Abstract
Aims
The aim of this meta‐analysis is to examine the impact of in‐hospital pharmacist‐led medication reviews in paediatric and adult patients.
Methods
Relevant studies were identified from the Medline and Cochrane Library databases. Studies were included if they met the following criteria (without any language or date restrictions): design: randomized controlled trial; intervention: in‐hospital pharmacist‐led medication review (experimental group) vs. usual care (control group); participants: paediatric or adult population. The primary outcome was all‐cause readmissions and/or emergency department (ED) visits at different time points. The secondary outcomes were all‐cause readmissions, all‐cause ED visits, drug‐related readmissions, mortality, length of hospital stay, adherence and quality of life. We calculated the relative risk (RR) or mean differences (MD) with 95% confidence intervals (CIs) for each study. We used fixed and/or random effects models. Heterogeneity was assessed using the I 2 statistic.
Results
We systematically reviewed 19 randomized controlled trials (4805 participants). The readmission rates did not differ between the experimental group and the control group (RR = 0.97, 95% CI 0.89; 1.05, p = 0.470). The secondary outcomes did not differ between the two groups, except for in drug‐related readmissions, which were lower in the experimental group (RR = 0.25, 95% CI 0.14; 0.45, p < 0.001), and all‐cause ED visits (RR = 0.70, 95% CI 0.59; 0.85 p = 0.001).
Conclusion
The low‐quality evidence in this analysis suggests an impact of pharmacist‐led medication reviews on drug‐related readmissions and all‐cause ED visits. Few studies reported on adherence and quality of life. More high‐quality randomized clinical trials are needed to assess the impact of pharmacist‐led medication reviews on patient‐relevant outcomes, including adherence and quality of life.
Keywords: clinical pharmacy, medication reconciliation, medication review, meta‐analysis, pharmacists, systematic review
What is Already Known about this Subject
Pharmacist‐led medication reconciliation interventions are an effective strategy to reduce medication discrepancies, and have a greater impact when conducted at either hospital admission or discharge. However, pharmacist‐led medication reviews at hospital settings programmes have not shown great interest in the use of post‐hospital healthcare.
What this Study Adds
In this article, we further explore the clinical impact of pharmacist‐led medication review at the hospital setting. We show that pharmacist‐led medication reviews have an impact on drug‐related readmission, all‐cause ED visits and adherence. We also show the quality and strength of evidence from these studies is low.
Introduction
Early hospital readmission of patients discharged from hospital are a public health problem. The ENEIS 2 study reported that the number of hospital admissions caused by preventable serious adverse drug events (ADEs) represented 1.3% of hospital admissions in France in 2009 1. Medication reviews are considered a key element in improving the quality of prescriptions and in preventing ADEs among hospitalized patients 2. A medication review is ‘a structured, critical examination of a patient's medicines with the objective of reaching an agreement with the patient about treatment, optimising the impact of medicines, minimising the number of medication‐related problems and reducing waste’ 3. When performed by pharmacists, this process is called a pharmacist‐led medication review. Pharmacist‐led medication reviews have been widely introduced in hospitals based on three types of interventions: (i) prescription review (type 1), (ii) adherence review (AR) (type 2) and (iii) clinical medication review (CMR) (type 3).
However, the impact of in‐hospital pharmacist‐led medication reviews has not been clearly demonstrated. To our knowledge, four meta‐analyses have studied the impact of hospital pharmacist‐led medication reviews 4, 5, 6, 7, but they did not explore several outcomes of clinical importance (i.e., all‐cause readmission, emergency department visits, drug‐related readmissions, all‐cause mortality, length of hospital stay, adherence and quality of life). Moreover, they varied in terms of the location of the intervention (hospital and community settings) 5, 6. The primary outcomes also varied, including reducing drug‐related problems, adverse drug reactions, and hospital readmissions and improving the appropriateness of medications. No studies to date have been sufficiently broad to test whether this type of intervention can reduce mortality, and although many studies have measured quality of life, this is rarely a primary outcome. Furthermore, the various analyses did not take into account the different timing of endpoints used to calculate the hospital readmission rates 4, 5, 6, 7. Indeed, such studies usually considered hospital readmissions at various time points (i.e., 30 days, 60 days, 6 months or 1 year) a common outcome. This could be very problematic, as the causes of readmissions may strongly differ according to these time points. Finally, some meta‐analyses have included randomized controlled trials (RCTs) and non‐RCTs 4.
The aim of the present review is to evaluate the impact of in‐hospital pharmacist‐led medication reviews on clinical outcomes at different time points.
Methods
Searching strategy
This meta‐analysis was based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) criteria 8. To identify relevant studies, we reviewed the following databases: Medline (from January 1990 to December 2015) and the Cochrane Library (from January 1990 to December 2015). A specific search strategy was developed based on a broad range of indexed terms and medical subject headings (i.e., medication reconciliation, medication therapy management, pharmaceutical services, drug therapy, drug utilization, patient education as topic, medication adherence, medication errors, patient medication knowledge, hospital, patient discharge, emergency medical services, hospitalization, ambulatory care, pharmacies, pharmacists and pharmacy). The algorithm used is listed in Appendices S1 and S2. Furthermore, the ProQuest dissertations and theses full‐text database was used to identify unpublished dissertations. The references of the included studies were examined to search for additional trials.
Selecting studies
Studies were included if they met the following criteria: design: RCT; intervention: pharmacist‐led medication review in a hospital (experimental group) vs. usual care (control group); participants: adult or paediatric participants; outcomes: evaluation of the selected outcomes based on a validated measure; and language: French or English. Because prescription review (type 1) is usually considered a component of pharmacists' routine practice in dispensing medications, this service was considered the usual care or control group.
The interventions had to be principally delivered by a pharmacist. The pharmacist‐led medication reviews were categorized by type based on objective descriptions (see Table 1).
Table 1.
Description of the types of medication review led by pharmacists
| Type | Name of service | Definition/Objective | Possible intervention provided |
|---|---|---|---|
| 1 | Prescription review | Definition: addresses issues related to patient prescriptions or medications. Objective: to address the technical issues related to a patient's prescriptions such as anomalies or changed items. | ✓ Drug interactions |
| ✓ Side effects | |||
| ✓ Dosage | |||
| ✓ Drug availability | |||
| 2 | Adherence review | Definition: a comprehensive and systematic evaluation of patient's understanding of and adherence to prescribed medication treatment. Objective: to improve understanding and adherence to prescribed medication treatment: identify and address factors linked to non‐adherent behaviours as well as minimize pharmaceutical waste. | ✓ Medication review of drug adherence |
| ✓ Therapeutic education | |||
| ○ Therapeutic goals | |||
| ○ Management of adverse events | |||
| 3 | Clinical medication review | Definition: a systematic and patient‐centred clinical assessment of all medicines currently taken by a patient. Objective: to identify, resolve and prevent medication‐related problems as well as optimize the effectiveness of medication treatment. | ✓ Medication reconciliation |
| ✓ Treatment review (dosages, drug interactions, side effects and therapeutic objectives, etc.) | |||
| ✓ Pharmaceutical interventions | |||
| ✓ Multidisciplinary revision of drug prescriptions | |||
| ✓ Medication liaison service | |||
| ▪ Comprehensive medication history, | |||
| ▪ Discharge letter faxed to general practitioner and community pharmacist, | |||
| ▪ Discharge counselling |
Manuscripts that met the following criteria were excluded: reports of qualitative data only, user‐satisfaction surveys, conference presentations, opinion pieces, letters, non‐RCTs, and interventions delivered principally by a community pharmacist. Interventions that were delivered by combinations of health professionals (e.g., physicians, nurses) in which the pharmacist was only partly involved were also excluded.
Outcome measures
The primary outcome was all‐cause readmissions and/or ED visits, i.e., the number of hospitalized patients regardless of the cause of hospitalization and the number of non‐hospitalized patients who visited an emergency department. The secondary outcomes were all‐cause readmissions (the number of hospitalized patients regardless of the cause of hospitalization), all‐cause ED visits (the number of non‐hospitalized patients who visited an emergency department), drug‐related readmissions, all‐cause mortality, length of hospital stay, adherence and quality of life.
Selection of studies and data extraction
Two authors (PR and SH) screened the titles and abstracts of the database records, retrieved full texts to assess their eligibility and independently checked the full‐text records for eligibility. Disagreements were resolved by consensus. The manuscripts of the studies were then independently reviewed by two of the authors (PR and SH). The following data were independently extracted into a standard electronic form: first author name, date of publication, country, design, sample size, number of patients, mean age, type of pharmacist‐led medication review, follow‐up after discharge, readmission rate collection, intervention details, all‐cause readmissions and/or ED visits, all‐cause readmissions, all‐cause ED visits, drug‐related readmissions, all‐cause mortality, length of hospital stay, adherence and quality of life. We developed a data extraction form (based on the Cochrane Consumers and Communication Review Group's data extraction template), pilot‐tested it on ten randomly‐selected included studies, and refined it accordingly 9. Data extraction was performed based on the intention‐to‐treat (ITT) principle. For all events, we considered the shortest available follow‐up period because the impact of pharmacist‐led medication reviews is probably the most substantial during the first 30 days following discharge. After this period, external factors can become predominant, and readmissions could thus be more influenced by these external factors than by pharmacist‐led medication reviews. Any discrepancies were resolved by consensus with a third reviewer (LB).
Assessing the methodological quality of included studies
The methodological quality of the included studies was assessed independently by two authors (PR and SH). Any discrepancies were resolved by consensus with a third reviewer (LB). We used indicators of internal validity from the Cochrane Risk of Bias Tool 10. The risk of selection bias was assessed at the study level (sequence generation, allocation sequence concealment), the risk of performance bias at the comparison level (i.e., blinding of participants and personnel), and the risk of detection bias as well as attrition bias at the outcome level (blinding of outcome assessors, handling of incomplete outcome data). The studies' risk of bias was then qualified as low, unclear or high.
Statistical analysis
All‐cause readmissions and/or ED visit rates, all‐cause readmission rates, all‐cause ED visit rates, drug‐related readmission rates and all‐cause mortality rates were analysed using relative risks (RRs), defined as the ratio of the probability of an event occurring between two groups.
The mean length of hospital stay was analysed using the mean difference (MD) with 95% confidence intervals (CIs) for each study. We used fixed effects 11 and random effects models 12, which account for the between‐study heterogeneity by weighting studies similarly. Heterogeneity was assessed using the I 2 statistic, which represents the percentage of variance due to between‐study factors rather than to sampling error 13. We considered values of I 2 > 50% as indicative of large heterogeneity 14. We used funnel plots (i.e., plots of effect estimates against sample size) to estimate the risk of bias: an asymmetry in these plots may indicate publication bias in the meta‐analysis 15. The robustness of the findings was investigated using the following sensitivity analyses when possible: (i) the use of random effects methods, (ii) risk of bias 10, (iii) follow‐up after discharge, (iv) medication reconciliation, (v) treatment review, (vi) medication service liaison, (vii) full MR and (viii) CMR or AR. Analyses were performed with RevMan software version 5.3.
Results
Search results and study characteristics
In total, 303 titles were identified in the MEDLINE and Cochrane Library databases and in the search for additional references. After removing duplicates, 239 studies were identified. After reading the titles and abstracts, 159 studies were excluded according to the inclusion and exclusion criteria. After reading the full studies, we selected 19 randomized controlled studies. Details on the article locations and the studies included/excluded from the systematic review are shown in Figure 1. The characteristics of the included studies are presented in Table 1.
Figure 1.
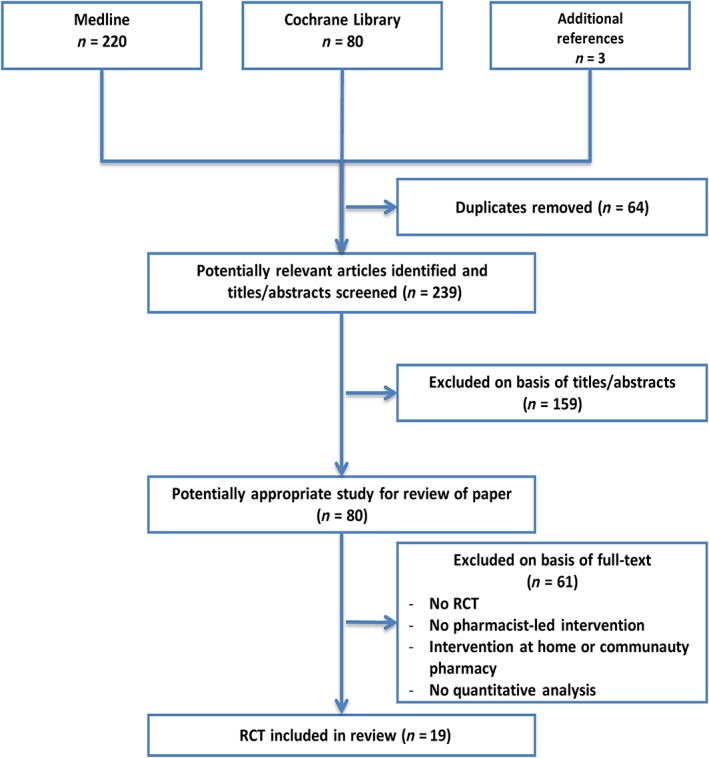
Systematic review inclusion and exclusion flowchart
One of the studies was conducted in a paediatric population 16, and the others were conducted with adults. The average age of the adults was between 58.6 and 87.6 years, with the proportion of men ranging from 23% to over 70% (see Table 2).
Table 2.
Characteristics of included studies
| Study author | Country | Design | No. of patients | Mean age, years | % male | Type of medication review | Follow‐up after discharge | Outcomes | Results | Readmission rates collection | Intervention a |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bladh 42 | Sweden | RCT | 345 | 82 | 39% | CMR | No | Quality of life (EQ‐5D)
Quality of life (HRQoL) Length of stay |
I = C
I = C I = C |
Medication service liaison Multidisciplinary revision of drug prescriptions | |
| Bolas 43 | Ireland | RCT | 162 | 74 | 50% | CMR | No | Readmission Length of stay | I = C I = C | 3 months | Medication service liaison |
| Farris 17 | USA | RCT | 945 | 61 | CMR | Yes | Readmission | I = C | 30 days | Minimal and enhanced group: | |
| – Medication reconciliation, Medication service liaison (minus faxed care plan) | |||||||||||
| Enhanced group: | |||||||||||
| – Telephone call 3–5 days post‐discharge, | |||||||||||
| – Care plan faxed to primary care physician/community pharmacist | |||||||||||
| Frankenthal 44 | Israel | RCT | 306 | >65 | 35% | CMR | No | Readmission Quality of life (SF‐12) | I = C I = C | 12 months | Treatment review Multidisciplinary revision of drug prescriptions |
| Gillespie 28 | Sweden | RCT | 400 | 86.4 | 23% | CMR | Yes | Readmission Length of stay | I = C I = C | 12 months | Medication reconciliation
Treatment review Medication service liaison Telephone follow‐up during 2 months. If patient is re‐admitted to the hospital, can be reinstated once into the interventional group |
| Jarab 33 | UK | RCT | 127 | 62.5 | 40% | AR | No | Quality of life (SGRQ) Adherence | I = C I > C | Medication review of drug adherence
Motivational interviewing Drug action plan Therapeutic education programme for smokers Medication service liaison |
|
| Koehler 29 | USA | RCT | 41 | 78 | 25% | CMR | Yes | Readmission Readmission | I > C I = C | 30 days 2 months | Medication reconciliation
Treatment review Medication service liaison Telephone follow‐up to 5–7 days |
| Lipton 26 | USA | RCT | 706 | 74.6 | CMR | Yes | Adherence Readmission | I > C I = C | 3 months | Medication reconciliation
Treatment review Medication service liaison Telephone follow‐up at 2–4 weeks and 2, 3 and 6 months. |
|
| Lisby 20 | Denmark | RCT | 99 | ≥70 | 70% | CMR | No | Length of stay Readmission | I = C I = C | 3 months | Medication reconciliation
Treatment review Pharmaceutical intervention on drug pharmacology |
| Lisby 21 | Denmark | RCT | 108 | 80.5 | CMR | No | Length of stay | I = C | Medication reconciliation Treatment review | ||
| McMullin 45 | USA | RCT | 259 | >61 | 36% | CMR | No | Readmission | I = C | 30 days | Prescription review Pharmaceutical intervention on antibiotics |
| Morgado 22 | Portugal | RCT | 197 | 59 | 45% | AR | No | Adherence | I > C | Medication review of drug adherence motivational interviewing
Drug action plan Therapeutic education |
|
| Nazareth 18 | UK | RCT | 362 | 84 | 38% | AR | Yes | Readmission Readmission | I = C I = C | 3 months 6 months | Medication review of drug adherence
Drug action plan Therapeutic education Medication service liaison Pharmacist community follow‐up at home with therapeutic education and review of drug action plan |
| Sadik 23 | UK | RCT | 221 | 58.6 | 45% | CMR | No | Readmission
Quality of life (MLHFQ) Quality of life (SF‐36) |
I > C
I > C I = C |
12 months | Treatment review
Multidisciplinary revision of drug prescriptions Medication service liaison |
| Schnipper 19 | USA | RCT | 178 | 59.2 | 34% | AR | Yes | Readmission Adherence | I = C I = C | 30 days | Medication review of drug adherence
Therapeutic education Telephone follow‐up to 3–5 days |
| Scullin 27 | North Ireland | RCT | 762 | 71.3 | 55% | CMR | No | Length of stay Readmission | I > C I > C | 30 days (and each month until 12 months) | Medication reconciliation Medication service liaison |
| Spinewine 24 | Belgium | RCT | 186 | 82.4 | 28% | CMR | No | Readmission | I = C | 12 months | Medication reconciliation Medication service liaison |
| Stowasser 25 | Australia | RCT | CMR | No | Readmission Length of stay | I = C I = C | 30 days | Medication liaison service Medication history confirmation with community healthcare professionals (telephone, faxing) | |||
| Zhang 16 | China | RCT | 150 | < 18 | 43% | CMR | Yes | Length of stay
Adherence Readmission |
I > C
I > C I = C |
14 days | Treatment review
Multidisciplinary revision of drug prescriptions Telephone follow‐up to 3–4 days |
Results based on significant findings reported in the individual study. I > C, intervention is significantly better than control; I = C, no significant difference between intervention and control; C > I, control is significantly better than intervention. AR, adherence review; CMR, clinical medication review; SF‐12, Short‐form 12 health survey; MLHFQ, Minnesota Living With Heart Failure Questionnaire; SF‐36, Short‐form 13 health survey; SGRQ, St. George's respiratory questionnaire; EQ‐5D, EuroQol‐5D; HRQoL, Health‐related quality of life.
All interventions were performed by a hospital pharmacist working directly in the care units (n = 19); 15 studies concerned clinical medication review (CMR, type 3) and four concerned adherence review (AR, type 2) (n = 4). In the studies assessing CMRs, the pharmacists delivered various interventions: medication reconciliation (n = 9), treatment review (n = 9) and medication liaison services (n = 9). Nine studies included a follow‐up after hospital discharge (five for CMR and four for AR).
Methodological quality of included studies
Table 3 summarizes the assessments of the risk of bias. All 19 trials were at high risk of performance bias because the nature of the intervention meant that the personnel and participants could not be blinded, and thus performance bias was not included in the calculation of global risk. Three out of 19 RCTs were classified as good quality 17, 18, 19. The allocation concealment procedures of six studies was unclear 20, 21, 22, 23, 24, 25, and two studies were considered at high risk of bias 26, 27. There was a high risk of other bias assessed in six trials, including a possible contamination bias with the same pharmacists caring for both the control and intervention groups, a lack of power to detect changes in admission rates, and the recruitment of only half of the eligible patients into the trial 21, 24, 26, 27, 28, 29.
Table 3.
Risk of bias regarding outcomes of the studies included in the systematic review
| Random sequence generation | Allocation concealment | Blinding: participants, personnel | Blinding: outcomes | Incomplete outcome assessment | Selective reporting | Other | Global risk | |
|---|---|---|---|---|---|---|---|---|
| Bladh 42 | Unclear | Low | High | Unclear | Low | High | High risk of bias | |
| Bolas 43 | Low | Low | High | High | High | High | Low | High risk of bias |
| Farris 17 | Low | Low | High | Low | Low | Low | Low | Low risk of bias |
| Frankenthal 44 | Low | Low | High | Low | Low | Low | Unclear | Unclear risk of bias |
| Gillepsie 28 | Low | Low | High | Low | Low | Low | High | High risk of bias |
| Jarab 33 | Low | Low | High | Low | Low | Low | Unclear | Unclear risk of bias |
| Koehler 29 | Low | Low | High | Low | Low | Unclear | High | High risk of bias |
| Lipton 26 | Low | High | High | Low | Low | Low | High | High risk of bias |
| Lisby 20 | Low | Unclear | High | Low | Low | High | Unclear | High risk of bias |
| Lisby 21 | Unclear | Unclear | High | Unclear | Low | Low | High | High risk of bias |
| McMullin 45 | Low | Low | High | High | Low | Low | Low | High risk of bias |
| Morgado 22 | High | Unclear | High | Low | Low | Low | Unclear | High risk of bias |
| Nazareth 18 | Low | Low | High | Low | Low | Low | Low | Low risk of bias |
| Sadik 23 | Low | Unclear | High | Low | Low | Low | Unclear | Unclear risk of bias |
| Scullin 27 | Low | High | High | Low | High | Low | High | High risk of bias |
| Schnipper 19 | Low | Low | High | Low | Low | Low | Low | Low risk of bias |
| Spinewine 24 | High | Unclear | High | Low | Low | Low | High | High risk of bias |
| Stowasser 25 | Low | Unclear | High | Low | Low | Low | Low | Unclear risk of bias |
| Zhang 16 | Low | Low | High | Low | Low | High | Unclear | High risk of bias |
All‐cause readmission and/or ED visits
We identified 13 studies that compared the effects of pharmacist‐led medication reviews (n = 2385) to those of usual care (n = 2420) on rates of all‐cause readmissions and/or ED visits. There were no significant differences between the two groups (RR = 0.97, 95% CI 0.90; 1.05, P = 0.44, I 2 = 0%) (see Figure 2). Moreover, there were no significant differences between all‐cause readmission and/or ED visits at 30 days (RR = 0.98, 95% CI 0.86; 1.12, P = 0.80, I 2 = 0%) or between 2 months and 12 months (RR = 0.96, 95% CI 0.87; 1.05, P = 0.38, I 2 = 22%) after hospital discharge. The associated funnel plot was symmetrical (Appendix S3). Moreover, we did not find any significant differences in the sensitivity analyses (see Table 4). The sensitivity analysis did not show significant differences between the studies assessing full medication reviews (medication reconciliation, treatment review, medication liaison service and telephone follow‐up) and those without full medication review. Only one study, which almost reached significance (0.26 [0.06; 1.09]) in this outcome, included a full medication review and the readmission rate at 30 days 29. However, these results concerned only 41 patients, and the effects of sampling may thus be important.
Figure 2.
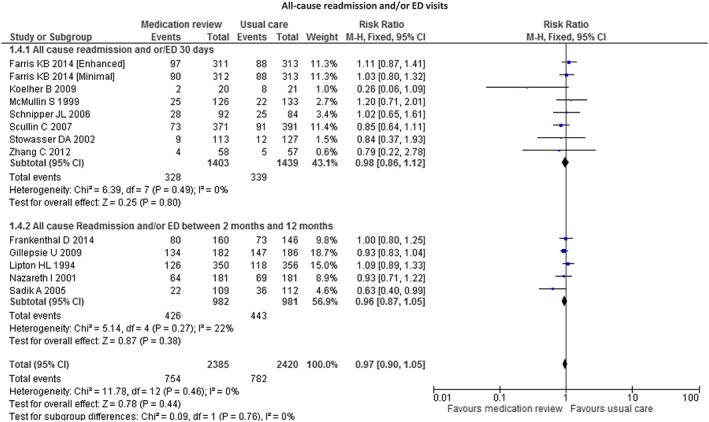
Forest plot of the effect of medication review on 30 days' all‐cause readmissions and/or ED visits and all cause readmission and/or ED visits between 2 months and 12 months after hospital discharge
Table 4.
Sensitivity analysis of primary outcome (all‐cause readmission and/or ED visits) and secondary outcome (length of hospital stay)
| All‐cause readmission and/or ED visits | Length of hospital stay | |||||||
|---|---|---|---|---|---|---|---|---|
| No. of patients (no. of trials) | Relative risk (95% CI) | P‐value of overall effect | I 2 ‐value of heterogeneity (%) | No. of patients (no. of trials) | MD (95% CI) | P‐value of overall effect | I 2 ‐value of heterogeneity (%) | |
| Random vs. Fixed effects | ||||||||
| Random effects | 4805 (12) | 0.97 (0.90, 1.05) | 0.44 | 0 | 1887 (6) | −0.0.6 (−0.23, 0.11) | 0.50 | 65 |
| Fixed effects | 4805 (12) | 0.97 (0.90, 1.04) | 0.33 | 0 | 1887 (6) | −0.08 (−0.17, −0.01) | 0.09 | 65 |
| Level quality | ||||||||
| High quality 17, 18, 19 | 1787 (3) | 1.03 (0.89; 1.18) | 0.70 | 0 | — | — | — | — |
| Low and unclear quality | 3018 (9) | 0.94 (0.86; 1.03) | 0.18 | 0 | 1887 (6) | −0.0.6 (−0.23, 0.11) | 0.50 | 65 |
| Medication reconciliation | ||||||||
| Yes 20, 21, 24, 26, 27, 28, 29 | 1877 (4) | 0.95 (0.85; 1.05) | 0.31 | 47 | 1337 (4) | −0.03 (−0.24; 0.19) | 0.81 | 66 |
| No | 2928 (8) | 0.99 (0.89. 1.11) | 0.89 | 36 | 550 (2) | −0.14 (−0.57; 0.29) | 0.54 | 81 |
| Treatment review | ||||||||
| Yes 16, 20, 23, 26, 28, 44, 45 | 1975 (6) | 1.98 (0.88; 1.08) | 0.62 | 16 | 617 (3) | −0.07 (−0.38; 0.25) | 0.67 | 70 |
| No | 2830 (6) | 0.96 (0.85; 1.09) | 0.55 | 0 | 1270 (3) | −0.06 (−0.29; 0.18) | 0.64 | 70 |
| Medication service liaison | ||||||||
| Yes 17, 18, 23, 24, 25, 26, 27, 29, 33, 42 | 2956 (6) | 0.95 (0.85; 1.07) | 0.41 | 42 | — | — | — | — |
| No | 1849 (6) | 0.99 (0.89; 1.10) | 0.84 | 0 | — | — | — | — |
| Full medication review | ||||||||
| Yes 26, 28, 29 | 1115 (3) | 0.96 (0.77; 1.21) | 0.75 | 69 | — | — | — | — |
| No | 3690 (9) | 0.96 (0.87; 1.07) | 0.49 | 0 | — | — | — | — |
| Inpatient only vs. with follow up | ||||||||
| Inpatient without follow up | 1788 (5) | 0.90 (0.77, 1.05) | 0.16 | 14 | — | — | — | — |
| Inpatient with follow up 16, 17, 18, 19, 26, 28, 29 | 3017 (7) | 1.00 (0.92, 1.10) | 0.98 | 0 | — | — | — | — |
| Clinical medication review 16, 17, 20, 21, 23, 24, 25, 26, 27, 28, 29, 42, 43, 44, 45 | 4267 (10) | 0.97 (0.89; 1.06) | 0.50 | 14 | 1887 (6) | −0.06 (−0.23, 0.11) | 0.50 | 65 |
| Adherence review 18, 19, 22, 33 | 538 (2) | 0.95 (0.76; 1.20) | 0.69 | 0 | — | — | — | — |
All‐cause readmission
We identified 12 studies that compared the effects of pharmacist‐led medication reviews (n = 2331) to usual care (n = 2382) on all‐cause readmission rate. There were no significant differences between the two groups (RR = 0.98, 95% CI 0.90; 1.06, P = 0.59, I 2 = 0%) (see Figure 3). Moreover, there were no significant differences between all‐cause readmissions 30 days (RR = 0.95, 95% CI 0.80; 1.13, P = 0.56, I 2 = 0%) or 2 months to 12 months (RR = 0.99, 95% CI 0.90; 1.09, P = 0.83, I 2 = 0%) after hospital discharge. The associated funnel plot was symmetrical (Appendix S3).
Figure 3.
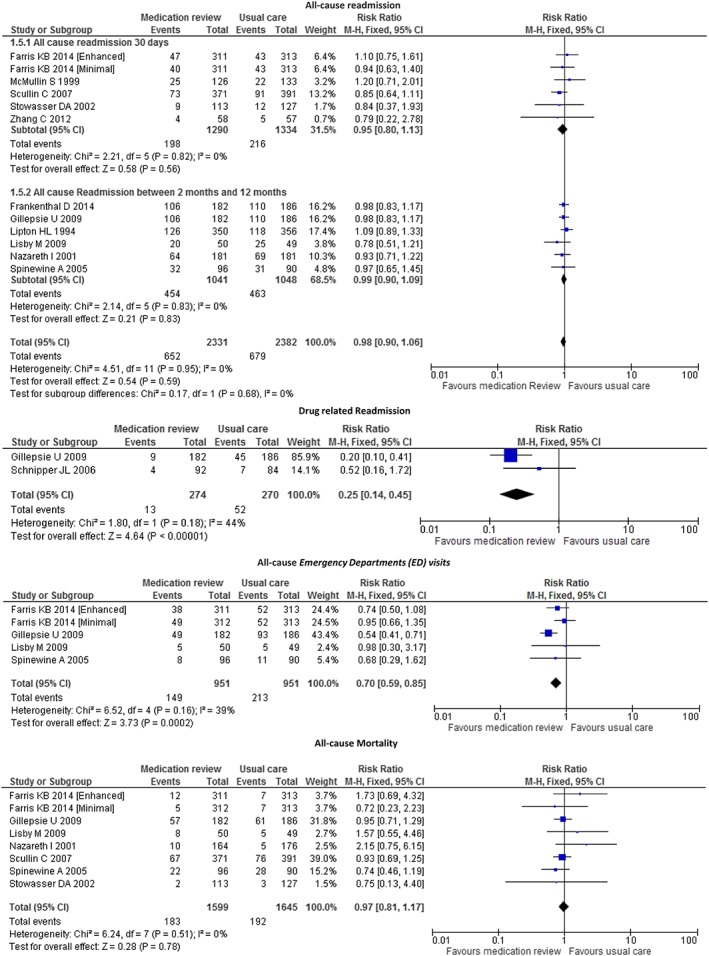
Forest plot of the effect of medication review on 30 days' all‐cause readmissions, all‐cause readmission between 2 months and 12 months after hospital discharge, all‐cause ED visits, drug‐related readmission and all‐cause mortality
All‐cause emergency department visits
We identified four studies that compared the effects of pharmacist‐led medication reviews (n = 951) to those of usual care (n = 951) on all‐cause ED visit rates. There was a significant difference between the two groups (RR = 0.70, 95% CI 0.59; 0.85 P = 0.0002, I 2 = 39%) (see Figure 3).
Drug‐related readmissions
We identified two studies that compared the effects of pharmacist‐led medication review (n = 274) to usual care (n = 270) on drug‐related readmission rates. There was a significant difference between the two groups (RR = 0.25, 95% CI 0.14; 0.45, P < 0.0001, I 2 = 44%) (see Figure 3).
All‐cause mortality
We identified seven studies that compared the effects of pharmacist‐led medication review (n = 1599) to usual care (n = 1645) on all‐cause mortality rate. There was no significant difference between the two groups (RR = 0.97, 95% CI 0.81; 1.17, P = 0.86, I 2 = 0%) (see Figure 3).
Length of hospital stay
We identified six studies comparing the effects of pharmacist‐led medication review (n = 931) to usual care (n = 956) on length of hospital stay. There was no significant difference between the two groups (MD −0.45 days, 95% CI −1.73; 0.82, P = 0.48, I 2 = 58%) (see Figure 4). Moreover, we did not find any significant differences in the sensitivity analyses (see Table 4).
Figure 4.
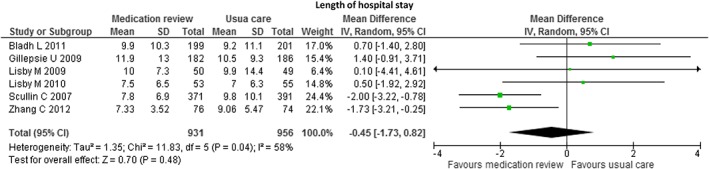
Forest plot of the effect of medication review on the length of hospital stay
Adherence and quality of life
Formal pooling was not possible for the adherence and quality of life variables due to the small number of studies and the diversity of assessments used. Nevertheless, qualitative reviews of the results were performed in terms of the number of studies showing a significant positive effect, a nonsignificant positive effect, no or negative effect. Five studies reported adherence to medications as an outcome of their research (see Table 5). The majority of the studies (n = 80%) reported a significant improvement in adherence to medication as a result of the pharmacist‐led medication review. Patients' quality of life was measured in four studies, and one study with CMR as the intervention reported a significant impact on one of the two scales of quality of life used 23.
Table 5.
Summary of reported findings on adherence and quality of life outcomes
| Outcomes | Number of trials | Favours medication review | Favours usual care | No significant differences between groups | Study reported both a significant and non‐significant differences |
|---|---|---|---|---|---|
| Adherence | 5 | 4 | — | 1 | — |
| Quality of life | 4 | 0 | — | 3 | 1b |
Outcomes were measured using two different methods, for example self‐reported adherence and medication refill.
Analysis using the MLHFQ but not SF‐36 showed a significant impact.
Discussion
Our study found no significant reductions in the rate of all‐cause readmissions and/or ED visits due to pharmacist‐led medication reviews in hospitals. However, pharmacist‐led medication reviews were associated with a decrease in the number of ED visits and drug‐related readmissions. One primary component of the pharmacist‐led medication review is pharmacist‐led medication reconciliation. To our knowledge, only one meta‐analysis has investigated the effectiveness of pharmacist‐led medication reconciliation programmes on clinical outcomes in hospital transitions and found a substantial reduction in the rate of all‐cause readmissions (19%), all‐cause ED visits (28%) and ADE‐related hospital revisits (67%) 30. However, that meta‐analysis, conducted by Mekonnen et al., included not only RCTs but also before/after studies and non‐RCTs. The inclusion of non‐RCTs in meta‐analyses is not recommended because it decreases the level of evidence of the meta‐analysis 31. Our meta‐analysis did not show any significant reductions in the rate of all‐cause readmissions (RR = 0.98, 95% CI 0.90; 1.06, P = 0.59). This difference in our findings from those of Mekonnen et al. may be due to a study by Hawes et al. 32, which included a pharmacist‐led medication reconciliation after discharge at home that did not meet our inclusion criteria. The rate of all‐cause readmissions and/or ED visits was significantly different at 30 days (0% vs. 40.5%, P < 0.001). Moreover, our results are comparable to most pharmacist‐led medication review or pharmacist‐led medication reconciliation meta‐analyses that have studied the rate of readmissions 4, 5, 6, 7.
To our knowledge, the various pharmacist‐led medication review or pharmacist‐led medication reconciliation meta‐analyses that examined all‐cause readmission rates pooled the different results and did not study the effect of the time points used in the calculation of readmission rates. Our meta‐analysis identified no differences when including only the studies that investigated the all‐cause readmission rates and all‐cause readmission and/or ED visits within 30 days.
However, pharmacist‐led medication reviews significantly reduced all‐cause ED visits (RR = 0.70; 95% CI 0.59; 0.85, P = 0.0002) using the 30‐day and the 12‐month endpoint.
It is difficult to study the impact of medication reviews alone because the process is affected by the patient's overall care and many intervening factors. Moreover, the interventions differ depending on the studies. For example, the interventions of Scullin et al. 27 and Spinewine et al. 24 consist of a medication reconciliation and medication service liaison. Other studies 16, 17, 18, 19, 26, 28, 29 have included, for example, a follow‐up post‐discharge by telephone or by a community pharmacist for a more or less lengthy period of time. The sensitivity analyses showed no significant differences depending on the presence or absence of medication reconciliation, treatment review, follow‐up post‐discharge or medication service liaison.
This systematic review on in‐hospital pharmacist‐led medication reviews did not identify an effect on the length of hospital admission (−0.50 (CI) −0.20, 0.10, P = 0.49). Another related meta‐analysis also did not identify an effect on the length of hospital stay (−0.04 days (−1.63; 1.55), P = 0.96). This can be explained by the incomplete medication reviews performed in the interventions. Indeed, the overall results are conflicting, with two trials providing significant results on the length of hospital stay in favour of the intervention 16, 27. It should be noted that the study by Zhang et al. 16 included a paediatric population and that Scullin et al. 27 integrated medication reconciliation into their intervention.
One RCT study has been conducted with children 16, whereas most studies have been conducted in patients over 65 years of age. It is currently difficult to review whether medication reviews have an impact on all‐cause readmissions in the paediatric population, although it is likely that it has an impact on the length of hospital stay 32.
Our study found that clinical medication reviews 16, 26 and adherence reviews 22, 33 had an impact on adherence. Adherence was measured by several scales, such as the Morisky scale 34, the method of Williford and Johnson 35, or by asking patients whether they had taken each medication exactly as prescribed during the previous day and on how many days during the previous week 19. Our study did not find a clear impact of pharmacist‐led medication review on quality of life. Indeed, only one study found an impact on patient's quality of life. This may be due to the range of assessments used: the SF‐12 36, SF‐36 37, EQ‐5D 38, MLHFQ 39, HRQL 40 or SGRQ 41 specific to chronic obstructive pulmonary disease. The variety of the scales used does not enable us to confirm the utility of pharmacist‐led clinical services as an activity to improve patient's quality of life.
Limitations of the study
There are a number of limitations to this study. The first is the small number of subjects that were included in some studies, which may raise concerns about the study sampling. Moreover, many studies were single centre, which raises the issue of replicability. It is necessary to cautiously interpret the impact of pharmacist reviews on all‐cause ED visits and drug‐related readmissions because we found few studies that examined these endpoints. These results were based on only four and two studies, respectively.
Only articles published in English or French were assessed in this review. There may have been studies published in non‐English language journals that involved interventions for improving care transitions. In addition, research disseminated in the grey literature, such as conference papers and unpublished reports, was not considered. This may have resulted in an over‐representation of studies with statistically significant findings, an inflation of effect size estimates, and less precise effect size estimates than meta‐analyses including grey literature.
Conclusion
The impact of pharmacist‐led medication reviews on all‐cause readmissions is not clear, but these clinical pharmacist services have a significant impact on all‐cause ED visits and drug‐related readmissions. However, the latter two results are based on only four and two studies, respectively. The impact of medication reviews on the length of hospital stay and adherence remains unclear. Based on the results of this meta‐analysis and other meta‐analyses, it seems very unlikely, as might be expected, that medication reviews have an impact on mortality. However, the impact on patient quality of life may be more in question; indeed, the variety of the assessments used did not enable us to determine any effects.
It is important to consider the timing of endpoints when studying readmission rates in future investigations. Indeed, medication reviews appeared to impact early hospital readmissions, i.e., at 30 days post‐discharge. The majority of studies investigated the elderly; it is important to demonstrate whether medication reviews can affect the paediatric population. Finally, the global quality of the studies is low, and, to our knowledge, there are no randomized controlled trials involving a large number of subjects; this type of study is necessary to demonstrate a significant impact on readmission rates after hospital discharge. Indeed, to demonstrate a reduction of at least 6% in readmission rates, future trials should include over 1400 subjects. An RCT with a large number of subjects using a standardized medication review in populations at risk for drug‐related readmission is necessary.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work.
Supporting information
Appendix S1 Medline search strategy
Appendix S2 Cochrane Library search strategy
Appendix S3 All‐cause readmission and/or ED visits; All‐cause readmission
Supporting info item
Supporting info item
Supporting info item
Renaudin, P. , Boyer, L. , Esteve, M. ‐A. , Bertault‐Peres, P. , Auquier, P. , and Honore, S. (2016) Do pharmacist‐led medication reviews in hospitals help reduce hospital readmissions? A systematic review and meta‐analysis. Br J Clin Pharmacol, 82: 1660–1673. doi: 10.1111/bcp.13085.
References
- 1. Michel P, others. Enquête nationale sur les événements indésirables liés aux soins (ENEIS). Paris: Direction de la Recherche, des Études et de l’Évaluation et des Statistiques (DREES) du Ministère de la Santé, 2011.
- 2. Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database Syst Rev 2013; CD008986. [DOI] [PubMed] [Google Scholar]
- 3. Clyne W, Blenkinsopp A, Seal R. A Guide to Medication Review 2008. Liverpool: National Prescribing Centre and Medicines Partnership Programme, 2008.
- 4. Hohl CM, Wickham ME, Sobolev B, Perry JJ, Sivilotti MLA, Garrison S, et al. The effect of early in‐hospital medication review on health outcomes: a systematic review. Br J Clin Pharmacol 2015; 80: 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holland R, Desborough J, Goodyer L, Hall S, Wright D, Loke YK. Does pharmacist‐led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta‐analysis. Br J Clin Pharmacol 2008; 65: 303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hatah E, Braund R, Tordoff J, Duffull SB. A systematic review and meta‐analysis of pharmacist‐led fee‐for‐services medication review. Br J Clin Pharmacol 2014; 77: 102–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thomas R, Huntley AL, Mann M, Huws D, Elwyn G, Paranjothy S, et al. Pharmacist‐led interventions to reduce unplanned admissions for older people: a systematic review and meta‐analysis of randomised controlled trials. Age Ageing 2014; 43: 174–187. [DOI] [PubMed] [Google Scholar]
- 8. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. Ann Intern Med 2009; 151: 264–269. [DOI] [PubMed] [Google Scholar]
- 9. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151: W–65. [DOI] [PubMed] [Google Scholar]
- 10. Higgins JPT, Altman DG, Gotzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies. J Natl Cancer Inst 1959; 22: 719–748. [PubMed] [Google Scholar]
- 12. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 13. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vassos E, Collier DA, Fazel S. Systematic meta‐analyses and field synopsis of genetic association studies of violence and aggression. Mol Psychiatry 2014; 19: 471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borenstein M, Hedges LV, Higgins J, Rothstein HR. A basic introduction to fixed‐effect and random‐effects models for meta‐analysis. Res Synth Meth 2010; 1: 97–111. [DOI] [PubMed] [Google Scholar]
- 16. Zhang C, Zhang L, Huang L, Luo R, Wen J. Clinical pharmacists on medical care of pediatric inpatients: a single‐center randomized controlled trial. PLoS One 2012; 7: e30856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Farris KB, Carter BL, Xu Y, Dawson JD, Shelsky C, Weetman DB, et al. Effect of a care transition intervention by pharmacists: an RCT. BMC Health Serv Res 2014; 14: 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nazareth I, Burton A, Shulman S, Smith P, Haines A, Timberall H. A pharmacy discharge plan for hospitalized elderly patients – a randomized controlled trial. Age Ageing 2001; 30: 33–40. [DOI] [PubMed] [Google Scholar]
- 19. Schnipper JL, Kirwin JL, Cotugno MC, Wahlstrom SA, Brown BA, Tarvin E, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med 2006; 166: 565–571. [DOI] [PubMed] [Google Scholar]
- 20. Lisby M, Thomsen A, Nielsen LP, Lyhne NM, Breum‐Leer C, Fredberg U, et al. The effect of systematic medication review in elderly patients admitted to an acute ward of internal medicine. Basic Clin Pharmacol Toxicol 2010; 106: 422–427. [DOI] [PubMed] [Google Scholar]
- 21. Lisby M, Bonnerup DK, Brock B, Gregersen PA, Jensen J, Rungby J. Systematic medication review and health‐related outcome in elderly patients acutely admitted to an orthopaedic ward: a randomised controlled study. Scand J Trauma Resusc Emerg Med 2010; 18 (Suppl 1): P31. [Google Scholar]
- 22. Morgado M, Rolo S, Castelo‐Branco M. Pharmacist intervention program to enhance hypertension control: a randomised controlled trial. Int J Clin Pharm 2011; 33: 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sadik A, Yousif M, McElnay JC. Pharmaceutical care of patients with heart failure. Br J Clin Pharmacol 2005; 60: 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spinewine A, Swine C, Dhillon S, Lambert P, Nachega JB, Wilmotte L, et al. Effect of a collaborative approach on the quality of prescribing for geriatric inpatients: a randomized controlled trial. J Am Geriatr Soc 2007; 55: 658–665. [DOI] [PubMed] [Google Scholar]
- 25. Stowasser DA, Stowasser M, Collins DM. A randomised controlled trial of medication liaison services – patient outcomes. J Pharm Pract Res 2002; 32: 133. [Google Scholar]
- 26. Lipton HL, Bird JA. The impact of clinical pharmacists' consultations on geriatric patients' compliance and medical care use: a randomized controlled trial. Gerontologist 1994; 34: 307–315. [DOI] [PubMed] [Google Scholar]
- 27. Scullin C, Scott MG, Hogg A, McElnay JC. An innovative approach to integrated medicines management. J Eval Clin Pract 2007; 13: 781–788. [DOI] [PubMed] [Google Scholar]
- 28. Gillespie U, Alassaad A, Henrohn D, Garmo H, Hammarlund‐Udenaes M, Toss H, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med 2009; 169: 894–900. [DOI] [PubMed] [Google Scholar]
- 29. Koehler BE, Richter KM, Youngblood L, Cohen BA, Prengler ID, Cheng D, et al. Reduction of 30‐day postdischarge hospital readmission or emergency department (ED) visit rates in high‐risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med 2009; 4: 211–218. [DOI] [PubMed] [Google Scholar]
- 30. Mekonnen AB, McLachlan AJ, Brien JE. Effectiveness of pharmacist‐led medication reconciliation programmes on clinical outcomes at hospital transitions: a systematic review and meta‐analysis. BMJ Open 2016; 6: e010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bossard N, Boissel F, Boissel J. Level of evidence and therapeutic evaluation: need for more thoughts. Fundam Clin Pharmacol 2004; 18: 365–372. [DOI] [PubMed] [Google Scholar]
- 32. Hawes EM, Maxwell WD, White SF, Mangun J, Lin F‐C. Impact of an outpatient pharmacist intervention on medication discrepancies and health care resource utilization in posthospitalization care transitions. J Prim Care Community Health 2014; 5: 14–18. [DOI] [PubMed] [Google Scholar]
- 33. Jarab AS, AlQudah SG, Khdour M, Shamssain M, Mukattash TL. Impact of pharmaceutical care on health outcomes in patients with COPD. Int J Clin Pharm 2011; 34: 53–62. [DOI] [PubMed] [Google Scholar]
- 34. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self‐reported measure of medication adherence. Med Care 1986; 24: 67–74. [DOI] [PubMed] [Google Scholar]
- 35. Williford SL, Johnson DF. Impact of pharmacist counseling on medication knowledge and compliance. Mil Med 1995; 160: 561–564. [PubMed] [Google Scholar]
- 36. Ware JE Jr, Kosinski M, Keller SD. A 12‐item short‐form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34: 220–233. [DOI] [PubMed] [Google Scholar]
- 37. Leplège A, Ecosse E, Verdier A, Perneger TV. The French SF‐36 health survey: translation, cultural adaptation and preliminary psychometric evaluation. J Clin Epidemiol 1998; 51: 1013–1023. [DOI] [PubMed] [Google Scholar]
- 38. Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ‐5D health states: development and testing of the D1 valuation model. Med Care 2005; 43: 203–220. [DOI] [PubMed] [Google Scholar]
- 39. University of Minnesota Office for Technology Commercialization . Minnesota Living With Heart Failure Questionnaire [Online]. Available at http://license.umn.edu/technologies/94019_minnesota‐living‐with‐heart‐failure‐questionnaire (last accessed 21 August 2016).
- 40. Centers for Disease Control and Prevention . CDC HRQOL‐14 ‘Healthy Days Measure’ [Online]. Available at http://www.cdc.gov/hrqol/hrqol14_measure.htm (last accessed 21 August 2016).
- 41. Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self‐complete measure of health status for chronic airflow limitation: the St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992; 145: 1321–1327. [DOI] [PubMed] [Google Scholar]
- 42. Bladh L, Ottosson E, Karlsson J, Klintberg L, Wallerstedt SM. Effects of a clinical pharmacist service on health‐related quality of life and prescribing of drugs: a randomised controlled trial. BMJ Qual Saf 2011; 20: 738–746. [DOI] [PubMed] [Google Scholar]
- 43. Bolas H, Brookes K, Scott M, McElnay J. Evaluation of a hospital‐based community liaison pharmacy service in Northern Ireland. Pharm World Sci 2004; 26: 114–120. [DOI] [PubMed] [Google Scholar]
- 44. Frankenthal D, Lerman Y, Kalendaryev E, Lerman Y. Intervention with the screening tool of older persons potentially inappropriate prescriptions/screening tool to alert doctors to right treatment criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. J Am Geriatr Soc 2014; 62: 1658–1665. [DOI] [PubMed] [Google Scholar]
- 45. McMullin S, Hennenfent JA, Ritchie DJ, Huey W, Lonergan T, Schaiff R, et al. A prospective, randomized trial to assess the cost impact of pharmacist‐initiated interventions. Arch Intern Med 1999; 159: 2306–2309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Medline search strategy
Appendix S2 Cochrane Library search strategy
Appendix S3 All‐cause readmission and/or ED visits; All‐cause readmission
Supporting info item
Supporting info item
Supporting info item


