Abstract
Aims
The biological response to clopidogrel is highly variable and a poor responsiveness is associated with major adverse cardiac events. Adherence to therapy is a major cause of poor responsiveness but its impact on long‐term platelet inhibition is unknown. The objective of the present study was to evaluate the effect of different programmes monitoring adherence to clopidogrel on platelet reactivity.
Methods
The study took the form of a monocentric, parallel group, randomized controlled trial. Adults treated with clopidogrel 75 mg after elective coronary stenting were randomized into one of three groups: (i) a standard of care group; (ii) a standard of care + adherence electronic monitoring group, in which drug intake was recorded but kept blinded until the study end; or (iii) an integrated care group, with regular feedback on recorded adherence. Clopidogrel response was assessed with the vasodilator‐stimulated phosphoprotein–platelet reactivity index (VASP‐PRI) at randomization, 3 months and 6 months.
Results
A total of 123 adults were enrolled and randomized. Baseline VASP‐PRI was highly variable, with a mean of 48 ± 18.8%. No difference between groups in VASP‐PRI was found at 6 months (P = 0.761), despite better adherence to clopidogrel in the integrated care group. However, adherence (P = 0.035) and baseline VASP‐PRI (P = 0.015) were associated with VASP‐PRI at 3 months and 6 months. The association between adherence and VASP‐PRI was lost in patients with baseline VASP‐PRI > 50%. Diabetes, CYP2C19*2 carrier status and body mass index were significant predictors of VASP‐PRI.
Conclusions
The platelet response to clopidogrel during chronic therapy remained highly variable, despite high adherence. Different adherence monitoring programmes did not affect VASP‐PRI at 6 months. Poor adherence is associated with lower VASP‐PRI only in initial good responders to clopidogrel.
Keywords: clopidogrel responsiveness, CYP450 2C19 loss‐of‐function polymorphism, drug adherence, elective coronary stenting, VASP‐PRI
What is Known About this Subject
The biological response to clopidogrel is highly variable, and poor responsiveness is associated with major adverse cardiac events.
So far, no randomized controlled trial has investigated whether adherence influences clopidogrel's effect on platelet inhibition.
What this Study Adds
Our data show that the pharmacokinetics and the pharmacogenetics of clopidogrel have a greater influence than drug adherence on platelet reactivity.
High adherence is important for patients who respond to clopidogrel, in terms of inhibition of platelet aggregation.
However, in nonresponders, poor adherence has little, if any, impact because genotype is a more important determinant of the response.
Tables of Links
| TARGETS | |
|---|---|
| G protein‐coupled receptors 2 | Enzymes 3 |
| P2Y12 receptor | CYP2C19 |
| LIGANDS |
|---|
| Clopidogrel |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 2, 3.
Introduction
Oral antiplatelet therapy associating aspirin with P2Y, G protein‐coupled 12 (P2Y12) antagonists constitutes a cornerstone of therapy after elective percutaneous coronary interventions (PCIs) to prevent stent thrombosis. Despite improvement in stent design, such as polymer biodegradable/free drug‐eluting stents (DESs), stent thrombosis still occurs and is associated with a high mortality and morbidity. Poor biological response to pharmacotherapy has been proposed as a possible explanation.
High on‐treatment platelet reactivity (HPR) after a loading dose of clopidogrel 4 has been described in the acute setting as well as on standard maintenance therapy 5, 6, 7. It has been associated with an increased risk of recurrence of cardiac complications 8, 9, 10, 11, 12. In clinical situations in which therapies do not provide the expected results, problems of drug adherence are always important to consider 13. In the case of clopidogrel, 7several demographic variables 10, 14, 15, genetic characteristics [cytochrome P450 (CYP) polymorphisms) 16, 17, 18, 19, 20, 21, 22, 23 and therapy‐related factors 16, 24, 25 have been found to interfere with the biological response in the acute setting. However, the impact of adherence on long‐term clopidogrel responsiveness has not been studied comprehensively.
The main objective of the present study was to investigate the role of drug adherence on the long‐term clopidogrel response by assessing whether an adherence monitoring programme combining the monitoring of adherence and professional feedback is associated with improved clopidogrel responsiveness compared with the standard of care approach. We also explored the relative impact of demographics, and clinical and genetic factors on the long‐term platelet response to clopidogrel.
Methods
Study population
We screened all consecutive adult patients admitted to the cardiology interventional unit of the Lausanne University Hospital for elective PCI. Patients were enrolled if they had undergone PCI with implantation of at least one stent and had been prescribed maintenance treatment with clopidogrel 75 mg day–1 for at least 6 months. The main exclusion criteria were: ST‐segment elevation and non‐ST‐segment elevation myocardial infarction within 30 days prior to randomization.
The study was approved by the local Ethics Committee of the University of Lausanne, Switzerland, and was carried out in accordance with the principles of the Declaration of Helsinki. The study was registered (Current Controlled Trials ISRCTN85949729). All patients gave their written informed consent.
Study design
This was a monocentric, randomized controlled trial with three parallel arms. Eligible patients were recruited by a study nurse 7–60 days after PCI and randomly assigned (block sizes 2:1:2) to one of three groups: (i) a standard of care (SOC) group, without medication adherence monitoring; (ii) a standard of care plus adherence electronic monitoring (SOC + EM) group, in which dosing history data were compiled using an electronic monitor [Medication Event Monitoring System (MEMS®)] but the dosing history data were kept blinded to the patient and investigators until the end of the trial; or (iii) an integrated care (IC) group, which implied a regular feedback discussion on MEMS®‐compiled adherence data with the patient. The vasodilator‐stimulated phosphoprotein (VASP) platelet reactivity index (PRI) assessed at baseline, 3 months and 6 months was used as marker of clopidogrel response. The primary endpoint of the study was the VASP‐PRI at 6 months.
Study procedures
Platelet function assay and evaluation of clopidogrel responsiveness
Clopidogrel response was assessed with citrated whole blood, using the platelet VASP/P2Y12 assay (VASP assay, Platelet VASP/P2Y12, Biocytex, Marseille, France), a flow cytometry assay that specifically assesses the effect of platelet P2Y12 antagonists 6. The VASP assay was expressed with the platelet reactivity index (PRI) using a FACS‐track flow cytometer (Becton Dickinson, Meylan, France), according to the manufacturer's instruction. Patients were instructed to take clopidogrel in the morning and blood samples were collected the following morning, approximately 24 h after the last clopidogrel dose. The laboratory technician was blinded to the randomization group. The VASP assay was performed within 48 h after blood collection. Clopidogrel poor response (HPR) was defined as a VASP‐PRI ≥50%, whereas a good response was defined as an on‐treatment VASP‐PRI <50%. This value has been shown to be the optimal cutoff to exclude major cardiovascular events after PCI 26. Missing VASP‐PRI values were replaced by carrying the last observation forward.
Genotyping
Genomic DNA was extracted from ethylenediamine tetra‐acetic acid (EDTA) blood samples using the FlexiGene DNA extraction kit (QIAGEN, Basel, Switzerland), according to the manufacturer's protocol. The following single nucleotide polymorphisms (SNPs) were detected using real‐time polymerase chain reaction and 5'‐nuclease allelic discrimination assays (ABI PRISM 7000; Applied Biosystems, Luzern, Switzerland), according to the manufacturer's protocols 27: CYP2C19*2, CYP2C19*3, CYP2C19*17. Patients were classified into six predicted phenotypes: carriers of two functional (*1) alleles (*1/*1, n = 55; extensive metabolizers), carriers of one functional allele and one nonfunctional (*2) allele (*1/*2, n = 18; intermediate metabolizers), carriers of one gain‐of‐function allele (*17) and one nonfunctional allele (*17/*2, n = 10; intermediate metabolizers), carriers of only nonfunctional alleles (*2/*2, n = 2; poor metabolizers), carriers of only gain‐of‐function alleles (*17/*17, n = 3; rapid metabolizers) and carriers of one functional and one gain‐of‐function allele (*1/*17, n = 35; rapid metabolizers). Subjects were also dichotomously classified as ‘carriers’ of at least one nonfunctional allele (*1/*2*, *17/*2 and *2/*2) and ‘noncarriers’ (*1/*1, *1/*17 and *17/*17).
Adherence monitoring strategies
The MEMS® system was used to compile clopidogrel dosing history data in the SOC + EM and IC‐groups during the 6‐month study period. This system entails the use of a tablet bottle cap device that records electronically the time and date when the cap is removed. The data are collected, recorded and can be processed to generate a graphical representation of the dates and times of bottle openings. The utility of the device has been shown in long‐term studies assessing drug adherence 28. In the IC group, adherence data were downloaded every 6 weeks and thus were available for therapeutic follow‐up and feedback. Medication adherence results were discussed in semi‐structured motivational interviews with the study nurse or pharmacist and the patients. In the SOC + EM approach, adherence data were recorded electronically but neither the patient nor the study collaborators had access to the adherence results until the end of the study (blinding).
Within the execution phase of adherence to the prescribed dosage regimen, multiple summary statistics can be calculated to evaluate group characteristics 29. These summary statistics include: taking adherence (Tac) and correct dosing (Cod). The percentage of prescribed doses taken (Tac) was calculated as: number of openings/number of prescribed doses ×100. This measure reflects both the average dose received over a given period and the total dose received over that period. However, it fails to distinguish between a patient who takes their medication regularly and a patient who balances periods of underdosing with periods of overdosing, and it captures no information about the precise timing of drug intake. The percentage of days with the correct number of doses taken (Cod) was calculated as: number of days with number of openings as prescribed/number of monitored days ×100. This statistic captures some measure of the closeness to ‘correct adherence’. It reflects the degree of regularity in lifestyle.
Statistical analysis
Sample size calculations were based on the assumption of a mean ± standard deviation VASP‐PRI of 40 ± 20% 30 in the SOC group. In order to detect a difference of at least 10% between the control (SOC) and intervention (IC) groups at 6 months (α = 0.05, β = 0.2), the inclusion of 85 patients per group was estimated to be necessary. Assuming a study dropout rate of 10%, we planned to include 93 patients per group.
The adherence summary statistics were compared between groups using Wilcoxon rank sum tests. Two analyses of VASP‐PRI data were performed. The first included the measures of VASP‐PRI at baseline, 3 months and 6 months as dependent variables, and demographic characteristics, genetic data, randomization group and time as covariates. As the monitoring of adherence started only at baseline and covered the follow‐up until month 6, a second analysis including VASP‐PRI levels collected at 3 months and 6 months, incorporating baseline covariates and adherence, was performed to investigate the association between VASP‐PRI and adherence. To this end, medication adherence was summarized over a time window directly preceding each VASP‐PRI measure. Linear mixed‐effects models were used to model longitudinal VASP‐PRI data. A visual inspection of the conditional residuals was used to assess the quality of fit of the models. For the analysis of the second VASP‐PRI dataset, the length of the time window used to summarize adherence prior to VASP‐PRI was selected using the Akaike information criterion (AIC). An α level of 0.05 was used for all statistical tests. The analyses were performed using SAS for Windows (version 9.1; SAS Institute Inc, Cary, NC, USA).
Results
From April 2010 to December 2012, a total of 519 patients were screened, and 123 patients were recruited and randomly assigned to the SOC group (n = 48), SOC + EM group (n = 25) and IC group (n = 50) (Figure 1). All 123 randomized patients were included in the intent‐to‐treat analysis. The baseline demographic and clinical characteristics of the patients were similar in the three groups (Table 1).
Figure 1.
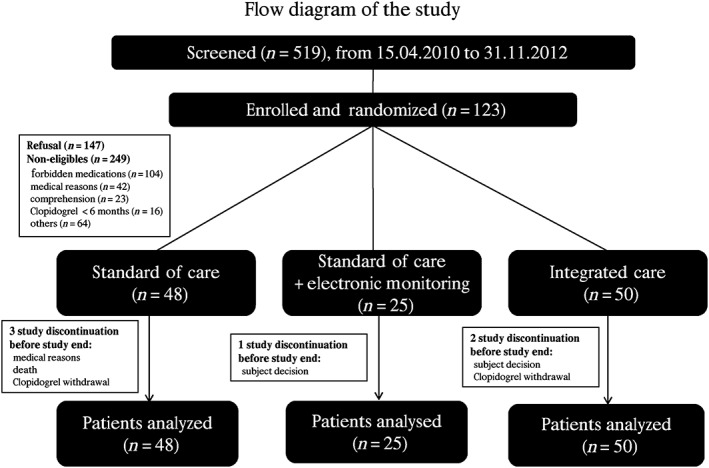
Flow diagram of the study
Table 1.
Baseline demographic, clinical and procedural characteristics of the study patients
| SOC (n = 48) | SOC + EM (n = 25) | IC (n = 50) | |
|---|---|---|---|
| Demographics | |||
| Race (Caucasian) | 43 (89.6) | 22 (88.0) | 49 (98.0) |
| Age, years | 64.0 ± 10.6 | 66.7 ± 8.3 | 65.5 ± 11.3 |
| Gender (male) | 42 (87.5) | 19 (76.0) | 43 (86.0) |
| BMI, kg m –2 | 28.2 ± 3.7 | 28.3 ± 3.4 | 26.8 ± 5.2 |
| Cardiovascular risks factors | |||
| Diabetes | 14 (29.2) | 7 (28.0) | 8 (16.0) |
| Hypertension | 40 (83.3) | 21 (84.0) | 38 (76.0) |
| Dyslipidaemia | 45 (93.8) | 22 (88.0) | 46 (92.0) |
| Smoker (current or former) | 35 (72.9) | 18 (72.0) | 31 (62.0) |
| Indication for revascularization | |||
| Stable angina | 21 (43.8) | 9 (36.0) | 15 (30.0) |
| Unstable angina | 4 (9.1) | 6 (25.0) | 8 (16.7) |
| Positive functional test | 13 (27.1) | 4 (16.0) | 9 (18.0) |
| Elective stent post‐ACS | 14 (29.2) | 8 (32.0) | 22 (44.0) |
| Number of stents | 1.5 ± 0.5 | 1.3 ± 0.7 | 1.4 ± 0.6 |
| Number of drug‐eluted stents | 1.5 ± 0.5 | 1.3 ± 0.8 | 1.4 ± 0.6 |
| Number of bare metal stents | 0.7 ± 0.6 | 1.3 ± 0.6 | 1.0 ± 0 |
| Prior cardiac history | |||
| ACS | 26 (54.2) | 11 (44.0) | 28 (56.0) |
| Coronary artery bypass graft | 7 (14.6) | 3 (12.0) | 8 (16.0) |
| PCI without stent | 11 (22.9) | 4 (16.0) | 7 (14.0) |
| PCI with stent | 27 (56.3) | 13 (52.0) | 25 (50.0) |
| Pharmacotherapy | |||
| Aspirin | 48 (100.0) | 24 (96.0) | 50 (100.0) |
| Beta‐blocker | 35 (72.9) | 16 (64.0) | 38 (76.0) |
| RAAS ‐inhibitor | 41 (85.4) | 21 (84.0) | 42 (84.0) |
| Calcium channel blocker | 9 (18.8) | 5 (20.0) | 2 (4.0) |
| Diuretic | 15 (31.3) | 5 (20.0) | 9 (18.0) |
| Statin | 47 (97.9) | 23 (92.0) | 48 (96.0) |
| Pantoprazole | 14 (29.2) | 5 (20.0) | 13 (26.0) |
| Laboratory results | |||
| Serum creatinine, μmol l –1 | 84 ± 20 | 85 ± 19 | 92 ± 22 |
| eGFR ckd‐epi, ml min –1 1. 73m – 2 | 83.6 ± 16.5 | 78.5 ± 14.8 | 75.7 ± 18.1 |
| Total cholesterol, mmol l –1 | 4.3 ± 1.0 | 4.3 ± 0.7 | 4.1 ± 0.8 |
| LDL‐cholesterol, mmol l –1 | 2.3 ± 0.8 | 2.4 ± 0.6 | 2.2 ± 0.8 |
| HDL‐cholesterol, mmol l –1 | 1.3 ± 0.4 | 1.3 ± 0.2 | 1.3 ± 0.4 |
| Fasting glucose, mmol l –1 | 6.6 ± 1.9 | 6.4 ± 1.7 | 6.1 ± 1.5 |
| Haemoglobin, g l –1 | 144 ± 15 | 140 ± 11 | 145 ± 12 |
| Platelets, cells μl –1 | 219 ± 64 | 229 ± 64 | 232 ± 65 |
| Genotyping | |||
| CYP2C19*2 allele noncarriers | 38 (79.2) | 17 (68.0) | 38 (76.0) |
| CYP2C19*2 allele carriers | 10 (20.8) | 8 (32.0) | 12 (24.0) |
Values are expressed as means ± standard deviation or n, with percentages in parenthesis. There were no significant differences (at P < 0.05) between groups in any of the summarized characteristics, with the exception of the use of calcium channel blockers (P = 0.048). ACS, acute coronary syndrome; BMI, body mass index; CYP, cytochrome P450; eGFR ckd‐epi, estimated glomerular function rate – chronic kidney disease epidemiology collaboration; HDL, high‐density lipoprotein; IC, integrated care; LDL, low‐density lipoprotein; PCI, percutaneous coronary intervention; RAAS, renin–angiotensin–aldosterone system; SOC, standard of care; SOC + EM, standard of care + adherence electronic monitoring
Results of adherence summary statistics
The adherence to clopidrogel was high in both group assessed with MEMS®. Figure 2 shows adherence summary statistics estimated over the 6 months of monitoring, according to the randomization groups. Medication adherence was higher and less variable in the IC group: median (min–max) Tac 101 (94–102)% vs. 99 (83–101) % in the SOC + EM group; P < 0.001; median (min–max) correct adherence 99 (93–100) % vs. 98 (80–100) % in the SOC + EM group; P < 0.001.
Figure 2.
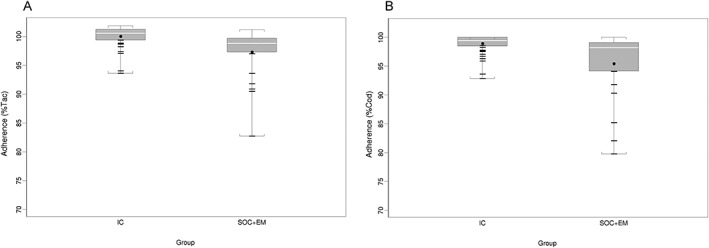
Boxplot of taking adherence (A) and correct adherence (B) over the 6‐month monitoring period according to the randomization group Boxes are defined by the 25th and 75th percentiles; the upper/lower whiskers represent maximum/minimum values; the medians are horizontal white segments; the means are black dots; and values below the 25th percentiles are represented as horizontal segments. Correct adherence (Cod) = proportion of days with correct dosing during the time window; taking adherence (Tac) = proportion of prescribed drug taken during the time window; IC = integrated‐care; SOC + EM = Standard of care + electronic monitoring
VASP‐PRI results, HPR and predictors of the on‐treatment clopidogrel response
Baseline mean VASP‐PRI was 48.3 ± 18.8% and the proportion of HPR was 48.0% in all participants. No significant difference between groups was observed (P = 0.849 for absolute VASP‐PRI values, P = 0.618 for HPR). VASP‐PRI values at baseline, 3 months and 6 months for each group are shown in Table 2. No significant effect of the intervention was observed (P = 0.309 for absolute VASP‐PRI values, P =0.244 for HPR) at 6 months. We observed large between‐patient variability at each visit and in the three groups. The between‐subject variability in VASP‐PRI was much higher than the within‐subject variability (Figure 3A). The correlations between VASP‐PRI measured at baseline and at 3 and 6 months were high (Figure 3B).
Table 2.
VASP‐PRI according to the randomization group
| SOC (n = 48) | SOC + EM (n = 25) | IC (n = 50) | P‐value | |
|---|---|---|---|---|
| VASP‐PRI at baseline | 49.5 ± 19.6 | 48.2 ± 17.0 | 47.3 ± 19.2 | 0.849 |
| VASP‐PRI at 3 months | 50.1 ± 19.2 | 48.8 ± 16.1 | 45.4 ± 18.4 | 0.442 |
| VASP‐PRI at 6 months | 49.7 ± 18.4 | 47.0 ± 15.8 | 47.3 ± 19.2 | 0.761 |
Values are expressed as means ± standard deviation. IC, integrated care; SOC, standard of care; SOC + EM, standard of care + adherence electronic monitoring; VASP‐PRI, vasodilator‐stimulated phosphoprotein phosphorylation–platelet reactivity index
Figure 3.
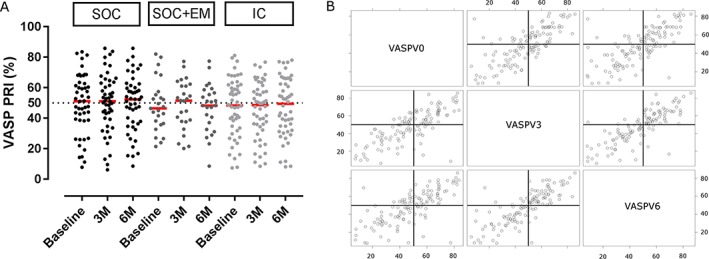
Inter‐ and intraindividual VASP‐PRI variability (A) and correlation of VASP‐PRI results over time (B). The dotted line represents the 50% cutoff. Red lines are medians. IC = integrated care (light grey); SOC = standard of care (black); SOC + EM = SOC + electronic monitoring (dark grey); VASP‐PRI = vasodilator‐stimulated phosphoprotein phosphorylation–platelet reactivity index; M = months
Neither randomization group nor visit time was found to be associated with the VASP‐PRI results. In the final model, diabetes (estimate 14.6, SE 3.1; P < 0.0001), CYP2C19*2 carrier status [estimate 8.8, standard error (SE) 3.1; P = 0.005) and body mass index (BMI, kg m‐2) (estimate 0.7, SE 0.4; P = 0.048) emerged as predictors of clopidogrel responsiveness. Figure 4 shows the association between VASP‐PRI and CYP2C19 genotype (panel A) and CYP2C19*2 carrier status (panel B).
Figure 4.
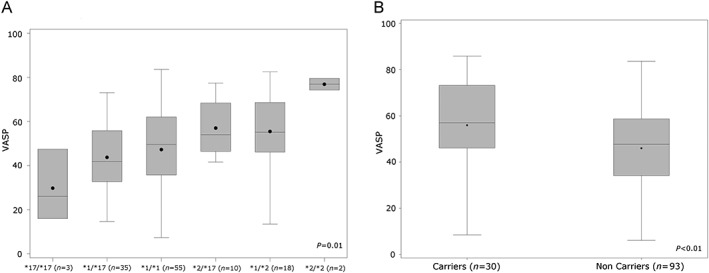
Association between VASP‐PRI and the CYP2C19 genotype (A) and the CYP2C19*2 carrier status (B) at baseline. Boxes are defined by the 25th and 75th percentiles; the upper/lower whiskers represent maximum/minimum values; medians are horizontal white segments; means are black dots. CYP = cytochrome P450; VASP‐PRI = vasodilator‐stimulated phosphoprotein phosphorylation–platelet reactivity index
Adherence and its associations with the on‐treatment clopidogrel response
Figure 5 shows the values of the AIC for different options for the time window. Given that lower AIC suggests a better model fit, it is graphically evident that a window of 2 weeks best describes the association of VASP‐PRI with adherence.
Figure 5.
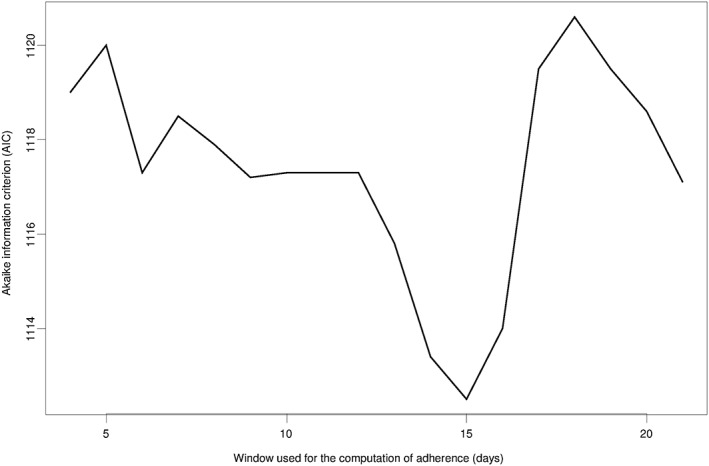
Selection of the window for the computation of adherence
The best model to describe the association between VASP‐PRI and medication adherence is a mixed‐effects model with a random intercept adjusted for baseline VASP‐PRI (estimate −2.8, SE 22.2; P = 0.015), the 2‐week Tac (Tac15) preceding the measure of VASP (estimate −48.0, SE 22.3; P = 0.035) and their interaction (estimate 3.6, SE 1.1; P = 0.003). No group or visit effects were significant.
To understand this interaction better, we investigated model predictions (Figure 6). To this end, we considered the first quartile (37%), the median (48%) and the third quartile (62%) of baseline VASP‐PRI. For each of these values, the predictions of the model were plotted in adherence summary statistics (Tac15) between 60% and 100%. We observed a slope for the first quartile and the median, but no relationship for a baseline VASP‐PRI of 62%. Further investigations of the model showed that the adherence slope became nonsignificant for a baseline VASP‐PRI of 50% (P = 0.055), indicating that the association between adherence and VASP‐PRI is lost when the VASP‐PRI at baseline is >50%.
Figure 6.
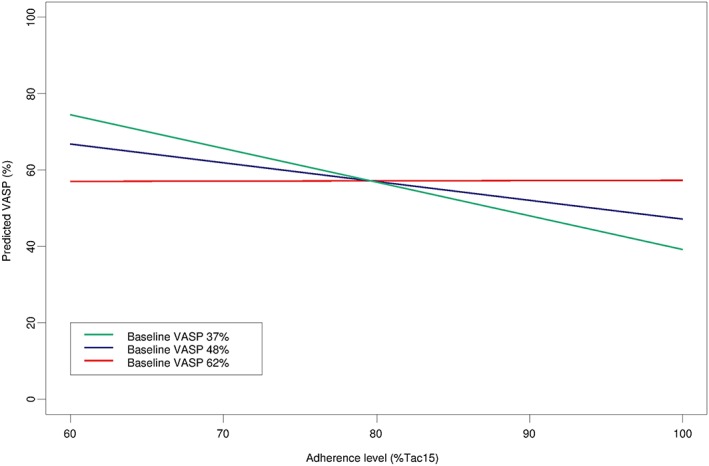
Predictions of VASP‐PRI as a function of the taking adherence level. Tac15 = 2‐week taking adherence preceding the measure of VASP‐PRI; VASP‐PRI = vasodilator‐stimulated phosphoprotein–phosphorylation platelet reactivity index
Safety
Table 3 reports all adverse events occurring during the 6‐month study period.
Table 3.
Adverse events at 6 months
| Total | SOC (n = 48) | SOC + EM (n = 25) | IC (n = 50) | P value | |
|---|---|---|---|---|---|
| Ischaemic events, N (%) | 4 (3.3) | 2 (4.2) | 1 (4.0) | 1 (2.0) | 0.814 |
| Unstable angina | 4 (3.3) | 2 (4.2) | 1 (4.0) | 1 (2.0) | 0.814 |
| ACS | 0 | 0 | 0 | 0 | |
| Stent thrombosis | 0 | 0 | 0 | 0 | |
| Cardiovascular mortality | 0 | 0 | 0 | 0 | |
| Coronary interventions, N (%) | 9 (7.3) | 2 (4.2) | 4 (16.0) | 3 (6.0) | 0.167 |
| Angioplasty | 7 (5.7) | 2 (4.2) | 4 (16.0) | 1 (2.0) | 0.040 |
| CABG | 2 (1.6) | 0 | 0 | 2 (4.0) | 0.231 |
| Bleeding (GUSTO criteria) N (%) | |||||
| Severe or life‐threatening | 1 (0.8) | 1 (2.1) | 0 | 0 | 0.461 |
| Moderate | 0 | 0 | 0 | 0 | 0 |
| Mild | 62 (50.4) | 29 (60.4) | 11 (44.0) | 22 (44.0) | 0.210 |
Values are expressed as n, with percentages in parenthesis. ACS, acute coronary syndrome; CABG, coronary artery bypass graft; IC, integrated care; SOC, standard of care; SOC + EM, standard of care + adherence electronic monitoring; GUSTO (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) definition of bleeding: severe or life‐threatening (intracerebral haemorrhage or bleeding resulting in substantial haemodynamic compromise requiring treatment), moderate (requiring blood transfusion but not resulting in haemodynamic compromise) and mild (bleeding that does not meet the above criteria)
Bleeding complications were characterized according to the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) definition for bleeding 31. Two patients had to end participation in the trial prematurely because of clopidogrel withdrawal: one patient in the context of recurrent vesical bleeding, and the second was switched to an alternative PY12 receptor inhibitor following a new revascularization procedure. We did not observe any between‐groups differences in the incidence of other adverse events, with the exception of more angioplasties with stent implantation in the control group (P = 0.040).
Discussion
The present study showed that adherence programmes were not associated with the long‐term response to clopidogrel as opposed to CYP2C19 polymorphisms, the presence of diabetes and obesity. The response to clopidogrel, assessed by VASP‐PRI, was highly variable, despite a high adherence in all groups assessed by electronic monitoring. HPR suggesting incomplete blockade of platelets occurred frequently, with almost 50% of patients in each group at each visit showing a VASP‐PRI ≥50%. The variability of VASP‐PRI was comparable in all groups, with the between‐subject variability much higher than the within‐subject variability. This finding was supported by the high correlation between the VASP‐PRI results measured within subjects at different times. The intervention on drug adherence did not affect the clopidogrel response over time. The adherence monitoring programme had an impact on the quality of platelet aggregation at 6 months in patients with a good initial VASP‐PRI (<50%). This suggests that adherence is also a determinant of the long‐term efficacy of clopidogrel but only in patients who actually respond to the drug. Our observation that patients with HPR are less likely to be affected by non‐adherence was hypothetical as the study was underpowered to draw conclusions in this regard. Further, we cannot exclude the possibility that our results were affected by the pharmacodynamic measurement chosen to measure clopidogrel response (VASP‐PRI), and could possibly have been different if other modalities based on the principle of platelet aggregometry had been used. One could question whether VASP‐PRI is the most adequate surrogate of clopidrogrel's response. Nevertheless, studies have shown reproducibility with the VASP assay has been found to be reproducible 32, and is considered to be the most specific assay for evaluating the effectiveness of clopidogrel (and other anti‐P2Y12 drugs) 33 as it assesses specifically the potency of the P2Y12 receptor using a standardized and commercially available method. Further, interventional trials 34 showing the clinical efficacy of clopidogrel have used the VASP assay to measure the P2Y12 receptor inhibitory effect 34, 35, 36.
Previous studies exploring the variability and clinical impact of clopidogrel‐induced platelet inhibition have focused on the acute phase (after the loading dose) or short‐term period (from a few days to several weeks) after PCI. To our knowledge, no study has investigated the long‐term (several months) platelet response to clopidogrel or determined the predictors of the long‐term clopidogrel responsiveness using long‐term adherence monitoring devices. Clarifying this issue is of major clinical interest because the factors affecting the long‐term drug efficacy may not be the same as those influencing the short‐term efficacy. Non‐adherence to treatment recommendations in patients with cardiovascular disease is a negligible factor in the acute hospitalization phase but may assume relevance in the chronic ambulatory setting, being identified as a factor contributing to poor outcomes, such as an increased risk of stent thrombosis after premature and permanent thienopyridine discontinuation following PCI with DES implantation 26.
In accordance with a previous study performed in the acute setting 16, we found an interaction between clinical variables and genomic traits with on‐clopidogrel platelet function in the long term. The major independent predictors for higher VASP‐PRI results emerged as diabetes mellitus, the CYP2C19*2 loss‐of‐function polymorphism and high BMI, after correction for age, race, dyslipidaemia, smoking, hypertension, cardiological antecedents and antecedents of acute coronary syndrome. In contrast to other studies, age and associated medications did not show associations with the clopidogrel response. The major finding of the present study was a significant interaction between VASP‐PRI at baseline and Tac15 measured prior to the VASP‐PRI at 6 months, which may explain the negative result in the primary outcome. Our observation suggests that the association between on‐treatment clopidogrel potency and adherence is dependent on the individual pharmacodynamic response to clopidogrel. Indeed, the association between adherence and VASP‐PRI disappears when the baseline VASP‐PRI is >50%, suggesting that poor responders are less sensitive to adherence behaviour and may have to be switched to a more potent P2Y12 antagonist. Importantly, the 50% threshold has been reported as the definition of clopidogrel resistance in other settings 30, 34, 37, 38. Previous trials have demonstrated that a VASP‐PRI <50% has a very high negative predictive value for thrombotic events after PCI, and this cutoff value has therefore been used to define HPR in clinical and research settings 34, 37, 38, 39.
The clinical impact of antiplatelet drug discontinuation after PCI with stent implantation is controversial. It has been shown to be harmful in several contexts 30, particularly concerning the risk of DES thrombosis after clopidogrel discontinuation 40. More recently published studies are less categorical. In the Adherence to Treatment of Coronary Patients After a Catheterization With DES Implantation (ACDC) trial 41, a prospective cohort study including coronary patients after DES implantation, over 14% of patients stopped taking at least one antiplatelet drug during the first year, mainly on the basis of their own or medical decisions, unrelated to major adverse events. In the subsequent analysis 42, it was showed that antiplatelet therapy discontinuation during the first year after DES implantation was in most instances a temporary interruption (median 7 days) and was not necessarily followed by major cardiovascular events, at least in patients discontinuing the drug later than 1 month after stenting, which was the most common situation.
Although there have been some sporadic studies showing the feasibility of remote home support programmers in the cardiovascular domain 43, only one study has tested interventions aimed at improving patient follow‐up after DES implantation. Through a simple approach that consisted of four telephone calls over the year after DES implantation 44, the authors achieved a significant one‐year medication adherence to antiaggregants and statins, achieving near‐perfect scores.
Limitations of the study
This study explored only a subset of factors potentially implicated in the long‐term VASP‐PRI variability – thus, in the clopidogrel maintenance setting. As yet unidentified clinical, demographic, technical and genomic factors will probably emerge in the future and further clarify these complex interactions. Procedural heterogeneity in sampling treatment could have influenced the VASP‐PRI results but the risk was minimized by a strict protocol for drug intake and blood sampling, and by centralizing the analysis within one laboratory.
The baseline imbalance in the prescription of calcium channel blockers (more frequently used in the SOC group) may have introduced a bias, increasing the VASP‐PRI results 24. Contamination between study arms might have occurred, owing to the open‐label nature of the trial and the fact that the same study nurse/pharmacist took care of both study groups. However, the nurse/pharmacist was blinded to medication adherence data from the SOC + EM group until the study end. Furthermore, an effort was made to standardize motivational interviews, in order to homogenize the intervention among individuals.
The protocol planed the inclusion of 93 patients in the control (SOC) and intervention (IC) groups to detect a 10% difference in the VASP‐PRI results, with α = 0.05 and β = 0.2. The study was stopped prematurely because the difference was smaller than expected but the variability was high. In fact, the variability of the response to clopidogrel was found to be so high and unpredictable that the number of patients that would have had to be enrolled to demonstrate an effect of the intervention increased beyond feasibility for one centre. After recalculating the sample size necessary to detect a 10% difference, we found that 1291 patients would have had to be included in each group, a number which was out of reach for a single centre. However, retrospectively, the inclusion of patients with HPR, possibly secondary to a nonfunctional CYP2C19 allele or diabetes, may have affected our primary outcome as adherence, whether good or bad, did not affect VASP‐PRI in early nonresponders. Thus, the high proportion of nonresponders masked the effect of poor adherence on the primary outcome. A posteriori, to avoid this issue, all patients should have been tested for their response to clopidogrel before being enrolled in the adherence programme.
The adherence results may have been influenced by participation in the trial and by the electronic monitoring of drug intake. All participants with the device were aware that their adherence was being monitored and they had regular appointments to attend, during which the adherence issue was discussed. This may have resulted in overestimation of the habitual adherence of the participants. However, compared with other methods assessing adherence, the MEMS® device tends to underestimate adherence if data for the device is analysed without an interview or pill count 45.
Conclusion
Adherence to clopidogrel after PCI was high, and adherence monitoring with an electronic device did not seem to influence clopidogrel responsiveness, as assessed by the VASP‐PRI at 6 months, in the whole patient group. However, the high interindividual variability in clopidogrel responsiveness may have masked an effect of adherence on the primary objective. Indeed, in patients with a baseline VASP‐PRI <50% – i.e. in responders to clopidogrel – an association between adherence and the long‐term VASP‐PRI clearly exists, and a poor adherence during the previous 15 days is associated with a poor clopidogrel response. By contrast, adherence does not appear to play a role in patients who are initially nonresponders to clopidogrel; in these cases, platelet aggregation is poor, irrespective of the adherence.
Competing Interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
This trial was made possible by the collaborative efforts of doctors, nurses, and administrators at the recruiting hospital. We thank everyone who contributed their time and expertise, in particular the trial participants. Their input and understanding were important in ensuring the success of this important trial. This work was supported by grants of the Swiss Heart Foundation and from the Swiss Federal Office of Technology and Professional Training (OFFT; Eurostar project n°4776). There was no commercial support for this study.
Contributors
The authors' responsibilities were as follows: G.W., V.F.O., B.V., I.M., O.M., E.T., P.F., E.E., C.B.E., B.V. and M.B. wrote the manuscript; C.B.E., G.W., M.B., V.F.O., O.M., P.F. and L.G. designed the research; G.W., V.F.O., I.M., L.G., M.B., O.M. and P.F. performed the research; B.V., C.B.E., E.T., G.W., O.M. and V.F.O analysed the data. All authors critically revised the manuscript for important intellectual content and approved the final version of the manuscript.
Forni Ogna, V. , Menetrey, I. , Muller, O. , Tousset, E. , Guihard, L. , Fontana, P. , Eeckhout, E. , Eap, C. B. , Vrijens, B. , Burnier, M. , and Wuerzner, G. (2016) Effect of long‐term adherence to clopidogrel on the VASP‐PRI after elective coronary stent implantation: a randomized controlled study. Br J Clin Pharmacol, 82: 1486–1497. doi: 10.1111/bcp.13071.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44 (Database Issue): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 2015; 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 2015; 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hochholzer W, Trenk D, Frundi D, Blanke P, Fischer B, Andris K, et al. Time dependence of platelet inhibition after a 600‐mg loading dose of clopidogrel in a large, unselected cohort of candidates for percutaneous coronary intervention. Circulation 2005; 111: 2560–2564. [DOI] [PubMed] [Google Scholar]
- 5. Lepantalo A, Virtanen KS, Heikkila J, Wartiovaara U, Lassila R. Limited early antiplatelet effect of 300 mg clopidogrel in patients with aspirin therapy undergoing percutaneous coronary interventions. Eur Heart J 2004; 25: 476–483. [DOI] [PubMed] [Google Scholar]
- 6. Aleil B, Ravanat C, Cazenave JP, Rochoux G, Heitz A, Gachet C. Flow cytometric analysis of intraplatelet VASP phosphorylation for the detection of clopidogrel resistance in patients with ischemic cardiovascular diseases. J Thromb Haemost 2005; 3: 85–92. [DOI] [PubMed] [Google Scholar]
- 7. Angiolillo DJ, Fernandez‐Ortiz A, Bernardo E, Alfonso F, Macaya C, Bass TA, et al. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol 2007; 49: 1505–1516. [DOI] [PubMed] [Google Scholar]
- 8. Angiolillo DJ, Bernardo E, Sabate M, Jimenez‐Quevedo P, Costa MA, Palazuelos J, et al. Impact of platelet reactivity on cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol 2007; 50: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 9. Hochholzer W, Trenk D, Bestehorn HP, Fischer B, Valina CM, Ferenc M, et al. Impact of the degree of peri‐interventional platelet inhibition after loading with clopidogrel on early clinical outcome of elective coronary stent placement. J Am Coll Cardiol 2006; 48: 1742–1750. [DOI] [PubMed] [Google Scholar]
- 10. Buonamici P, Marcucci R, Migliorini A, Gensini GF, Santini A, Paniccia R, et al. Impact of platelet reactivity after clopidogrel administration on drug‐eluting stent thrombosis. J Am Coll Cardiol 2007; 49: 2312–2317. [DOI] [PubMed] [Google Scholar]
- 11. Sibbing D, Braun S, Morath T, Mehilli J, Vogt W, Schomig A, et al. Platelet reactivity after clopidogrel treatment assessed with point‐of‐care analysis and early drug‐eluting stent thrombosis. J Am Coll Cardiol 2009; 53: 849–856. [DOI] [PubMed] [Google Scholar]
- 12. Combescure C, Fontana P, Mallouk N, Berdague P, Labruyere C, Barazer I, et al. Clinical implications of clopidogrel non‐response in cardiovascular patients: a systematic review and meta‐analysis. J Thromb Haemost 2010; 8: 923–933. [DOI] [PubMed] [Google Scholar]
- 13. Burnier M, Wuerzner G, Struijker‐Boudier H, Urquhart J. Measuring, analyzing, and managing drug adherence in resistant hypertension. Hypertension 2013; 62: 218–225. [DOI] [PubMed] [Google Scholar]
- 14. Matetzky S, Shenkman B, Guetta V, Shechter M, Beinart R, Goldenberg I, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation 2004; 109: 3171–3175. [DOI] [PubMed] [Google Scholar]
- 15. Hochholzer W, Trenk D, Mega JL, Morath T, Stratz C, Valina CM, et al. Impact of smoking on antiplatelet effect of clopidogrel and prasugrel after loading dose and on maintenance therapy. Am Heart J 2011; 162: 518–526. [DOI] [PubMed] [Google Scholar]
- 16. Hochholzer W, Trenk D, Fromm MF, Valina CM, Stratz C, Bestehorn HP, et al. Impact of cytochrome P450 2C19 loss‐of‐function polymorphism and of major demographic characteristics on residual platelet function after loading and maintenance treatment with clopidogrel in patients undergoing elective coronary stent placement. J Am Coll Cardiol 2010; 55: 2427–2434. [DOI] [PubMed] [Google Scholar]
- 17. Geisler T, Grass D, Bigalke B, Stellos K, Drosch T, Dietz K, et al. The Residual Platelet Aggregation after Deployment of Intracoronary Stent (PREDICT) score. J Thromb Haemost 2008; 6: 54–61. [DOI] [PubMed] [Google Scholar]
- 18. Trenk D, Hochholzer W, Fromm MF, Chialda LE, Pahl A, Valina CM, et al. Cytochrome P450 2C19 681G > A polymorphism and high on‐clopidogrel platelet reactivity associated with adverse 1‐year clinical outcome of elective percutaneous coronary intervention with drug‐eluting or bare‐metal stents. J Am Coll Cardiol 2008; 51: 1925–1934. [DOI] [PubMed] [Google Scholar]
- 19. Hulot JS, Bura A, Villard E, Azizi M, Remones V, Goyenvalle C, et al. Cytochrome P450 2C19 loss‐of‐function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood 2006; 108: 2244–2247. [DOI] [PubMed] [Google Scholar]
- 20. Sibbing D, Stegherr J, Latz W, Koch W, Mehilli J, Dorrler K, et al. Cytochrome P450 2C19 loss‐of‐function polymorphism and stent thrombosis following percutaneous coronary intervention. Eur Heart J 2009a; 30: 916–922. [DOI] [PubMed] [Google Scholar]
- 21. Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome p‐450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360: 354–362. [DOI] [PubMed] [Google Scholar]
- 22. Brandt JT, Close SL, Iturria SJ, Payne CD, Farid NA, Ernest CS, et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost 2007; 5: 2429–2436. [DOI] [PubMed] [Google Scholar]
- 23. Shuldiner AR, O'Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 2009; 302: 849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Siller‐Matula JM, Lang I, Christ G, Jilma B. Calcium‐channel blockers reduce the antiplatelet effect of clopidogrel. J Am Coll Cardiol 2008; 52: 1557–1563. [DOI] [PubMed] [Google Scholar]
- 25. Siller‐Matula JM, Spiel AO, Lang IM, Kreiner G, Christ G, Jilma B. Effects of pantoprazole and esomeprazole on platelet inhibition by clopidogrel. Am Heart J 2009; 157: 148–145. [DOI] [PubMed] [Google Scholar]
- 26. Tantry US, Bonello L, Aradi D, Price MJ, Jeong YH, Angiolillo DJ, et al. Consensus and update on the definition of on‐treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol 2013; 62: 2261–2273. [DOI] [PubMed] [Google Scholar]
- 27. Jaquenoud SE, Knezevic B, Morena GP, Harenberg S, Oneda B, Crettol S, et al. ABCB1 and cytochrome P450 polymorphisms: clinical pharmacogenetics of clozapine. J Clin Psychopharmacol 2009; 29: 319–326. [DOI] [PubMed] [Google Scholar]
- 28. Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ 2008; 336: 1114–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wessels AM, Jin Y, Pollock BG, Frank E, Lange AC, Vrijens B, et al. Adherence to escitalopram treatment in depression: a study of electronically compiled dosing histories in the ‘Depression: the search for phenotypes’ study. Int Clin Psychopharmacol 2012; 27: 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gurbel PA, Bliden KP, Hiatt BL, O'connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation 2003; 107: 2908–2913. [DOI] [PubMed] [Google Scholar]
- 31. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011; 123: 2736–2747. [DOI] [PubMed] [Google Scholar]
- 32. Reny JL, Berdague P, Poncet A, Barazer I, Nolli S, Fabbro‐Peray P, et al. Antiplatelet drug response status does not predict recurrent ischemic events in stable cardiovascular patients: results of the Antiplatelet Drug Resistances and Ischemic Events study. Circulation 2012; 125: 3201–3210. [DOI] [PubMed] [Google Scholar]
- 33. Cattaneo M. Resistance to antiplatelet drugs: molecular mechanisms and laboratory detection. J Thromb Haemost 2007; 5 (Suppl. 1): 230–237. [DOI] [PubMed] [Google Scholar]
- 34. Bonello L, Paganelli F, Arpin‐Bornet M, Auquier P, Sampol J, Dignat‐George F, et al. Vasodilator‐stimulated phosphoprotein phosphorylation analysis prior to percutaneous coronary intervention for exclusion of postprocedural major adverse cardiovascular events. J Thromb Haemost 2007; 5: 1630–1636. [DOI] [PubMed] [Google Scholar]
- 35. Bonello L, Camoin‐Jau L, Arques S, Boyer C, Panagides D, Wittenberg O, et al. Adjusted clopidogrel loading doses according to vasodilator‐stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J Am Coll Cardiol 2008; 51: 1404–1411. [DOI] [PubMed] [Google Scholar]
- 36. Bonello L, Camoin‐Jau L, Armero S, Com O, Arques S, Burignat‐Bonello C, et al. Tailored clopidogrel loading dose according to platelet reactivity monitoring to prevent acute and subacute stent thrombosis. Am J Cardiol 2009; 103: 5–10. [DOI] [PubMed] [Google Scholar]
- 37. Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug‐eluting stents. JAMA 2005; 293: 2126–2130. [DOI] [PubMed] [Google Scholar]
- 38. Airoldi F, Colombo A, Morici N, Latib A, Cosgrave J, Buellesfeld L, et al. Incidence and predictors of drug‐eluting stent thrombosis during and after discontinuation of thienopyridine treatment. Circulation 2007; 116: 745–754. [DOI] [PubMed] [Google Scholar]
- 39. Ingelman‐Sundberg M. Human drug metabolising cytochrome P450 enzymes: properties and polymorphisms. Naunyn Schmiedebergs Arch Pharmacol 2004; 369: 89–104. [DOI] [PubMed] [Google Scholar]
- 40. Barragan P, Bouvier JL, Roquebert PO, Macaluso G, Commeau P, Comet B, et al. Resistance to thienopyridines: clinical detection of coronary stent thrombosis by monitoring of vasodilator‐stimulated phosphoprotein phosphorylation. Catheter Cardiovasc Interv 2003; 59: 295–302. [DOI] [PubMed] [Google Scholar]
- 41. Frere C, Cuisset T, Quilici J, Camoin L, Carvajal J, Morange PE, et al. ADP‐induced platelet aggregation and platelet reactivity index VASP are good predictive markers for clinical outcomes in non‐ST elevation acute coronary syndrome. Thromb Haemost 2007; 98: 838–843. [PubMed] [Google Scholar]
- 42. Blindt R, Stellbrink K, de Taeye A, Muller R, Kiefer P, Yagmur E, et al. The significance of vasodilator‐stimulated phosphoprotein for risk stratification of stent thrombosis. Thromb Haemost 2007; 98: 1329–1334. [PubMed] [Google Scholar]
- 43. Ho PM, Tsai TT, Wang TY, Shetterly SM, Clarke CL, Go AS, et al. Adverse events after stopping clopidogrel in post‐acute coronary syndrome patients: insights from a large integrated healthcare delivery system. Circ Cardiovasc Qual Outcomes 2010; 3: 303–308. [DOI] [PubMed] [Google Scholar]
- 44. Rinfret S, Rodes‐Cabau J, Bagur R, Dery JP, Dorais M, Larose E, et al. Telephone contact to improve adherence to dual antiplatelet therapy after drug‐eluting stent implantation. Heart 2013; 99: 562–569. [DOI] [PubMed] [Google Scholar]
- 45. Liu H, Golin CE, Miller LG, Hays RD, Beck CK, Sanandaji S, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med 2001; 134: 968–977. [DOI] [PubMed] [Google Scholar]


