Abstract
By coating fibers with titania sol to generate a dual-size surface roughness, followed by hydrophobization with stearic acid, 1H,1H,2H,2H-perfluorodecyltrichlorosilane or their combination, hydrophilic cotton fabrics were made superhydrophobic. The surface wettability and topology of cotton fabrics were studied by contact angle measurement and scanning electron microscopy. The UV-shielding property of the treated fabrics was also characterized by UV-vis spectrophotometry.
Keywords: superhydrophobicity, titania sol, UV shielding, cotton fabrics
Introduction
In recent years, researchers have made impressive efforts to prepare superhydrophobic surfaces for potential applications in a variety of areas [1–8]. Among others, superhydrophobic textiles have been successfully obtained by a number of different approaches [9–16]. For example, Gao and McCarthy, according to a 1945 patent, grafted a silicone coating to a microfiber polyester fabric to render the fabric superhydrophobic [9], but the microfiber polyester fabric (with a single fiber as small as 2–5 μ m) must be tightly woven, and this approach may not be suited to cotton textiles. Gold particles have been incorporated into cotton fabrics to induce a dual-size surface topology by Wang et al [11], but there is obviously no chemical bond between the gold particles and the cotton fiber. Hoefnagels et al [14] reported biomimetic superhydrophobic and highly oleophobic cotton textiles. Silica particles with amine groups at the surface, covalently bonded to the cotton fibers, were in situ generated by either a one-step or two-step reaction; the amine groups were then used to hydrophobize the surface via the reaction with mono-epoxy-functionalized polydimethylsiloxane. For oleophobic textiles, perfluoralkyl silane was used for surface modification. Michielsen and Lee [16] used mechanical and chemical surface modifications with further grafting of 1H,1H-perfluorooctylamine or octadecylamine to poly(acrylic acid) chains, which was previously grafted onto a nylon 6,6 woven fabric surface, to prepare artificial superhydrophobic surfaces. Li et al [15] prepared superhydrophobic surfaces from water glass and nonfluorinated alkylsilane on cotton substrates.
Similarly, particles have been introduced to various substrates to achieve water repellency by sol–gel methods [8,12,13,17]. A sol–gel process is an important method for preparing inorganic oxide-modified textiles [18–25]. Hribernik et al [18] grew a layer of silica on the surface of a regenerated cellulose fiber via a sol–gel process and studied the flame retardant activity of the coated regenerated cellulose fibers. Abidi et al [23] modified lightweight cotton fabrics with titania and titania-silica nanosol for improved UV radiation protection. Wang et al [24] reported a low-temperature growth approach for growing hexagonally oriented ZnO nanorod arrays onto cotton fabrics. The treated cotton fabrics provided an excellent UV protection.
Increasing global competition in textiles has created many challenges for worldwide dyers and finishers. High-performance textiles are greatly appreciated by a more discerning and demanding consumer market [26, 27]. Thus, great efforts have been made in order to produce high-value-added textiles [27].
In this work, bifunctional cotton textiles with superhydrophobic and UV-shielding properties were prepared by the sol–gel coating of TiO2 and surface hydrophobization. Fabrics with superhydrophobic and UV-shielding properties are greatly appreciated for such applications as those for technical, industrial, medical and military end-uses, as well as those for everyday life uses, such as beach umbrellas, shade shelters, camps, garments, and advertisement materials. Superhydrophobicity can not only provide protection from a wide variety of liquids but also prolong the lifetime of the fabrics owing to the prevention of water wetting that causes degradation, and UV shielding can protect people from burning caused by UV from the sun.
Experimental procedure
Materials
A white pure cotton fabric was purchased from a local fabric store, which was cleaned with deionized water and ethanol before it was dried for use. Tetrabutyl titanate (98%), acetic acid, toluene, acetone, stearic acid, and anhydrous ethanol were purchased from Sinopharm Chemical Reagent Co., Ltd. 1H,1H,2H,2H-Perfluorodecyltrichlorosilane (PFTDS) was purchased from Aldrich. All chemicals were used without further purification.
Preparation of TiO2sol
TiO2 sol was prepared by a typical controlled hydrolysis process modified from the reported method [28]. Solution A was prepared by adding 8.5 ml of tetrabutyl titanate slowly into 20 ml of anhydrous ethanol with continuous stirring. Solution B was prepared by mixing 1.5 ml of deionized water, 5 ml of acetic acid, and 20 ml of anhydrous ethanol together with stirring. Solution A was added slowly into solution B for 1 h. The reaction mixture was stirred for 24 h at room temperature. Thus, transparent bright yellowish TiO2 sol was obtained.
Surface coating of cotton fabrics
For coating fabrics with TiO2, the cotton fabrics were immersed in the ethanol solution of the as-prepared TiO2 sol for 10 min and then padded with a wet pickup of 70–80%. This process was repeated two times. The samples were dried at 110 °C for 5 min and then washed with ethanol.
Surface energy lowering
To lower the surface energy of the fabrics or TiO2-coated fabrics, stearic acid and PFTDS were used individually or in combination. The treatment with stearic acid was conducted by immersing the fabrics in a 0.5 wt.% stearic acid solution of acetone for 10 min, then padded, and cured at 110 °C for 1 h. The samples were then rinsed with acetone and dried. Similarly, the treatment with PFTDS was conducted by immersing the fabrics in a 1 vol.% PFTDS solution of toluene for 1 h. Then, the fabrics were dried at room temperature. The samples were rinsed with toluene and dried.
For combination treatment, the samples were treated with stearic acid, followed by PFTDS. All the conditions are shown in detail in table 1.
Table 1.
Water contact angles on modified cotton fabrics.
| Sample | TiO2 | steric acid | PFTDS | Static CA |
|---|---|---|---|---|
| ID | (wt.%) | (wt.%) | (vol.%) | (°) |
| 0 | 0 | 0 | 0 | 0 |
| 1 | 3.2 | 95.2 ± 2.1 | ||
| A0 | 0 | 1 | 142.1 ± 1.4 | |
| A1 | 3.2 | 1 | 151.5 ± 0.9 | |
| A2 | 1.6 | 1 | 152.0 ± 0.8 | |
| A3 | 0.8 | 1 | 151.2 ± 0.9 | |
| A4 | 0.4 | 1 | 150.5 ± 0.7 | |
| A5 | 0.2 | 1 | 149.5 ± 1.1. | |
| B0 | 0 | 1 | 153.0 ± 0.5 | |
| B1 | 3.2 | 1 | 163.5 ± 0.6 | |
| B2 | 1.6 | 1 | 160.3 ± 0.5 | |
| B3 | 0.8 | 1 | 159.9 ± 0.5 | |
| B4 | 0.4 | 1 | 157.7 ± 0.6 | |
| B5 | 0.2 | 1 | 156.0 ± 1.1 | |
| C0 | 0 | 0.5 | 0.5 | 154.0 ± 0.5 |
| C1 | 3.2 | 0.5 | 0.5 | 163.0 ± 1.3 |
| C2 | 1.6 | 0.5 | 0.5 | 160.7 ± 0.6 |
| C3 | 0.8 | 0.5 | 0.5 | 160.0 ± 0.9 |
| C4 | 0.4 | 0.5 | 0.5 | 160.3 ± 0.8 |
| C5 | 0.2 | 0.5 | 0.5 | 158.2 ± 1.1 |
Characterization
Contact angles (CAs) were measured with a 5 μ l deionized water droplet on a Dataphysics OCA 20 (Dataphysics, Germany) instrument at room temperature. All the contact angles were determined by averaging the values obtained at five-six different points on each sample surface. Scanning electron microscopy (SEM) images were obtained using a Hitachi S-4800 field emission scanning electron microscope. UV-vis characterization was conducted using a CARY 100 Bio UV-visible spectrophotometer with a CARY dual-cell Peltier accessory (Varian, Walnut Creek, CA).
Results and discussion
Surface wettability and topolopy of cotton fabrics
Surface wettability was examined by contact angle measurements. The obtained results are shown in table 1. Pure cotton sample 0 as a control can be completely wetted with water, which is common and well known for cotton fabrics. Sample 1 indicates that the fabric treated with TiO2 sol presents a weak water repellency. These samples modified with stearic acid (A0), PFTDS (B0), or their combination (C0) were made highly hydrophobic, with a water static CA larger than 140°. By the pretreatment of the TiO2 rough coating on the fabric samples, the hydrophobicity is further enhanced, as shown in table 1. The water static CAs range from 151.5° to 163.5° for a 5 μ l droplet, and the modified cotton samples are completely water nonwettable.
When a water droplet sits on a hydrophobic cotton fabric surface, its wetting behavior can be described by the equation of Cassie and Baxter [14, 29]:
where θ CB is the observed water CA on a rough, porous surface, θ 0 is the intrinsic water CA on the corresponding smooth surface, fls is the liquid/solid contact area divided by the projected area, and flv is the liquid/vapor contact area divided by the projected area. Equation (1) has recently been modified to account for the local surface roughness on the wetted area as follows [14,16, 30]:
where f is the fraction of the projected area of the solid surface wetted by water (thus, we have flv = 1 - f) and rf is the surface roughness of the wetted area. Generally, water CAs on smooth surfaces cannot exceed 120° through tailoring surface chemistry [31,32]. For the stearic acid- or PFTDS-modified cotton fiber in the absence of TiO2 particles, the curvature of the cotton fiber renders rf> 1, which, in comparison with a smooth wetted area, can enhance the surface hydrophobicity. When TiO2 particles are chemically incorporated onto the cotton fiber surface in our study, rf further increases; the higher the roughness of the TiO2 coating on the cotton fiber is, the greater rf would become, thus the CA would be larger, as shown in figure 2 with (d) versus (f). Once rf reaches a certain level, air may become trapped between TiO2 particles underneath a water droplet, which would further enhance the surface hydrophobicity.
Figure 2.
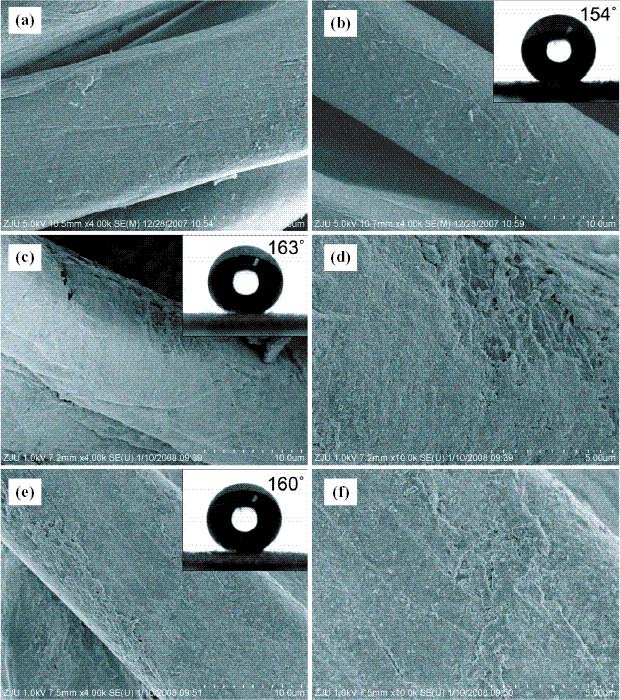
SEM images for samples (a) 0, (b) C0, and (c) C1. (d) Higher-magnification image for (c), (e) C3, and (f) higher-magnification image for (e). Shown in the insets are the images of static water droplets on the respective samples.
SEM was used to determine the topology of the cotton samples. The as-received cotton sample has a tightly woven, fibrous structure, as shown in figure 1. From figure 2(a), a typical longitudinal fibril structure can be observed in sample 0. When the cotton sample was only modified with stearic acid, PFTDS or their combination, no significant change was observed, as shown in figure 2(b). After a sol–gel treatment, the surface of the cotton fiber exhibited a compact coating with a number of small TiO2 clusters, as shown in figures 2(c)–(e), which made the surface rough, thus generating a dual-size surface structure on the fabrics.
Figure 1.

SEM image of as-received cotton fabric.
It should be noted that, owing to the fibers sticking out from the cotton sample as depicted in the optical images in the insets in figure 2, the measurement of CAs is often not straightforward, in terms of the difficulty in determining the baseline of the water droplet, which may in turn lead to the possible underestimation of the contact angle data [14]. Different baseline sets would cause a difference of more than 10° between the CAs of the same sample. To obtain consistent CAs, the baseline was set manually in parallel with the surface of the samples and at the same distance vertically from the top point of the water in the measurement of all the samples. The measurement of advancing and receding contact angles was also attempted. We measured each sample several times. It was found that the differences between the advancing and receding contact angles are within the range of 0.05°–0.3° for the fabrics with static contact angles larger than 150°. We suppose that the obtained advancing and receding contact angles are not the true accurate values owing to the effect of the protruding fibers. Because the protruding fibers exhibit elasticity and also forces on the water droplet [11], it is difficult to yield accurate values for the advancing and receding water contact angles [14, 16]. Therefore, only static CAs are reported here, as shown in table 1.
From the CA data in table 1, it was observed that the concentration of TiO2 in the sol negligibly affects the hydrophobicity, because the CAs of the samples are not markedly different, particularly when the concentration of TiO2 in the sol is larger than 0.2 wt.%.
From C samples in table 1, it was observed that using the combination of stearic acid and PFTDS, CAs larger than 160° can be obtained. Such a combination was used to decrease the amount of PFTDS used in lowering the surface energy. Generally, fluorochemicals have an extremely low surface free energy. However, such compounds are expensive. Hence, decreasing their concentration using combinations of long train alkyl compounds is useful for industrial application.
UV-shielding properties of treated fabrics
Figure 3 shows the transmittance plotted against the wavelength for the treated fabrics. Curve 0 in figure 3 shows that the transmittance of the samples is lower than 0.35%, indicating that the tightly woven fabrics have a high sun shielding property. After being treated with stearic acid followed by PFTDS, the transmittance decreases in the wavelength range of 200–700 nm, as shown in curve C0, which might be caused by an increase in the density of the fabrics. Curves of samples C1–C5 show a marked transmittance decrease in the wavelength range below 350 nm, indicating a better UV protection, particularly in the most critical range of 289–315 nm for the fabric treated with titania sol. The reason for this is that the formation of TiO2 particles on the fabric surface imparts a very good and efficient UV scattering because of the large refractive index of TiO2 particles [23, 33]. It is interesting to observe that curve C5 shows an obvious blue shift. This observation might be related to the nanoeffect of the smaller particles in the thinner film due to the low concentration of titania, which prevents the formation of large floccules of TiO2 particles. In this study, sample C3 treated with a TiO2 concentration of 0.8 wt.% showed the best UV protection property, indicating that the UV-shielding property of the treated fabrics does not linearly depend on the TiO2 concentration. This is because the light scattering of TiO2 depends on both the individual crystal size and the cluster size [34]. It is known that large floccules of TiO2 particles scatter less light than well-dispersed TiO2 particles [34]. A lower TiO2 concentration might produce well-dispersed TiO2 particles on the surfaces of the fibers, but far too low TiO2 concentration would cause a weak TiO2 loading. In the present study, the increased TiO2 concentrations higher than 0.8 wt.%, in contrast, show decreased UV-shielding properties. This is because a higher TiO2 concentration would induce the aggregation of TiO2 particles titania sol coating, thus producing a lower light scattering efficiency.
Figure 3.
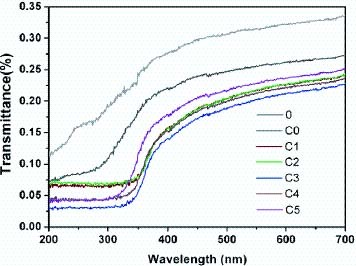
UV-vis spectra of samples 0, C0, C1, C2, C3, C4, and C5. For the descriptions of the sample codes, refer to table 1.
Conclusion
Superhydrophobic cotton fabrics have been successfully prepared. The incorporation of TiO2 particles by titania sol coating can not only cause a dual-size surface roughness for enhancing the hydrophobicity but also result in good UV-shielding property. In addition, such bifunctional fabrics can be fabricated at low temperatures without expensive equipment and tedious processes.
Acknowledgments
This work was supported by the Ministry of Education Foundation of Shaanxi Province, China (No. 07JK186) and the Project of Science Innovation Foundation of Shannxi University of Science and Technology (No. SUST-B15). The authors also thank Youwen Wang and Linshen Chen of Zhejiang University for SEM and TGA measurements.
References
- Erbil H Y, Demirel A L, Avci Y. and Mert O. Science. 2003;299:1377. doi: 10.1126/science.1078365. [DOI] [PubMed] [Google Scholar]
- Xie Q D, Xu J, Feng L, Jiang L, Tang W H, Luo X D. and Han C C. Adv. Mater. 2004;16:302. doi: 10.1002/adma.200306281. [DOI] [Google Scholar]
- Tuteja A, Choi W, Ma M L, Mabry J M, Mazzella S A, Rutledge G C, McKinley G H. and Cohen R E. Science. 2007;318:1618. doi: 10.1126/science.1148326. [DOI] [PubMed] [Google Scholar]
- Zhai L, Cebeci F C, Cohen R E. and Rubner M F. Nano Lett. 2004;4:1349. doi: 10.1021/nl049463j. [DOI] [PubMed] [Google Scholar]
- Furstner R, Barthlott W, Neinhuis C. and Walzel P. Langmuir. 2005;21:956. doi: 10.1021/la0401011. [DOI] [PubMed] [Google Scholar]
- Han W, Wu D, Ming W H, Niemantsverdriet H. and Thune P C. Langmuir. 2006;22:7956. doi: 10.1021/la061414u. [DOI] [PubMed] [Google Scholar]
- Kulkarni S A, Ogale S B. and Vijayamohanan K P. J. Colloid Interface Sci. 2008;318:372. doi: 10.1016/j.jcis.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Li X M, Reinhoudt D. and Crego-Calama M. Chem. Soc. Rev. 2007;36:1350. doi: 10.1039/b602486f. [DOI] [PubMed] [Google Scholar]
- Gao L C. and McCarthy T J. Langmuir. 2006;22:5998. doi: 10.1021/la061237x. [DOI] [PubMed] [Google Scholar]
- Nystrom D, Lindqvist J, Ostmark E, Hult A. and Malmstrom E. Chem. Commun. 2006;34:3594. doi: 10.1039/b607411a. [DOI] [PubMed] [Google Scholar]
- Wang T, Hu X G. and Dong S J. Chem. Commun. 2007:1849. doi: 10.1039/b616778k. [DOI] [PubMed] [Google Scholar]
- Yu M, Gu G T, Meng W D. and Qing F L. Appl. Surf. Sci. 2007;253:3669. doi: 10.1016/j.apsusc.2006.07.086. [DOI] [Google Scholar]
- Daoud W A, Xin J H. and Tao X M. J. Am. Ceram. Soc. 2004;87:1782. [Google Scholar]
- Hoefnagels H F, Wu D, de With G. and Ming W. Langmuir. 2007;23:13158. doi: 10.1021/la702174x. [DOI] [PubMed] [Google Scholar]
- Li Z, Xing Y. and Dai J. Appl. Surf. Sci. 2008;254:2131. doi: 10.1016/j.apsusc.2007.08.083. [DOI] [Google Scholar]
- Michielsen S. and Lee H J. Langmuir. 2007;23:6004. doi: 10.1021/la063157z. [DOI] [PubMed] [Google Scholar]
- Hikita M, Tanaka K, Nakamura T, Kajiyama T. and Takahara A. Langmuir. 2005;21:7299. doi: 10.1021/la050901r. [DOI] [PubMed] [Google Scholar]
- Hribernik S, Smole M S, Kleinschek K S, Bele M, Jamnik J. and Gaberscek M. Polym. Degrad. Stab. 2007;92:1957. doi: 10.1016/j.polymdegradstab.2007.08.010. [DOI] [Google Scholar]
- Uddin M J, Cesano F, Bonino F, Bordiga S, Spoto G, Scarano D. and Zecchina A. J. Photochem. Photobiol. A-Chem. 2007;189:286. doi: 10.1016/j.jphotochem.2007.02.015. [DOI] [Google Scholar]
- Daoud W A. and Xin J H. J. Am. Ceram. Soc. 2004;87:953. [Google Scholar]
- Meilert K T, Laub D. and Kiwi J. J. Mol. Catal. A-Chem. 2005;237:101. doi: 10.1016/j.molcata.2005.03.040. [DOI] [Google Scholar]
- Bozzi A, Yuranova T, Guasaquillo I, Laub D. and Kiwi J. J. Photochem. Photobiol. A-Chem. 2005;174:156. doi: 10.1016/j.jphotochem.2005.03.019. [DOI] [Google Scholar]
- Abidi N, Hequet E, Tarimala S. and Dai L L. J. Appl. Polym. Sci. 2007;104:111. doi: 10.1002/app.24572. [DOI] [Google Scholar]
- Wang R H, Xin J H, Tao X M. and Daoud W A. Chem. Phys. Lett. 2004;398:250. doi: 10.1016/j.cplett.2004.09.077. [DOI] [Google Scholar]
- Han K Q. and Yu M H. J. Appl. Polym. Sci. 2006;100:1588. doi: 10.1002/app.23312. [DOI] [Google Scholar]
- Qian L. J. Text. Apparel. Technol. Manag. 2004;4:1. [Google Scholar]
- Holme I. Color. Technol. 2007;123:59. doi: 10.1111/j.1478-4408.2007.00064.x. [DOI] [Google Scholar]
- Chen W, Zhang J Y, Fang Q, Li S, Wu J X, Li F Q. and Jiang K. Sens. Actuator B-Chem. 2004;100:195. doi: 10.1016/j.snb.2003.12.053. [DOI] [Google Scholar]
- Cassie A B D. and Baxter S. Trans. Faraday Soc. 1944;40:546. doi: 10.1039/tf9444000546. [DOI] [Google Scholar]
- Marmur A. Langmuir. 2003;19:8343. doi: 10.1021/la0344682. [DOI] [Google Scholar]
- Song X Y, Zhai J, Wang Y L. and Jiang L. J. Colloid Interface Sci. 2006;298:267. doi: 10.1016/j.jcis.2005.11.048. [DOI] [PubMed] [Google Scholar]
- Genzer J. and Efimenko K. Science. 2000;290:2130. doi: 10.1126/science.290.5499.2130. [DOI] [PubMed] [Google Scholar]
- McNeil L E. and French R H. Acta Mater. 2000;48:4571. doi: 10.1016/S1359-6454(00)00243-3. [DOI] [Google Scholar]
- Nelson K. and Deng Y L. J. Colloid Interface Sci. 2008;319:130. doi: 10.1016/j.jcis.2007.09.037. [DOI] [PubMed] [Google Scholar]


