Abstract
Aims
The UK Medicines and Healthcare products Regulatory Agency (MHRA) runs a national spontaneous reporting system (Yellow Card [YC] Scheme) to collect ‘suspected’ adverse drug reaction (ADR) data. We aim to describe the content and utility of YC reports received for patients aged <2 years.
Methods
Data on all ADRs reported using YC in infants aged <2 years from the years 2001–10 were supplied by the MHRA.
Results
For infants age <2 years, 3496 suspected ADRs were reported using YC (paternal medication pre‐conception n = 3, transplacental n = 246, transmammary n = 30, neonates n = 97, infant n = 477, and vaccinations n = 2673), averaging 0.96 YC per day. There was a male preponderance (male 49.1%, female 44.4%, unknown 6.5%), and only 34 (1.0%) of YC reports stated a gestational age. The medications most frequently reported were: transplacental and transmammary (fluoxetine, n = 21 and n = 4 respectively), neonate (swine flu vaccine, n = 8) infant (oseltamivir, n = 37) and vaccines (meningococcal vaccine, n = 693). Paternal, transmammary, neonatal and infant YC did not reflect clinical concerns raised by the UK regulator. Transplacental and vaccination reports did correlate with some of the changes in practice and clinical alerts received.
Conclusions
The frequency of YC reports for those <2 years is low, neonates are poorly represented, and recording of gestational age is poor. With the exception of vaccinations, spontaneous reports alone are not currently generating the data required, and important safety messages from the regulator do not match reporting patterns. Additional reporting strategies are required to improve the quantity and quality of suspected ADR data in young children.
Keywords: adverse drug reaction, fetal, neonate, paediatric, pharmacology, spontaneous reporting scheme
What is Already Known about this Subject
Neonates and infants have different adverse drug reaction (ADR) profiles compared to adults and older children
What this Study Adds
The overall frequency of ADR reporting for those aged <2 years is low, and safety messages from the regulator do not match reporting patterns
Gestation is poorly recorded in spontaneous ADRs
The frequency of reporting for transplacental, transmammary and paternal suspected ADRs is extremely low
New systems of generating data regarding ADRs from specific groups such as sentinel sites may be required to improve the quality and quantity of reports
Table of Links
This Table lists key ligands in this article, which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1.
Introduction
The World Health Organization defines an adverse drug reaction (ADR) as ‘a response to a drug which is noxious, and unintended, and which occurs at doses normally used in man for prophylaxis, diagnosis or therapy of disease, or for the modification of physiological function’ 2. ADRs are responsible for 2% of paediatric hospital admissions (compared to 4–7% of adult admissions) 3, 4, 5, 6, but there is considerable variation depending on the paediatric specialty involved (0.2% of neonatal admissions compared to 22% of paediatric oncology admissions). Paediatric inpatient stays are complicated by an ADR in 15–18% of cases 7, 8, with 28% of these inpatient ADRs classified as severe 9. Medication is also required in 22% of pregnant women, and fathers may be on medication at the time of conception, so many infants are born following medication exposure 10. Furthermore, within the UK in 2010, 73.7% of new mothers initiated breastfeeding 10, 11, generating another potential route of exposure. All these medications are prescribed to achieve therapeutic benefit, but it is important to ensure that consideration is given to any potential ADRs exhibited by the baby.
Neonates have a very high rate of use of unlicensed and off‐label medications 12, the use of which may increase the risk of an ADR 13. Profound changes in development and rapid maturation of organ and enzyme systems in both premature babies and new‐borns mean that prescribing in this group poses different risks compared to older children and adults with respect to drug action and toxicity 14.
Fifty years after thalidomide, a transplacental ADR that led to the creation of the UK Yellow Card (YC) Scheme, post‐marketing surveillance through spontaneous reporting remains the major means of obtaining ADR data in this population. During the period this study was conducted, the YC scheme applied to all ADRs in children. The Medicines and Healthcare Regulatory Agency (MHRA) recently altered guidance for paediatric YC reporting to match the reporting guidelines to that of adult patients 15.
Previous work describing spontaneous ADR reports amongst children indicated the highest number of reports to be in those <1 year of age 16, and that maternal use of medications may cause ADRs 17. Previous work has not specifically addressed the youngest children 16, 18, focused on individual medicines 19 or ADRs 20, been unable to stratify by source of exposure, or used unlinked data (therefore unable to determine outcomes of individual ADRs 16).
Other spontaneous reporting schemes aid identification of significant ADRs in neonates 21, 22, but significant under‐reporting is recognized (up to 95% of all ADRs are not reported) 23, 24. While spontaneous reporting is unlikely to be complete, we speculated that the YC Scheme would have useful functionality in neonates and infants. We postulated that functional spontaneous ADR reporting would: (1) reflect uncertainties about medicines (e.g. more reactions when new medications or formulations are introduced (as exemplified by the increased reporting after the introduction of a new brand of BCG in 2001 16) and (2) contribute to the recognition of new signals so that information about ADRs to professionals would reflect YC reports.
The objectives of the study were therefore to describe the content of YC reports in those <2 years (drug suspected, age, source, type and outcome of ADR) in key groups (neonates, infants, vaccinations, transplacental exposure, transmammary exposure and paternal exposure) and assess the utility of these reports by comparing them with information received from the regulator by healthcare professionals.
Methods
Yellow Card data
Data were requested from the UK national medicines regulator MHRA. Data were supplied to the researchers in anonymized format. Approval was prospectively obtained from the Independent Scientific Advisory Committee for MHRA database research (ISAC) (Reference AYCD031), and all data received by the research team were anonymized.
Data on all YC reports submitted from 2001–2010, on which the age of the patient was listed as being <2 years of age, from all sources (health professionals, parents, patients, industry and other reporters), were supplied by the MHRA.
Compared to our previous study of childhood ADRs reported via the YC Scheme 16, these data were different in the following ways:
All YC data for the population were included (previous data published were limited to the most common drugs and reactions)
Complete linked data (including suspected drug, age, and outcomes for each YC received in the target population)
Different 10‐year timeframe
Specific focus on the youngest children (<2 years of age)
Reporting via the YC reporting scheme was initially restricted to doctors. Hospital pharmacists were introduced to the scheme in 1997, and in 1999 all community pharmacists were included. As part of the National Meningococcal C vaccine immunization campaign in 2000, nurses were also able to report ADRs via the YC Scheme, and from October 2002 the scheme was extended generally to all nurses, midwives and health visitors. Parents, carers and patients have been able to report ADRs since 2003, initially through the telephone helpline NHS direct. Following an independent review of access to the YC Scheme, a nationwide pilot scheme was launched for parents, carers and patients to report ADRs directly to the MHRA in January 2005, with formal implementation in February 2008, although uptake has been low 16. The final analysis therefore consists of reports from all of these groups.
Population definitions
The term ‘neonates’ can refer to more than one group of babies. The internationally accepted epidemiological definition of a neonate, and International Conference on Harmonization suggestion for this age group, is a baby within 28 days of birth. However, this definition ignores the impact of prematurity. We have therefore used the more clinically relevant definition of a baby within 28 days of expected date of delivery. Prematurity was defined as birth before 37 completed weeks of gestation. Stillbirth was defined as delivery of a dead fetus ≥ 24 weeks gestation and intrauterine death < 24 weeks.
YC are classified according to exposure to the medication as follows:
Paternal: The suspected medication was taken at the time of conception by the father
Transplacental: The suspected medication was taken by the mother during pregnancy
Transmammary: The suspected medication was taken by a breastfeeding mother after delivery
Neonatal: Suspected medication taken by patient with a corrected gestational age of less than 28 days, or labelled ‘neonate’ on the YC (includes vaccinations)
Infant: Suspected medication taken by patients older than 28 days corrected gestational age (excludes vaccinations)
Vaccination: All ADRs where a vaccine was listed as a suspected medication
Comparator data
Birth rates were obtained from UK national maternity statistics 25. Rates of adverse events related to individual medications were obtained from Summary of Product Characteristics, recognized formularies (e.g. British National Formulary for Children), or from the medical literature. Frequency of HIV infection was obtained from UK government data (Health Protection Agency) 26. The number of deliveries and number of women taking antiepileptic medications were retrieved from hospital computerized records system. Drug alerts were collated from ‘Drug Safety Update’ (DSU), a monthly update for healthcare professionals produced by the MHRA 27.
Statistics
Data were described as frequencies, percentages, medians, or means with 95% confidence intervals. Statistical analysis was undertaken using Excel 2007 (Microsoft Corporation, USA).
Results
Between 2001 and 2010, the MHRA received 3509 YC for children aged <2 years. Five YC were excluded as therapeutic failure or ‘misclassified’ adult data after review of the drug suspected and reaction(s) (e.g., Varenicline smoking cessation therapy), leaving 3504 YC (0.96 YC per day). Table 1 gives characteristics of these YC. Overall, there was a slight male preponderance with 1718 (49.0%) categorized as male, 1548 (44.2%) as female, and 238 (6.8%) not recorded.
Table 1.
Demographics of patients aged <2 years for which a report of a suspected adverse drug reaction was received by the Yellow Card Scheme 2001–10 excluding misclassified adult yellow cards
| Total | Paternal | Transplacental | Transmammary | Neonates | Infant | Vaccines | ||
|---|---|---|---|---|---|---|---|---|
| Number of reports | 3504 | 3 | 248 | 30 | 97a | 471 | 2673a | |
| Total number of drugs (can be >1 per card) | 4587 | 4 | 304 | 33 | 108 | 514 | 3646 | |
| Total number of different medications | 273 | 4 | 221 | 24 | 68 | 223 | 31 | |
| Median number of drugs per YC (range) | 1 (1–15) | 1 (1–2) | 1 (1–14) | 1 (1–3) | 1 (1–5) | 1 (1–3) | 1 (1–15) | |
| Total Number of Reactions (can be >1 per YC) | 6663 | 6 | 486 | 88 | 184 | 837 | 5090 | |
| Median number of reactions (range) per YC) | 1 (1–15) | 1 | 1 (1–14) | 1 (1–5) | 1 (1–5) | 1 (1–12) | 1 (1–15) | |
| Gender | ||||||||
| Male | 1718 | 1 | 89 | 12 | 52 | 254 | 1318 | |
| Female | 1548 | 1 | 70 | 12 | 42 | 197 | 1236 | |
| Not recorded | 238 | 1 | 89 | 6 | 3 | 20 | 119 | |
| Outcomes b | ||||||||
| Fatal | 65 | 0 | 28 | 2 | 3 | 13 | 19 | |
| Not recovered/Not resolved | 507 | 0 | 75 | 2 | 16 | 65 | 350 | |
| Recovered/Resolved | 2413 | 0 | 49 | 21 | 67 | 350 | 1939 | |
| Unknown | 519 | 3 | 96 | 5 | 11 | 44 | 365 | |
| Reporter | ||||||||
| Doctor | 1445 | 3 | 188 | 22 | 54 | 251 | 934 | |
| Nurse | 1086 | 0 | 8 | 1 | 10 | 41 | 1033 | |
| Pharmacist | 138 | 0 | 14 | 2 | 15 | 87 | 20 | |
| Parent/Carer | 173 | 0 | 8 | 3 | 2 | 42 | 118 | |
| Other healthcare | 652 | 0 | 30 | 2 | 16 | 47 | 563 | |
| Unknown | 8 | 0 | 0 | 0 | 0 | 3 | 5 | |
YC that contain vaccines given to those infants on the neonatal age range are included in both neonates and vaccinations data (n = 18).
Outcomes shows the worst recorded on each YC.
There were 6663 suspected ADRs reported across the entire cohort, with a median of one reaction per YC (range 1–15). Details of the YC reactions reported, suspected medications, outcomes and reporter are shown in Table 1, and the most commonly reported medications per category are shown in Table 2.
Table 2.
The most common medications causing spontaneous reports of suspected adverse drug reactions received by the MHRA Yellow Card Scheme in children age < 2 years
| Paternal | Transplacental | Transmammary | Neonates | Infant | Vaccinations | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Reports | Drug | Reports | Drug | Reports | Drug | Reports | Drug | Reports | Drug | Reports |
| Azathioprine | 1 | Fluoxetine | 21 | Fluoxetine | 4 | Swine Flu Vaccine | 8 | Oseltamivir | 37 | Meningococcal Vaccine | 693 |
| Sulphasalazine | 1 | Citalopram | 20 | Erythromycin | 2 | Caffeine | 5 | Tetracaine | 22 | DTaP/IPV /Hib vaccine | 600 |
| Methotrexate | 1 | Paroxetine | 19 | Sertraline | 2 | Acyclovir | 3 | Amoxycillin | 17 | DTwP/ Hib vaccine | 540 |
| Etanercept | 1 | Venlafaxine | 18 | Citalopram | 2 | Chlorhexidine | 3 | Ibuprofen | 12 | Pneumococcal vaccine | 496 |
| Sertraline | 11 | Lamotrigine | 2 | DTPw HIB vaccine | 3 | Palivizumab | 12 | Bacillus Calmette Guerin Vaccine | 309 | ||
| Olanzapine | 7 | Fluconazole | 2 | Ibuprofen | 3 | Domperidone | 10 | MMR vaccine | 257 | ||
| Valproate | 7 | Desogestrel | 2 | Indomethacin | 3 | Cefotaxime | 9 | Haemophilus influenzae type b vaccine | 190 | ||
| Lamotrigine | 6 | Trimethoprim | 3 | Montelukast | 9 | Poliomyelitis vaccine | 174 | ||||
| Vitamin K substances | 3 | Trimethoprim | 9 | DTwP vaccine | 104 | ||||||
| Ranitidine | 8 | Hib/MEN C conjugate vaccine | 87 | ||||||||
For transmammary, transplacental and paternal all reported medications shown. For neonates, all drugs with n > 2 shown, vaccinations and infants, 10 most commonly reported shown. Vaccinations given to those in neonatal period are shown in both neonates and vaccination columns. DTaP, Diptheria, tetanus and (acellular) Pertussis; DTwP, Diptheria, Tetanus and (whole cell) Pertussis; HIb, Haemophilus influenzae type b; MEN C, Meningococcal group C; MMR, Measles/Mumps/Rubella. Full dataset for all groups in supplementary data section
Paternal
Four drugs, all for the management of autoimmune conditions, were recorded on three YCs. The reactions listed on these YC were growth retardation, diarrhoea, developmental delay, hypotonia, fetal cardiac disorder and development of a birth mark. Gestation of the child was not documented in any of the cases and the ADR outcomes were all ‘unknown’.
Transplacental
Reporting improved across the decade; during the first five years the mean YC received per year was 14.6, but for the latter five years it increased to a mean of 34.4 (Figure 1A). A total of 221 different medications were listed with 81 (36.6%) generating more than one report. The most commonly reported medication suspected of causing an ADR was fluoxetine (n = 21) and overall, 16 different psychotropic drugs were associated with 120 reported reactions. The most commonly reported medications are shown in Table 2; however, under‐reporting appears widespread.
Figure 1.
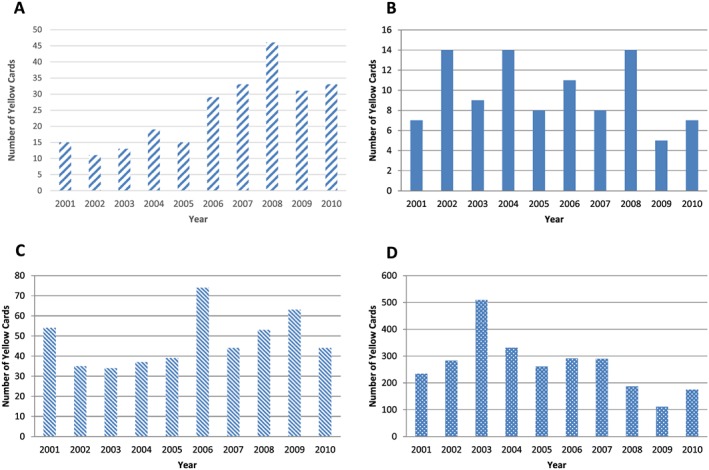
Number of Yellow Card reports of suspected adverse drug reactions received by the Medicines and Healthcare Regulatory Agency (MHRA) per year for (A) transplacental, (B) neonates, (C) infants and (D) vaccinations
Most of the 11 000 HIV‐positive women who became pregnant in the UK during this time period will have received antiretroviral drugs 28. Current evidence suggests no increase in congenital abnormalities reported with these drugs, either in the UK or internationally (2.8 birth defects per 100 live births) 29, 30. We would therefore estimate, based on the standard frequency of congenital anomalies, that approximately 300 children within this population would be affected. Over the 10‐year period, only seven reports associated with maternal antiretroviral drug use in pregnancy were reported via the YC Scheme, an under‐reporting rate of 97.7%.
It has been known for some time that there are significant adverse effects of anti‐epileptic medications in pregnancy, but use is continued as the benefits to the mother outweigh the risks to the child. Epidemiological estimates put the number of pregnancies exposed to anti‐epileptic drugs (AEDs) at 0.3–0.4% 31, 32, while the frequency of major congenital malformations in pregnancies exposed to AEDs has been shown to be 4.2% overall (with some variation between different AEDs) 33. Extrapolating from the average number of deliveries per year in the UK 2001–10 (659 551), we would expect 0.35% (2300) pregnancies per year to be to women using AEDs, with approximately 100 children per year with major congenital malformations. The YC system collected 29 reports of suspected ADRs to anti‐epileptics over the 10‐year period, suggesting under‐reporting rates of approximately 98.8%.
The most commonly reported reactions (for all transplacental YC) were ‘irritability/jittery/agitation’ (n = 21), ‘congenital abnormality’ (n = 17), withdrawal syndrome (n = 12), cleft lip/palate (n = 11), death (stillbirth, intrauterine or neonatal) (n = 11), prematurity (n = 11) and cardiac septal defect (n = 10).
For psychotropic drugs, the most commonly reported reactions were congenital hand malformation (n = 7), dextrocardia (n = 4), cardiac septal defect (n = 2), stillbirth/intrauterine death (n = 16), withdrawal syndrome (n = 14), spina bifida (n = 4) and neonatal agitation (n = 11) (full list in supplementary data).
Gestational age was recorded in 45 out of 246 (18%) cases with the highest proportion documented in 2004. Not all were specific, with ‘premature’ written in four cases and ‘term’ in one. The mean recorded gestational age was 32.8 weeks.
Transmammary
From the 30 transmammary reports, no discernible trend in reporting frequency was observed (range: 0 reports in 2003 to 10 reports in 2010). Twenty‐four different medications were listed, with fluoxetine being the most frequently reported drug, featuring on four YCs. Seven drugs were reported more than once (Table 2, full list in the supplementary data section). The most commonly reported reactions were irritability/agitation (n = 5), sleep disorder/somnolence (n = 4), dyskinesias (n = 4), abdominal pain (n = 3), diarrhoea (n = 3) and failure to thrive (n = 3). One gestation was provided as ‘full term’ with another documented as ‘premature’ but no accurate gestational ages were given.
Neonates
No pattern was noted in the neonatal reporting rates over the time period (Figure 1B). The most commonly reported medication suspected of causing an ADR was swine flu vaccine (n = 8), followed by caffeine (n = 5). There were 68 different medications listed on neonatal YC as being suspected of causing an ADR (full list in supplementary data section), although only eight (11.7%) generated more than one report (Table 2, full list in the supplementary data section). There were 10 YC reports for antibiotics (trimethoprim [n = 3], benzylpenicllin [n = 2], gentamicin [n = 2], ceftazidime [n = 2], clindamycin, erythromycin and metronidazole [n = 1 for each]. Despite well publicized safety concerns, there were no reports for codeine, ceftriaxone or ritonavir‐boosted lopinavir (Kaletra) 21 over the 10‐year period.
Approximately 7% of neonates receive antibiotics to treat suspected early onset neonatal infection, so between 42 000 and 51 000 babies a year were exposed to antibiotics, totalling 450 000 babies exposed to antibiotics during the study period. This implies a reporting rate of one YC per 4500 babies receiving antibiotics. There were no reports relating to surfactant, but during the study period almost all live births born at 28 weeks gestation or less would have received at least one dose of surfactant 34. This represents 1.4% of the total births (93 000 babies), and other babies born closer to term would have been be eligible for, and received, surfactant. We therefore estimate that at least 100 000 babies received surfactant without prompting a YC report. Other medications with well‐recognized ADRs that are commonly used on neonatal units were not commonly found among YC reports, e.g. corticosteroids.
The most commonly reported reactions for neonates were rashes (n = 14), erythema (n = 7), bradycardia (n = 6), convulsion (n = 5), tachycardia (n = 5), local reaction (n = 4), cardiac arrest, dyspnoea, hypotension, necrotizing colitis, decreased oxygen saturations and pruritus (n = 3 for all).
Of the 97 YC that were categorized as neonatal, 88 were categorized on the basis of age since delivery as stated on the YC (6–24 hours, 0–27 days, or 1–3 weeks), five stated ‘neonate’ and gave clinical details that put the affected child in the neonatal period, and four gave an age >27 days but recorded gestation in the clinical details section enabling a calculation of corrected gestational age. Overall, only 25 (0.8%) YC reports stated a gestational age at birth for the affected neonate. Twenty‐three provided an exact gestation, one stated ‘preterm’ and one ‘9 weeks premature’.
Infants
There was no discernible pattern to the reporting frequency in infants (Figure 1C). In 2006 there was an apparent ‘spike’ in reporting in the infant cohort, with 74 YC submitted. The most frequently reported medication that year was domperidone (n = 5), and of the 61 different medications reported in 2006, only 11 of them were reported on more than one occasion (for full list, see supplementary data section). Overall, the most common suspected drug was oseltamivir (n = 37). Overall, there were 223 different medications listed in this cohort. There was a single report for codeine in the infant category. Gestation was recorded in nine YC in this cohort (1.9%).
Vaccinations
Reporting of vaccination‐related ADRs peaked in 2003 (n = 506) (Figure 1D). This year contained 256 reports (50.6%) for various preparations and combinations of primary immunizations containing diptheria, pertussis and tetanus (DTP). Over the 10‐year period, the percentage of reports in children <2 years of age related to vaccinations was 82.8%. There was a decrease in vaccine‐related reports from 2006, likely related to the introduction of the acellular pertussis vaccine (associated with fewer ADRs than the previously used whole cell vaccines 35). From the vaccination cohort, the most commonly suspected drug on a YC was meningococcal C vaccine (n = 693). There were 31 different medications reported in the vaccination cohort, 29 of which were vaccinations (the remaining medications in addition were ketotifen and mesalazine, reported at the same time as swine flu vaccination). A complete list is contained in the supplementary data section. Most reports of ADRs after immunization were local or generalized reactions, including anaphylaxis (n = 20). However, there were a few reports of vaccine failure (Haemophilus influenzae type b 8, Meningococcal group C 1, Pneumococcal vaccine 1, Pertussis 1), vaccines not being given according to the schedule (e.g. single antigen measles, mumps or rubella) or hospitalization due to inter‐current illness or ADR (upper respiratory tract infection, urinary tract infection, pneumonia, febrile convulsion).
Reporter
Overall, doctors reported the largest number of YC (1445), followed by nurses (1086), parents/carers (173), and pharmacists (138) (Table 1). Of note, the proportion of parent/carer reports was greater in the infant group (8.9%) than in any other group.
Outcome
There was a fatal outcome in 65 (1.9%) of YC submitted in this cohort. The drugs suspected of causing a fatal outcome are summarized in Table 3. The proportion of fatal reports for each category were: 0% paternal, 11.2% transplacental, 6.7% transmammary, 3.1% neonatal, 2.8% infant and 0.7% for vaccinations. Some ongoing issues remained in patients in 513 (14.5%) YC, while there was complete recovery reported in 2413 YC (68.8%). The outcome was unknown in 507 (14.5%) YC.
Table 3.
Most commonly reported medications listed on Yellow card reports from children age <2 years between 2001 and 2010 with fatal outcomes
| Drug(s) listed on Yellow Card | Category | Reports |
|---|---|---|
| DTaP/IPV/Hib vaccine, pneumococcal vaccine | Vaccination | 4 |
| Palivizumab | Infant | 4 |
| Citalopram | Transplacental | 4 |
| DTaP/IPV/Hib vaccine, meningococcal vaccine | Vaccination | 3 |
| DTwP/Hib vaccine, meningococcal vaccine, polio vaccine | Vaccination | 2 |
| MMR vaccine | Vaccination | 2 |
| Olanzapine, Venlafaxine | Transplacental | 2 |
| Lamivudine and zidovudine, lopinavir with ritonavir | Transplacental | 2 |
DTaP, diptheria, tetanus and (acellular) pertussis; DTwP, diptheria, tetanus and (whole cell) pertussis; HIb, haemophilus influenzae type b; IPV, inactivated polio vaccine; MMR, measles/mumps/rubella
Review of drug safety update
Review and analysis of the monthly MHRA publication Drug Safety Update (DSU) between October 2007 and September 2014 has shown that alerts were provided on 405 drugs (or classes of drug) over this period. Of these, 97 were medications with potential applicability to the population in whom YC reports were available (92 paternal, 96 transplacental, 90 transmammary, 50 neonates, 134 infant and 12 vaccinations [some applicable to more than one category]). Selected drugs for which DSU alerts were produced, and the number of YC reports for that medication during the study period are shown in Table 4 (the full table of drugs featured in DSU and relevance to this population are available from the authors on request). DSU alerts, while clinically important, were not well correlated with YC reports before or after the alert. There were two examples where some correlation was found: a single DSU report highlighting agitation in a baby following anti‐psychotic use in pregnancy was preceded by a corresponding YC; advice on avoiding sodium valproate in pregnancy unless no alternative was available was preceded by five YC reports for a range of suspected ADRs (Table 4).
Table 4.
Comparison of selected drugs from Drug Safety Update alerts produced for the populations of interest and the YC reports received during the years 2001–10
| Medication | Cohort | Drug alert reason (date) | ADR (number and date) reported to YC from that cohort |
|---|---|---|---|
| Lopinavir and ritonavir | Neonate | Neonatal administration error (October 2007) | 0 |
| Codeine | Neonate and infant | Transplacental fatality (November 2007), Restricted use in children <12 years (June and July 2013) | 0 |
| Ceftriaxone | Neonate | Incompatible with calcium infusions (October 2009) | 0 |
| Domperidone | Neonate and infant | Cardiac side effects (May 2012, May and September 2014) | 10 (2001 (diarrhoea), 2004 (dystonia) 2005 (irritability & gynaecomastia), 2006 × 5 (death out of hospital; trembling and muscle spasm; deranged LFTs; drowsiness with O2 requirement; worsening dystonia), 2010 × 2 (pruritis and increased instability)) |
| Caffeine | Neonate | Check dose as two forms (August 2008, September 2008, June 2012), Single form now used (August 2013) | 5 (2001 × 3 (all extravasations with tissue injury), 2007 (tachycardia and tachypnoea), 2010 (vasoconstriction)) |
| Corticosteroids (including individual drug names) | Neonate | Psychiatric side effects (September 2007) | 3 (2007 × 3 (Cushing's, Benign Intracranial Hypertension × 2)) |
| Codeine | Transmammary | Neonatal death following maternal use (November 2007, October 2010, December 2012, June 2013, July 2013) | None |
| ACE inhibitors and angiotensin II receptor antagonists | Transmammary | Updated advice during breastfeeding – not recommended but not contraindicated (July 2009) | None |
| Antipsychotics | Transplacental | Increased extrapyramidal and/or withdrawal ADRs in infants if used in third trimester (September 2011) | 9 (2004 (Lethargy [olanzapine]), 2005 (gastroschisis [haloperidol]), 2006 (agitation [olanzapine]), 2007 (macrosomia [olanzapine]), 2008 × 2 (both congenital anomalies [olanzapine]), 2009 × 2 (failure to thrive [quetiapine], congenital anomaly [quetiapine]), 2010 (congenital anomaly [quetiapine) |
| Sodium Valproate | Transplacental | Avoid in pregnant women unless no alternative (November 2013) | 2001 (congenital anomaly), (2005 × 2 (jittery, floppy with abnormal LFTs), 2006 (congenital anomaly), 2007 (fetal anticonvulsant syndrome) |
Discussion
The MHRA spontaneous reporting scheme has been collecting data on suspected ADRs in the UK for 50 years. It originated following the devastating consequences of a transplacental ADR in newborn babies (phocomelia) secondary to in utero exposure to thalidomide. We present the most detailed examination of YC reports for children aged <2 years of age, covering a 10‐year period from 2001 to 2010. Previous studies of paediatric YC reports were unable to examine outcomes, and were limited to the most commonly reported medications within the entire paediatric population. To our knowledge there are no previous studies examining spontaneous ADR reports in neonates. These data show that the UK generates very few reports for paternal, transmammary and transplacental ADRs, and that key data (such as gestational age and outcome) are missing from many YC reports. The data used to create alerts from the regulator to clinicians is derived from many sources (including UK‐specific and international data), but in this population, during the period studied, spontaneous YC generated in the UK do not appear to be a significant source of data.
Under‐reporting is a problem for all spontaneous ADR reporting schemes, and estimates of the frequency of under‐reporting are approximately 95% 36. This may be an under‐estimate of reporting frequency for some sections of our cohort, as evidenced by the lack of reports for HIV‐positive and epileptic women. However, reporter fatigue and/or confusion may also have had a role (children previously having different reporting rules to adults, where all suspected ADRs were requested). Recently the MHRA have been actively promoting the reporting of suspected ADRs in children, and updated the paediatric reporting rules to align them with adults 15. Future work will be required to see the response to these initiatives, and if they increase the quality and/or quantity of reports. Finally, these data only contain information on YC where the age was given by the reporter, and will omit YC submitted without ages provided.
Data from the British Paediatric Surveillance Unit found four infant deaths associated with ADRs in a single year (2002–3) 37, while equivalent ADRs (where the child received the medication postnatally) in this cohort are reported with a mean frequency of 3.7 per year, suggesting that clinicians are reporting these most serious of suspected ADRs to the YC Scheme.
Paternal
Given the paucity of reports of paternal adverse drug reactions, it is hard to draw any firm conclusions from these. The time delay between paternal use of the medication and the identification of the suspected ADR makes using a spontaneous reporting scheme for these ADRs difficult. Other mechanisms may therefore have to be considered to generate suspected ADR reports from this population.
Transplacental
The importance of ongoing active surveillance to reassure pregnant women that these immunizations are safe and beneficial for them and their babies 38, 39, 40 is highlighted by the very small number of reports of congenital anomalies in infants born to women given vaccines during pregnancy. These data contain nothing that would suggest the routinely recommended vaccines of pregnancy are unsafe for the fetus when given to healthy pregnant women.
More concerning is the report of fatally disseminated BCG following BCG vaccination in an infant whose mother received infliximab during pregnancy 41, 42. TNF antagonists are known to increase the risk of tuberculosis 43 and are known to cross the placenta. It is recommended that infants born to women given anti‐TNF agents in pregnancy are not given live vaccines for up to 7 months. National guidance is now being updated to include this advice.
Despite the low frequency of YC reports, those related to transplacental ADRs were the best correlated with the clinical concerns of the UK regulatory agency.
Transmammary
The data received over 10 years for transmammary suspected ADRs is limited, and while there were several alerts from the regulatory agency regarding transmammary ADRs, none of the reports received reflected this. New strategies will be needed to improve this neglected area of reporting to the YC Scheme.
Neonates
The classification of infants as neonates requires either an accurate age at the time of the reaction accompanied by the gestational age at birth, or a corrected gestational age at the time of the reaction. Very few YC reports contained a gestational age at birth (0.8%). If more infants had accurate gestational ages included in the YC then it is probable that some infant ADRs would have been reclassified as neonatal, increasing interpretable reports in this neglected group.
It is disappointing that despite the regulatory agency producing many different clinical warnings about potential ADRs for various neonatal medications via the DSU (and other routes), there were no relevant reports from the UK preceding these reports. This suggests that the current rate or quality of YC reports (or both) needs to improve in order to ensure that the UK contributes meaningfully to international efforts to identify ADRs from spontaneous reports.
Infants
The infant cohort is the most heterogeneous, encompassing those 28 days old, to those 1 year 11 months old as the YC reports only give an age in years, so if the reporter gives an accurate age in years only, the child may still be nearly 2. An improvement to the YC system in the UK would therefore be to request reporters provide an age in completed months until the age of two years. The first two years after birth represent the most dynamic stage of ex utero ontogeny 44.
Vaccinations
Vaccine reports varied by year, and are associated with the introduction, or removal, of various vaccines from the national programme. As in our previous study of YC data in all children, meningococcal C vaccine was the most frequently reported vaccine. Reports for BCG vaccine were less than our previous paper, because older children were given BCG until 2005. However, the same ‘spike’ in reports was seen when the SSI BCG was introduced into the UK in November 2002 16. These data suggest that YC reports for vaccinations were responsive to the underlying pattern of medicine use. Vaccination therefore represents an area of success for the YC system for patients <2 years of age.
Most reports associated with a vaccine described local or generalized reactions. However, reporters also used the YC Scheme to report vaccine failures (especially the Hib vaccine failures that occurred in 2003 45). Reports also described inadvertent use of the wrong vaccine in some infants (such as giving the diptheria, tetanus, pertussis and polio vaccine without Hib, instead of the vaccine with Hib included (Pediacel)).
Improving reporting
The value of the YC Scheme has been demonstrated many times, and it has helped to identify numerous important safety issues (including in this population), many of which were not previously recognized 46. However, the shortcomings identified in the UK's spontaneous ADR reporting system for this population currently suggest that both improvements in the YC system, and consideration of alternative approaches, are needed. Since obtaining these data, we do note the active promotion of reporting in children, the amendments to the reports required to align with adults, and the updates to the online YC form (requesting gestational age, and improving the capture of pregnancy‐related data). Additional simple measures to improve the quality of YC reports would include making the gestational age of children <1 year a mandatory field on the online YC reporting, as well as asking for all ages <2 years in completed months.
We believe that there is also a place for additional systems in parallel with YC reports, including some or all of the following: sentinel sites (undertaking active surveillance of ADRs); changed recommendations about reporting; increased professional education about reporting ADRs; a focus on specific questions (e.g., a couple of medicines a year, such as a neonatal black triangle, etc.). Internationally, there is an example of active surveillance in Canada 47. Given the issues with causality, polypharmacy and multiple inter‐current illnesses, spontaneous reporting may not be appropriate and registry‐based data may be more useful. This does not only apply to non‐intensive care situations: a large number of ‘healthy’ neonates receive medicines 47.
We further speculate that the paucity of reporting we found is not limited to the UK. Recent legislative changes will stimulate the development of medicines for neonates 48. Post‐marketing surveillance will become increasingly important, so that reporting by clinicians needs to be strengthened. Our findings may be informative for other jurisdictions.
Conclusions
Analysis of the content of YC reports suggests that gaps in reporting are not random: the YC Scheme is used more effectively in some therapeutic areas than others. Medications suspected of causing ADRs in those <2 years are reported to the MHRA through the YC Scheme, but the frequency of reports is low, with less than one per day overall. Paternal, transplacental, transmammary and neonatal ADRs are particularly poorly represented, and recording of gestational age is poor. The regulator draws attention to serious ADRs in the neonatal and infant populations, but these ADRs are not represented in YCs before regulatory action is taken. Action is required to stimulate ADR reporting for the youngest patients to improve the quality and quantity of YC reports, including the consideration of additional systems to run in parallel with YC reporting.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work.
The authors would like to thank Mitul Jadeja and Juliana Min, Vigilance Intelligence & Research Group, Vigilance and Risk Management of Medicines, MHRA.
DH is part funded by the NIHR‐Alder Hey Clinical Research Facility.
Supporting information
Table S1 Complete list of drugs reported as causing suspected ADRs for children of age <2 years between 2001 and 2010.
Supporting info item
Hawcutt, D. B. , Russell, N. ‐J. , Maqsood, H. , Kouranloo, K. , Gomberg, S. , Waitt, C. , Sharp, A. , Riordan, A. , and Turner, M. A. (2016) Spontaneous adverse drug reaction reports for neonates and infants in the UK 2001–2010: content and utility analysis. Br J Clin Pharmacol, 82: 1601–1612. doi: 10.1111/bcp.13067.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44: D1054–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Safety of medicines: a guide to detecting and reporting adverse drug reactions. Geneva: World Health Organization, 2002. [Google Scholar]
- 3. Gallagher RM, Mason JR, Bird KA, Kirkham JJ, Peak M, Williamson PR, et al. Adverse drug reactions causing admission to a paediatric hospital. PLoS One 2012; 7: e50127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ 2004; 329: 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta‐analysis of prospective studies. JAMA 1998; 279: 1200–1205. [DOI] [PubMed] [Google Scholar]
- 6. Mitchell AA, Lacouture PG, Sheehan JE, Kauffman RE, Shapiro S. Adverse drug reactions in children leading to hospital admission. Pediatrics 1988; 82: 24–29. [PubMed] [Google Scholar]
- 7. Gonzalez‐Martin G, Caroca CM, Paris E. Adverse drug reactions (ADRs) in hospitalized pediatric patients: a prospective study. Int J Clin Pharmacol Ther 1998; 36: 530–533. [PubMed] [Google Scholar]
- 8. Thiesen S, Conroy EJ, Bellis JR, Bracken LE, Mannix HL, Bird KA, et al. Incidence, characteristics and risk factors of adverse drug reactions in hospitalized children? A prospective observational cohort study of 6,601 admissions. BMC Med 2013; 11: 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martínez‐Mir I, García‐López M, Palop V, Ferrer JM, Rubio E, Morales‐Olivas FJ. A prospective study of adverse drug reactions in hospitalized children. Br J Clin Pharmacol 1999; 47: 681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tinker SC, Broussard CS, Frey MT, Gilboa SM. Prevalence of prescription medication use among non‐pregnant women of childbearing age and pregnant women in the United States: NHANES, 1999–2006. Matern Child Health J 2015; 19: 1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Department of Health . Indicators on breastfeeding: quarter 4 2012/13. London: Department of Health, 2013. [Google Scholar]
- 12. Choonara I, Conroy S. Unlicensed and off‐label drug use in children – implications for safety. Drug Saf 2002; 25: 1–5. [DOI] [PubMed] [Google Scholar]
- 13. Horen B, Montastruc JL, Lapeyre‐Mestre M. Adverse drug reactions and off‐label drug use in paediatric outpatients. Br J Clin Pharmacol 2002; 54: 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ward RM, Benitz WE, Benjamin DK, Blackmon L, Giacoia GP, Hudak M, et al. Criteria supporting the study of drugs in the newborn. Clin Ther 2006; 28: 1385–1398. [DOI] [PubMed] [Google Scholar]
- 15. Barton C, Hawcutt DB. When to report adverse drug reactions in children? Arch Dis Child 2015; 100: 682–683. [DOI] [PubMed] [Google Scholar]
- 16. Hawcutt DB, Mainie P, Riordan A, Smyth RL, Pirmohamed M. Reported paediatric adverse drug reactions in the UK 2000–2009. Br J Clin Pharmacol 2012; 73: 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moore TJ, Weiss SR, Kaplan S, Blaisdell CJ. Reported adverse drug events in infants and children under 2 years of age. Pediatrics 2002; 110: e53. [DOI] [PubMed] [Google Scholar]
- 18. Li H, Guo X‐J, Ye X‐F, Jiang H, Du W‐M, Xu J‐F, et al. Adverse drug reactions of spontaneous reports in Shanghai pediatric population. PLoS One 2014; 9: e89829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Toska A, Mary G, Kyriakos S, Maria S, Costas D. Adverse drug reaction reporting related to the administration of antibiotics in hospitalized pediatric patients in Greece. Curr Drug Saf 2014; 9: 49–55. [DOI] [PubMed] [Google Scholar]
- 20. Ribeiro‐Vaz I, Marques J, Demoly P, Polónia J, Gomes ER. Drug‐induced anaphylaxis: a decade review of reporting to the Portuguese Pharmacovigilance Authority. Eur J Clin Pharmacol 2013; 69: 673–681. [DOI] [PubMed] [Google Scholar]
- 21. Hawcutt DB, O'Connor O, Turner MA. Adverse drug reactions in neonates: could we be documenting more? Expet Rev Clin Pharmacol 2014; 7: 807–820. [DOI] [PubMed] [Google Scholar]
- 22. Zanardi LR, Haber P, Mootrey GT, Niu MT, Wharton M. Intussusception among recipients of rotavirus vaccine: reports to the vaccine adverse event reporting system. Pediatrics 2001; 107: e97. [DOI] [PubMed] [Google Scholar]
- 23. Mittmann N, Knowles SR, Gomez M, Fish JS, Cartotto R, Shear NH. Evaluation of the extent of under‐reporting of serious adverse drug reactions – the case of toxic epidermal necrolysis. Drug Saf 2004; 27: 477–487. [DOI] [PubMed] [Google Scholar]
- 24. Fletcher AP. Spontaneous adverse drug reaction reporting vs. event monitoring – a comparison. J R Soc Med 1991; 84: 341–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Office for National Statistics . Live births in England and Wales by characteristics of birth, 2010 [online]. 2011. Available at: http://www.ons.gov.uk/ons/rel/vsob1/characteristics‐of‐birth‐2‐‐england‐and‐wales/2010/stb‐characteristics‐of‐birth.html (last accessed 19 June 2014).
- 26. Health Protection Agency . HIV in the United Kingdom: 2011 Report. London: Health Protection Services, Colindale, 2011. [Google Scholar]
- 27. Medicines and Healthcare Regulatory Agency . Drug Safety Update [online]. 2014. Available at: http://www.mhra.gov.uk/Safetyinformation/DrugSafetyUpdate/index.htm (last accessed 31 October 2014).
- 28. Townsend CL, Byrne L, Cortina‐Borja M, Thorne C, de Ruiter A, Lyall H, et al. Earlier initiation of ART and further decline in mother‐to‐child HIV transmission rates, 2000–2011. AIDS 2014; 28: 1049–1057. [DOI] [PubMed] [Google Scholar]
- 29. Townsend CL, Willey BA, Cortina‐Borja M, Peckham CS, Tookey PA. Antiretroviral therapy and congenital abnormalities in infants born to HIV‐infected women in the UK and Ireland, 1990–2007. AIDS 2009; 23: 519–524. [DOI] [PubMed] [Google Scholar]
- 30. Antiretroviral Pregnancy Registry Steering Committee. Wilmington NRCC . Antiretroviral Pregnancy Registry International Interim Report. Wilmington, NC: Antiretroviral Pregnancy Registry, 2015. [online]. Available at: http://www.apregistry.com/forms/exec‐summary.pdf (last accessed 17 April 2015). [Google Scholar]
- 31. Dansky LV, Finnell RH. Parental epilepsy, anticonvulsant drugs, and reproductive outcome: epidemiologic and experimental findings spanning three decades; 2: Human studies. Reprod Toxicol 1991; 5: 301–335. [DOI] [PubMed] [Google Scholar]
- 32. Olafsson E, Hallgrimsson JT, Hauser WA, Ludvigsson P, Gudmundsson G. Pregnancies of women with epilepsy: a population‐based study in Iceland. Epilepsia 1998; 39: 887–892. [DOI] [PubMed] [Google Scholar]
- 33. Morrow J, Russell A, Guthrie E, Parsons L, Robertson I, Waddell R, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry 2006; 77: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sweet D, Bevilacqua G, Carnielli V, Greisen G, Plavka R, Didrik Saugstad O, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome. J Perinat Med 2007; 35: 175–186. [DOI] [PubMed] [Google Scholar]
- 35. Rosenthal S, Chen R, Hadler S. The safety of acellular pertussis vaccine vs. whole‐cell pertussis vaccine: a postmarketing assessment. Arch Pediatr Adolesc Med 1996; 150: 457–460. [DOI] [PubMed] [Google Scholar]
- 36. Hazell L, Shakir SA. Under‐reporting of adverse drug reactions. Drug Saf 2006; 29: 385–396. [DOI] [PubMed] [Google Scholar]
- 37. Cheng K, Masters S, Stephenson T, Cooke R, Ferner R, Ashworth M, et al. Identification of suspected fatal adverse drug reactions by paediatricians: a UK surveillance study. Arch Dis Child 2008; 93: 609–611. [DOI] [PubMed] [Google Scholar]
- 38. Donegan K, King B, Bryan P. Safety of pertussis vaccination in pregnant women in UK: observational study. BMJ 2014; 349: g4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McMillan M, Porritt K, Kralik D, Costi L, Marshall H. Influenza vaccination during pregnancy: a systematic review of fetal death, spontaneous abortion, and congenital malformation safety outcomes. Vaccine 2015; 33: 2108–2117. [DOI] [PubMed] [Google Scholar]
- 40. Fell D, Platt R, Lanes A, Wilson K, Kaufman J, Basso O, et al. Fetal death and preterm birth associated with maternal influenza vaccination: systematic review. BJOG: Int J Obstet Gynecol 2015; 122: 17–26. [DOI] [PubMed] [Google Scholar]
- 41. Cheent K, Nolan J, Shariq S, Kiho L, Pal A, Arnold J. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn's disease. J Crohns Colitis 2010; 4: 603–605. [DOI] [PubMed] [Google Scholar]
- 42. Heller MM, Wu JJ, Murase JE. Fatal case of disseminated BCG infection after vaccination of an infant with in utero exposure to infliximab. J Am Acad Dermatol 2011; 65: 870. [DOI] [PubMed] [Google Scholar]
- 43. Solovic I, Sester M, Gomez‐Reino J, Rieder H, Ehlers S, Milburn H, et al. The risk of tuberculosis related to tumour necrosis factor antagonist therapies: a TBNET consensus statement. Eur Respir J 2010; 36: 1185–1206. [DOI] [PubMed] [Google Scholar]
- 44. Allegaert K, Vanhaesebrouck S, Verbesselt R, van den Anker JN. In vivo glucuronidation activity of drugs in neonates: extensive interindividual variability despite their young age. Ther Drug Monit 2009; 31: 411–415. [DOI] [PubMed] [Google Scholar]
- 45. McVernon J, Andrews N, Slack M, Ramsay M. Risk of vaccine failure after Haemophilus influenzae type b (Hib) combination vaccines with acellular pertussis. Lancet 2003; 361: 1521–1523. [DOI] [PubMed] [Google Scholar]
- 46. Medicines and Healthcare Regulatory Agency . Contribution of Yellow Cards to identifying safety issues [online]. 2014. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/396811/Contribution_of_Yellow_Cards_to_identifying_safety_issues.pdf (last accessed 4 February 2016).
- 47. Carleton BC, Poole RL, Smith MA, Leeder JS, Ghannadan R, Ross CJD, et al. Adverse drug reaction active surveillance: developing a national network in Canada's children's hospitals. Pharmacoepidemiol Drug Saf 2009; 18: 713–721. [DOI] [PubMed] [Google Scholar]
- 48. Turner M, Catapano M, Hirschfeld S, Giaquinto C. Paediatric drug development: the impact of evolving regulations. Adv Drug Deliv Rev 2014; 73: 2–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Complete list of drugs reported as causing suspected ADRs for children of age <2 years between 2001 and 2010.
Supporting info item


