thrombolysis among stroke patients taking new oral anticoagulants–case series and systematic review of reported cases. J Stroke Cerebrovasc Dis 2015;24:2685–2693. [DOI] [PubMed] [Google Scholar]
- 7. Bladin C, Curnow J, Levi C, et al. Thrombolysis for acute ischaemic stroke in patients treated with dabigatran: practical guidance. In: SSo Australasia, editor. Victoria, Australia: Stroke Society of Australasia & The Australasian Society of Thrombosis and Haemostasis, New South Wales; 2014. [Google Scholar]
- 8. Diener HC, Foerch C, Riess H, et al. Treatment of acute ischaemic stroke with thrombolysis or thrombectomy in patients receiving anti‐thrombotic treatment. Lancet Neurol 2013;12:677–688. [DOI] [PubMed] [Google Scholar]
- 9. Hankey GJ, Norrving B, Hacke W, Steiner T. Management of acute stroke in patients taking novel oral anticoagulants. Int J Stroke 2014;9:627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kate M, Szkotak A, Witt A, et al. Proposed approach to thrombolysis in dabigatran‐treated patients presenting with ischemic stroke. J Stroke Cerebrovasc Dis 2014;23:1351–1355. [DOI] [PubMed] [Google Scholar]
- 11. Rybinnik I, Mullen MT, Messe S, et al. Treatment of acute stroke in patients on dabigatran: a survey of US stroke specialists. J Stroke Cerebrovasc Dis 2013;22:1312–1316. [DOI] [PubMed] [Google Scholar]
- 12. Tummala R, Kavtaradze A, Gupta A, Ghosh RK. Specific antidotes against direct oral anticoagulants: a comprehensive review of clinical trials data. Int J Cardiol 2016;1:292–298. [DOI] [PubMed] [Google Scholar]
- 13. Pfeilschifter W, Abruscato M, Hovelmann S, Baas H. Thrombolysis in a stroke patient on dabigatran anticoagulation: case report and synopsis of published cases. Case Rep Neurol 2013;5:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heidbuchel H, Verhamme P, Alings M, et al. Updated European heart rhythm association practical guide on the use of non‐vitamin‐k antagonist anticoagulants in patients with non‐valvular atrial fibrillation: executive summary. Eur Heart J 2016; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berrouschot J, Stoll A, Hogh T, Eschenfelder CC. Intravenous thrombolysis with recombinant tissue‐type plasminogen activator in a stroke patient receiving dabigatran anticoagulant after antagonization with idarucizumab. Stroke 2016;47:1936–1938. [DOI] [PubMed] [Google Scholar]
- 16. Schäfer N, Müller A, Wüllner U. Systemic thrombolysis for ischemic stroke after antagonizing dabigatran with idarucizumab‐a case report. J Stroke Cerebrovasc Dis 2016;25(8):e126–7. [DOI] [PubMed] [Google Scholar]


