I. Catalytic Hydrogenation – A Brief Historical Perspective
The first metal catalyzed additions of elemental hydrogen to π-unsaturated reactants were reported by James F. Boyce of the Nathaniel Kellogg Fairbank Soap Company in connection with the processing of vegetable oils.1 Subsequently, Paul Sabatier of the University of Toulouse developed general protocols for the hydrogenation of alkenes employing heterogeneous nickel catalysts.2 Foreshadowing the merger hydrogenation and carbonyl addition described in the present account, Paul Sabatier and Victor Grignard jointly received the Nobel Prize in Chemistry in 1912.3 Following Sabatier’s pioneering work, numerous noble metal catalysts for heterogeneous hydrogenation emerged. Among these, the platinum oxide catalyst developed by Roger Adams in 1922 is still one of the most active and readily prepared catalysts for heterogeneous hydrogenation.4
The first examples of homogeneous hydrogenation were reported by Melvin Calvin at the University of California at Berkeley in 1938.5 Calvin showed that under one atmosphere of hydrogen, copper acetate catalyzed the reduction of 1,4-benzoquinone to 1,4-hydroquinone in quinoline solution at 100 °C. It was not until 1961 that Halpern’s group at the University of British Columbia performed the first homogeneous hydrogenation of an olefin.6 In Halpern’s system, ruthenium catalysts were used to reduce activated alkenes such as maleic acid. In 1962, Vaska subsequently found that IrCl(CO)(PPh3)2 reacts reversibly with elemental hydrogen to form isolable dihydrides.7 This result solidified the conceptual foundation of catalytic hydrogenation by establishing hydrogen “oxidative addition” as a key mechanistic feature.
Finally, in 1965, Wilkinson’s group at Imperial College reported the homogeneous hydrogenation of unactivated alkenes and alkynes catalyzed by RhCl(PPh3)3.8,9 This finding ultimately led William S. Knowles of Monsanto Company in St. Louis to discover the first enantioselective hydrogenation in 1968.10 Knowles’ discovery was made possible by Horner and Mislow’s disclosure of methods for the preparation of nonracemic P-stereogenic phosphines.11,12 In Knowles’ initially reported asymmetric hydrogenation, a maximum enantiomeric excess of 15% was obtained. Subsequent work by Kagan in 1971 using chiral bis(phosphines) derived from tartaric acid (i.e. “DIOP”) gave up to 72% enantiomeric excess.13 Using a P-stereogenic bis(phosphine) known as DiPAMP, chemists at Monsanto performed the first industrial catalytic asymmetric synthesis for the production of L-DOPA, a treatment for Parkinson’s disease. Lastly, in 1980 Ryoji Noyori reported the synthesis of BINAP and demonstrated the broad utility of this ligand in asymmetric hydrogenation.14,15
The purpose of this review is to provide a comprehensive summary of ruthenium catalyzed C-C couplings induced via alcohol-mediated transfer hydrogenation.16–20 These studies build on several important milestones in the area of ruthenium catalyzed hydrogenation and transfer hydrogenation (Scheme 1). In 1971, one decade beyond the seminal work of Halpern on ruthenium catalyzed hydrogenation,6 transfer hydrogenations employing ruthenium catalysts were described.21 The ruthenium catalyzed acceptorless dehydrogenation of alcohols was reported by Robinson in 1977,22 and ruthenium catalyzed oxidative esterifications were reported by Shvo in 1981.23,24 These studies were followed by the first highly enantioselective ruthenium catalyzed hydrogenations and transfer hydrogenations, reported by Noyori in 1986 and 1995, respectively.25,26 As documented in this account, ruthenium catalyzed transfer hydrogenation now serves as the basis for C-C bond constructions that directly convert lower alcohols to higher alcohols.16–20 This body of work was preceded by studies on metal catalyzed carbonyl reductive couplings mediated by elemental hydrogen, as initially described in 2002 by the present author,27 and as documented in the review literature.28,29
Scheme 1.
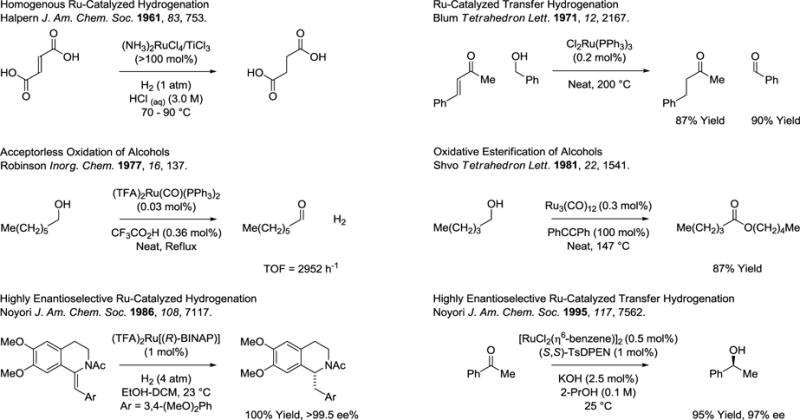
Selected milestones in homogeneous ruthenium catalyzed hydrogenation and transfer hydrogenation.a
aBINAP = 2,2′-bis-(diphenylphosphino)-1,1′-binaphthalene. TsDPEN = N-p-Tosyl-1,2-diphenylethylenediamine.
II. Conversion of Primary Alcohols to Secondary Alcohols
In 2007, our laboratory reported the first transfer hydrogenative couplings of alcohols with π-unsaturated reactants using iridium-based catalysts.30 In 2008, the first ruthenium catalyzed reactions of this type were developed (Scheme 2). Specifically, it was found that exposure of alcohols to 1,3-dienes in the presence of catalysts derived from HClRu(CO)(PPh3)3 and various phosphine ligands resulting in hydrogen transfer to furnish aldehyde-allylruthenium pairs that combine to form homoallylic alcohols as single regioisomers.31 The coupling of isoprene to d2-benzyl alcohol results in transfer of a benzylic deuteride to the allylic methyl (19% 2H) and allylic methine (32% 2H). These data are consistent with reversible hydrometalation of the less substituted olefin to form a secondary σ-allyl. Conversion to the more stable primary σ-allyl haptomer occurs in advance of carbonyl addition, which proceeds through the indicated closed six-centered transition state with allylic inversion to deliver the product of carbonyl allylation.
Scheme 2.
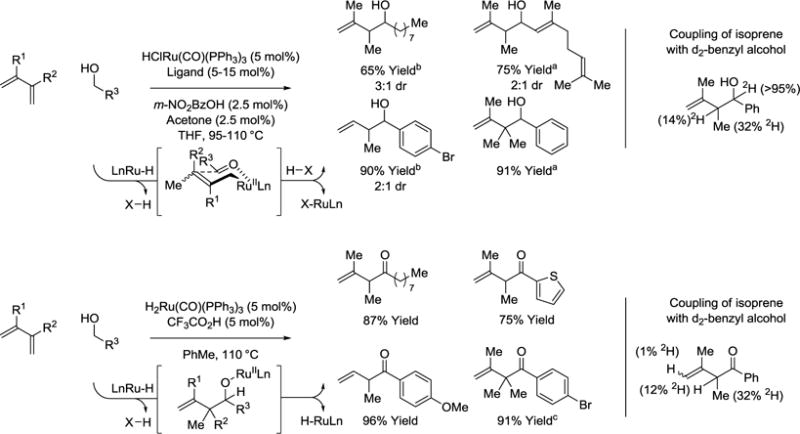
Ruthenium catalyzed C-C coupling of primary alcohols with 1,3-dienes to form homoallylic alcohols or β,γ-enones.a
Yields are of material isolated by flash chromatography on silica gel. aLigand = (p-MeOPh)3P, bLigand = rac-BINAP, 2,2′-bis-(diphenylphosphino)-1,1′-binaphthalene. c120 °C.
Remarkably, while the primary alcohol reactants readily dehydrogenate, the secondary homoallylic alcohol products resist further oxidation due to chelation of homoallylic olefin to ruthenium to generate a coordinatively saturated complex. Indeed, in the coupling of isoprene to d2-benzyl alcohol, deuterium is completely retained at the carbinol position, suggesting the product is completely unreactive toward alcohol dehydrogenation. However, the ruthenium catalyst (F3CCO2)(H)Ru(CO)(PPh3)2, which is generated in situ through the acid base reaction of H2Ru(CO)(PPh3)3 and F3CCO2H, possesses a higher degree of coordinative unsaturation, enabling β-hydride elimination at the stage of the homoallylic ruthenium alkoxide to form the β,γ-unsaturated enones (Scheme 2).32 Notably, both transformations, diene hydrohydroxyalkylation or hydroacylation, may be conducted from the alcohol or aldehyde (not shown) oxidation level of the reactant.31,32
Initial studies aimed at directing relative and absolute stereochemistry in alcohol mediated diene hydrohydroxyalkylation relied on the use of 2-trialkylsilyl-butadienes.33 Hydrometalation of 2-trialkylsilyl-substituted dienes gives rise to crotylmetal species that exist as single geometrical isomers due to allylic strain.34–36 In the event, using the chiral ruthenium catalyst generated in situ from HClRu(CO)(PPh3)3 and (R)-DM-SEGPHOS, the indicated 2-trialkylsilyl-butadiene couples with reactant alcohols to furnish the branched products of hydrohydroxyalkylation with complete syn-diastereoselectivity and uniformly high levels of enantioselectivity (Scheme 3).
Scheme 3.
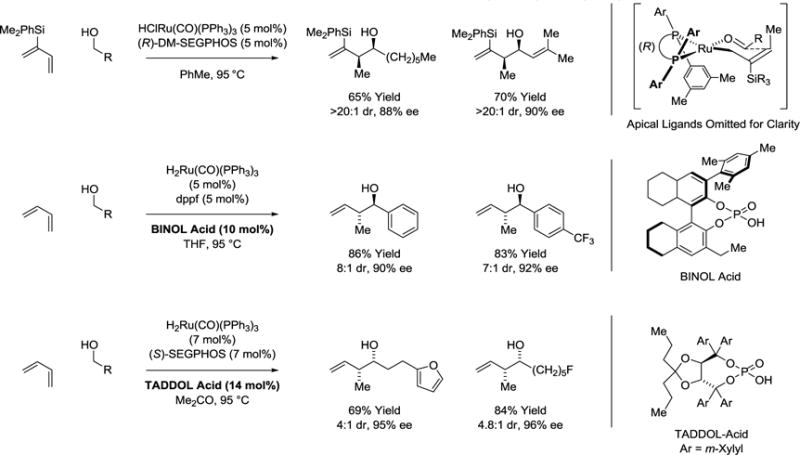
Diastereo- and enantioselective alcohol mediated hydrohydroxyalkylation of butadienes.a
aYields are of material isolated by flash chromatography on silica gel. Diastereoselectivity was determined through 1H NMR analysis of crude reaction mixtures. Enantiomeric excess was determined by chiral stationary phase HPLC analysis. DM-SEGPHOS = 5,5′−bis-[di(3,5-xylyl)phosphino]-4,4′-bi-1,3-benzodioxole. dppf = 1,1-bis-(diphenylphosphino)ferrocene. SEGPHOS = 5,5′-bis-(diphenylphosphino)-4,4′-bi-1,3-benzodioxole
Direct diastereo- and enantioselective hydrohydroxyalkylations of butadiene, an abundant petrochemical feedstock, required use of a ruthenium catalyst modified by a chiral phosphate counterion derived from H8-BINOL. The anion is attached to the metal center through the acid-base reaction of H2Ru(CO)(PPh3)3 with the indicated chiral phosphoric acid. With the chiral counterion as the sole chiral inducing element, primary benzylic alcohols hydrohydroxyalkylate butadiene with good levels of anti-diastereo- and enantioselectivity (Scheme 3).37
The corresponding syn-diastereomers are formed upon use of the ruthenium catalyst generated in situ from RuH2(CO)(PPh3)3, (S)-SEGPHOS and the indicated TADDOL-derived phosphoric acid (Scheme 3).38 It is postulated that the s-cis-conformer of butadiene hydrometalates to form a (Z)-σ-crotylruthenium intermediate. The relatively Lewis basic TADDOL-derived phosphate counterion preserves the kinetic selectivity of diene hydrometalation by attenuating the degree of coordinative unsaturation, decelerating isomerization to the (E)-σ-crotylruthenium haptomer with respect to carbonyl addition. Additionally, computational studies suggest a formyl hydrogen bond between the transient aldehyde and the phosphate oxo-moiety assists in stabilizing the (Z)-σ-crotylruthenium intermediate.36
A divergence in regioselectivity is observed upon use of neutral vs cationic ruthenium complexes in alcohol-mediated hydrohydroxyalkylations of 2-substituted dienes. For example, in 2-propanol mediated reductive couplings of 2-substituted dienes with paraformaldehyde (Scheme 4),39–41 neutral ruthenium complexes favor coupling at the C3 position,40 whereas ruthenium catalysts with greater cationic character favor coupling at the C2 position, resulting in formation of an all-carbon quaternary center.39 An erosion in C2-regioselectivity is observed when cationic ruthenium catalysts are applied in reactions of higher carbonyl partners with 2-substituted dienes, as illustrated in couplings with ethanol (Scheme 4).42
Scheme 4.
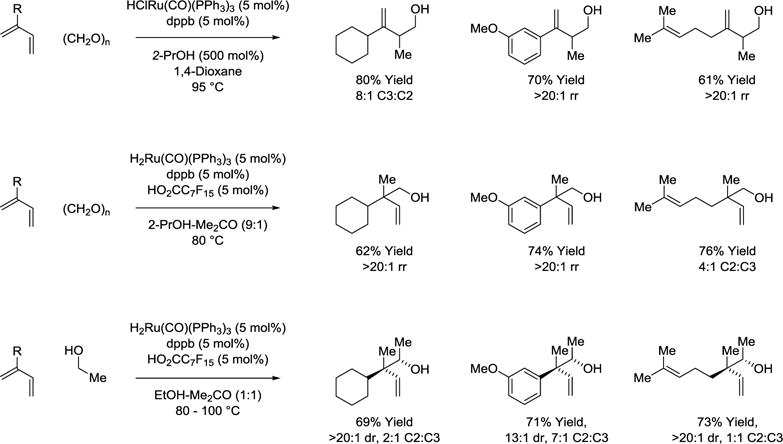
Divergent regioselectivity in 2-propanol mediated reductive couplings of dienes with paraformaldehyde and redox neutral couplings of ethanol.a
aYields are of material isolated by flash chromatography on silica gel. Diastereoselectivity was determined through 1H NMR analysis of crude reaction mixtures. dppb = bis-(diphenylphosphino)butane.
The collective data, including deuterium labeling experiments,39,40 are consistent with the following mechanistic interpretation (Scheme 5). Hydroruthenation to form allylruthenium complex A is kinetically preferred. For neutral ruthenium catalysts, hydrometalation is less reversible and strongly favors formation of allylruthenium complex A. Hence, formation of C3-coupling products is preferred. For cationic ruthenium complexes, hydrometalation becomes highly reversible, enabling access to both allylruthenium complex A and allylruthenium complex B. It now appears that a Curtin-Hammett scenario is operative. For small aldehyde partners (R1 = H), the transition state leading to C2-adducts is lower in energy. However, as the aldehyde increases in size (R1 = Me), the formation of a more congested C-C bond raises the energy of the transition state for formation of C3-adducts, eroding regioselectivity.
Scheme 5.
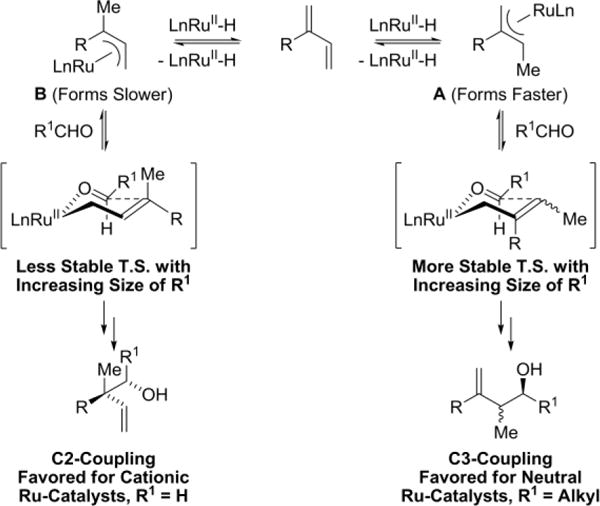
Divergent regioselectivity in the hydrohydroxyalkylation of 2-substituted dienes.
Hydrogen transfer from primary alcohols to allenes represents an alternate means of accessing allylruthenium-carbonyl pairs that deliver products of hydrohydroxyalkylation (Scheme 6).43–47 Interestingly, whereas 2-propanol-mediated reductive couplings of 1,1-disubstituted allenes display poor levels of diastereoselectivity,43 related redox-neutral couplings with primary alcohols delivers branched homoallylic allylic alcohols bearing all-carbon quaternary centers with good to complete control of relative stereochemistry.45 In reactions conducted from the alcohol oxidation level, diastereoselectivities are highly concentration dependent. At lower concentrations higher diastereoselectivities are observed. These data suggest a Curtin-Hammett scenario wherein turn-over limiting carbonyl addition preferentially consumes the (E)-σ-allylruthenium haptomer via stereospecific carbonyl addition from an equilibrating mixture of transient (Z)- and (E)-σ-allylruthenium isomers. At lower concentration, the (E)-isomer can be replenished via isomerization of the (Z)-σ-allylruthenium isomer. These conditions have been applied to the coupling of allenes with fluorinated alcohols (not shown).47
Scheme 6.
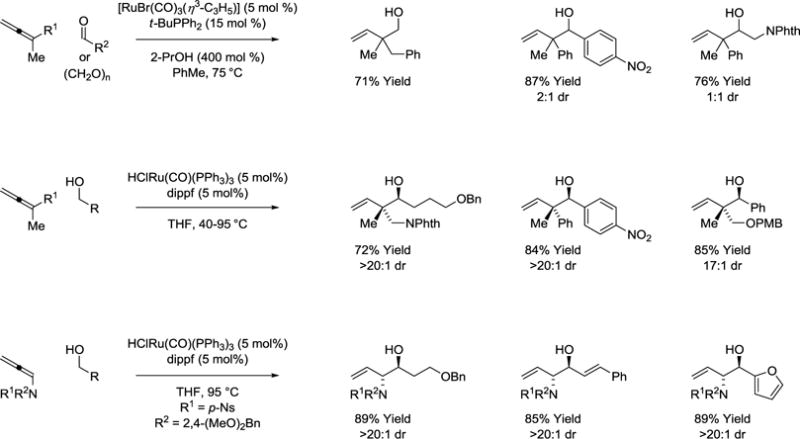
Alcohol-mediated hydrohydroxyalkylation of allenes.a
aYields are of material isolated by flash chromatography on silica gel. Diastereoselectivity was determined through 1H NMR analysis of crude reaction mixtures. dippf = bis-(diisopropylphosphino)ferrocene
In the case of mono-substituted allenes, the steric demand of an appropriately defined substituent can direct exclusive formation of (E)-σ-allylruthenium intermediates that participate in stereospecific carbonyl addition to deliver single diastereomers. For example, hydrogen transfer from primary alcohols to allenamides provides geometrically defined (amino)allylruthenium-aldehyde pairs that combine to form vicinal anti-aminoalcohols as single diastereomers (Scheme 6).45 Identical products are accessible as single diastereomers via 2-propanol-mediated reductive coupling allenamides and aldehydes (not shown).44
Isomerization of alkynes to allenes under the conditions of ruthenium catalyzed hydrohydroxyalkylation enables transformations that are otherwise inaccessible, including the conversion of primary alcohols to (Z)-homoallylic secondary alcohols (Scheme 7).48 Isomerization is promoted through the use of cationic ruthenium catalysts generated through the acid-base reaction of H2Ru(CO)(PPh3)3 and 2,4,6-(2-Pr)3PhSO3H. As corroborated by deuterium labelling studies, the cationic ruthenium complex appears to exist in equilibrium with zero-valent species that promote isomerization via propargyl C-H oxidative addition. Allene-aldehyde oxidative coupling mediated by ruthenium(0) then forms an oxaruthenacycle, which upon transfer hydrogenolysis delivers the (Z)-homoallylic alcohols with good to complete levels of stereocontrol. Oxidative coupling pathways are suppressed upon introduction of iodide ion and a chelating phosphine ligand, the Josiphos ligands SL-J009-1 or SL-J002-1, yet alkyne-to-allene isomerization pathways persist. Under these conditions, the transient allenes accept hydrogen from primary alcohols to form chiral allylruthenium-aldehyde pairs that deliver enantiomerically enriched branched homoallylic alcohols as single diastereomers.49 In this way, alkynes serve as chiral allylmetal equivalents.50–57
Scheme 7.
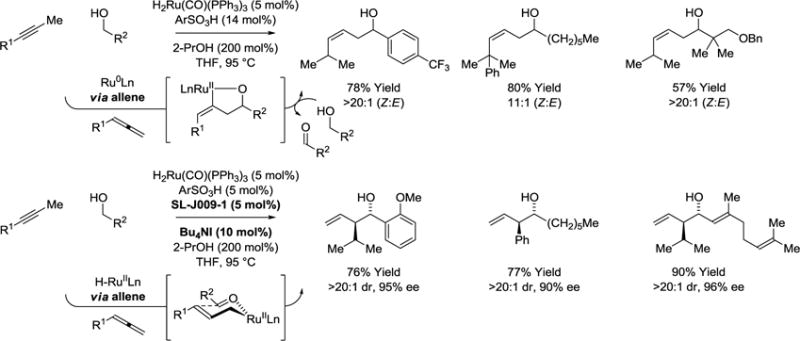
Alkynes as latent allenes in alcohol-mediated hydrohydroxyalkylation to form linear or branched homoallylic alcohols.a
aYields are of material isolated by flash chromatography on silica gel. Diastereoselectivity was determined through 1H NMR analysis of crude reaction mixtures. Enantiomeric excess was determined by chiral stationary phase HPLC analysis. SL-J009-1 = (R)-1-[(SP)-2-(dicyclohexylphosphino)ferrocenyl]ethyldi-tert-butylphosphine. Ar = 2,4,6-triisopropylphenyl.
A third mechanism for the coupling of primary alcohols with alkynes becomes operative when these conditions are applied to the propargyl ether, MeC≡CCH2OTIPS (TIPS = triisopropylsilyl) (Scheme 8).58 Unlike closely related ruthenium catalyzed alkyne-alcohol C-C couplings, deuterium labeling studies corroborate a novel 1,2-hydride shift mechanism that converts metal-bound alkynes to vinyl carbenes that protonate to form siloxy-π-allylruthenium nucleophiles in the absence of intervening allenes. Due to the negative inductive effect of the siloxy moiety, carbonyl addition occurs through a closed transition structure from the σ-allylruthenium haptomer where ruthenium resides at the oxygen-bearing carbon. Using a Josiphos (SL-J009-1) modified ruthenium(II) catalyst, the resulting products of siloxy-crotylation form as single regioisomers with complete levels of anti-diastereoselectivity and high levels of enantioselectivity. Although mixtures of enol geometrical isomers are produced, the (Z)- and (E)-selectivity is inconsequential as fluoride assisted cleavage of the enol in the presence of NaBH4 converts both isomers to the same 1,4-diol.
Scheme 8.
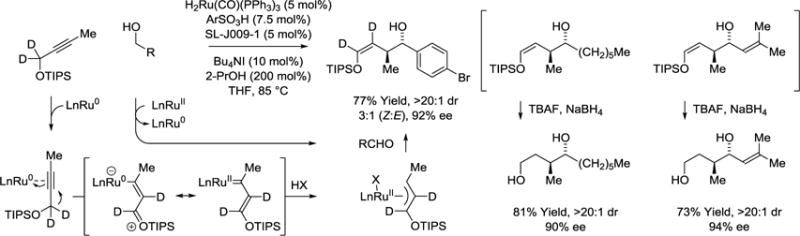
anti-Diastereo- and enantioselective siloxy-crotylation in the transfer hydrogenative coupling of primary alcohols with alkynes via hydride-shift enabled π-allyl formation.a
aYields are of material isolated by flash chromatography on silica gel. Diastereoselectivity was determined through 1H NMR analysis of crude reaction mixtures. Enantiomeric excess was determined by chiral stationary phase HPLC analysis. SL-J009-1 = (R)-1-[(SP)-2-(dicyclohexylphosphino)ferrocenyl]ethyldi-tert-butylphosphine.
Remarkably, a fourth mechanism for the coupling of primary alcohols with alkynes is evident in couplings that form allylic alcohols59 or conjugated enones (Scheme 9).60 These processes are catalyzed by (TFA)2Ru(CO)(PPh3)2 in the absence of added phosphine ligand. It is postulated that coordinative unsaturation, the presence of a π-acidic carbonyl ligand and the reducing environment provided by 2-propanol promote equilibration between ruthenium(II) and ruthenium(0) complexes. Thus, while the experimental data cannot exclude hydrometalative pathways involving vinylruthenium-aldehyde pairs, another possible mechanism involves ruthenium(0)-mediated alkyne-carbonyl oxidative coupling to form a ruthenacyclopentene that suffers alcohol-mediated transfer hydrogenolysis to release the allylic alcohol and regenerate the zero valent catalyst. Under more forcing conditions (higher temperatures, longer reaction times) and in the absence of 2-propanol, the initially formed allylic alcohols undergo further dehydrogenation to form the conjugated enones. Resubjection of the allylic alcohols to the reaction conditions results in formation of the enone, suggesting β-hydride elimination may not occur at the stage of the intermediate ruthenacycle.
Scheme 9.
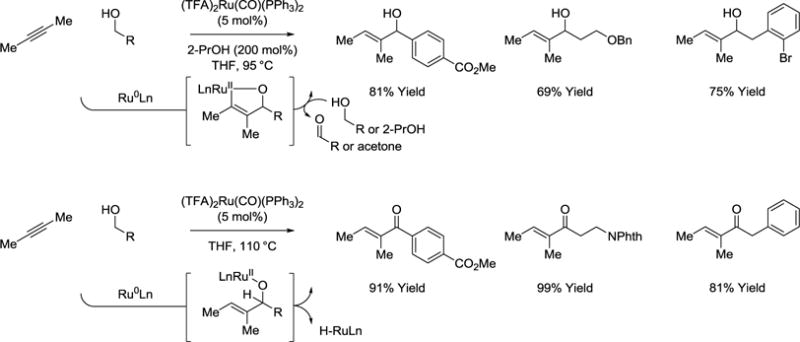
Transfer hydrogenative couplings of 2-butyne to form allylic alcohols and conjugated enones.a
aYields are of material isolated by flash chromatography on silica gel.
Hydrogen transfer from primary alcohols to 1,3-enynes delivers allenylruthenium-aldehyde pairs that combine to form products of carbonyl propargylation (Scheme 10).61–63 Initially developed conditions provided products of α-methyl-propargylation as diastereomeric mixtures.61 Identical products of propargylation are generated upon 2-propanol mediated 1,3-enyne-aldehyde reductive coupling.62 In subsequent work, it was found that anti-diastereoselectivity improves upon use of sterically demanding reactants.63 More recently, the chiral ruthenium complex formed in situ from (TFA)2Ru(CO)(PPh3)2 and (R)-BINAP was found to catalyze the C-C coupling of primary alcohols with the 1,3-enyne, TMSC≡CC(Me)=CH2, to form secondary homopropargyl alcohols bearing gem-dimethyl groups. These conditions deliver products of C-C coupling with uniformly high levels of enantioselectivity and are applicable to aliphatic, allylic and benzylic alcohols. One may view these protocols as an alternative to the use of stoichiometric allenylmetal reagents in carbonyl propargylation.64,65
Scheme 10.
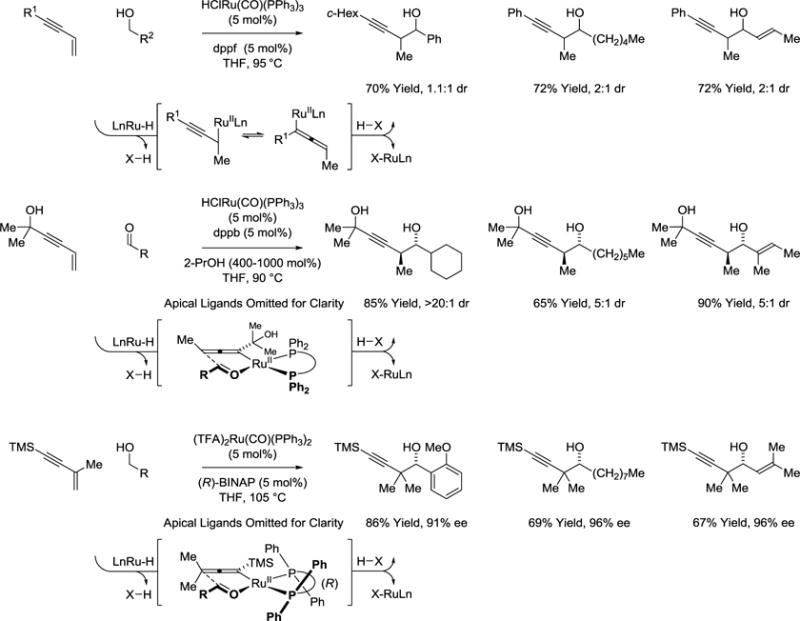
Carbonyl propargylation via 1,3-enyne hydrohydroxyalkylation.a
aYields are of material isolated by flash chromatography on silica gel. Diastereoselectivity was determined through 1H NMR analysis of crude reaction mixtures. Enantiomeric excess was determined by chiral stationary phase HPLC analysis. dppb = bis-(diphenylphosphino)butane. dppf = 1,1-bis-(diphenylphosphino)ferrocene. BINAP = 2,2′-bis-(diphenylphosphino)-1,1′-binaphthalene.
III. Conversion of Secondary Alcohols to Tertiary Alcohols
In 2012, it was found that ruthenium(0) complexes catalyze the C-C coupling of activated secondary alcohols with feedstock dienes such isoprene and myrcene to furnish products of carbinol C-H prenylation and geranylation, respectively (Scheme 11).66–68 Mechanistic studies corroborate a catalytic mechanism involving diene-carbonyl oxidative coupling to form an oxaruthenacycle. The transfer of hydrogen from the secondary alcohol reactant mediates transfer hydrogenolysis to release the products of C-C coupling and regenerate the activated ketone to close the catalytic cycle. The regioselectivity of C-C bond formation for the diene C4-position is unique among diene-carbonyl reductive couplings.41,69 Beyond α-hydroxy esters,66 these conditions are applicable to 3-hydroxy-2-oxindoles67 and secondary alcohols substituted by certain heteroaromatic moieties.68 In the latter case, the putative oxaruthenacycle intermediate was isolated, characterized and reversible metalacycle formation was demonstrated through experiments involving diene exchange.
Scheme 11.
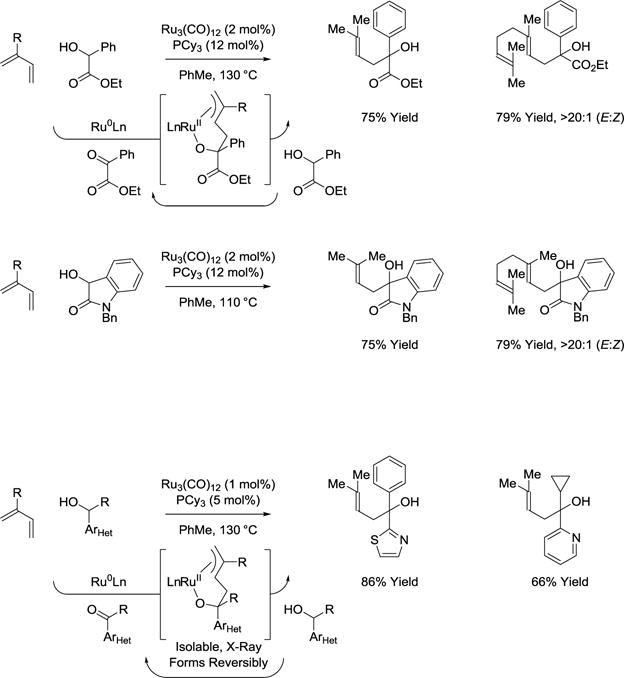
Conversion of secondary to tertiary alcohols via ruthenium(0) catalyzed C-C bond forming transfer hydrogenation with conjugated dienes.a
aYields are of material isolated by flash chromatography on silica gel. PCy3 = tricyclohexylphosphine.
The transient 1,2-dicarbonyl motifs required for oxidative coupling are also accessible from vicinal diols. For example, in the presence of the ruthenium(0) catalyst derived from Ru3(CO)12 and PCy3, vicinal diols and alkynes react to form α-hydroxy β,γ-unsaturated ketones as single geometrical isomers (Scheme 12).70 Here, it was found that carboxylic acid cocatalysts dramatically increase rate and conversion. A catalytic mechanism that accounts for the effect of the carboxylic acid cocatalysts is as follows. A mononuclear ruthenium(0) complex71 promotes alkyne-dione oxidative coupling to form the indicated oxaruthenacycle.72,73 Direct protonation of the oxaruthenacycle by the diol or ketol is postulated to be slow compared to protonolytic cleavage of the oxaruthenacycle by the carboxylic acid. The resulting ruthenium carboxylate exchanges with the diol or ketol to form a ruthenium alkoxide, which upon β-hydride elimination releases the ketol or dione, respectively, and a vinylruthenium hydride. C-H Reductive elimination furnishes the product of C-C coupling and returns ruthenium to its zero-valent form. Conventional diol-alkyne transfer hydrogenation provides the initial quantities of dione required for entry into the catalytic cycle.23,24,74
Scheme 12.
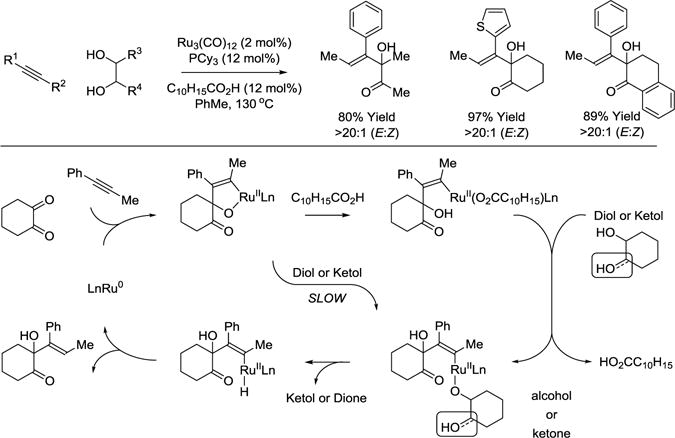
Ruthenium(0) catalyzed C-C coupling of diols with alkynes via transfer hydrogenation.a
aYields are of material isolated by flash chromatography on silica gel. C10H15CO2H = 1-adamantanecarboxylic acid. PCy3 = tricyclohexylphosphine.
Intermolecular catalytic reductive couplings of α-olefins with unactivated aldehydes and ketones remains an unmet challenge.75–77 In a significant step toward this goal, it was found that ruthenium(0) catalysts promote the transfer hydrogenative C-C coupling of 3-hydroxy-2-oxindoles with α-olefins, including feedstocks such as ethylene, propylene and styrene, to furnish the branched adducts as single regio- and diastereomers. In the absence of carboxylic acid cocatalyst, only trace quantities of product were formed (Scheme 13).78
Scheme 13.
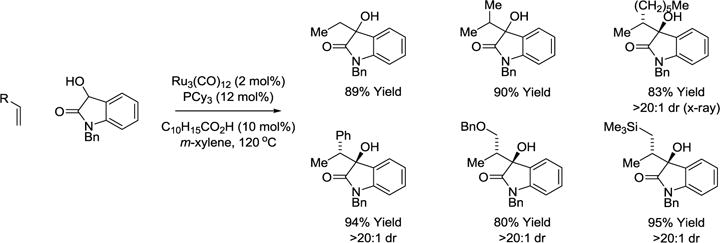
Ruthenium(0) catalyzed C-C coupling of diols with α-olefins via transfer hydrogenation.a
aYields are of material isolated by flash chromatography on silica gel. C10H15CO2H = 1-adamantanecarboxylic acid. PCy3 = tricyclohexylphosphine.
IV. Transfer Hydrogenative Cycloaddition
Intermolecular hydrogen transfer reactions that result in the formation of rings represent a new class of metal catalyzed cycloadditions.79,80 Ruthenium catalyzed C-C bond forming transfer hydrogenation contributes a new dimension to this emerging area. Using a ruthenium(0) catalyst, diols react with acrylates to form spiro-γ-butyrolactones (Scheme 14).81 Ethyl 2-(hydroxymethyl)acrylate reacts with diols by way of transient oxaruthenacycles that engage in E1cB elimination to furnish α-methylene-spiro-γ-butyrolactones.81 As illustrated in couplings with 3-hydroxy-2-oxindoles, β-substituted acrylic esters provides spiro-γ-butyrolactones as single diastereomers.81 Remarkably, the cycloadditions may be conducted in oxidative, redox-neutral or reductive modes using diols, ketols or diones, respectively, as reactants. To illustrate, ethyl acrylate reacts with hydrobenzoin, benzoin or benzil to form an identical γ-lactone. For the latter reaction involving benzyl, 2-propanol (300 mol%) is employed as terminal reductant (Scheme 15).
Scheme 14.
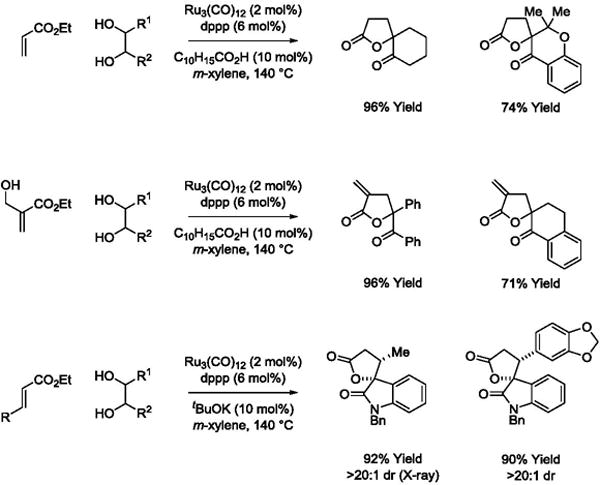
Ruthenium(0) catalyzed C-C coupling of acrylic esters with diols and α-hydroxycarbonyl compounds via transfer hydrogenation.a
aYields are of material isolated by flash chromatography on silica gel. C10H15CO2H = 1-adamantanecarboxylic acid. dppp = bis-(diphenylphosphino)propane.
Scheme 15.
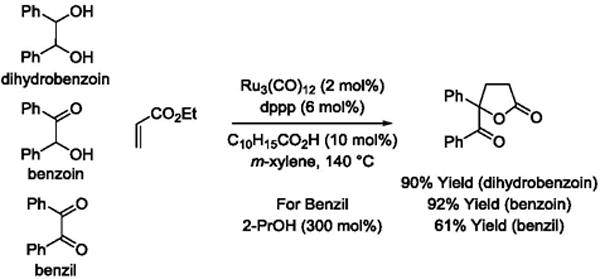
Redox level-independent cycloaddition to form a γ-lactone.a
aYields are of material isolated by flash chromatography on silica gel. C10H15CO2H = 1-adamantanecarboxylic acid. dppp = bis-(diphenylphosphino)propane.
In the presence of a ruthenium(0) catalyst, vicinal diols transfer hydrogen to conjugated dienes to furnish diones that engage in diene-carbonyl oxidative coupling. The resulting oxaruthenacycles incorporate an allylruthenium moiety that engages the pendant ketone in intramolecular allylruthenation to form products of formal [4+2] cycloaddition as single diastereomers (Scheme 16).82,83 The cycloadducts are readily transformed to the 9–12 membered 1,6-diketones upon exposure to iodosobenzene diacetate.83 Alternatively, the cycloadducts can be dehydrated to form products of benzannulation.84 Two-directional benzannulation is especially powerful. For example, exposure of the indicated pyracylene-based tetraol to butadiene in the presence of the ruthenium(0) catalyst delivers the double [4+2] cycloadduct, which is directly dehydrated to form the indeno[1,2,3-cd]-fluoranthene in a single “one-pot” operation.
Scheme 16.
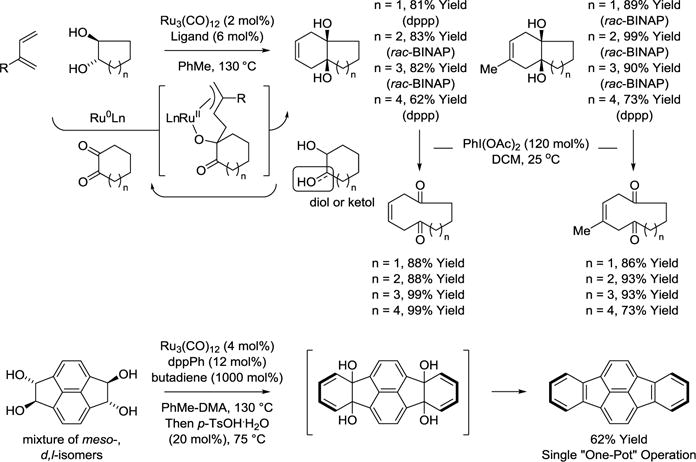
Transfer hydrogenative diene-diol [4+2] cycloaddition.a
aYields are of material isolated by flash chromatography on silica gel. dppp = bis-(diphenylphosphino)propane. BINAP = 2,2′-bis-(diphenylphosphino)-1,1′-binaphthalene. dppPh = bis-(1,2-diphenylphosphino)benzene.
Exposure of 3,4-benzannulated 1,5-diynes (benzo-endiynes) to α-ketols in the presence of ruthenium(0) catalysts derived from Ru3(CO)12 and RuPhos results in successive redox-triggered C-C coupling to generate products of [4+2] cycloaddition (Scheme 17).85 Here, redox-neutral couplings using α-ketols are essential, as diols require a sacrificial hydrogen acceptor, which contributes to partial reduction of the diyne reactant. Regioselective cycloaddition is achieved using nonsymmetric diynes with alkyne termini substituted by n-propyl and t-butyl groups. This strategy for cycloaddition has been extended to the reaction of ortho-acetylenic benzaldehydes with α-ketols. Using ruthenium(0) catalysts modified by CyJohnPhos, the indicated products of [4+2] cycloaddition form as single regio- and diastereomers.86 This methodology enables convergent construction of ring systems characteristic of type II polyketides, specifically those of the angucycline class.87–89
Scheme 17.
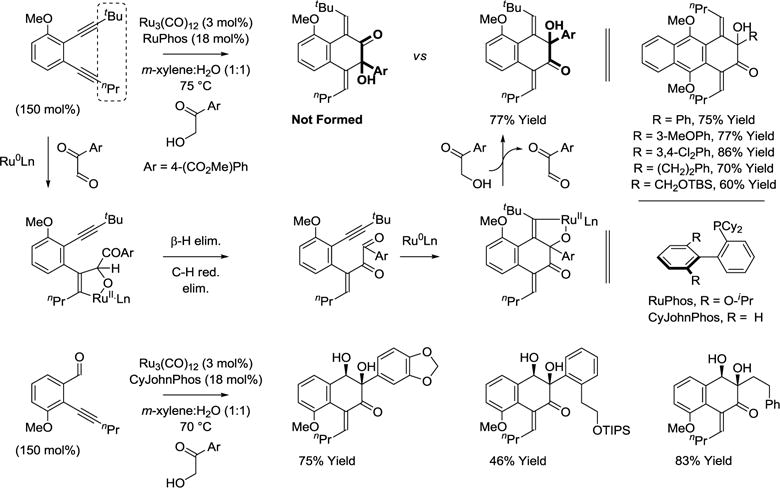
Transfer hydrogenative cycloaddition of α-ketols with benzannulated 1,5-diynes or ortho-acetylenic benzaldehydes.a
aYields are of material isolated by flash chromatography on silica gel.
V. Hydroaminoalkylation
Since the initial discovery of metal catalyzed hydroaminoalkylation Maspero90 and Nugent91 in the early 1980’s, significant advances in the field of hydroaminoalkylation using early transition metal catalysts have been made. In contrast, the development of corresponding late transition metal catalyzed amine C-H functionalizations has proven challenging.92–94 Indeed, with the exception of the present authors work,95–99 all other late transition metal catalyzed hydroaminoalkylations require pyridyl directing groups in combination with mono-olefin reactants.100–108 In a significant departure from prior art, it was found that ruthenium catalyzed hydrogen transfer from 4-aminobutanol to 1-substituted-1,3-dienes results in the generation of dihydropyrrole-allylruthenium pairs, which combine to form products of hydroaminoalkylation with good to complete control of anti-diastereoselectivity (Scheme 18).95 As corroborated by deuterium labeling experiments, kinetically preferred hydrometalation of the terminal olefin of the 1-substituted-1,3-diene delivers a 1,1-disubstituted π-allylruthenium complex that isomerizes to the more stable monosubstituted π-allylruthenium complex. Imine addition then occurs with allylic inversion through a closed transition structure. Using a carboxylic acid cocatalyst, pyrrolidine itself can be engaged in direct ruthenium catalyzed diene hydroaminoalkylations. Finally, 2-propanol mediated reductive coupling of butadiene with the dihydropyrrole trimer provides the identical product of diene hydroaminoalkylation. All three reaction types proceed through a common set of reactive intermediates, as shown in the indicated stereochemical model.
Scheme 18.
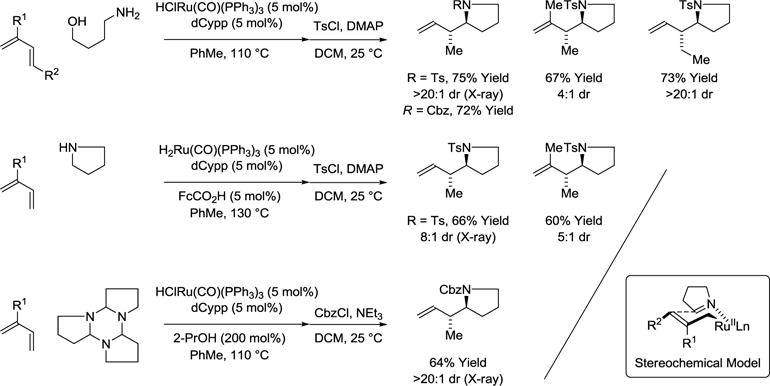
Transfer hydrogenative imine addition and hydroaminoalkylation.a
aYields are of material isolated by flash chromatography on silica gel. FcCO2H = ferrocene carboxylic acid. dCypp = bis-(dicyclohexylphosphino)propane.
Carbonylative hydroaminomethylation (hydroformylation-reductive amination)109–115 has only been reported for mono-olefin reactants, as hydroformylation of dienes and allenes suffers from poor regioselectivity and “over-hydroformylation” to form dialdehyde products. In contrast, 2-propanol-mediated reductive couplings of allenes or dienes with formaldimines (generated in situ from saturated 1,3,5-triazines) are efficient and selective processes.95–98 Specifically, ruthenium catalyzed transfer hydrogenation of allenes in the presence of 1,3,5-tris(4-methoxyphenyl)-hexahydro-1,3,5-triazine provides products of hydroaminomethylation as single regioisomers.96 Under similar conditions, butadiene and related 2-substituted dienes engage in regioselective reductive C-C coupling to furnish products of hydroaminomethylation.97 Here, higher temperatures (140 °C) are required to supress the competing aza-Diels-Alder reaction of formaldimine. Regioselective 2-propanol mediated reductive coupling of dienes with iminoacetates also have been described (not shown).98
Whereas ruthenium(II) catalysts promote hydroaminoalkylation through hydrometalative pathways, ruthenium(0) catalysts derived from Ru3(CO)12 and triphos enable catalytic mechanism involving diene-imine oxidative coupling (Scheme 20).99 Presently, transformations of this type are restricted to the hydroaminoalkylation of isoprene with aryl substituted hydantoins. The catalytic mechanism involves hydrogenolytic cleavage of the azaruthenacyclopentane intermediate through hydrogen transfer from the hydantoin reactant, which releases product and regenerates the requisite imine for oxidative coupling.
Scheme 20.
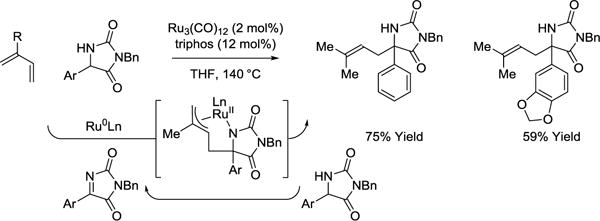
Regioselective ruthenium(0) catalyzed hydroaminoalkylation of isoprene with hydantoins.a
aYields are of material isolated by flash chromatography on silica gel. triphos = bis-(diphenylphosphinoethyl)phenylphosphine.
VI. Conclusion and Outlook
Since the seminal work of Sabatier and Grignard, hydrogenation and carbonyl addition have found longstanding use as methods for chemical synthesis. Merging the chemistry of transfer hydrogenation and carbonyl or imine addition, we have developed a broad, new family of reductive and redox-neutral hydrohydroxyalkylations and hydroaminomethylations – processes in which the transfer or redistribution of hydrogen is accompanied by C-C bond formation. We have just begun to exploit the potential of this novel reactivity mode, yet already one may see that carbonyl additions traditionally employing stoichiometric organometallic reagents can now be conducted catalytically via hydrogen transfer. Perhaps most importantly, this new reactivity has enabled transformations that have no counterpart in the current lexicon of synthetic methods.
Scheme 19.
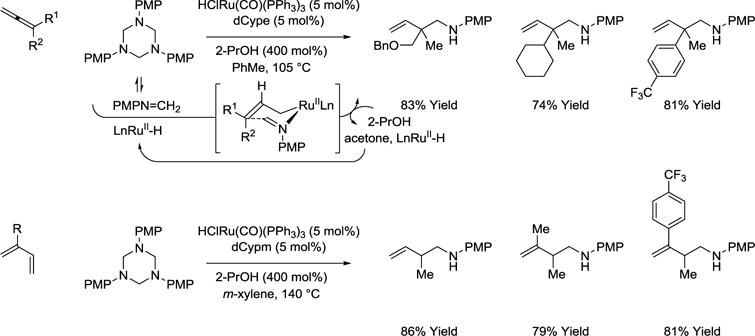
Regioselective hydroaminomethylation of allenes and dienes via 2-propanol mediated reductive coupling with formaldimines.a
aYields are of material isolated by flash chromatography on silica gel. dCypm = bis-(dicyclohexylphosphino)methane. dCype = bis-(dicyclohexylphosphino)ethane.
Acknowledgments
Acknowledgment is made to the Robert A. Welch Foundation (F-0038) and the NIH-NIGMS (RO1 GM-069445) for partial financial support.
Bibliography
- 1.Boyce J. Process of producing an edible compound. US1061254 A. 1913 May 6; James F. Boyce is credited with the hydrogenation of vegetable oils prior to Sabatier’s seminal work. To our knowledge, the earliest written record of Boyce’s work is a patent filed in 1911.
- 2.Sabatier P, Senderens J-B. Action du Nickel sur l’Éthyléne. Synthéses de l’Éthane. C R Acad Sci Paris. 1897;124:1358–1360. [Google Scholar]
- 3.Kagan HB. Victor Grignard and Paul Sabatier: Two Showcase Laureates of the Nobel Prize for Chemistry. Angew Chem Int Ed. 2012;51:7376–7382. doi: 10.1002/anie.201201849. [DOI] [PubMed] [Google Scholar]
- 4.Voorhees V, Adams R. The Use of the Oxides of Platinum for the Catalytic Reduction of Organic Compounds. J Am Chem Soc. 1922;44:1397–1405. [Google Scholar]
- 5.Calvin M, Polyani M. Homogeneous Catalytic Hydrogenation. Trans Faraday Soc. 1938;34:1181–1191. [Google Scholar]
- 6.Halpern J, Harrod JF, James BR. Homogeneous Catalytic Hydrogenation of Olefinic Compounds. J Am Chem Soc. 1961;83:753–754. [Google Scholar]
- 7.Vaska L, DiLuzio JW. Activation of Hydrogen by a Transition Metal Complex at Normal Conditions Leading to a Stable Molecular Dihydride. J Am Chem Soc. 1962;84:679–680. [Google Scholar]
- 8.Jardine FH, Osborn JA, Wilkinson G, Young JF. Homogeneous Catalytic Hydrogenation and Hydroformylation of Acetylenic Compounds. Chem Ind. 1965:560–561. [Google Scholar]
- 9.Young JF, Osborn JA, Jardine FH, Wilkinson G. Hydride Intermediates in Homogeneous Hydrogenation Reactions of Olefins and Acetylenes using Rhodium Catalysts. Chem Commun. 1965:131–132. [Google Scholar]
- 10.Knowles WS, Sabacky MJ. Catalytic Asymmetric Hydrogenation Employing a Soluble, Optically Active, Rhodium Complex. Chem Commun. 1968:1445–1446. [Google Scholar]
- 11.Horner L, Winkler H, Rapp A, Mentrup A, Hoffmann H, Beck P. Phosphororganische Verbindungen Optisch Aktive Tertiare Phosphine aus Optisch Aktiven Quartaren Phosphoniumsalzen. Tetrahedron Lett. 1961;2:161–166. [Google Scholar]
- 12.Korpiun O, Mislow K. New Route to the Preparation and Configurational Correlation of Optically Active Phosphine Oxides. J Am Chem Soc. 1967;89:4784–4786. [Google Scholar]
- 13.Dang TP, Kagan HB. The Asymmetric Synthesis of Hydratropic Acid and Amino-acids by Homogeneous Hydrogenation. J Chem Soc D Chem Commun. 1971:481–481. [Google Scholar]
- 14.Miyashita A, Yasuda A, Takaya H, Toriumi K, Ito T, Souchi T, Noyori R. Synthesis of 2,2′-Bis(diphenylphosphino)-1,1-binapthyl (BINAP), an Atropisomeric Chiral Bis(triaryl)phosphine, and Its Use in the Rhodium(I)-Catalyzed Asymmetric Hydrogenation of α-(Acylamino)acrylic Acids. J Am Chem Soc. 1980;102:7932–7934. [Google Scholar]
- 15.Noyori R, Takaya H. BINAP: An Efficient Chiral Element for Asymmetric Catalysis. Acc Chem Res. 1990;23:345–350. [Google Scholar]
- 16.Shibahara F, Krische MJ. Formation of C-C Bonds via Ruthenium Catalyzed Transfer Hydrogenation: Carbonyl Addition from the Alcohol or Aldehyde Oxidation Level. Chem Lett. 2008;37:1102–1107. doi: 10.1246/cl.2008.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bower JF, Krische MJ. Formation of C-C Bonds via Iridium Catalyzed Hydrogenation and Transfer Hydrogenation. Top Organomet Chem. 2011;34:107–138. doi: 10.1007/978-3-642-15334-1_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassan A, Krische MJ. Unlocking Hydrogenation for C-C Bond Formation: A Brief Overview of Enantioselective Methods. Org Process Res Dev. 2011;15:1236–1242. doi: 10.1021/op200195m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moran J, Krische MJ. Formation of C-C Bonds via Ruthenium Catalyzed Transfer Hydrogenation. Pure Appl Chem. 2012;84:1729–1739. doi: 10.1351/PAC-CON-11-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ketcham JM, Shin I, Montgomery TP, Krische MJ. Catalytic Enantioselective C-H Functionalization of Alcohols by Redox-Triggered Carbonyl Addition: Borrowing Hydrogen, Returning Carbon. Angew Chem Int Ed. 2014;53:9142–9150. doi: 10.1002/anie.201403873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasson Y, Blum J. Homogenous Catalytic Transfer-Hydrogenation of α,β-Unsaturated Carbonyl Compounds by Dichlorotris(triphenylphosphine)ruthenium(II) Tetrahedron Lett. 1971;12:2167–2170. [Google Scholar]
- 22.Dobson A, Robinson SD. Complexes of the platinum metals. 7. Homogeneous Ruthenium and Osmium Catalysts for the Dehydrogenation of Primary and Secondary Alcohols. Inorg Chem. 1977;16:137–142. [Google Scholar]
- 23.Blum Y, Reshef D, Shvo Y. H-Transfer Catalysis with Ru3(CO)12. Tetrahedron Lett. 1981;22:1541–1544. [Google Scholar]
- 24.Shvo Y, Blum Y, Reshef D, Menzin M. Catalytic Oxidative Coupling of Diols by Ru3(CO)12. J Organomet Chem. 1982;226:C21–C24. [Google Scholar]
- 25.Noyori R, Ohta M, Hsiao Y, Kitamura M, Ohta T, Takaya H. Asymmetric Synthesis of Isoquinoline Alkaloids by Homogeneous Catalysis. J Am Chem Soc. 1986;108:7117–7119. [Google Scholar]
- 26.Hashiguchi S, Fujii A, Takehara J, Ikariya T, Noyori R. Asymmetric Transfer Hydrogenation of Aromatic Ketones Catalyzed by Chiral Ruthenium(II) Complexes. J Am Chem Soc. 1995;117:7562–7563. [Google Scholar]
- 27.Jang H-Y, Huddleston RR, Krische MJ. Reductive Generation of Enolates from Enones Using Elemental Hydrogen: Catalytic C–C Bond Formation under Hydrogenative Conditions. J Am Chem Soc. 2002;124:15156–15157. doi: 10.1021/ja021163l. [DOI] [PubMed] [Google Scholar]
- 28.Iida H, Krische MJ. Catalytic Reductive Coupling of Alkenes and Alkynes to Carbonyl Compounds and Imines Mediated by Hydrogen. Top Curr Chem. 2007;279:77–104. [Google Scholar]
- 29.Skucas E, Ngai M-Y, Komanduri V, Krische MJ. Enantiomerically Enriched Allylic Alcohols and Allylic Amines via C-C Bond Forming Hydrogenation: Asymmetric Carbonyl and Imine Vinylation. Acc Chem Res. 2007;40:1394–1401. doi: 10.1021/ar7001123. [DOI] [PubMed] [Google Scholar]
- 30.Bower JF, Skucas E, Patman RL, Krische MJ. Catalytic C-C Coupling via Transfer Hydrogenation: Reverse Prenylation, Crotylation and Allylation from the Alcohol or Aldehyde Oxidation Level. J Am Chem Soc. 2007;129:15134–15135. doi: 10.1021/ja077389b. [DOI] [PubMed] [Google Scholar]
- 31.Shibahara F, Bower JF, Krische MJ. Ruthenium Catalyzed C-C Bond Forming Transfer Hydrogenation: Carbonyl Allylation from the Alcohol or Aldehyde Oxidation Level Employing Acyclic 1,3-Dienes as Surrogates to Preformed Allyl Metal Reagents. J Am Chem Soc. 2008;130:6338–6339. doi: 10.1021/ja801213x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shibahara F, Bower JF, Krische MJ. Diene Hydroacylation from the Alcohol or Aldehyde Oxidation Level via Ruthenium-Catalyzed C-C Bond-Forming Transfer Hydrogenation: Synthesis of β,γ-Unsaturated Ketones. J Am Chem Soc. 2008;130:14120–14122. doi: 10.1021/ja805356j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zbieg JR, Moran J, Krische MJ. Diastereo- and Enantioselective Ruthenium Catalyzed Hydrohydroxyalkylation of 2-Silyl-Butadienes: Carbonyl syn-Crotylation from the Alcohol Oxidation Level. J Am Chem Soc. 2011;133:10582–10586. doi: 10.1021/ja2046028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato F, Kusakabe M, Kobayashi Y. Highly Diastereofacial Selective Addition of Nucleophiles to 2-Alkyl-3-Trimethylsilylalk-3-enyl Carbonyl Compounds. Stereoselective Preparation of β-Methylhomoallyl Alcohols and β-Hydroxy-α-Methyl Ketones. J Chem Soc Chem Commun. 1984:1130–1132. [Google Scholar]
- 35.Helm MD, Mayer P, Knochel P. Preparation of Silyl Substituted Crotylzinc Reagents and Their Highly Diastereoselective Addition to Carbonyl Compounds. Chem Commun. 2008:1916–1917. doi: 10.1039/b802157k. [DOI] [PubMed] [Google Scholar]
- 36.Grayson MN, Krische MJ, Houk KN. Ruthenium-Catalyzed Asymmetric Hydrohydroxyalkylation of Butadiene: The Role of the Formyl Hydrogen Bond in Stereochemical Control. J Am Chem Soc. 2015;137:8838–8850. doi: 10.1021/jacs.5b04844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zbieg JR, Yamaguchi E, McInturff EL, Krische MJ. Enantioselective C-H Crotylation of Primary Alcohols via Hydrohydroxyalkylation of Butadiene. Science. 2012;336:324–327. doi: 10.1126/science.1219274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McInturff EL, Yamaguchi E, Krische MJ. Chiral Anion Dependent Inversion of Diastereo- and Enantioselectivity in Carbonyl Crotylation via Ruthenium Catalyzed Butadiene Hydrohydroxyalkylation. J Am Chem Soc. 2012;134:20628–20631. doi: 10.1021/ja311208a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smejkal T, Han H, Breit B, Krische MJ. All Carbon Quaternary Centers via Ruthenium Catalyzed Hydroxymethylation of 2-Substituted Butadienes Mediated by Formaldehyde: Beyond Hydroformylation. J Am Chem Soc. 2009;131:10366–10367. doi: 10.1021/ja904124b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Köpfer A, Sam B, Breit B, Krische MJ. Regiodivergent Reductive Coupling of 2-Substituted Dienes to Formaldehyde Employing Ruthenium or Nickel Catalysts: Hydrohydroxymethylation via Transfer Hydrogenation. Chem Sci. 2013;4:1876–1880. [Google Scholar]
- 41.Sam B, Breit B, Krische MJ. Paraformaldehyde and Methanol as C1-Feedstocks in Metal Catalyzed C-C Couplings of π-Unsaturated Reactants: Beyond Hydroformylation. Angew Chem Int Ed. 2015;54:3267–3274. doi: 10.1002/anie.201407888. [DOI] [PubMed] [Google Scholar]
- 42.Han H, Krische MJ. Direct Ruthenium Catalyzed C-C Coupling of Ethanol: Diene Hydro-Hydroxyethylation to Form All Carbon Quaternary Centers. Org Lett. 2010;12:2844–2846. doi: 10.1021/ol101077v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ngai M-Y, Skucas E, Krische MJ. Ruthenium Catalyzed C-C Bond Formation via Transfer Hydrogenation: Branch-Selective Reductive Coupling of Allenes to Paraformaldehyde and Higher Aldehydes. Org Lett. 2008;10:2705–2708. doi: 10.1021/ol800836v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skucas E, Zbieg JR, Krische MJ. anti-Aminoallylation of Aldehydes via Ruthenium Catalyzed Transfer Hydrogenative Coupling of Sulfonamido-Allenes: 1,2-Aminoalcohols. J Am Chem Soc. 2009;131:5054–5055. doi: 10.1021/ja900827p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zbieg JR, McInturff EL, Krische MJ. Allenamide Hydro-Hydroxyalkylation: 1,2-Aminoalcohols via Ruthenium Catalyzed Carbonyl anti-Aminoallylation. Org Lett. 2010;12:2514–2516. doi: 10.1021/ol1007235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zbieg JR, McInturff EL, Leung JC, Krische MJ. Amplification of anti-Diastereoselectivity via Curtin-Hammett Effects in Ruthenium Catalyzed Hydrohydroxyalkylation of 1,1-Disubstituted Allenes: Diastereoselective Formation of All-Carbon Quaternary Centers. J Am Chem Soc. 2011;133:1141–1144. doi: 10.1021/ja1104156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sam B, Luong T, Krische MJ. Ruthenium-Catalyzed C-C Coupling of Fluorinated Alcohols with Allenes: Dehydrogenation at the Energetic Limit of β-Hydride Elimination. Angew Chem Int Ed. 2015;54:5465–5469. doi: 10.1002/anie.201500238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park BY, Nguyen KD, Chaulagain MR, Komanduri V, Krische MJ. Alkynes as Allylmetal Equivalents in Redox-Triggered C-C Couplings to Primary Alcohols: (Z)-Homoallylic Alcohols via Ruthenium Catalyzed Propargyl C-H Oxidative Addition. J Am Chem Soc. 2014;136:11902–11905. doi: 10.1021/ja505962w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang T, Nguyen KD, Zhang W, Krische MJ. Enantioselective Ruthenium Catalyzed Carbonyl Allylation via Alkyne-Alcohol C-C Bond Forming Transfer Hydrogenation: Allene Hydrometallation vs. Oxidative Coupling. J Am Chem Soc. 2015;137:3161–3164. doi: 10.1021/jacs.5b00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramachandran PV. Pinane-Based Versatile Allyl Boranes. Aldrichimica Acta. 2002;35:23–35. [Google Scholar]
- 51.Kennedy JWJ, Hall DG. Recent Advances in the Activation of Boron and Silicon Reagents for Stereocontrolled Allylation Reactions. Angew Chem Int Ed. 2003;42:4732–4739. doi: 10.1002/anie.200301632. [DOI] [PubMed] [Google Scholar]
- 52.Denmark SE, Fu J. Catalytic Enantioselective Addition of Allylic Organometallic Reagents to Aldehydes and Ketones. Chem Rev. 2003;103:2763–2794. doi: 10.1021/cr020050h. [DOI] [PubMed] [Google Scholar]
- 53.Yu C-M, Youn J, Jung H-K. Regulation of Stereoselectivity and Reactivity in the Inter- and Intramolecular Allylic Transfer Reactions. Bull Korean Chem Soc. 2006;27:463–472. [Google Scholar]
- 54.Marek I, Sklute G. Creation of Quaternary Stereocenters in Carbonyl Allylation Reactions. Chem Commun. 2007:1683–1691. doi: 10.1039/b615042j. [DOI] [PubMed] [Google Scholar]
- 55.Hall DG. Lewis and Brønsted Acid Catalyzed Allylboration of Carbonyl Compounds: From Discovery to Mechanism and Applications. Synlett. 2007:1644–1655. [Google Scholar]
- 56.Lachance H, Hall DG. Allylboration of Carbonyl Compounds. Org React. 2008;73:1–573. [Google Scholar]
- 57.Yus M, González-Gómez JC, Foubelo F. Catalytic Enantioselective Allylation of Carbonyl Compounds and Imines. Chem Rev. 2011;111:7774–7854. doi: 10.1021/cr1004474. [DOI] [PubMed] [Google Scholar]
- 58.Liang T, Zhang W, Chen T-Y, Nguyen KD, Krische MJ. Ruthenium Catalyzed Diastereo- and Enantioselective Coupling of Propargyl Ethers with Alcohols: Siloxy-Crotylation via Hydride Shift Enabled Conversion of Alkynes to π-Allyls. J Am Chem Soc. 2015;137:13066–13071. doi: 10.1021/jacs.5b08019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patman RL, Chaulagain MR, Williams VM, Krische MJ. Direct Vinylation of Alcohols or Aldehydes Employing Alkynes as Vinyl Donors: A Ruthenium Catalyzed C-C Bond Forming Transfer Hydrogenation. J Am Chem Soc. 2009;131:2066–2067. doi: 10.1021/ja809456u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams VM, Leung JC, Patman RL, Krische MJ. Hydroacylation of 2-Butyne from the Alcohol or Aldehyde Oxidation Level via Ruthenium Catalyzed C-C Bond Forming Transfer Hydrogenation. Tetrahedron. 2009;65:5024–5029. doi: 10.1016/j.tet.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patman RL, Williams VM, Bower JF, Krische MJ. Carbonyl Propargylation from the Alcohol or Aldehyde Oxidation Level Employing 1,3-Enynes as Surrogates to Preformed Allenylmetal Reagents: A Ruthenium Catalyzed C-C Bond Forming Transfer Hydrogenation. Angew Chem Int Ed. 2008;47:5220–5223. doi: 10.1002/anie.200801359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geary LM, Leung JC, Krische MJ. Ruthenium Catalyzed Reductive Coupling of 1,3-Enynes and Aldehydes via Transfer Hydrogenation: anti-Diastereoselective Carbonyl Propargylation. Chem Eur J. 2012;18:16823–16827. doi: 10.1002/chem.201202446. [DOI] [PubMed] [Google Scholar]
- 63.Nguyen KD, Herkommer D, Krische MJ. Ruthenium-BINAP Catalyzed Alcohol C-H tert-Prenylation via 1,3-Enyne Transfer Hydrogenation: Beyond Stoichiometric Carbanions in Enantioselective Carbonyl Propargylation. J Am Chem Soc. 2016;138 doi: 10.1021/jacs.6b02279. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ding C-H, Lou X-L. Catalytic Asymmetric Propargylation. Chem Rev. 2011;111:1914–1937. doi: 10.1021/cr100284m. [DOI] [PubMed] [Google Scholar]
- 65.Wisniewska HM, Jarvo ER. Enantioselective Propargylation and Allenylation Reactions of Ketones and Imines. J Org Chem. 2013;78:11629–11636. doi: 10.1021/jo4019107. [DOI] [PubMed] [Google Scholar]
- 66.Leung JC, Geary LM, Chen T-Y, Zbieg JR, Krische MJ. Direct, Redox Neutral Prenylation and Geranylation of Secondary Carbinol C-H Bonds: C4 Regioselectivity in Ruthenium Catalyzed C-C Couplings of Dienes to α-Hydroxy Esters. J Am Chem Soc. 2012;134:15700–15703. doi: 10.1021/ja3075049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen T-Y, Krische MJ. Regioselective Ruthenium Catalyzed Hydrohydroxyalkylation of Dienes with 3-Hydroxy-2-Oxindoles: Prenylation, Geranylation and Beyond. Org Lett. 2013;15:2994–2997. doi: 10.1021/ol401184k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park BY, Montgomery TP, Garza VJ, Krische MJ. Ruthenium Catalyzed Hydrohydroxyalkylation of Isoprene with Heteroaromatic Secondary Alcohols: Isolation and Reversible Formation of the Putative Metallacycle Intermediate. J Am Chem Soc. 2013;135:16320–16323. doi: 10.1021/ja4087193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kimura M, Tamaru Y. Nickel-Catalyzed Reductive Coupling of Dienes and Carbonyl Compounds. Top Curr Chem. 2007;279:173–207. [Google Scholar]
- 70.McInturff EL, Nguyen KD, Krische MJ. Redox-Triggered C-C Coupling of Diols and Alkynes: Synthesis of β,γ-Unsaturated α-Hydroxyketones and Furans by Ruthenium Catalyzed Hydrohydroxyalkylation. Angew Chem Int Ed. 2014;53:3232–3235. doi: 10.1002/anie.201311130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ru3(CO)12 reacts with dppe in benzene solvent to provide Ru(CO)3(dppe):; Sanchez-Delgado RA, Bradley JS, Wilkinson G. Further Studies on the Homogeneous Hydroformylation of Alkenes by Use of Ruthenium Complex Catalysts. J Chem Soc Dalton Trans. 1976:399–404. [Google Scholar]
- 72.Chatani N, Tobisu M, Asaumi T, Fukumoto Y, Murai S. Ruthenium Carbonyl-Catalyzed [2+2+1]-Cycloaddition of Ketones, Olefins, and Carbon Monoxide, Leading to Functionalized γ-Butyrolactones. J Am Chem Soc. 1999;121:7160–7161. [Google Scholar]
- 73.Tobisu M, Chatani N, Asaumi T, Amako K, Ie Y, Fukumoto Y, Murai S. Ru3(CO)12-Catalyzed Intermolecular Cyclocoupling of Ketones, Alkenes or Alkynes, and Carbon Monoxide. [2+2+1] Cycloaddition Strategy for the Synthesis of Functionalized γ-Butyrolactones. J Am Chem Soc. 2000;122:12663–12674. [Google Scholar]
- 74.Meijer RH, Ligthart GBWL, Meuldijk J, Vekemans JAJM, Hulshof LA, Mills AM, Kooijman H, Spek AL. Triruthenium Dodecacarbonyl/Triphenylphosphine Catalyzed Dehydrogenation of Primary and Secondary Alcohols. Tetrahedron. 2004;60:1065–1072. [Google Scholar]
- 75.Crowe WE, Rachita MJ. Titanium-Catalyzed Reductive Cyclization of δ,ε-Unsaturated Ketones and Aldehydes. J Am Chem Soc. 1995;117:6787–6788. [Google Scholar]
- 76.Kablaoui NM, Buchwald SL. Development of a Method for the Reductive Cyclization of Enones by a Titanium Catalyst. J Am Chem Soc. 1996;118:3182–3191. [Google Scholar]
- 77.Kablaoui NM, Buchwald SL. Reductive Cyclization of Enones by a Titanium Catalyst. J Am Chem Soc. 1995;117:6785–6786. [Google Scholar]
- 78.Yamaguchi E, Mowat J, Luong T, Krische MJ. Regio- and Diastereoselective C-C Coupling of α-Olefins and Styrenes to 3-Hydroxy-2-Oxindoles by Ru-Catalyzed Hydrohydroxyalkylation. Angew Chem Int Ed. 2013;52:8428–8431. doi: 10.1002/anie.201303552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lautens M, Klute W, Tam W. Transition Metal-Mediated Cycloaddition Reactions. Chem Rev. 1996;96:49–92. doi: 10.1021/cr950016l. [DOI] [PubMed] [Google Scholar]
- 80.Nandakumar A, Midya SP, Landge VG, Balaraman E. Transition-Metal-Catalyzed Hydrogen-Transfer Annulations: Access to Heterocyclic Scaffolds. Angew Chem Int Ed. 2015;54:11022–11034. doi: 10.1002/anie.201503247. [DOI] [PubMed] [Google Scholar]
- 81.McInturff EL, Mowat J, Waldeck AR, Krische MJ. Ruthenium Catalyzed Hydrohydroxyalkylation of Acrylates with Diols and α-Hydroxycarbonyl Compounds to Form Spiro- and α-Methylene-γ-Butyrolactones. J Am Chem Soc. 2013;135:17230–17235. doi: 10.1021/ja410533y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Geary LM, Glasspoole BW, Kim MM, Krische MJ. Successive C-C Coupling of Dienes to Vicinally Dioxygenated Hydrocarbons: Ruthenium Catalyzed [4+2] Cycloaddition across the Diol, Hydroxycarbonyl or Dione Oxidation Levels. J Am Chem Soc. 2013;135:3796–3799. doi: 10.1021/ja400691t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kasun ZA, Geary LM, Krische MJ. Ring Expansion of Cyclic 1,2-Diols to form Medium Sized Rings via Ruthenium Catalyzed Transfer Hydrogenative [4+2] Cycloaddition. Chem Commun. 2014;50:7545–7547. doi: 10.1039/c4cc03983a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Geary LM, Chen TY, Montgomery TP, Krische MJ. Benzannulation via Ruthenium Catalyzed Diol-Diene [4+2] Cycloaddition: One- and Two-Directional Syntheses of Fluoranthenes and Acenes. J Am Chem Soc. 2014;136:5920–5922. doi: 10.1021/ja502659t. [DOI] [PubMed] [Google Scholar]
- 85.Saxena A, Perez F, Krische MJ. Ruthenium(0) Catalyzed Endiyne-α-Ketol [4+2] Cycloaddition: Convergent Assembly of Type II Polyketide Substructures. J Am Chem Soc. 2015;137:5883–5886. doi: 10.1021/jacs.5b02755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saxena A, Perez F, Krische MJ. Ruthenium(0) Catalyzed [4+2] Cycloaddition of Acetylenic Aldehydes with α-Ketols: Convergent Construction of Angucycline Ring Systems. Angew Chem Int Ed. 2016;55:1493–1497. doi: 10.1002/anie.201509646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krohn K, Rohr J. Angucyclines: Total Syntheses, New Structures, and Biosynthetic Studies of an Emerging New Class of Antibiotics. Top Curr Chem. 1997;188:127–195. [Google Scholar]
- 88.Carreño MC, Urbano A. Recent Advances in the Synthesis of Angucyclines. Synlett. 2005:1–25. [Google Scholar]
- 89.Kharel MK, Pahari P, Shepherd MD, Tibrewal N, Nybo SE, Shaaban KA, Rohr J. Angucyclines: Biosynthesis, Mode-of-Action, New Natural Products, and Synthesis. Nat Prod Rep. 2012;29:264–325. doi: 10.1039/c1np00068c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clerici MG, Maspero F. Catalytic C-Alkylation of Secondary Amines with Alkenes. Synthesis. 1980:305–306. [Google Scholar]
- 91.Nugent WA, Ovenall DW, Holmes SJ. Catalytic C-H Activation in Early Transition-Metal Dialkylamides and Alkoxides. Organometallics. 1983;2:161–162. [Google Scholar]
- 92.Campos KR. Direct sp3 C-H Bond Activation Adjacent to Nitrogen in Heterocycles. Chem Soc Rev. 2007;36:1069–1084. doi: 10.1039/b607547a. [DOI] [PubMed] [Google Scholar]
- 93.Roesky PW. Catalytic Hydroaminoalkylation. Angew Chem Int Ed. 2009;48:4892–4894. doi: 10.1002/anie.200900735. [DOI] [PubMed] [Google Scholar]
- 94.Chong E, Garcia P, Schafer LL. Hydroaminoalkylation: Early-Transition-Metal-Catalyzed α-Alkylation of Amines. Synthesis. 2014:2884–2896. [Google Scholar]
- 95.Chen T-Y, Tsutsumi R, Montgomery TP, Volchkov I, Krische MJ. Ruthenium Catalyzed C-C Coupling of Amino Alcohols with Dienes via Transfer Hydrogenation: Redox-Triggered Imine Addition and Related Hydroaminoalkylations. J Am Chem Soc. 2015;137:1798–1801. doi: 10.1021/ja5130258. [DOI] [PubMed] [Google Scholar]
- 96.Oda S, Sam B, Krische MJ. Hydroaminomethylation Beyond Carbonylation: Allene-Imine Reductive Coupling by Ruthenium-Catalyzed Transfer Hydrogenation. Angew Chem Int Ed. 2015;54:8525–8528. doi: 10.1002/anie.201503250. [DOI] [PubMed] [Google Scholar]
- 97.Oda S, Franke J, Krische MJ. Diene Hydroaminomethylation via Ruthenium-Catalyzed C-C Bond Forming Transfer Hydrogenation: Beyond Carbonylation. Chem Sci. 2016;7:136–141. doi: 10.1039/c5sc03854e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhu S, Lu X, Luo Y, Zhang W, Jiang H, Yan M, Zeng W. Ruthenium(II)-Catalyzed Regioselective Reductive Coupling of α-Imino Esters with Dienes. Org Lett. 2013;15:1440–1443. doi: 10.1021/ol4006079. [DOI] [PubMed] [Google Scholar]
- 99.Schmitt DC, Lee J, Dechert-Schmitt A-MR, Yamaguchi E, Krische MJ. Ruthenium Catalyzed Hydroaminoalkylation of Isoprene via Transfer Hydrogenation: Byproduct-free Prenylation of Hydantoins. Chem Commun. 2013;49:6096–6098. doi: 10.1039/c3cc43463j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jun C-H. Chelation-Assisted Alkylation of Benzylamine Derivatives by Ru0 Catalyst. Chem Commun. 1998:1405–1406. [Google Scholar]
- 101.Chatani N, Asaumi T, Yorimitsu S, Ikeda T, Kakiuchi F, Murai S. Ru3(CO)12-Catalyzed Coupling Reaction of sp3 C-H Bonds Adjacent to a Nitrogen Atom in Alkylamines with Alkenes. J Am Chem Soc. 2001;123:10935–10941. doi: 10.1021/ja011540e. [DOI] [PubMed] [Google Scholar]
- 102.Bergman SD, Storr TE, Prokopcová H, Aelvoet K, Diels G, Meerpoel L, Maes BUW. The Role of the Alcohol and Carboxylic Acid in Directed Ruthenium-Catalyzed C(sp3)-H α-Alkylation of Cyclic Amines. Chem Eur J. 2012;18:10393–10398. doi: 10.1002/chem.201201072. [DOI] [PubMed] [Google Scholar]
- 103.Schinkel M, Wang L, Bielefeld K, Ackermann L. Ruthenium(II)-Catalyzed C(sp3)-H α-Alkylation of Pyrrolidines. Org Lett. 2014;16:1876–1879. doi: 10.1021/ol500300w. [DOI] [PubMed] [Google Scholar]
- 104.Kulago AA, Van Steijvoort BF, Mitchell EA, Meerpoel L, Maes BUW. Directed Ruthenium-Catalyzed C(sp3)-H α-Alkylation of Cyclic Amines Using Dioxolane-Protected Alkenones. Adv Synth Catal. 2014;356:1610–1618. [Google Scholar]
- 105.Tsuchikama K, Kasagawa M, Endo K, Shibata T. Cationic Ir(I)-Catalyzed sp3 C-H Bond Alkenylation of Amides with Alkynes. Org Lett. 2009;11:1821–1823. doi: 10.1021/ol900404r. [DOI] [PubMed] [Google Scholar]
- 106.Pan S, Endo K, Shibata T. Ir(I)-Catalyzed Enantioselective Secondary sp3 C-H Bond Activation of 2-(Alkylamino)pyridines with Alkenes. Org Lett. 2011;13:4692–4695. doi: 10.1021/ol201907w. [DOI] [PubMed] [Google Scholar]
- 107.Pan S, Matsuo Y, Endo K, Shibata T. Cationic Iridium-Catalyzed Enantioselective Activation of Secondary sp3 C-H Bond Adjacent to Nitrogen Atom. Tetrahedron. 2012;68:9009–9015. [Google Scholar]
- 108.Lahm G, Opatz T. Unique Regioselectivity in the C(sp3)-H α-Alkylation of Amines: The Benzoxazole Moiety as a Removable Directing Group. Org Lett. 2014;16:4201–4203. doi: 10.1021/ol501935d. [DOI] [PubMed] [Google Scholar]
- 109.Eilbracht P, Bärfacker L, Buss C, Hollmann C, Kitsos-Rzychon BE, Kranemann CL, Rishe T, Roggenbuck R, Schmidt A. Tandem Reaction Sequences under Hydroformylation Conditions: New Synthetic Applications of Transition Metal Catalysis. Chem Rev. 1999;99:3329–3366. doi: 10.1021/cr970413r. [DOI] [PubMed] [Google Scholar]
- 110.Breit B, Seiche W. Recent Advances on Chemo-, Regio- and Stereoselective Hydroformylation. Synthesis. 2001:1–36. [Google Scholar]
- 111.Eilbracht P, Schmidt AM. Synthetic Applications of Tandem Reaction Sequences Involving Hydroformylation. Top Organomet Chem. 2006;18:65–95. [Google Scholar]
- 112.Crozet D, Urrutigoïty M, Kalck P. Recent Advances in Amine Synthesis by Catalytic Hydroaminomethylation of Alkenes. ChemCatChem. 2011;3:1102–1118. [Google Scholar]
- 113.Behr A, Vorholt AJ. Hydroformylation and Related Reactions of Renewable Resources. Top Organomet Chem. 2012;39:103–128. [Google Scholar]
- 114.Raoufmoghaddam S. Recent Advances in Catalytic C-N Bond Formation: A Comparison of Cascade Hydroaminomethylation and Reductive Amination Reactions with the Corresponding Hydroamidomethylation and Reductive Amidation Reactions. Org Biomol Chem. 2014;12:7179–7193. doi: 10.1039/c4ob00620h. [DOI] [PubMed] [Google Scholar]
- 115.Wu X-F, Fang X, Wu L, Jackstell R, Neumann H, Beller M. Transition-Metal-Catalyzed Carbonylation Reactions of Olefins and Alkynes: A Personal Account. Acc Chem Res. 2014;47:1041–1053. doi: 10.1021/ar400222k. [DOI] [PubMed] [Google Scholar]


