Abstract
Aim
The aim of the present study was to describe the occurrence and determinants of angiotensin‐converting enzyme (ACE) inhibitor (ACEI) intolerance and angioedema (AE) among patients initiating ACEI therapy in a real‐world primary care population.
Methods
Two nested case–control studies were conducted in a cohort of 276 977 patients aged ≥45 years initiating ACEIs from 2007 to 2014 in the UK Clinical Practice Research Datalink (CPRD). Cases of AE occurring for the first time during ACEI therapy (n = 416) were matched with AE‐free controls (n = 4335) on the duration of ACEI treatment. Documented switches to angiotensin‐II receptor blockers in the prescription records were used to identify ACEI‐intolerance cases (n = 24 709), and these were matched with continuous ACEI users (n = 84 238) on the duration of ACEI therapy. Conditional logistic regression was used to assess the associations of demographic factors, comorbidities and comedication with AE and ACEI intolerance.
Results
AE during ACEI therapy was associated with age over 65 years [odds ratio (OR) 1.36, 95% confidence interval (CI) 1.07, 1.73], history of allergy (OR 1.53, 95% CI 1.19, 1.96), use of calcium channel blockers (OR 1.57, 95% CI 1.23; 2.01), use of antihistamines (OR 21.25, 95% CI 16.44, 27.46) and use of systemic corticosteroids (OR 4.52, 95% CI 3.26, 6.27). ACEI intolerance was significantly associated with more comorbidities and comedication compared with AE, including allergy (OR 2.02, 95% CI 1.96, 2.09), use of antiasthmatic drugs (OR 1.51, 95% CI 1.42, 1.61) and use of antihistamines (OR 1.53, 95% CI 1.43, 1.63).
Conclusions
Among ACEI users developing AE or ACEI intolerance, several comorbidities and comedication classes were significantly more prevalent compared with ACEI users not developing these adverse reactions.
Keywords: ACE inhibitors, ACE inhibitor intolerance, angioedema, angiotensin II receptor blockers, case–control studies, drug‐related side effects and adverse reactions
What is Already Known about this Subject
Angioedema (AE) and dry cough due to angiotensin‐converting enzymes (ACEIs) often result in the discontinuation of ACEI treatment. Knowledge of potential risk factors could be helpful in identifying patients more likely to develop these adverse reactions and assist in prescribing decisions. Previous studies identified a number of risk factors, including, for instance, female gender, ethnicity and older age.
What this Study Adds
We investigated whether history of chronic disease and comedication use were associated with ACEI intolerance (defined by switching to angiotensin II receptor blockers (ARBs) in prescription records) and AE during ACEI therapy in two explorative case–control studies.
We found that age over 65 years, history of allergy and prescriptions for calcium channel blockers, antihistamines and systemic corticosteroids were more prevalent in ACEI users developing AE than in AE‐free ACEI users.
The number of observed associations with comorbidities and comedication was higher in those developing ACEI intolerance than in those developing AE. A history of allergy, and the use of antihistamines, antiasthmatic drugs and calcium channel blockers were more frequent in switchers to ARBs compared with continuous ACEI users. A history of diabetes or chronic obstructive pulmonary disease, and use of statins were less prevalent among switchers to ARBs.
Tables of links
| TARGETS | |
|---|---|
| Enzymes 2 | G protein‐coupled receptors 3 |
| ACE1, angiotensin converting enzyme | AT1 receptor |
| LIGANDS | |
|---|---|
| Captopril | Lisinopril |
| Cilazapril | Perindopril |
| Enalapril | Quinapril |
| Fosinopril | Ramipril |
| Imidapril | Trandolapril |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 2, 3.
Introduction
Angiotensin‐converting enzyme inhibitors (ACEIs) are among the most commonly prescribed antihypertensive agents to date used for hypertension, heart failure, diabetic nephropathy and secondary prevention following a myocardial infarction (MI). The estimated number of ACEI prescriptions has substantially increased over the past few years, from 35–40 million worldwide in 2001 to approximately 160 million in 2011 in the USA alone 4, 5. In the UK, ramipril is the leading drug among medications for hypertension and heart failure, with almost 26 million prescriptions dispensed in 2014, which shows an increase of 17 million prescriptions since 2004 6.
ACEIs have been proven to reduce all‐cause mortality in patients with hypertension, as well as major cardiovascular (CV) events, and all‐cause and CV mortality in patients with diabetes mellitus (DM) 7, 8. A beneficial effect of ACEIs on the risk of subsequent CV events and mortality was also found in secondary prevention after MI 9. However, according to observational studies, 19–30% of patients initiating ACEIs discontinue treatment owing to adverse effects 10, 11. One of the most common adverse effects of ACEIs is a persistent dry cough, described in 9.9–35% of the patients in randomized clinical trials 12, 13. A far more uncommon, but potentially life‐threatening adverse effect of ACEIs is angioedema (AE) of the head and neck region and the viscera. In randomized clinical trials, the incidence of ACEI‐induced AE was estimated to be 0.3–0.7% 14. In emergency care, ACEI‐induced AE of the larynx accounts for a third of all hospitalizations for AE 4, 15, 16.
Most cases of ACEI‐induced cough occur early in the course of treatment, while ACEI‐induced AE may develop either in the first weeks or several years after the start of treatment 4. Although the exact mechanisms of ACEI‐induced AE and cough are not known, they have been proposed to be similar, and to involve a reduction in the catabolism of vasoactive substances (bradykinin and substance P) as a result of ACE inhibition 17. Furthermore, ACEI‐induced AE and cough share some similar clinical predictors 18. For instance, ethnical origin is an important risk factor, with African American patients having an almost threefold higher risk for ACEI‐induced AE and East Asians being at a higher risk for ACEI‐induced cough 19, 20.
Some of the previous studies on predictors of ACEI‐related adverse effects were limited by a relatively small sample size and an incomplete registering of adverse effects outside randomized clinical trials. One way of enabling this limitation to be bypassed is to use a large patient database and to ascertain a drug prescribing pattern indicative of adverse drug reactions (ADRs). Generally, ACEI‐intolerant patients are advised to avoid ACEIs, and frequently switch to angiotensin‐II receptor blockers (ARBs) 12, 21. A recent study found that approximately half of patients with ACEI‐induced cough discontinued ACEIs and switched to ARBs 22. The decision to use ARBs in patients with a history of ACEI‐induced AE should be weighed against the therapeutic need for angiotensin inhibition in each patient because a risk of recurrent AE while on ARBs remains 23, 24. An analysis of medical records identified a prescription pattern reflecting ACEI intolerance in a Dutch population 22. Switching to ARBs within a 6‐month interval from the end of an ACEI prescription in the latter study was an indicator for definitive ACEI‐related adverse events, with a positive predictive value (PPV) of 56.1% 22. The PPV for the combined probable and definitive ACEI‐related adverse effects was 68.3%, and combined for possible, probable and definitive adverse effects it was 90.5%.
Given the increasing utilization of ACEIs, the purpose of the present study was, firstly, to describe the occurrence of AE and ACEI intolerance, defined by a switch to ARBs, among primary care patients newly treated with ACEIs. Secondly, we assessed the associated demographic factors, comorbidities and comedications to gain more insight into patient groups more likely to experience ACEI‐related adverse reactions.
Methods
Data source
Data for the present study were obtained from the UK Clinical Practice Research Datalink (CPRD), an anonymized database containing approximately 12 million complete electronic medical records from over 600 participating general practices across the UK 25. Primary care diagnoses, prescriptions, laboratory test results, referrals, patient demographics and lifestyle information are recorded in the CPRD using a hierarchical clinical coding system (read codes) 26. Hospital diagnoses are available for a subgroup of patients and are coded according to the International Classification of Diseases (ICD‐10). Validity and a complete description of available CPRD data have been reported elsewhere 25, 26. The protocol for the present study was reviewed and approved by the independent scientific advisory committee (ISAC) of CPRD (protocol number: 14_030R).
Study design and population
As a source population, we identified all new users of ACEIs of 45 years of age or older, registered between 1 January 2007 and 1 January 2014 in the CPRD. The date of the first ACEI prescription within this time period was considered as the cohort entry date. A new ACEI user was defined as a subject without ACEI prescription records in the CPRD prior to the cohort entry date. All included ACEI users had at least 12 months of valid prescription history available before the start of ACEI use. To identify patients with AE and switching to ARBs during follow‐up, subjects were followed until the end of the study, death or moving out of the practice area, whichever came first.
Within the cohort of new ACEI users, we conducted two retrospective nested case–control studies to identify determinants of the occurrence of AE during ACEI therapy and on switching to ARBs, as a proxy for an ACEI‐induced adverse reaction. In the AE study, the index date was defined by the AE diagnosis date. For ACEI intolerance, the date of switching to ARBs was considered as the index date.
The first ever registered AE episode among ACEI users was assumed to be an ACEI‐related AE, if the AE diagnosis was entered into the CPRD at any time during ACEI therapy or within a maximum of 3 months after expiration of the last ACEI prescription. Thus, AE cases were individuals in whom AE occurred for the first time during ACEI therapy. Individuals who had a diagnosis of AE at any time while not receiving ACEI therapy were excluded. Cases had no AE records, either before the start or after discontinuation of ACEI treatment. An individual also became a case if multiple episodes of AE had occurred while on ACEI therapy, but only the first AE during ACEI therapy was considered as an event. For each case, up to 20 controls were selected from new ACEI users who did not have a diagnosis of AE in CPRD records. Controls were ACEI users at the time of AE of the corresponding cases, and were matched to cases on the duration of ACEI therapy. Cases were excluded if no matching controls were available in the cohort.
ACEI‐intolerant cases were defined by the switching from ACEIs to ARBs in the prescription records, allowing a 6‐month interval between the theoretical last use of ACEIs and the start of ARBs, as described previously 22. ACEI users continuously filling ACEI prescriptions, without discontinuing ACEIs or switching to another antihypertensive drug at the index date of the relevant case, were selected as controls. For each case of ACEI intolerance, up to four controls were sampled. Controls were matched to cases on the duration of ACEI treatment at the index date.
Information on general practitioner‐prescribed medications was extracted using appropriate British National Formulary medicine codes. The theoretical duration of an ACEI prescription was calculated as a ratio between the quantity of medication and the defined daily dose, estimated according to the World Health Organization. The duration of ACEI treatment was defined as the time between the start of the first ACEI prescription until the end of the last ACEI prescription, allowing a gap of less than 6 months between two consecutive ACEI prescriptions. Discontinuation of ACEI therapy was defined as the absence of a new ACEI prescription record for at least 6 months after the theoretical end date of the last ACEI prescription.
Determinants
As possible determinants of AE during ACEI therapy and a switch to ARBs, we considered gender, age over 65 years at the index date, the use of comedication and medical history of chronic comorbidities. The use of comedication, including antidiabetic drugs, antihistamines, antiasthmatic medications, nonsteroidal anti‐inflammatory drugs (NSAIDs), systemic corticosteroids, calcium channel blockers and statins, was assessed by any prescription record within a 3‐month time window before the index date, regardless of the duration of that prescription. Therefore, drugs for which the theoretical end date of the previous prescription would occur in this time window were not included in the analysis. We did not discriminate between various product names, but rather investigated the association between different classes of comedication and AE or ACEI intolerance. Exposure to each comedication class was included in the models as a dichotomous variable (use vs. no use). The history of comorbidities, including asthma, allergy, chronic obstructive pulmonary disease (COPD), DM and rheumatoid arthritis (RA), was retrieved from medical records using read codes any time before the index date. Drug prescriptions were not used to classify individuals on disease status when ascertaining comorbidities. Additionally, the occurrence of any type of cough within 3 months before the AE date was included in the analyses.
For descriptive purposes, the demographic characteristics of the study population were determined on the cohort entry date. Information on lifestyle factors was not available for multiple subjects at the cohort entry date; therefore, the most recent recording of body mass index (BMI) was retrieved within a time interval of 365 days on either side of the cohort entry date. When assessing baseline characteristics of study populations, we selected records of alcohol consumption and smoking closest to the index date from those entered into the CPRD at any stage before the index date. In association analyses we only considered the presence or the absence of information regarding smoking status in the CPRD at any stage before the index date. The indication for ACEI therapy was obtained from medical records any time before the cohort entry date or at any time within 1 year after this date.
Statistical analyses
The results are presented as means and standard deviations for continuous variables, and as proportions for categorical variables. Differences in baseline characteristics between cases and controls were assessed using Student's t‐test for continuous variables and using the chi‐squared test for categorical variables. Kaplan–Meier curves were constructed to estimate the time to AE and switching to ARBs. Odds ratios and 95% confidence intervals for the association of AE and switching to ARBs with age, gender, smoking, comorbidities and comedication were estimated by univariate logistic regression. The analyses with comorbidities and comedications were further adjusted for age and gender. Subsequently, forward stepwise multivariable logistic regression was performed, including all determinants significantly (P < 0.05) associated with the outcomes in univariate analyses. Additionally, a stratified analysis was performed to compare the occurrence of AE and ACEI intolerance, depending on the type of ACEI. A two‐sided P‐value of less than 0.05 was considered statistically significant. Data analyses were performed IBM SPSS for Windows, version 23.0 (IBM SPSS Statistics for Windows Version 23.0. IBM Corporation, Armonk, NY, USA).
Results
The cohort comprised 276 977 ACEI users aged 45 years or older initiating ACEI therapy between 2007 and 2014. Among these individuals, we identified 416 cases of AE occurring for the first time during ACEI therapy and matched them with 4335 controls. We determined that 24 709 individuals switched to ARBs within 6 months of the end of the last ACEI prescription. Switchers to ARBs were matched with 84 238 continuous ACEI users, after excluding those who stopped ACEI therapy or switched to another antihypertensive drug.
Clinical characteristics of the study populations are presented in Table 1. The proportion of women was statistically significantly higher among the switchers compared with the continuous ACEI users (58.6% vs. 45.5%). Hypertension as the only indication for ACEI therapy was used most frequently (approximately 60% in cases and controls of each study). About 20% of study participants in the AE study and 18% of participants in the ACEI intolerance study had more than one indication. Myocardial infarction, renal disease and heart failure as isolated indications were used much less frequently. The most frequent ACEI prescribed was ramipril, followed by lisinopril, perindopril and enalapril. There were no major differences in the frequency of use of these ACEI's between cases and controls (Table 1). Figure 1 depicts the time to AE and switching to ARBs among ACEI users. The mean time to AE was 76.7 months, while the mean time to switching to ARBs was 71.6 months. There were no statistically significant differences in the occurrence of AE and switching to ARBs in the analyses stratified by type of ACEI (see Tables S3 and S4 ).
Table 1.
Baseline characteristics of study populations (at cohort entry date)
| AE cases (n = 416) | Controls (n = 4335) | P | Switchers to ARBs (n = 24 709) | Continuous users of ACEIs (n = 84 238) | P | |
|---|---|---|---|---|---|---|
| Gender, n (%) | 0.472 | <0.005 | ||||
| Female | 204 (49.0) | 2045 (47.2) | 14 482 (58.6) | 38 297 (45.5) | ||
| Male | 212 (51.0) | 2290 (52.8) | 10 227(41.4) | 45 941 (54.5) | ||
| Age (years), mean ± SD | 67.8 ± 11.6 | 65.6 ± 11.8 | <0.005 | 65.2 ± 11.8 | 65.7 ± 11.1 | <0.005 |
| BMI ( kg m –2 ), mean ± SD | 29.3 ± 5.8 | 29.4 ± 5.8 | 0.706 | 29.4 ± 5.9 | 29.5 ± 5.8 | 0.001 |
| BMI unknown, n (%) | 129 (31.0) | 1249 (28.8) | 23 482 (27.9) | 6963 (28.2) | ||
| Alcohol consumption, n (%) | 0.335 | <0.001 | ||||
| No | 86 (20.7) | 771 (17.8) | 4136 (16.7) | 13 857 (16.4) | ||
| Yes | 296 (71.2) | 3210 (74.0) | 18 630 (75.4) | 62 760 (74.5) | ||
| Unknown | 34 (8.2) | 354 (8.2) | 1943 (7.9) | 7621 (9.0) | ||
| Smoking status, n (%) | 0.156 | <0.001 | ||||
| No | 212 (51.0) | 2412 (55.6) | 15 292 (61.9) | 46 584 (55.3) | ||
| Yes | 186 (44.7) | 1729 (39.9) | 8502 (34.4) | 34 208 (40.6) | ||
| Unknown | 18 (4.3) | 194 (4.5) | 915 (3.7) | 3446 (4.1) | ||
| Indications for ACEI therapy, n (%) | 0.031 | <0.005 | ||||
| Heart failure | 6 (1.4) | 50 (1.2) | 319 (1.3) | 1244 (1.5) | ||
| Hypertension | 246 (59.1) | 2468 (56.9) | 15 160 (61.4) | 50 354 (59.8) | ||
| Myocardial infarction | 18 (4.3) | 196 (4.5) | 765 (3.1) | 3060 (3.6) | ||
| Renal disease | 17 (4.1) | 159 (3.7) | 901 (3.6) | 2843 (3.4) | ||
| More than one of the above | 96 (23.1) | 866 (20.0) | 4492 (18.2) | 14 736 (17.5) | ||
| Unknown | 33 (7.9) | 596 (13.7) | 3072 (12.4) | 12 001 (14.2) | ||
| Type of ACEI used, n (%) | 0.992 | <0.001 | ||||
| Captopril, n (%) | 0 (0) | 1 (0) | 5 (0) | 26 (0) | ||
| Cilazapril, n (%) | 0 (0) | 0 (0) | 0 (0) | 5 (0) | ||
| Enalapril, n (%) | 9 (2.2) | 89 (2.2) | 433 (1.8) | 1794 (2.1) | ||
| Fosinopril, n (%) | 0 (0) | 1 (0) | 2 (0) | 7 (0) | ||
| Imidapril, n (% | 0 (0) | 1 (0) | 0 (0) | 0 (0) | ||
| Lisinopril, n (%) | 118 (28.0) | 1213 (28.4) | 6079 (24.6) | 19 110 (22.7) | ||
| Perindopril, n (%) | 56 (13.5) | 526 (12.1) | 1890 (7.6) | 6807 (8.1) | ||
| Quinapril, n (%) | 0 (0) | 1 (0) | 4 (0) | 26 (0) | ||
| Ramipril, n (%) | 233 (57.7) | 2503 (56.0) | 16 280 (65.9) | 56 377 (66.9) | ||
| Trandolapril, n (%) | 0 (0) | 0 (0) | 16 (0.1) | 86 (0.1) |
ACEI, angiotensin‐converting enzyme inhibitor; AE, angioedema; ARBs, angiotensin II receptor blockers; BMI, body mass index; SD, standard deviation. Smoking status and alcohol consumption were determined at any stage before the cohort entry date (start of ACEI therapy). BMI was retrieved within a time interval of 365 days around the cohort entry date. Indications for ACEI therapy were assessed at any time prior to the start of ACEI. Age was assessed at the date of the first ACEI prescription.
Figure 1.
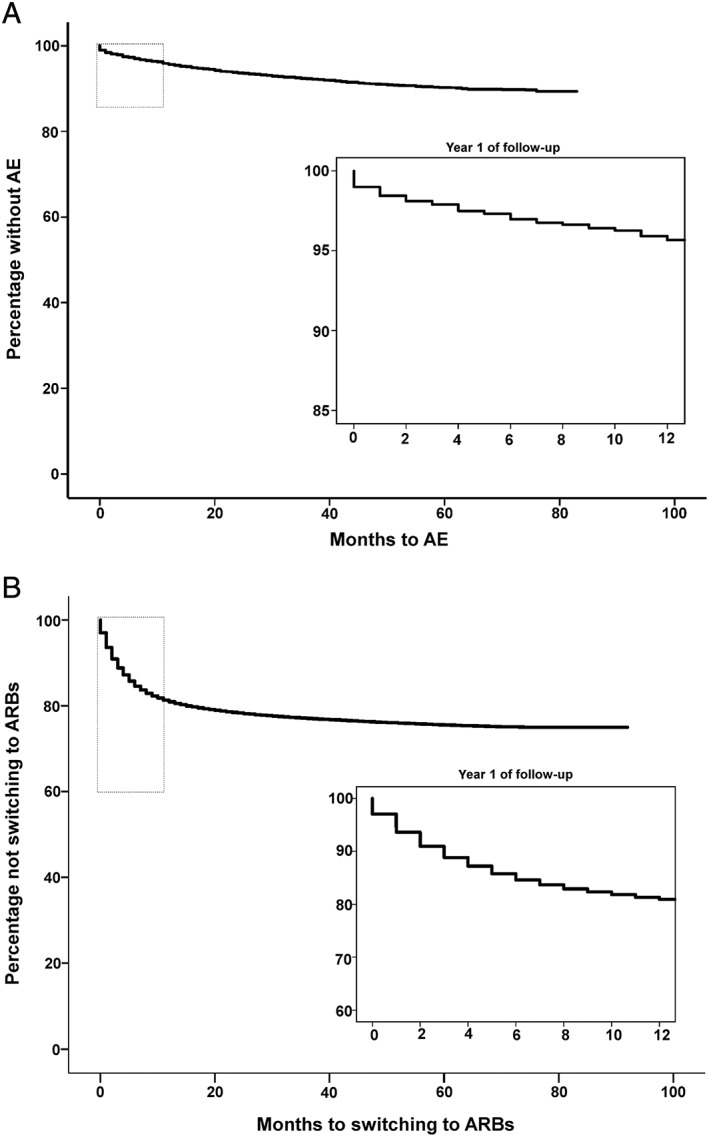
Time to the development of angiotensin‐converting enzyme inhibitor (ACEI) intolerance and angioedema during ACEI therapy. (A) Kaplan ‐Meier curves for time to the development of AE during ACEI therapy. Kaplan‐Meier curves were constructed for cases only. The top left rectangle indicates the area of Kaplan‐Meier curve depicted in the bottom right panel. The bottom right panel shows time to event during the first year of follow‐up. (B) Kaplan ‐Meier curves for time to switching to ARBs. Kaplan‐Meier curves were constructed for cases only. The top left rectangle indicates the area of Kaplan‐Meier curve depicted in the bottom right panel. The bottom right panel shows time to event within the first year of follow‐up. AE, angioedema; ARB, angiotensin II receptor blocker
Crude and adjusted ORs for the association of comorbidities and comedications with AE during ACEI therapy and ACEI intolerance are provided in Table 2 and Table 3. Overall, univariate analyses yielded similar associations for AE and switching to ARB. Age over 65 years at index date was statistically significantly associated with an increased risk of both AE and ACEI intolerance in univariate models [odds ratio (OR) 1.51, 95% confidence interval (CI) 1.23, 1.86; OR 1.15, 95% CI 1.12, 1.18]. Female gender was not associated with AE (OR 1.08, 95% CI 0.88, 1.32) but was associated with an increased risk of ACEI intolerance (OR 1.70, 95% CI 1.65, 1.75). History of asthma and allergy were associated with both AE and ACEI intolerance (OR 1.83, 95% CI 1.42, 2.37; OR 1.21, 95% CI 1.17, 1.27 for asthma and OR 2.03, 95% CI 1.64, 2.52; OR 2.17, 95% CI 2.10, 2.23 for allergy, respectively). History of COPD appeared to increase the risk of AE (OR 2.08, 95% CI 1.52, 2.85) but not of ACEI intolerance (OR 0.91, 95% CI 0.85, 0.97). Patients with DM had a lower risk of AE and ACEI intolerance (OR 0.73, 95% CI 0.54, 0.98 and OR 0.77, 95% CI 0.74, 0.80, respectively). The proportion of patients with RA was higher in AE cases than in the controls (OR 2.83, 95% CI 1.69, 4.75).
Table 2.
Determinants of angioedema during angiotensin‐converting enzyme inhibitor therapy
| No. (%) | ||||||
|---|---|---|---|---|---|---|
| Cases (n = 416) | Controls (n = 4335) | Crude OR (95% CI) | P | Adjusted OR c (95% CI) | P | |
| Gender | ||||||
| Male | 212 (51.0) | 2290 (52.8) | reference | ‐ | ‐ | ‐ |
| Female | 204 (49.0) | 2045 (47.2) | 1.08 (0.88; 1.32) | 0.470 | ‐ | ‐ |
| Age > 65 years | ||||||
| No | 158 (38.0) | 2086 (48.1) | reference | ‐ | ‐ | ‐ |
| Yes | 258 (62.0) | 2249 (51.9) | 1.51 (1.23; 1.86) | <0.001 | ‐ | ‐ |
| Smoking | ||||||
| No | 215 (51.7) | 2455 (56.6) | reference | ‐ | reference | ‐ |
| Yes | 184 (44.2) | 1735 (40.0) | 1.21 (0.89; 1.49) | 0.069 | 1.27 (1.03; 1.56) | 0.027 |
| History of co‐morbidities a | ||||||
| Asthma | ||||||
| No | 333 (80.0) | 3816 (88.0) | reference | ‐ | reference | ‐ |
| Yes | 83 (20.0) | 519 (12.0) | 1.83 (1.42; 2.37) | <0.001 | 1.84 (1.42; 2.39) | <0.001 |
| Allergy | ||||||
| No | 134 (32.2) | 2129 (49.1) | reference | ‐ | reference | ‐ |
| Yes | 282 (67.8) | 2206 (50.9) | 2.03 (1.64; 2.52) | <0.001 | 2.02 (1.62;2.50) | <0.001 |
| COPD | ||||||
| No | 363 (87.3) | 4051 (93.4) | reference | ‐ | reference | ‐ |
| Yes | 53 (12.7) | 284 (6.6) | 2.08 (1.52; 2.85) | <0.001 | 1.96 (1.43;2.68) | <0.001 |
| Diabetes mellitus | ||||||
| No | 360 (86.5) | 3573 (82.4) | reference | ‐ | reference | ‐ |
| Yes | 56 (13.5) | 762 (17.6) | 0.73 (0.54; 0.98) | 0.034 | 0.73 (0.55; 0.98) | 0.037 |
| Rheumatoid arthritis | ||||||
| No | 397 (95.4) | 4263 (98.3) | reference | ‐ | reference | ‐ |
| Yes | 19 (4.6) | 72 (1.7) | 2.83 (1.69; 4.75) | <0.001 | 2.68 (1.59; 4.49) | <0.001 |
| Co‐medications b | ||||||
| Anti‐diabetic drugs | ||||||
| No | 378 (90.9) | 3773 (87.0) | reference | ‐ | reference | ‐ |
| Yes | 38 (9.1) | 562 (13.0) | 0.67 (0.48; 0.95) | 0.026 | 0.69 (0.49; 0.98) | 0.036 |
| Anti‐histamines | ||||||
| No | 202 (48.6) | 4163 (96.0) | reference | ‐ | reference | ‐ |
| Yes | 214 (51.4) | 172 (4.0) | 25.64 (20.06; 32.77) | <0.001 | 26.62 (20.72; 34.20) | <0.001 |
| Anti‐asthmatic drugs | ||||||
| No | 326 (78.4) | 3849 (88.8) | reference | ‐ | reference | ‐ |
| Yes | 90 (21.6) | 486 (11.2) | 2.19 (1.70; 2.81) | <0.001 | 2.14 (1.66; 2.75) | <0.001 |
| Calcium channel blockers | ||||||
| No | 257 (61.8) | 3143 (72.5) | reference | reference | ‐ | |
| Yes | 159 (38.2) | 1192 (27.5) | 1.63 (1.32; 2.01) | <0.001 | 1.59 (1.29; 1.96) | <0.001 |
| NSAIDs | ||||||
| No | 375 (90.1) | 3920 (90.4) | reference | ‐ | reference | ‐ |
| Yes | 41 (9.9) | 415 (9.6) | 1.03 (0.74; 1.45) | 0.852 | 1.07 (0.76; 1.51) | 0.687 |
| Systemic corticosteroids | ||||||
| No | 312 (75.0) | 4142 (95.5) | reference | ‐ | reference | ‐ |
| Yes | 104 (25.0) | 193 (4.5) | 7.15 (5.49; 9.32) | <0.001 | 6.93 (5.31; 9.05) | <0.001 |
| Statins | ||||||
| No | 215 (51.7) | 2104 (48.5) | reference | ‐ | reference | ‐ |
| Yes | 201 (48.3) | 2231 (51.5) | 0.88 (0.72; 1.08) | 0.220 | 0.85 (0.69; 1.04) | 0.112 |
| Any type of cough b | ||||||
| No | 383 (92.1) | 4123 (95.1) | reference | ‐ | reference | ‐ |
| Yes | 33 (7.9) | 212 (4.9) | 1.68 (1.14; 2.45) | 0.008 | 1.63 (1.11; 2.39) | 0.012 |
CI, confidence interval; COPD, chronic obstructive pulmonary disease; NSAIDs, nonsteroidal anti‐inflammatory drugs; OR, odds ratio. Smoking was assessed at any stage before the index date. The number of individuals with unknown smoking status 3 months before the index date was 17 (4.1%) out of AE cases and 145 (3.3%) out of AE controls
History of comorbidities was assessed at any time before the AE date
Comedication was assessed 3 months before the AE date
Gender and age‐adjusted ORs
Table 3.
Determinants of angiotensin‐converting enzyme inhibitor (ACEI) intolerance defined by a switch to angiotensin II receptor blockers (ARBs) in prescription records
| No. (%) | Crude OR (95% CI) | P | Adjusted OR c (95% CI) | P | ||
|---|---|---|---|---|---|---|
| Cases (n = 24 709) | Controls (n = 84 238) | |||||
| Gender | ||||||
| Male | 10 227 (41.4) | 45 941 (54.5) | reference | – | – | – |
| Female | 14 482 (58.6) | 38 297 (45.5) | 1.70 (1.65; 1.75) | <0.001 | – | – |
| Age >65 years | ||||||
| No | 11 839 (47.9) | 43 331 (51.4) | reference | – | – | – |
| Yes | 12 870 (52.1) | 40 907 (48.6) | 1.15 (1.12; 1.18) | <0.001 | – | – |
| Smoking | ||||||
| No | 15 360 (62.2) | 46 959 (55.7) | reference | – | reference | – |
| Yes | 8577 (34.7) | 34 289 (40.7) | 0.77 (0.74; 0.79) | <0.001 | 0.83 (0.81. 0.86) | <0.001 |
| History of comorbidities a | ||||||
| Asthma | ||||||
| No | 21 150 (85.6) | 73 986 (87.8) | reference | – | reference | – |
| Yes | 3559 (14.4) | 10 252 (12.2) | 1.21 (1.17; 1.27) | <0.001 | 1.17 (1.12. 1.22) | <0.001 |
| Allergy | ||||||
| No | 7840 (31.7) | 42 265 (50.2) | reference | – | reference | – |
| Yes | 16 869 (68.3) | 41 973 (49.8) | 2.17 (2.10; 2.23) | <0.001 | 2.06 (1.99. 2.12) | <0.001 |
| COPD | ||||||
| No | 23 417 (94.8) | 79 412 (94.3) | reference | – | – | – |
| Yes | 1292 (5.2) | 4826 (5.7) | 0.91 (0.85; 0.97) | 0.003 | 0.90 (0.84. 0.96) | <0.001 |
| Diabetes mellitus | ||||||
| No | 21 210 (85.8) | 69 361 (82.3) | reference | – | reference | – |
| Yes | 3499 (14.2) | 14 877 (17.7) | 0.77 (0.74; 0.80) | <0.001 | 0.80 (0.77. 0.83) | <0.001 |
| Rheumatoid arthritis | ||||||
| No | 24 263 (98.2) | 82 766 (98.3) | reference | – | reference | – |
| Yes | 446 (1.8) | 1472 (1.7) | 1.03 (0.93; 1.15) | 0.545 | 0.93 (0.83.1.03) | 0.171 |
| Comedications b | ||||||
| Antidiabetic drugs | ||||||
| No | 22 317 (90.3) | 73 938 (87.8) | reference | – | reference | – |
| Yes | 2392 (9.7) | 10 300 (12.2) | 0.77 (0.73; 0.81) | <0.001 | 0.81 (0.77. 0.85) | <0.001 |
| Antihistamines | ||||||
| No | 23 119 (93.6) | 81 213 (96.4) | reference | – | reference | – |
| Yes | 1590 (6.4) | 3025 (3.6) | 1.85 (1.73; 1.97) | <0.001 | 1.77 (1.66. 1.88) | <0.001 |
| Antiasthmatic drugs | ||||||
| No | 21 266 (86.1) | 75 412 (89.5) | reference | – | reference | – |
| Yes | 3443 (13.9) | 8826 (10.5) | 1.38 (1.33; 1.44) | <0.001 | 1.34 (1.28. 1.39) | <0.001 |
| Calcium channel blockers | ||||||
| No | 17 660(71.5) | 63 210 (76.2) | reference | – | reference | – |
| Yes | 7049 (28.5) | 21 028 (23.8) | 1.20 (1.16; 1.24) | <0.001 | 1.21 (1.17. 1.25) | <0.001 |
| NSAIDs | ||||||
| No | 22 416 (90.7) | 77 169 (91.6) | reference | – | reference | – |
| Yes | 2293 (9.3) | 7069 (8.4) | 1.12 (1.06; 1.17) | <0.001 | 1.10 (1.05. 1.16) | <0.001 |
| Systemic corticosteroids | ||||||
| No | 23 514 (95.2) | 81 144 (96.3) | reference | – | reference | – |
| Yes | 1195 (4.8) | 3094 (3.7) | 1.33 (1.24; 1.43) | <0.001 | 1.25 (1.17. 1.34) | <0.001 |
| Statins | ||||||
| No | 14 064 (56.9) | 44 426 (52.7) | reference | – | reference | – |
| Yes | 10 645 (43.1) | 39 812 (47.3) | 0.84 (0.82; 0.87) | <0.001 | 0.89 (0.86. 0.92) | <0.001 |
CI, confidence interval; COPD, chronic obstructive pulmonary disease; NSAID, nonsteroidal anti‐inflammatory drug; OR, odds ratio. Smoking was assessed at any stage before the index date. The number of individuals with unknown smoking status 3 months before the index date was 772 (3.1%) of the switchers to ARBs and 2990 (3.5%) of continuous ACEI users
History of comorbidities was assessed at any time before the switch to ARBs.
Comedication was assessed 3 months before the switch to ARBs.
Gender and age‐adjusted ORs
The strongest associations for AE in univariate models were found with antihistamines and systemic corticosteroids within 3 months before the index date (OR 25.64, 95% CI 20.06, 32.77, and OR 7.15, 95% CI 5.49, 9.32, respectively). These associations were also significant but less strong in ACEI intolerance (OR 1.85, 95% CI 1.73, 1.97, and OR 1.33, 95% CI 1.24, 1.43, respectively). Furthermore, when these determinants were examined at the cohort entry date instead of the index date, the association with AE remained statistically significant, with an OR of 4.48 (95% CI 3.41, 5.88) for antihistamines and an OR of 2.90 (95% CI 2.16, 3.91) for systemic corticosteroids (Tables S1 , and S2). Antidiabetic drugs contributed to a lower risk of both AE and ACEI intolerance in univariate analyses (Tables S2 and S3). Recent NSAID use was associated with a higher risk of ACEI intolerance (OR 1.12, 95% CI 1.06, 1.17). We observed a similar effect size for statin use and ACEI intolerance (OR 0.84, 95% CI 0.82, 0.87) and AE during ACEI therapy (OR 0.88, 95% CI 0.72, 1.08), but the association was not statistically significant for AE.
To evaluate whether having cough during ACEI therapy could be predictive of developing ACEI‐related AE in our dataset, we assessed the association of any type of cough with AE during ACEI therapy. We chose any type of cough because it was not possible to specify adverse effects, such as ACEI‐induced cough, in the CPRD. Indeed, any type of cough was associated with AE during ACEI therapy (OR 1.68, 95% CI 1.14, 2.45).
Compared to AE, switching to ARBs was associated with more risk factors in the forward stepwise multivariable analysis. Age over 65 years (OR 1.36, 95% CI 1.07, 1.73), history of allergy (OR 1.53, 95% CI 1.19, 1.96), and the use of antihistamines (OR 21.25, 95% CI 16.44, 27.46), systemic corticosteroids (OR 4.52, 95% CI 3.26, 6.27) and calcium channel blockers (OR 1.57, 95% CI 1.23, 2.01) were associated with AE during ACEI therapy (Table 4). Age over 65 years (OR 1.06, 95% CI 1.03, 1.09), history of allergy (OR 2.02, 95% CI 1.96, 2.09), and the use of antihistamines (OR 1.53, 95% CI 1.43, 1.63) and calcium channel blockers (OR 1.19, 95% CI 1.15, 1.23) were also associated with ACEI intolerance in a multivariable model (Table 5). Other determinants associated with ACEI intolerance were female gender (OR 1.49, 95% CI 1.44, 1.53), smoking (OR 0.83, 95% CI 0.81, 0.86), asthma (OR 0.88, 95% CI 0.83, 0.93), COPD (OR 0.69, 95% CI 0.64, 0.75), DM (OR 0.80, 95% CI 0.77, 0.84) and the use of antiasthmatic drugs (OR 1.51, 95% CI 1.42, 1.61), NSAIDs (OR 1.07, 95% CI 1.02, 1.13) and statins (OR 0.92, 95% CI 0.89, 0.95).
Table 4.
Determinants of angioedema (AE) during angiotensin‐converting enzyme inhibitor therapy in the multivariable model
| Determinants | OR | 95% CI | P‐value |
|---|---|---|---|
| Age > 65 years | 1.36 | 1.07, 1.73 | 0.013 |
| History of comorbidities | |||
| Allergy | 1.53 | 1.19, 1.96 | <0.001 |
| Comedications | |||
| Antihistamines | 21.25 | 16.44, 27.46 | <0.001 |
| Systemic corticosteroids | 4.52 | 3.26, 6.27 | <0.001 |
| Calcium channel blockers | 1.57 | 1.23, 2.01 | <0.001 |
CI, confidence interval; OR, odds ratio. Chronic comorbidities were assessed at any stage within a period of time from the start of available CPRD records until the date of AE entered into the CPRD. Comedication was assessed within 3 months before the date of AE entered into the CPRD. Smoking was defined as presence or absence of information regarding smoking status in the CPRD at any stage before the index date (date of AE).
Table 5.
Determinants of angiotensin‐converting enzyme inhibitor intolerance (defined by switching to angiotensin II receptor blockers) in the multivariable model
| Determinants | OR | 95%CI | P‐value |
|---|---|---|---|
| Female gender | 1.49 | 1.44, 1.53 | <0.001 |
| Age >65 years | 1.06 | 1.03, 1.09 | <0.001 |
| Smoking | 0.83 | 0.81, 0.86 | <0.001 |
| History of comorbidities | |||
| Allergy | 2.02 | 1.96, 2.09 | <0.001 |
| Asthma | 0.88 | 0.83, 0.93 | <0.001 |
| COPD | 0.69 | 0.64, 0.75 | <0.001 |
| Diabetes mellitus | 0.80 | 0.77, 0.84 | <0.001 |
| Comedications | |||
| Antiasthmatic drugs | 1.51 | 1.42, 1.61 | <0.001 |
| Antihistamines | 1.53 | 1.43, 1.63 | <0.001 |
| NSAIDs | 1.07 | 1.02, 1.13 | 0.008 |
| Statins | 0.92 | 0.89, 0.95 | <0.001 |
| Calcium channel blockers | 1.19 | 1.15, 1.23 | <0.001 |
CI, confidence interval; COPD, chronic obstructive pulmonary disease; NSAID, Nonsteroidal anti‐inflammatory drug; OR, odds ratio. Chronic comorbidities were assessed at any stage within a period of time from the start of available CPRD records until the date of switching to ARBs. Comedication was assessed within 3 months before the date of switching to ARBs. Smoking was defined as presence or absence of information regarding smoking status in the CPRD at any stage before the index date (date of switching to ARBs).
Discussion
We conducted two exploratory case–control studies in a cohort of patients in an extensive real‐world primary care database to evaluate the association between a history of comorbidities and comedication use, and ACEI intolerance (defined by switching to ARBs) and AE during ACEI therapy. The main finding of both studies was that several comorbidities and prescriptions for different comedication classes within 3 months before the event were significantly more prevalent in ACEI starters developing AE and ACEI intolerance, compared with ACEI users who did not develop these adverse reactions. Moreover, although some of the associations were similar for both outcomes, we observed a larger number of associations with switching to ARBs than with AE. The knowledge gained through these studies might be helpful for further research by using the history of comorbidities and recent comedication as potential risk factors for ACEI‐related adverse reactions.
Several of the risk factors for AE which we report here have been described earlier, including, among others, age, female gender, smoking, allergies and some drug exposures 10, 18, 19, 27, 28, 29, 30, 31, 32, 33. We replicated the association of AE with older age but, contrary to prior observations, did not replicate an increased risk for ACEI‐related AE in females and in smokers 10, 18, 27. Furthermore, our finding of a positive association of AE with allergies is also in accordance with previous observations, which showed that seasonal allergies, a history of drug rash, sensitization to certain food components and pollen season were all associated with a higher number of AE episodes 28, 29.
It was evident from univariate analyses that a history of asthma, COPD and RA were more frequent and DM less frequent among ACEI users who developed AE during ACEI therapy. However, in multivariable analyses, none of these comorbidities remained associated with AE during ACEI therapy. To the authors' knowledge, no reports on associations between asthma or COPD, and ACEI‐related AE have been published. A study by Byrd et al. found no association between RA and ACEI‐related AE 29. An explanation for a possible higher number of episodes of ACEI‐related AE in RA could be the observation by Habibagahi et al. that patients with autoimmune disorders (such as systemic lupus erythematosus) might have an acquired antibody‐mediated c1 inhibitor (c1‐INH) deficiency contributing to the development of AE 34. In this scenario, it is probable that starting an ACEI in RA could more likely trigger AE. It has been suggested that AE is less likely to develop in diabetic patients owing to the higher dipeptidyl peptidase‐4 (DPP‐IV) activity that is seen in hyperglycaemia 35. A number of studies also reported that simultaneous use of DPP‐IV inhibitors and ACEIs increases the risk of AE 36, 37.
Our results showed that asthma and allergies occurred more frequently in switchers to ARBs, while DM and COPD were less common in these patients. A study by Wyskida et al. found that asthma and COPD were associated with ACEI‐related cough, with age‐adjusted ORs of 1.60 and 1.70, respectively 38. Based on validation of the database marker for ACEI intolerance used in our analyses, at least half of the cases of ACEI intolerance in the present study might be considered as having had ACEI‐related cough 22. However, it is important to acknowledge the possibility that there were other undesirable side effects of ACEIs or different reasons leading to a switch to ARBs. Therefore, only an indirect comparison with the results of previous studies on ACEI‐induced cough is possible. While we confirmed the association with asthma, the association with COPD was in the opposite direction to that found in the above‐mentioned study 38. A potential explanation for this could be that ACEI users with COPD who already experience cough as a symptom of COPD might be less likely to attribute cough to ACEIs, and therefore less likely to be switched to ARBs. Similarly, we could not replicate a recently reported finding that statin use was independently associated with a higher risk for ACEI‐induced cough 20.
We observed that prescriptions for antihistamines, systemic corticosteroids and calcium channel blockers within 3 months prior to the index date were most prevalent in the AE study population. Although the association with antihistamines and systemic corticosteroids could be attributed to prescription of these drugs for the treatment of AE (reverse causation), it persisted irrespective of the moment when drug exposure was assessed – i.e. recent to the event date and at baseline, and after adjusting for gender and age. Similar results for corticosteroids and other immunosuppressants have been reported in previous studies and are thought to be due to reduced DPP‐IV activity during immunosuppressant use 18, 29, 31. In the setting of DPP‐IV suppression, its normal function – to degrade substance P and bradykinin – is compromised, causing the accumulation of these substances, ultimately leading to AE 39. Furthermore, there are data suggesting that ACEIs may affect local microvascular perfusion in the skin by a bradykinin‐dependent mechanism 40. In animal models and in humans, captopril was shown to increase skin microvascular blood flow owing to an increase in endogenous tissue bradykinin and the subsequent release of prostaglandins and nitric oxide 40.
The strengths of the present study included using a real‐world primary care patient population, a large number of events, complete data on the medication prescriptions and comorbidities, and the use of a validated marker for ACEI intolerance in prescription databases. We acknowledge that there were a number of limitations to our study. Firstly, we could not assess the causal relationship in this observational exploratory study because the actual reason behind AE cannot be retrieved directly from the CPRD. The read coding system does not allow for the differentiation of hereditary AE, drug‐induced AE or AE secondary to acquired c1 esterase deficiency. We believe that ACEI treatment is likely to contribute to AE because we considered only AE occurring during ACEI therapy. However, in cases of AE occurring after several years of ACEI treatment, we cannot completely exclude another trigger for AE. Furthermore, there is a possibility that diagnostic codes for allergy were entered into the CPRD together with diagnostic codes for AE to indicate the AE event, which could have affected the associations described in the present study. Another reason for possible errors in the ascertainment of AE, possibly compromising the relationship with ACEI use, is the difference between the time of the actual AE episode and the time that it was entered into the CPRD. Secondly, the CPRD provides information on drug prescriptions but not drug dispensing, and it is not possible to verify the actual intake of a drug. Thirdly, we could not assess the influence of comorbidities and comedication on ACEI‐induced cough as information on this adverse reaction was not available. Using a prescription pattern for the identification of ACEI‐induced cough could have resulted in misclassification of the outcome of ACEI intolerance. In particular, an increased number of switchers to ARBs among patients with COPD, asthma and users of systemic corticosteroids (a marker of an exacerbation) might indicate a preventive measure for patients more prone to cough, rather than the presence of the ACEI‐related adverse effect itself.
In conclusion, the present study showed that several comorbidities and recently prescribed comedication were significantly more prevalent in ACEI starters developing AE and ACEI intolerance as opposed to ACEI users who did not develop these adverse reactions. Attention to the history of comorbidities and comedication when ACEI treatment is required might assist in identifying patients potentially at a higher risk for ACEI‐related adverse effects.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: A.H.M. had support from grants from the European Union FP7 Collaborative grant EU‐PACT, during the conduct of the study and an unrestricted research grant from GSK, outside the submitted work; P.S. had grants from Top‐Institute Pharma, grants from EU Innovative Medicines Initiative (IMI) , grants from Respiratory Effectiveness Group, outside the submitted work; S.H.M. and E.V.B. had support from European Union FP7 Grant no. 602 108 during the conduct of the study. F.W.A. and A.D.B. had no support from any organization for the submitted work.
This research was conducted as a part of the Personalisation of treatment In Cardiovascular disease through next generation sequencing in Adverse Drug Reactions (PREDICTION‐ADR) consortium. The PREDICTION‐ADR project is supported by the European Union FP7 Grant no. 602108. The authors would like to thank the members of PREDICTION‐ADR consortium, particularly Colin NA Palmer (University of Dundee, Dundee, UK), Ana Alfirevic (University of Liverpool, Liverpool, UK) Mia Wadelius (Uppsala University, Uppsala, Sweden), Alun McCarthy (Pharmacogenomic Innovative Solutions Ltd, UK) and Anu Aaspollu (Asper Biotech Ltd, Tartu, Estonia) for their support and contribution to this work. F.W.A. is supported by the UCL Hospitals NIHR Biomedical Research Centre and by a Dekker scholarship (Junior Staff Member 2014 T001) from the Dutch Heart Foundation.
Contributors
E.V.B. wrote the manuscript; S.H.M. performed the analysis; P.C.S. and S.H.M. managed the data; E.V.B., S.H.M., P.C.S, F.W.A., A.d.B. and A.H.M. designed the research and critically revised the manuscript.
Supporting information
Table S1 Crude and adjusted odds ratios for determinants of ACEI‐related angioedema. OR – odds ratio. ^ Sex and age adjusted ORs. CI – confidence interval. COPD ‐ Chronic obstructive pulmonary disease. NSAIDs ‐ Non‐steroidal anti‐inflammatory drugs. History of co‐morbidities was assessed at any stage before the start date of the first ACEI prescription. Co‐medications use was assessed 3 months on either side of the start date of the first ACEI prescription.
Table S2 Crude and adjusted odds ratios for determinants of switching to ARBs. OR – odds ratio. ^ Sex and age adjusted ORs. CI – confidence interval. COPD ‐ Chronic obstructive pulmonary disease. NSAIDs ‐ Non‐steroidal anti‐inflammatory drugs. History of co‐morbidities was assessed at any stage before the start date of the first ACEI prescription. Co‐medications use was assessed 3 months on either side of the start date of the first ACEI prescription.
Table S3 Odds ratios for angioedema by type of angiotensin‐converting enzyme inhibitor.
Table S4 Odds ratios for angiotensin‐converting enzyme inhibitor (ACEI) intolerance by type of ACEI.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Mahmoudpour, S. H. , Baranova, E. V. , Souverein, P. C. , Asselbergs, F. W. , de Boer, A. , Maitland‐van der Zee, A. H. , and on behalf of the PREDICTION‐ADR consortium (2016) Determinants of angiotensin‐converting enzyme inhibitor (ACEI) intolerance and angioedema in the UK Clinical Practice Research Datalink. Br J Clin Pharmacol, 82: 1647–1659. doi: 10.1111/bcp.13090.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl. Acids Res 2016; 44 (Database Issue): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 2015; 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 2015; 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan NJ, Soliman AM. Angiotensin converting enzyme inhibitor‐related angioedema: onset, presentation, and management. Ann Otol Rhinol Laryngol 2015; 124: 89–96. [DOI] [PubMed] [Google Scholar]
- 5. Soo Hoo GW, Lin HK, Junaid I, Klaustermeyer WB. Angiotensin‐converting enzyme inhibitor angioedema requiring admission to an intensive care unit. Am J Med 2015; 128: 785–789. [DOI] [PubMed] [Google Scholar]
- 6. National Statistics Prescriptions Dispensed in the Community , Statistics for England – 2004–2014 [online]. Available at http://hscic.gov.uk/prescribing (last accessed 12 January 2016).
- 7. Cheng J, Zhang W, Zhang X, Han F, Li X, He X, et al. Effect of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers on all‐cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus: a meta‐analysis. JAMA Intern Med 2014; 174: 773–785. [DOI] [PubMed] [Google Scholar]
- 8. van Vark LC, Bertrand M, Akkerhuis KM, Brugts JJ, Fox K, Mourad JJ, et al. Angiotensin‐converting enzyme inhibitors reduce mortality in hypertension: a meta‐analysis of randomized clinical trials of renin‐angiotensin‐aldosterone system inhibitors involving 158,998 patients. Eur Heart J 2012; 33: 2088–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jorgensen CH, Gislason GH, Ahlehoff O, Andersson C, Torp‐Pedersen C, Hansen PR. Use of secondary prevention pharmacotherapy after first myocardial infarction in patients with diabetes mellitus. BMC Cardiovasc Disord 2014; 14: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morimoto T, Gandhi TK, Fiskio JM, Seger AC, So JW, Cook EF, et al. An evaluation of risk factors for adverse drug events associated with angiotensin‐converting enzyme inhibitors. J Eval Clin Pract 2004; 10: 499–509. [DOI] [PubMed] [Google Scholar]
- 11. Ng LP, Goh PS. Incidence of discontinuation of angiotensin‐converting enzyme inhibitors due to cough, in a primary healthcare centre in Singapore. Singapore Med J 2014; 55: 146–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bezalel S, Mahlab‐Guri K, Asher I, Werner B, Sthoeger ZM. Angiotensin‐converting enzyme inhibitor‐induced angioedema. Am J Med 2015; 128: 120–125. [DOI] [PubMed] [Google Scholar]
- 13. Matchar DB, McCrory DC, Orlando LA, Patel MR, Patel UD, Patwardhan MB, et al. Systematic review: comparative effectiveness of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann Intern Med 2008; 148: 16–29. [DOI] [PubMed] [Google Scholar]
- 14. Makani H, Messerli FH, Romero J, Wever‐Pinzon O, Korniyenko A, Berrios RS, et al. Meta‐analysis of randomized trials of angioedema as an adverse event of renin‐angiotensin system inhibitors. Am J Cardiol 2012; 110: 383–391. [DOI] [PubMed] [Google Scholar]
- 15. Gandhi J, Jones R, Teubner D, Gabb G. Multicentre audit of ACE‐inhibitor associated angioedema (MAAAA). Aust Fam Physician 2015; 44: 579–583. [PubMed] [Google Scholar]
- 16. Rasmussen ER, Mey K, Bygum A. Angiotensin‐converting enzyme inhibitor‐induced angioedema – a dangerous new epidemic. Acta Derm Venereol 2014; 94: 260–264. [DOI] [PubMed] [Google Scholar]
- 17. Dykewicz MS. Cough and angioedema from angiotensin‐converting enzyme inhibitors: new insights into mechanisms and management. Curr Opin Allergy Clin Immunol 2004; 4: 267–270. [DOI] [PubMed] [Google Scholar]
- 18. Hoover T, Lippmann M, Grouzmann E, Marceau F, Herscu P. Angiotensin converting enzyme inhibitor induced angio‐oedema: a review of the pathophysiology and risk factors. Clin Exp Allergy 2010; 40: 50–61. [DOI] [PubMed] [Google Scholar]
- 19. Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens 2004; 17: 103–111. [DOI] [PubMed] [Google Scholar]
- 20. Brugts JJ, Arima H, Remme W, Bertrand M, Ferrari R, Fox K, et al. The incidence and clinical predictors of ACE‐inhibitor induced dry cough by perindopril in 27,492 patients with vascular disease. Int J Cardiol 2014; 176: 718–723. [DOI] [PubMed] [Google Scholar]
- 21. Dicpinigaitis PV. Angiotensin‐converting enzyme inhibitor‐induced cough: ACCP evidence‐based clinical practice guidelines. Chest 2006; 129: 169S–173S. [DOI] [PubMed] [Google Scholar]
- 22. Mahmoudpour SH, Asselbergs FW, de Keyser CE, Souverein PC, Hofman A, Stricker BH, et al. Change in prescription pattern as a potential marker for adverse drug reactions of angiotensin converting enzyme inhibitors. Int J Clin Pharmacol 2015; 37: 1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beavers CJ, Dunn SP, Macaulay TE. The role of angiotensin receptor blockers in patients with angiotensin‐converting enzyme inhibitor‐induced angioedema. Ann Pharmacother 2011; 45: 520–524. [DOI] [PubMed] [Google Scholar]
- 24. Haymore BR, Yoon J, Mikita CP, Klote MM, DeZee KJ. Risk of angioedema with angiotensin receptor blockers in patients with prior angioedema associated with angiotensin‐converting enzyme inhibitors: a meta‐analysis. Ann Allergy Asthma Immunol 2008; 101: 495–499. [DOI] [PubMed] [Google Scholar]
- 25. Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 2010; 69: 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matcho A, Ryan P, Fife D, Reich C. Fidelity assessment of a clinical practice research datalink conversion to the OMOP common data model. Drug Saf 2014; 37: 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Campo P, Fernandez TD, Canto G, Mayorga C. Angioedema induced by angiotensin‐converting enzyme inhibitors. Curr Opin Allergy Clin Immunol 2013; 13: 337–344. [DOI] [PubMed] [Google Scholar]
- 28. Straka B, Nian H, Sloan C, Byrd JB, Woodard‐Grice A, Yu C, et al. Pollen count and presentation of angiotensin‐converting enzyme inhibitor‐associated angioedema. J Allergy Clin Immunol Pract 2013; 1: 468–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Byrd JB, Woodard‐Grice A, Stone E, Lucisano A, Schaefer H, Yu C, et al. Association of angiotensin‐converting enzyme inhibitor‐associated angioedema with transplant and immunosuppressant use. Allergy 2010; 65: 1381–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De Marchi S, Perale L, Cecchin E, Sechi LA. Nickel and sulfites food allergy in patients with angioedema associated with ACE Inhibitor use. Arch Intern Med 2005; 165: 814–815. [DOI] [PubMed] [Google Scholar]
- 31. Duerr M, Glander P, Diekmann F, Dragun D, Neumayer HH, Budde K. Increased incidence of angioedema with ACE inhibitors in combination with mTOR inhibitors in kidney transplant recipients. Clin J Am Soc Nephrol 2010; 5: 703–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kostis JB, Kim HJ, Rusnak J, Casale T, Kaplan A, Corren J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med 2005; 165: 1637–1642. [DOI] [PubMed] [Google Scholar]
- 33. Miller DR, Oliveria SA, Berlowitz DR, Fincke BG, Stang P, Lillienfeld DE. Angioedema incidence in US veterans initiating angiotensin‐converting enzyme inhibitors. Hypertension 2008; 51: 1624–1630. [DOI] [PubMed] [Google Scholar]
- 34. Habibagahi Z, Ruzbeh J, Yarmohammadi V, Kamali M, Rastegar MH. Refractory angioedema in a patient with systemic lupus erythematosus. Iran J Med Sci 2015; 40: 372–375. [PMC free article] [PubMed] [Google Scholar]
- 35. Mannucci E, Pala L, Ciani S, Bardini G, Pezzatini A, Sposato I, et al. Hyperglycaemia increases dipeptidyl peptidase IV activity in diabetes mellitus. Diabetologia 2005; 48: 1168–1172. [DOI] [PubMed] [Google Scholar]
- 36. Millot I, Plancade D, Hosotte M, Landy C, Nadaud J, Ragot C, et al. Treatment of a life‐threatening laryngeal bradykinin angio‐oedema precipitated by dipeptidylpeptidase‐4 inhibitor and angiotensin‐I converting enzyme inhibitor with prothrombin complex concentrates. Br J Anaesth 2012; 109: 827–829. [DOI] [PubMed] [Google Scholar]
- 37. Brown NJ, Byiers S, Carr D, Maldonado M, Warner BA. Dipeptidyl peptidase‐IV inhibitor use associated with increased risk of ACE inhibitor‐associated angioedema. Hypertension 2009; 54: 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wyskida K, Jura‐Szoltys E, Smertka M, Owczarek A, Chudek J. Factors that favor the occurrence of cough in patients treated with ramipril – a pharmacoepidemiological study. Med Sci Monit 2012; 18: PI21–P128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Byrd JS, Minor DS, Elsayed R, Marshall GD. DPP‐4 inhibitors and angioedema: a cause for concern? Ann Allergy Asthma Immunol 2011; 106: 436–438. [DOI] [PubMed] [Google Scholar]
- 40. Warren JB, Loi RK. Captopril increases skin microvascular blood flow secondary to bradykinin, nitric oxide, and prostaglandins. FASEB J 1995; 9: 411–418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Crude and adjusted odds ratios for determinants of ACEI‐related angioedema. OR – odds ratio. ^ Sex and age adjusted ORs. CI – confidence interval. COPD ‐ Chronic obstructive pulmonary disease. NSAIDs ‐ Non‐steroidal anti‐inflammatory drugs. History of co‐morbidities was assessed at any stage before the start date of the first ACEI prescription. Co‐medications use was assessed 3 months on either side of the start date of the first ACEI prescription.
Table S2 Crude and adjusted odds ratios for determinants of switching to ARBs. OR – odds ratio. ^ Sex and age adjusted ORs. CI – confidence interval. COPD ‐ Chronic obstructive pulmonary disease. NSAIDs ‐ Non‐steroidal anti‐inflammatory drugs. History of co‐morbidities was assessed at any stage before the start date of the first ACEI prescription. Co‐medications use was assessed 3 months on either side of the start date of the first ACEI prescription.
Table S3 Odds ratios for angioedema by type of angiotensin‐converting enzyme inhibitor.
Table S4 Odds ratios for angiotensin‐converting enzyme inhibitor (ACEI) intolerance by type of ACEI.
Supporting info item
Supporting info item
Supporting info item
Supporting info item


