Abstract
Aims
Adverse drug reactions (ADRs) contribute to poorer patient outcomes and additional burden to the healthcare system. However, data on the true burden, relevant types and drugs causing ADRs are lacking. The aim of this study was to determine the prevalence of ADR‐related hospitalization in the general adult population in Singapore and to investigate their characteristics.
Methods
We prospectively recruited 1000 adult patients with unplanned admission to a large tertiary‐care hospital. Two independent reviewers evaluated all suspected ADRs for causality, type, severity and avoidability. The prevalence of ADR‐related hospitalization was calculated based on ‘definite’ and ‘probable’ ADRs. Logistic regression was used to evaluate predictors for having an ADR at admission.
Results
The prevalence of all ADRs at admission was 12.4% (95% CI: 10.5–14.6%) and ADRs causing admission was 8.1% (95% CI: 6.5–10.0%). The most common ADRs were gastrointestinal‐related. The most common drug category causing ADRs were cardiovascular drugs. Patients with ADRs had a longer length of stay than those who did not (median 4 vs. 3 days, P = 1.70 × 10−3). About 30% of ADRs at admission were caused by at least one drug with a clinical annotation in the Pharmacogenomics KnowledgeBase (PharmGKB), suggesting that some of these ADRs may have been predicted by pharmacogenetic testing.
Conclusions
We have quantified the burden and characteristics of clinically impactful ADRs in the Singaporean general adult population. Our results will provide vital information for efforts in reducing ADRs through targeted vigilance, patient education and pharmacogenomics in Singapore.
Keywords: adverse drug reactions, hospital admission, prevalence, pharmacogenomic testing
What is Already Known about this Subject
Adverse drug reactions (ADRs) contribute to adverse clinical outcomes and add to healthcare costs
The true burden and their characteristics in adult Singaporeans are unknown.
What this Study Adds
The prevalence of ADRs at admission in Singaporean adults was 12.4% and that causing admission was 8.1%.
Patients with ADRs have a longer length of stay than those who do not.
The most common ADRs were gastrointestinal ADRs and most common drugs causing ADRs were cardiovascular drugs.
Introduction
Adverse drug reactions (ADRs) are a major cause of morbidity and mortality, and leading cause of hospital admission 1. ADRs negatively affect patients' quality of life and confidence in medications, which consequently leads to worse treatment outcomes 2. A major limitation in ADR research is that we lack reliable data on the true prevalence and burden of ADRs. This relates to the fact that information about ADRs is traditionally captured through voluntary reporting systems, which vastly under‐report the true number of ADRs 3, and do not capture information about the number of patients at risk, making assessment of the relative frequencies of different ADRs impossible. Singapore has one of the highest per capita rates of spontaneous ADR reporting in the world 4, but nonetheless, under‐reporting, incomplete information and reporting bias lead to an inability to calculate the true prevalence of ADRs 3. Understanding the healthcare burden of ADRs as well as the relevant types and drugs involved represents an important gap in our knowledge.
Studies based on medical records review in the UK and other countries have reported that the prevalence of hospital admissions caused by ADRs range from 2.3 to 21.2%, and that a substantial proportion of these ADRs are potentially preventable 5, 6, 7, 8, 9, 10, 11, 12, 13, 14. In Singapore, similar studies have been conducted in the paediatric population (2.6%) 15, patients on polypharmacy (2.3%) 16 and oncology patients (12.2%) 17. However, data from the general adult population in Singapore or other Asian countries are lacking.
Pharmacogenomic markers have been identified for a growing number of drugs for serious ADRs, and implementation of pharmacogenomic testing has been advanced as a means to prevent the occurrence of some of these ADRs. A notable example is the recommendation for pharmacogenetic testing of HLA‐B*1502 allele prior to the prescription of carbamazepine as risk stratification for the prevention of severe skin allergic reactions in Singapore and Taiwan, leading to a significant drop in cases of carbamazepine‐induced Stevens‐Johnsons syndrome and toxic epidermal necrolysis (SJS/TENS) 18, 19. Examining ADRs for association with pharmacogenomic markers would provide an additional layer of information on the potential preventability of these ADRs.
To address these gaps in knowledge, we undertook a prospective observational study of ADRs occurring among patients at the time of admission to a large tertiary care hospital in Singapore. The key objectives of this study were to determine the prevalence, specific types and characteristics of ADRs that occur among patients at the time of admission to hospital, and to explore the role of pharmacogenomic markers associated with these ADRs.
Methods
We prospectively recruited 1000 patients admitted to Singapore General Hospital (SGH), a 1600‐bed tertiary teaching hospital, from December 2015 to February 2016 by randomly selecting 20 patients every weekday from patients admitted the preceding day. This was done by selecting the patients with the 20 smallest random numbers generated against their names using Excel RAND() function in a list based on admission date in which patients discharged on the same day were also included. Elective admissions, obstetric cases, patients aged <21 years old and patients directly transferred from other hospitals were excluded. The sample size of 1000 was expected to allow us to estimate the prevalence with 95% confidence interval (CI) of 4.6–7.7%, based on the reported prevalence of 6% from a large UK study 5. This study was approved by the ethics review committee of SGH with waiver of informed consent.
A trained pharmacist recorded clinical information for the selected patients each day from electronic medical records and case notes, and cumulatively for all 1000 patients throughout the study period. Information collected include demographics, presenting complaints, past medical history, all drugs taken in the preceding 2 weeks (or 1 month for chemotherapeutic drugs), preliminary and final diagnoses, length of stay and mortality. If any information was incomplete at the time of admission, the patient was followed up until all information required was collected.
The same pharmacist assessed whether an ADR was present at the point of admission by matching the presenting complaints and findings to ADR profiles of drugs the patient was taking, using Lexi‐Comp 20 and Micromedex 21 as drug references. We used the Edwards and Aronson definition of ADR: ‘an appreciably harmful or unpleasant reaction, resulting from an intervention related to the use of a medicinal product, which predicts hazard from future administration and warrants prevention or specific treatment, or alteration of the dosage regimen, or withdrawal of the product’ 22. All suspected ADRs present at admission, whether or not they were the main reason for hospital admission, were captured. We excluded ADRs due to drug abuse, complementary alternative medicines or intentional poisoning as they are not amenable to the systematic clinical studies needed to investigate genetic factors modifying drug response. If an ADR was suspected, more details were recorded and each suspected ADR–drug(s) pair was further assessed for causality using the Liverpool Causality Assessment Tool (LCAT) 23, type, using the classification of Rawlins and Thompson 24 (type A: dose dependent and predictable from the known pharmacology of the drug, and type B: not dose dependent and unpredictable), severity, using the adapted Hartwig scale 25 (Table S1) and avoidability, using the Method of Hallas et al. 26 (Table S2).
The causality, type, severity and avoidability for each suspected ADR–drug(s) pair were assessed independently by a second study team member, and any disagreements were resolved by a third member by consensus. Both the second and third team members were also trained pharmacists. To ensure accurate classification and completeness, data entered for 10% of admissions deemed not to be ADR‐related were randomly sampled and reviewed by the second study team member.
Statistical analysis
All data was entered into the Epi Info 7.1.5.2 software 27, and the data subsequently exported and analysed in R version 3.1.0 28. The prevalence of ADR‐related hospitalizations was calculated based on ADRs with a consensus LCAT causality rating of ‘definite’ or ‘probable’.
While all prescribed or received items in the 2 week (or 1 month for chemotherapeutic drugs) window prior to admission were recorded, certain categories were excluded (cosmetic products, surgical products, nasal care, oral care and eye care) and only the number of active ingredients in the remaining items were used for analysis. Combination products were counted as one active ingredient unless each component was also available as a product separately, according to the database of registered medicinal products maintained by the Health Sciences Authority of Singapore 29. Drugs for which the patient was known to be non‐adherent to, based on documentation in the medical records by a doctor or pharmacist, were also excluded (<0.6% of all drug entries).
For ease of presentation, drugs were grouped into classes using the Anatomical Therapeutic Chemical (ATC) classification system 30. Drugs were also annotated with respect to whether they had a Pharmacogenomics Knowledgebase (PharmGKB) clinical annotation (CA), and associated details such as gene, level of evidence and type of outcome. PharmGKB CAs are manually curated with levels of evidence ranging from 1A (highest) to 4 (lowest) 31. Each drug was then further explored whether the ADRs were caused by drugs with PharmGKB CAs for a matching phenotype.
Differences in length of stay and mortality between patients with and without ADRs were tested using the Mann‐Whitney U and Fisher's exact tests, respectively. Risk factors for ADRs at admission were investigated using univariate logistic regression. For multivariate analysis, possibly collinear variables among significant variables from univariate analyses were first removed sequentially based on the variance inflation factor (VIF) (highest removed first), until all are <1.5 32. The cut‐off of 1.5 was arbitrary and took into account biological knowledge of the variables tested. The remaining variables were then put through an Akaike Information Criteria (AIC)‐based stepwise logistic regression to select the best set of predictors for having an ADR at admission (more details in Supplementary information). A P‐value of <0.05 was deemed statistically significant.
Results
Demographics
We studied 1000 patients admitted to hospital over a 3‐month period. The characteristics of these patients are shown in Table 1. Most patients (78.5%) were admitted to medical wards and 91.3% manifested at least one co‐morbidity. The median number of drugs prior to admission was seven (range 0–28). Two‐thirds of all patients were taking five or more drugs prior to admission.
Table 1.
Patient characteristics and association with presence of ADR at admission
| Characteristic | All patients | ADR at admission (n = 124) | No ADR at admission (n = 876) | OR (95% CI) | P‐value |
|---|---|---|---|---|---|
| Female gender, n (%) | 474 (47.4) | 59 (47.6) | 415 (47.4) | 1.01 (0.69–1.47) | 0.966 |
| Age, mean (SD) | 62.8 (16.9) | 67.1 (14.2) | 62.1 (17.2) | 1.02 (1.01–1.03) | 2.51 × 10−3 |
| Ethnicity, n (%) | |||||
| Chinese | 690 (69.0) | 88 (71.0) | 602 (68.7) | Ref | 0.512a |
| Malays | 127 (12.7) | 14 (11.3) | 113 (12.9) | 0.85 (0.45–1.50) | |
| Indians | 137 (13.7) | 19 (15.3) | 118 (13.5) | 1.10 (0.63–1.84) | |
| Others | 46 (4.6) | 3 (2.4) | 43 (4.9) | 0.48 (0.11–1.35) | |
| Has drug allergy, n (%) | 244 (24.4) | 34 (27.4) | 210 (24.0) | 1.20 (0.78–1.81) | 0.403 |
| Ward type, n (%) | |||||
| Surgical | 215 (21.5) | 13 (10.5) | 202 (23.1) | Ref | |
| Medical | 785 (78.5) | 111 (89.5) | 674 (76.1) | 2.56 (1.46–4.86) | 1.99 × 10−3 |
| Oncology | 92 (9.2) | 24 (19.4) | 68 (7.8) | 2.85 (1.69–4.69) | 5.57 × 10−5 |
| Admitted from, n (%) | |||||
| Emergency | 865 (86.5) | 93 (75.0) | 772 (88.1) | Ref | |
| Specialist outpatient clinics | 135 (13.5) | 31 (25.0) | 104 (83.9) | 2.47 (1.55–3.87) | 9.56 × 10−5 |
| Co‐morbidities, n (%) | 913 (91.3) | ||||
| CVS condition | 674 (67.4) | 107 (86.3) | 567 (64.7) | 3.43 (2.07–6.02) | 5.19 × 10−6 |
| Diabetes | 319 (31.9) | 45 (36.3) | 274 (31.3) | 1.25 (0.84–1.85) | 0.263 |
| Chronic kidney disease | 183 (18.3) | 24 (19.4) | 159 (18.2) | 1.08 (0.66–1.72) | 0.746 |
| Has cancer | 223 (22.3) | 45 (36.3) | 178 (20.3) | 2.23 (1.49–3.32) | 8.68 × 10−5 |
| No. of medications received, median (range) | 7 (0–28) | 9 (1–20) | 7 (0–28) | 1.08 (1.05–1.12) | 4.48 × 10−6 |
| Proportion of drugs with PharmKGB CA, median (range) | |||||
| Any level of evidence | 52.6 (0–100) | 57.1 (0–100) | 50.0 (0–100) | 1.01 (1.01–1.02) | 5.30 × 10‐4 |
| Level 1 only | 0 (0–100) | 7.7 (0–50) | 0 (0–100) | 1.01 (0.99–1.02) | 0.346 |
| Level 1 or 2 | 16.7 (0–100) | 18.5 (0–75) | 16.7 (0–100) | 1.01 (1.00–1.02) | 0.048 |
| Have at least 1 drug with PharmGKB CA, n (%) | |||||
| Any level of evidence | 829 (82.9) | 122 (98.4) | 707 (80.7) | 14.6 (4.58–88.9) | 1.90 × 10−4 |
| Level 1 only | 460 (46.0) | 73 (58.9) | 387 (44.2) | 1.81 (1.24–2.66) | 2.34 × 10−3 |
| Level 1 or 2 | 694 (69.4) | 106 (85.5) | 588 (67.1) | 2.88 (1.76–5.00) | 6.36 × 10−5 |
| Outcomes | |||||
| Length of stay (days), median (range) | 3 (1–112)b | 4 (1–44) | 3 (1–112) | NA | 1.70 × 10−3c |
| Mortality rate, n (%) | 30 (3.0)b | 4 (3.2) | 26 (3.0) | NA | 0.781c |
P‐values are logistic regression P values unless stated otherwise. Oncology (under medical ward type) indicates the patient had been admitted to the specialty for the current admission, while ‘has cancer’ (under co‐morbidities) indicate that the patient has ever had a cancer diagnosis. A CVS condition includes at least one of the following: hypertension, hyperlipidaemia, congestive heart failure, history of myocardial infarction, ischemic heart disease, rheumatic heart disease, atrial fibrillation, coronary artery disease and any arrhythmias
ADR, adverse drug reaction; CVS, cardiovascular; NA, not applicable; OR, odds ratio; PharmGKB CA, Pharmacogenomics knowledgebase clinical annotation; Ref, reference; SD, standard deviation
Global test of significance using Analysis of Variance
Censored on 31 Mar 2016 with 3 patients still not discharged
Mann‐Whitney U test
Fisher's exact test
Number and significance of ADRs
A total of 124 patients had at least one ‘definite’ or ‘probable’ ADR at the point of admission (12.4%, 95% CI: 10.5%–14.6%). In 81 of these patients, the ADR was the cause of hospital admission (8.1%, 95% CI: 6.5%–10.0%, or 65% of patients who experienced an ADR) (Table S3). To assess the sensitivity of our ADR detection, we randomly selected 10% of all non‐ADR cases to perform an additional quality control assessment. Of these cases, we detected that one patient had been misclassified, and we found no major omission or inaccuracy of recorded information. Inter‐rater agreement between the two assessors on causality was moderate (kappa = 0.544) and was within the range of pairwise agreements in the testing of the LCAT tool (0.51–0.85) 23.
A total of 135 ADRs occurred in these 124 patients. All but two of these ADRs were type A (dose dependent and predictable from the known pharmacology of the drug). Most (91.9%) had levels 1 to 3 severity and were possibly avoidable (94.1%). Of the 11 ADRs (8.1%) with severity levels 4 to 6, four involved bleeding due to warfarin or clopidogrel with level 4 severity. Four patients with ADRs died, and the cause of death was related to the ADR(s) in two of these patients. ADRs causing and occurring at admission were similar in their causality, type and avoidability (Figure 1). However, ADRs causing admission were more severe than those which were not causative of admission (Fisher's exact test P‐value = 2.19 × 10−5) (Figure 1). A detailed breakdown of the number of ADRs by causality, type, severity and avoidability is shown in Table S3.
Figure 1.
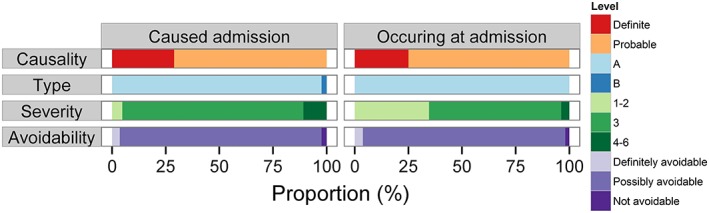
Characteristics of ADRs by causality, type, severity, avoidability and whether they caused admission. The figure shows the proportions of the 135 ‘definite’ and ‘probable’ ADRs by causality, type, severity, avoidability and by whether they caused or simply occurred at admission. The distribution of ADRs by causality, type and avoidability were similar in ADRs that caused or occurred at admission. However, more ADRs causing admission had a higher severity grade than those that occurred at admission (Fisher's exact test P = 2.19 × 10−5)
Patients with ADR(s) at admission had a significantly longer length of stay than those who did not experience an ADR (median 4 days vs. 3 days, respectively, P‐value = 1.70 × 10−3). There was no statistically significant difference in mortality rate between patients with or without ADRs (3.4% vs. 3.0%, respectively, P = 0.781). Compared to patients without an ADR on hospital admission, patients with an ADR were older (OR = 1.02, 95% CI = 1.01–1.03), more likely to be medical patients (OR = 2.56, 95% CI = 1.46–4.86), more likely to be admitted to oncology specialty (OR = 2.85, 95% CI = 1.69–4.69), more likely to be admitted from specialist outpatient clinics (OR = 2.47, 95% CI = 1.55–3.87), more likely to have a cardiovascular (CVS) condition (OR = 3.43, 95% CI = 2.07–6.02) or cancer (OR = 2.23, 95% CI = 1.49–3.32). Patients with an ADR also received more drugs before admission (OR = 1.08, 95% CI = 1.05–1.12), and were more likely to receive a drug with PharmGKB CA with level 1 or 2 evidence (OR = 2.88, 95% CI = 1.76–5.00) (Table 1).
Types of ADRs and drugs
The most common types of ADRs were gastrointestinal (GI)‐related, followed by electrolyte abnormalities and bleeding and/or supratherapeutic International Normalized Ratio (INR) (Figure 2 and Table S4). The most common ADR types that were causative of hospital admission were GI and bleeding and/or supratherapeutic INR (Figure 2). A substantial proportion of electrolyte abnormalities and renal impairment were detected at hospital admission but were not deemed to be the cause of hospital admission.
Figure 2.
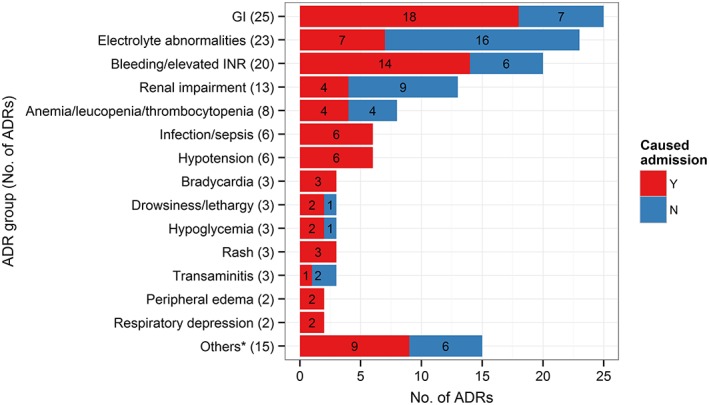
Most common ADRs. The figure shows the number of ‘definite’ and ‘probable’ ADRs grouped by ADRs. GI ADRs included nausea, vomiting, abdominal bloatedness, diarrhoea, constipation and dyspepsia. Electrolyte abnormalities included high or low serum sodium, potassium, magnesium, phosphate or calcium levels. *Acute gout flare, cytomegalovirus reactivation/viraemia, elevated creatine kinase, elevated haemoglobin, elevated T4/suppressed thyroid stimulating hormone, extrapyramidal side effects, headache, elevated white cell count, lactic acidosis, mucositis, peripheral neuropathy, premature ventricular complex, QTC prolongation, seizure and tremors (1 each). ADR: adverse drug reaction, GI: gastrointestinal, INR: international normalized ratio
The most common categories of drugs that cause ADRs were CVS, followed by anti‐coagulants/antiplatelets and chemotherapeutic drugs (Figure 3 and Table S5). This was similar among ADRs causing and occurring at admission (Figure 3). About 30% of the 135 ADRs included at least one drug with a PharmGKB CA associated with the ADR, suggesting that these ADRs may have been at least partially predictable based on patient genotype. Most of these drugs were chemotherapeutic drugs, warfarin and clopidogrel, and many had a high level of evidence (1A–2B) for the association with the reported ADRs (Table 2).
Figure 3.
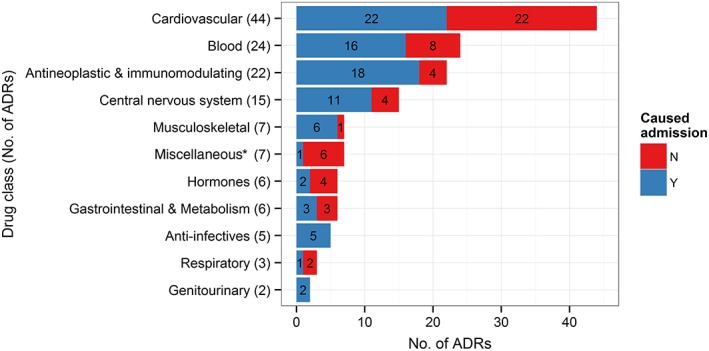
Most common drugs causing ADRs. The figure shows the number of ADRs caused by drugs in each anatomical therapeutic drug class. The total number exceeds 135 (total number of ‘definite’ and ‘probable’ ADRs) as an ADR can be counted more than once if the associated drugs fall under different drug classes. *Miscellaneous: phosphate binders (5), resonium (1), vitamin D (1). ADR: adverse drug reaction
Table 2.
Drug ADR pairs with associated genes
| ADR | Suspected drugs (PharmGKB CA) a | No. of drug–ADR pairs | ADR type associated with b | Associated genes c |
|---|---|---|---|---|
| Supratherapeutic INR ± bleeding | Warfarin (1A) | 11 | Dosage | CYP2C9, VKORC1 |
| Acenocoumarol (1B) | 1 | VKORC1 | ||
| Bleeding only | Warfarin (2A) | 2 | Bleeding | CYP2C9 |
| Aspirin (2B) | 1 | CYP2C19 | ||
| Aspirin (2B), clopidogrel (1A) | 1 | CYP2C19 | ||
| Clopidogrel (1A) | 2 | CYP2C19 | ||
| Anaemia | Gemcitabine, carboplatin (3) | 1 | Drug toxicity | CDA, ALDH1A1, ALG10 |
| 5‐FU (1A), epirubicin, cyclophoshamide (2A) | 1 | DPYD | ||
| Anaemia/thrombocytopenia | Vinblastine, cisplatin (1B), ifosfamide | 1 | ERCC1 MTR, MTHFR | |
| Anaemia/thrombocytopenia/ leucopenia | Cyclophosphamide (2A), carfilzomib | 1 | MTHFR, GSTP1 | |
| Neutropenia | Capecitabine (1A), oxaliplatin (3) | 1 | DPYD | |
| Neutropenia/ thrombocytopenia | Capecitabine (1A) | 1 | DPYD | |
| Neutropenic sepsis | Cyclophosphamide (2A), doxorubicin (3), etoposide, prednisolone | 1 | MTHFR, GSTP1 | |
| Cyclophosphamide (2A), doxorubicin (3) | 1 | MTHFR, GSTP1 | ||
| Paclitaxel (2B) | 1 | TP53 | ||
| Febrile neutropenia | Rituximab, cyclophosphamide (2A), doxorubicin (3), prednisolone | 1 | MTHFR, GSTP1 | |
| Sepsis (not neutropenic) | Paclitaxel (2B), trastuzumab | 1 | TP53 | |
| Hypotension | Lisinopril (3) | 1 | Efficacy (BP response) | ACE, MMP3 |
| Nifedipine, carvedilol, losartan (3) | 1 | ADRB1, CYP2C9, CAMK1D, STK39, SLC14A2 | ||
| Atenolol, amlodipine, lisinopril (3), tamsulosin, ISMN | 1 | ACE, CYP3A4, CYP3A5, EDN1, GNB3, AGT, ADRA2A, LDLR, ADRB1, CACNA1C, NR1H3, ABCB1, MMP3 | ||
| Atenolol, nifedipine (3) | 1 | EDN1, GNB3, AGT, ADRA2A, LDLR, ADRB1, CACNA1C, NR1H3, ABCB1, SLC14A2 | ||
| Losartan (3) | 1 | CYP2C9, CAMK1D, STK39 | ||
| Giddiness | Mirtazapine, quetiapine (3) | 1 | Toxicity | SLC6A4, GRIN2B |
| EPS | Risperidone (3) | 1 | EPS | DRD3 |
| Diarrhoea | Capecitabine (1A), oxaliplatin, bevacizumab | 1 | Diarrhea | DPYD |
| Peripheral neuropathy | Vincristine (3), MTX, cytarabine | 1 | Neurotoxicity | CAPG, CEP72, ABCB1, ACTG1 |
| Renal impairment | Cisplatin (2B) | 1 | Nephrotoxicity | ERCC1 |
| Transaminitis | Leflunomide, MTX (3) | 1 | Toxicity/liver toxicity | CYP1A2, SLC19A1, SHMT1 |
ADR, adverse drug reaction; BP, blood pressure; CA, clinical annotation; EPS, extrapyramidal side effects; 5‐FU, fluorouracil; ISMN, isosorbide mononitrate; MTX, methotrexate; PGx, pharmacogenomic
Drugs with PharmGKB CA appear in bold type
Extracted from PharmGKB
Only genes associated with the highest level of evidence are listed
Predictors of having ADRs at admission
We explored variables that were predictive of having an ADR at the time of hospital admission. Among the significant variables from univariate analysis (Table 1), four variables were eliminated sequentially based on variance inflation factor (VIF) [having at least one drug with PharmGKB CA (level 1 or 2 evidence), being admitted to oncology specialty, the proportion of drugs with PharmGKB CA (any level of evidence), and having at least one drug with PharmGKB CA (level 1 evidence)]. The remaining variables were entered into a stepwise regression and the best set of predictors for having an ADR at admission was place of admission (emergency department vs. specialist outpatient clinics), ward type, having a CVS condition, having cancer and having at least one drug with PharmGKB CA of any level of evidence (Table 3).
Table 3.
Factors associated with ADR at admission
| Variable | Odds ratio (95% CI) | P‐value |
|---|---|---|
| Admitted from SOC (vs. ED) | 2.21 (1.33–3.59) | 1.73 × 10−3 |
| Medical ward (vs. surgical) | 2.18 (1.22–4.20) | 0.013 |
| Have a CVS condition | 2.58 (1.50–4.70) | 1.03 × 10−3 |
| Have cancer | 1.87 (1.20–2.87) | 4.88 × 10−3 |
| Have at least one drug with a PharmGKB CA (any level of evidence) | 6.64 (1.98–41.4) | 0.010 |
This table shows the significant variables predicting having an ADR at admission after multivariate analysis
CI, confidence interval; CVS, cardiovascular; ED, emergency department; PharmGKB CA, Pharmacogenomics knowledgebase clinical annotation; SOC, specialist outpatient clinics
Discussion
Here we report the results of the first large prospective observational study of ADRs among the general hospitalized adult population in Singapore. The key findings from this study are that 12.4% of patients admitted to a large tertiary care hospital had an ADR, and that in 8.1% of hospital admissions, an ADR contributed to the reason for hospitalization.
Previous studies examining ADRs causing hospital admission have reported rates of 2.7–8.8% of hospital admission to be caused by ADRs 6, 7, 9, 10, 12. Our finding that 8.1% of admissions were caused at least in part by an ADR is therefore consistent with these prior data. However, our finding that 12.4% of patients had an ADR at time of hospital admission is higher than what has been reported previously, namely 2.3–7.9% 5, 16, 33, 34. Several factors likely contribute to this difference. Notably, patients in our study received a larger number of drugs than was reported in previous studies 34, 35. An Italian study surveying patients admitted through the emergency department to three hospitals found an initial diagnosis of an ADR in 21.2% of patients but 98% of those were deemed predictable and were not further analysed, thus this prevalence could be overestimated, as the causality of those ADRs were unknown 36. Studies which considered ADRs causing admission and focused on medical wards and/or older patients tended to report a higher prevalence of 3.3–12.8% 8, 11, 37, 38, 39, 40, 41, 42, 43, 44, which is in line with what we observed. Our choice of the causality assessment tool might also have resulted in a higher prevalence compared to other studies. Many previous ADR surveys have used the Naranjo tool, but difficulties in answering some of the questions in the tool may artificially lower the causality score, thus reducing its sensitivity 23. The LCAT is a modification of the Naranjo tool designed to address these problems, and its use in our study might have resulted in more ADRs being classified as ‘definite’ and ‘probable’ than if we had used the Naranjo tool.
Eleven ADRs were severe enough to warrant a higher level of care, cause permanent disability or contribute to patient death. However, all but three ADRs were ‘definitely’ or ‘possibly’ avoidable, suggesting a considerable opportunity to reduce healthcare burden and costs due to ADRs in Singapore. Four of these 11 severe ADRs involved bleeding due to warfarin or clopidogrel, highlighting the importance of reducing this type of ADR. The high number of ‘avoidable’ ADRs should be interpreted with caution as it could be partly an artefact of the avoidability scale used. We chose the method of Hallas et al. 26 as it is commonly used, but its validity and reliability relies heavily on the assessors' notion of ‘good medical practice’ and ‘obligatory demands’ 45.
In terms of healthcare burden, patients with ADRs on average stayed one day longer than those who did not, translating to about 9,400 extra hospital days per year at SGH. Extrapolated to all of Singapore, this would be an additional 48 000 hospital days per year caused by ADRs.
It is noteworthy that almost a third of ADRs detected in our study were caused by at least one drug with a PharmGKB CA for that ADR, suggesting that at least part of these ADRs could potentially be predicted or avoided using pharmacogenomic testing. The most notable drug–ADR pair with this potential is warfarin and bleeding and/or supratherapeutic INR, for which there is very strong evidence (PharmGKB CA level 1A–2A). Indeed, a recent meta‐analysis of randomized clinical trials showed that pharmacogenetic‐based dosing significantly reduced risk of adverse events and major bleeding compared to conventional dosing 46. Warfarin pharmacogenetic testing may therefore reduce warfarin‐related hospitalizations and their associated healthcare burden. The other main group of drugs for which ADRs might be predicted or prevented with pharmacogenomic testing is the chemotherapeutic agents. The strength of evidence, however, varies depending on the specific drug–ADR pair (level 1A–3).
Apart from a third of ADRs actually having a pharmacogenetic association, 69% of all patients have at least one drug with a level 1 or 2 PharmGKB CA (Table 1), suggesting a much higher potential for optimizing therapy and prevention of future ADRs. This is in line with a study arguing the case for pre‐emptive pharmacogenomic testing, which reported that 65% of patients in a medical centre would be exposed to at least one drug with an established pharmacogenetic association within a 5‐year period 47. At present, clinical utility has only been demonstrated for a handful of drug–ADR pairs, such as HLA‐B*57:01 and abacavir hypersensitivity 48, and HLA‐B*15:02 and carbamazepine‐induced SJS/TENS 19. The clinical significance of other pharmacogenomic associations is less clear, and the recommended action in the presence of a risk allele is not always well established. Nonetheless, the high percentage of ADR–drug pairs detected in our study for which pharmacogenomic associations exist suggests that at least a proportion of these ADRs could have been in principle predicted using pharmacogenetic testing. Future studies will be needed to determine the clinical benefit and cost‐effectiveness of pre‐emptive pharmacogenomic testing in Singapore.
In contrast to most studies in similar settings where the most common ADR was bleeding 5, 6, 7, 8, 11, 16, 34, 40, 41, 42, the most common ADR in our study affected the GI system (including nausea, vomiting, abdominal bloatedness, diarrhoea, constipation and dyspepsia). The reason for this is unclear but could be due to differences in recording of such subjective symptoms and the threshold different researchers have for considering these to be potential ADRs. Nevertheless, bleeding was also one of the most common ADRs, as these events were clinically significant and would lead to an unplanned admission. Cumulatively, CVS drugs contributed to the highest number of ADRs, consistent with several prior studies 6, 9, 10, 37, 40, 42. This could be due to the fact that the clinical condition of the patients often warranted several CVS drugs to be co‐prescribed despite their additive risk for ADRs, most commonly renal impairment and electrolyte disturbances. Interestingly, non‐steroidal anti‐inflammatory drugs were highlighted in several previous studies 5, 6, 8, 16, but these drugs were not a common cause of ADRs in our study.
Consistent with other studies 6, 7, 8, 9, 10, 11, 12, 16, 33, 37, 39, 40, 42, 43, 44, we found that patients with ADRs tended to be older, were more likely to be admitted to a medical ward and received more drugs. However, many variables including age and number of drugs were eliminated in the final set of predictors because of collinearity with other variables. For example, older patients, medical patients and patients with a CVS condition tended to receive more drugs, and patients with more drugs were more likely to have at least one of them with a PharmGKB CA (Supplementary information).
There are several strengths in our study. Firstly, this is the first ADR survey in the Singapore general adult population, an important population to study for the estimation of the burden of clinically impactful ADRs. Compared to spontaneous reporting, which captured approximately 11 500 ADRs from all Singapore public and private hospitals in 2014 49, we estimate the number of ADRs at the point of hospital admission alone in adults (not including those occurring during admission) across Singapore to be about 54 000 50. Secondly, this was a prospective study, a design that allowed the most complete and accurate capture of information possible. Lastly, we have a relatively large sample size for reasonably precise estimation of the prevalence of ADRs at admission.
There are a number of limitations of this study that merit consideration. Firstly, adjudication of a potential ADR was dependent on clinical judgement. Patients often have underlying conditions that may contribute to the presenting complaint or finding and the role of the suspected drug(s) can be difficult or impossible to establish. However, this is an inherent limitation of any ADR survey, even prospective ones. Therefore we employed independent observers who were trained pharmacists and used validated instruments to adjudicate the likelihood that a patient experienced an ADR. Secondly, patients' drug history may sometimes be inaccurate due to compliance issues or self‐medication. We endeavoured to collect the most accurate information available using patients' medical records and pharmacists' medication reconciliation reports, which included interviews with the patient and family members when necessary. An additional limitation relates to the fact that our study was conducted at a single centre. While we believe our data are likely to be broadly representative of other tertiary medical centres in Singapore, this would require additional validation in future studies. Nevertheless, our study took place at the largest acute care hospital in Singapore and we believe these findings are noteworthy to SGH and other healthcare institutions in Singapore and beyond.
In conclusion, we found the prevalence of ADRs at hospital admission among adults in Singapore to be 12.4%, highlighting a considerable potential for improvement in health outcomes and healthcare burden. Our study has for the first time quantified the burden of clinically impactful ADRs in the general adult population and identified the most common ADR types and drugs responsible, providing vital information for targeted efforts in pharmacovigilance, patient education, pharmacogenomic testing and future pharmacogenomic research for reduction of ADR‐related hospitalizations in Singapore.
Competing Interests
There are no competing interests to declare.
The authors would like to acknowledge Sir Munir Pirmohamed and Dr Ana Alfirevic from the University of Liverpool for their scientific input, and Ms Angie Yeo for her administrative support. This study was supported by the Biomedical Research Council of the Agency for Science, Technology and Research of Singapore ( SPF2014/001 ).
Contributors
LRB and AC are the senior co‐authors. SLC wrote the manuscript, designed and performed the research and analysed the data. XA designed and performed the research. LLS and HYN contributed data or clinical inputs. MDW, JJL, LRB and AC designed the research. SLC and XA contributed equally to this work. All authors reviewed the manuscript.
Supporting information
Table S1 Adapted Hartwig scale for severity rating of ADRs
Table S2 Method of Hallas for avoidability rating of ADRs
Table S3 Evaluation of all suspected ADRs
Table S4 ADR groups and their associated drugs
Table S5 Drugs causing ADRs
Table SI‐1 Overview of steps taken to explore risk factors for ADRs at admission and variables remaining at each step
Table SI‐2 Removal of collinear variables
Table SI‐3 Logistic regression of remaining variables after removal of collinear variables
Table SI‐4 Collinearity between variables entered into stepwise regression
Table SI‐5 Effect of age and/or number of medications alone on risk of ADRs at admission
Table SI‐6 Effect of categorized age and/or number of medications alone on risk of ADRs at admission
Figure SI‐1 Correlation between age and number of medications. Scatterplot of age and number of medications with a fitted line (blue) and 95% confidence interval region (shaded)
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Chan, S. L. , Ang, X. , Sani, L. L. , Ng, H. Y. , Winther, M. D. , Liu, J. J. , Brunham, L. R. , and Chan, A. (2016) Prevalence and characteristics of adverse drug reactions at admission to hospital: a prospective observational study. Br J Clin Pharmacol, 82: 1636–1646. doi: 10.1111/bcp.13081.
References
- 1. Sultana J, Cutroneo P, Trifirò G. Clinical and economic burden of adverse drug reactions. J Pharmacol Pharmacother 2013; 4: S73–S77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pirmohamed M, Breckenridge AM, Kitteringham NR, Park BK. Adverse drug reactions. BMJ 1998; 316: 1295–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wise L, Parkinson J, Raine J, Breckenridge A. New approaches to drug safety: a pharmacovigilance tool kit. Nat Rev Drug Discov 2009; 8: 779–782. [DOI] [PubMed] [Google Scholar]
- 4. Viklund A, Biriell C. VigiBase scales new heights. Uppsala Reports 2015; 70: 5. [Google Scholar]
- 5. Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ 2004; 329: 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hopf Y, Watson M, Williams D. Adverse‐drug‐reaction related admissions to a hospital in Scotland. Pharm World Sci 30: 854–862. [DOI] [PubMed] [Google Scholar]
- 7. van der Hooft CS, Dieleman JP, Siemes C, Aarnoudse A‐JLHJ, Verhamme KMC, Stricker BHCH, et al. Adverse drug reaction‐related hospitalisations: a population‐based cohort study. Pharmacoepidemiol Drug Saf 2008; 17: 365–371. [DOI] [PubMed] [Google Scholar]
- 8. Alexopoulou A, Dourakis SP, Mantzoukis D, Pitsariotis T, Kandyli A, Deutsch M, et al. Adverse drug reactions as a cause of hospital admissions: a 6‐month experience in a single center in Greece. Eur J Intern Med 2008; 19: 505–510. [DOI] [PubMed] [Google Scholar]
- 9. Ahern F, Sahm LJ, Lynch D, McCarthy S. Determining the frequency and preventability of adverse drug reaction‐related admissions to an Irish University Hospital: a cross‐sectional study. Emerg Med J 2014; 31: 24–29. [DOI] [PubMed] [Google Scholar]
- 10. Pedrós C, Quintana B, Rebolledo M, Porta N, Vallano A, Arnau JM. Prevalence, risk factors and main features of adverse drug reactions leading to hospital admission. Eur J Clin Pharmacol 2014; 70: 361–367. [DOI] [PubMed] [Google Scholar]
- 11. Bénard‐Laribière A, Miremont‐Salamé G, Pérault‐Pochat M‐C, Noize P, Haramburu F. Incidence of hospital admissions due to adverse drug reactions in France: the EMIR study. Fundam Clin Pharmacol 2015; 29: 106–111. [DOI] [PubMed] [Google Scholar]
- 12. Akbari Sari A, Doshmangir L, Torabi F, Rashidian A, Sedaghat M, Ghomi R, et al. The incidence, nature and consequences of adverse events in Iranian hospitals. Arch Iran Med 2015; 18: 811–815. [PubMed] [Google Scholar]
- 13. Kongkaew C, Noyce PR, Ashcroft DM. Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Ann Pharmacother 2008; 42: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 14. Leendertse AJ, Visser D, Egberts ACG, van den Bemt PMLA. The relationship between study characteristics and the prevalence of medication‐related hospitalizations: a literature review and novel analysis. Drug Saf 2010; 33: 233–244. [DOI] [PubMed] [Google Scholar]
- 15. Kidon MI, See Y. Adverse drug reactions in Singaporean children. Singapore Med J 2004; 45: 574–577. [PubMed] [Google Scholar]
- 16. Koh Y, Kutty FBM, Li SC. Drug‐related problems in hospitalized patients on polypharmacy: the influence of age and gender. Ther Clin Risk Manag 2005; 1: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan A, Soh D, Ko Y, Huang Y‐C, Chiang J. Characteristics of unplanned hospital admissions due to drug‐related problems in cancer patients. Support Care Cancer 2014; 22: 1875–1881. [DOI] [PubMed] [Google Scholar]
- 18. Toh DSL, Tan LL, Aw DCW, Pang SM, Lim SH, Thirumoorthy T, et al. Building pharmacogenetics into a pharmacovigilance program in Singapore: using serious skin rash as a pilot study. Pharmacogenomics J 2014; 14: 316–321. [DOI] [PubMed] [Google Scholar]
- 19. Chen P, Lin J‐J, Lu C‐S, Ong C‐T, Hsieh PF, Yang C‐C, et al. Carbamazepine‐induced toxic effects and HLA‐B*1502 screening in Taiwan. N Engl J Med 2011; 24: 1126–1133. [DOI] [PubMed] [Google Scholar]
- 20. Lexicomp Online®. Hudson, OH: Lexi‐Comp, Inc, 2016. [Google Scholar]
- 21. DRUGDEX® System. [Internet database]. Greenwood Village, CO: Updated periodicallyThomson Micromedex. [Google Scholar]
- 22. Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000; 356: 1255–1259. [DOI] [PubMed] [Google Scholar]
- 23. Gallagher RM, Kirkham JJ, Mason JR, Bird KA, Williamson PR, Nunn AJ, et al. Development and inter‐rater reliability of the Liverpool adverse drug reaction causality assessment tool. PLoS One 2011; 6: e28096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rawlins M, Thompson J. Mechanisms of adverse drug reactions In: Textbook of adverse drug reactions, ed Davies D. Oxford: Oxford University Press, 1991; 18–45. [Google Scholar]
- 25. Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm 1992; 49: 2229–2232. [PubMed] [Google Scholar]
- 26. Hallas J, Harvald B, Gram LF, Grodum E, Brøsen K, Haghfelt T, et al. Drug related hospital admissions: the role of definitions and intensity of data collection, and the possibility of prevention. J Intern Med 1990; 228: 83–90. [DOI] [PubMed] [Google Scholar]
- 27. Dean A, Arner T, Sunki G, Friedman R, Lantinga M, Sangam S, et al. Epi Info™, a database and statistics program for public health professionals. Atlanta, GA: CDC, 2011. [Google Scholar]
- 28. R Core Team . R: A Language and Environment for Statistical Computing [online]. R Foundation for Statistical Computing, 2014. Available at http://www.R‐project.org (last accessed 20 August 2016).
- 29. Health Sciences Authority . Database search for registered medicinal products [online]. Available at http://eservice.hsa.gov.sg/prism/common/enquirepublic/SearchDRBProduct.do?action=load (last accessed 20 August 2016).
- 30. WHO Collaborating Centre for Drug Statistics Methodology . Guidelines for ATC classification and DDD assignment 2015. Oslo: WHO Collaborating Centre for Drug Statistics Methodology, 2014. [Google Scholar]
- 31. Whirl‐Carrillo M, McDonagh EM, Hebert JM, Gong L, Sangkuhl K, Thorn CF, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther 2012; 92: 414–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zuur AF, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 2010; 1: 3–14. [Google Scholar]
- 33. Alsbou M, Alzubiedi S, Alzobi H, Samhadanah NA, Alsaraireh Y, Alrawashdeh O, et al. Adverse drug reactions experience in a teaching hospital in Jordan. Int J Clin Pharmacol 2015; 37: 1188–1193. [DOI] [PubMed] [Google Scholar]
- 34. Hallas J, Gram LF, Grodum E, Damsbo N, Brøsen K, Haghfelt T, et al. Drug related admissions to medical wards: a population based survey. Br J Clin Pharmacol 1992; 33: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Green CF, Mottram DR, Rowe PH, Pirmohamed M. Adverse drug reactions as a cause of admission to an acute medical assessment unit: a pilot study. J Clin Pharm Ther 2000; 25: 355–361. [DOI] [PubMed] [Google Scholar]
- 36. Ventura MT, Laddaga R, Cavallera P, Pugliese P, Tummolo RA, Buquicchio R, et al. Adverse drug reactions as the cause of emergency department admission: focus on the elderly. Immunopharmacol Immunotoxicol 2010; 32: 426–429. [DOI] [PubMed] [Google Scholar]
- 37. Onder G, Pedone C, Landi F, Cesari M, Della Vedova C, Bernabei R, et al. Adverse drug reactions as cause of hospital admissions: results from the Italian Group of Pharmacoepidemiology in the Elderly (GIFA). J Am Geriatr Soc 2002; 50: 1962–1968. [DOI] [PubMed] [Google Scholar]
- 38. Walsh D, Lavan A, Cushen A‐M, Williams D. Adverse drug reactions as a cause of admission to a Dublin‐based university teaching hospital. Ir J Med Sci 2015; 184: 441–447. [DOI] [PubMed] [Google Scholar]
- 39. Fattinger K, Roos M, Vergères P, Holenstein C, Kind B, Masche U, et al. Epidemiology of drug exposure and adverse drug reactions in two Swiss departments of internal medicine. Br J Clin Pharmacol 2000; 49: 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. von Euler M, Eliasson E, Ohlén G, Bergman U. Adverse drug reactions causing hospitalization can be monitored from computerized medical records and thereby indicate the quality of drug utilization. Pharmacoepidemiol Drug Saf 2006; 15: 179–184. [DOI] [PubMed] [Google Scholar]
- 41. Franceschi M, Scarcelli C, Niro V, Seripa D, Pazienza AM, Pepe G, et al. Prevalence, clinical features and avoidability of adverse drug reactions as cause of admission to a geriatric unit: a prospective study of 1756 patients. Drug Saf 2008; 31: 545–556. [DOI] [PubMed] [Google Scholar]
- 42. Brvar M, Fokter N, Bunc M, Mozina M. The frequency of adverse drug reaction related admissions according to method of detection, admission urgency and medical department specialty. BMC Clin Pharmacol 2009; 9: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Olivier P, Bertrand L, Tubery M, Lauque D, Montastruc J‐L, Lapeyre‐Mestre M. Hospitalizations because of adverse drug reactions in elderly patients admitted through the emergency department: a prospective survey. Drugs Aging 2009; 26: 475–482. [DOI] [PubMed] [Google Scholar]
- 44. Marcum ZA, Amuan ME, Hanlon JT, Aspinall SL, Handler SM, Ruby CM, et al. Prevalence of unplanned hospitalizations caused by adverse drug reactions in older veterans. J Am Geriatr Soc 2012; 60: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ferner RE, Aronson JK. Preventability of drug‐related harms – part I: a systematic review. Drug Saf 2010; 33: 985–994. [DOI] [PubMed] [Google Scholar]
- 46. Shi C, Yan W, Wang G, Wang F, Li Q, Lin N. Pharmacogenetics‐based versus conventional dosing of warfarin: a meta‐analysis of randomized controlled trials. PLoS One 2015; 10: e0144511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schildcrout JS, Denny JC, Bowton E, Gregg W, Pulley JM, Basford MA, et al. Optimizing drug outcomes through pharmacogenetics: a case for preemptive genotyping. Clin Pharmacol Ther 2012; 92: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cargnin S, Jommi C, Canonico PL, Genazzani AA, Terrazzino S. Diagnostic accuracy of HLA‐B*57:01 screening for the prediction of abacavir hypersensitivity and clinical utility of the test: a meta‐analytic review. Pharmacogenomics 2014; 15: 963–976. [DOI] [PubMed] [Google Scholar]
- 49. Health Sciences Authority . Analysis of adverse event reports for year 2014. Adverse Drug Reaction News, Singapore; 2–3.
- 50. Ministry of Health Singapore . 2014. Admissions and outpatient attendances [online]. Available at https://www.moh.gov.sg/content/moh_web/home/statistics/Health_Facts_Singapore/Admissions_and_Outpatient_Attendances.html (last accessed 22 April 2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Adapted Hartwig scale for severity rating of ADRs
Table S2 Method of Hallas for avoidability rating of ADRs
Table S3 Evaluation of all suspected ADRs
Table S4 ADR groups and their associated drugs
Table S5 Drugs causing ADRs
Table SI‐1 Overview of steps taken to explore risk factors for ADRs at admission and variables remaining at each step
Table SI‐2 Removal of collinear variables
Table SI‐3 Logistic regression of remaining variables after removal of collinear variables
Table SI‐4 Collinearity between variables entered into stepwise regression
Table SI‐5 Effect of age and/or number of medications alone on risk of ADRs at admission
Table SI‐6 Effect of categorized age and/or number of medications alone on risk of ADRs at admission
Figure SI‐1 Correlation between age and number of medications. Scatterplot of age and number of medications with a fitted line (blue) and 95% confidence interval region (shaded)
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item


