Abstract
The aims of the present study were to evaluate the bactericidal activity of a new antiseptic agent, ozone nano-bubble water (NBW3), against periodontopathogenic bacteria and to assess the cytotoxicity of NBW3 against human oral cells. The bactericidal activities of NBW3 against representative periodontopathogenic bacteria, Porphyromonas gingivalis (P. gingivalis) and Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans) were evaluated using in vitro time-kill assays. The cytotoxicity of NBW3 was evaluated using three-dimensional human buccal and gingival tissue models. The numbers of colony forming units (CFUs)/mL of P. gingivalis and A. actinomycetemcomitans exposed to NBW3 dropped to below the lower limit of detection (<10 CFUs mL−1) after only 0.5 min of exposure. There were only minor decreases in the viability of oral tissue cells after 24 h of exposure to NBW3. These results suggest that NBW3 possesses potent bactericidal activity against representative periodontopathogenic bacteria and is not cytotoxic to cells of human oral tissues. The use of NBW3 as an adjunct to periodontal therapy would be promising.
Keywords: ozone nano-bubble water, periodontitis, antiseptic, periodontopathic bacteria, cytotoxicity
1. Introduction
Periodontitis is a chronic inflammatory disease caused by microorganisms residing in subgingival biofilm. The disease is predominantly associated with colonization by Gram-negative anaerobic microorganisms. In particular, Porphyromonas gingivalis (P. gingivalis) and Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans) has been known to be the major etiological agents of periodontitis [1–4]. The primary goal of periodontitis treatment is thus elimination of pathogen-containing biofilms. Supra- and subgingival mechanical debridement is the traditional initial step in treating periodontitis, even though the process is laborious and the results technique-dependent. In addition, mechanical debridement rarely leads to the complete removal of putative periodontal pathogens.
A number of topical antiseptics and topical or systemic antibiotics have been employed to augment mechanical debridement [5, 6]. However, topical and systemic antibiotic therapies have several significant drawbacks, such as selectivity of antimicrobial action, possible development of resistant bacteria, and risk for adverse host reactions. For these reasons, the topical use of a low-cost, broad-spectrum antiseptic agent with low potential for adverse reactions is preferable in treating periodontitis. The most widely used adjunctive antiseptic in periodontal treatment is probably chlorhexidine digluconate (CHX), which is applied at concentrations of 0.1% to 2%. However, prolonged use of CHX may cause mucosal desquamation, tooth staining, and altered taste sensation. In addition, even low concentrations of CHX may be toxic to gingival fibroblasts, thereby reducing the production of collagen and non-collagen proteins and potentially impeding periodontal healing [7]. Moreover, an increasing number of immediate-type allergies to this agent have been reported, including contact urticaria, occupational asthma, and anaphylactic shock. Due to the potential for serious allergic reactions to CHX, topical application of this drug, especially to mucous membranes, is discouraged [8]. Therefore, an alternative adjunctive antiseptic with higher antimicrobial activity, a better safety profile, and fewer side effects would be valuable in treating periodontal disease.
Ozone (O3) is attracting attention as a possible alternative antiseptic in the dental field as well as food industries. Ozone has strong antimicrobial activity against bacteria, fungi, protozoa, and viruses [9] and does not induce microbial resistance, characteristics that were originally noted in the fields of water purification and food preservation [10–12]. Recent reports have shown that both the gaseous and aqueous forms of ozone have antimicrobial activity against oral pathogens associated with dental caries and endodontic infections [13–18]. It has also been reported that ozone therapy considerably reduces the growth of A. actinomycetemcomitans, P. gingivalis, and Tannerella forsythia [19]. Regarding the potential for cytotoxicity, ozone gas has been found to significantly decrease the viability of oral cells in the concentrations currently used in dentistry [20]. In contrast, other reports have indicated that aqueous ozone is highly biocompatible with fibroblasts, cementoblasts, and epithelial cells [20–22], suggesting that aqueous ozone would be useful in treating oral infectious diseases such as periodontal disease, apical periodontitis, and peri-implantitis. However, ozonated water has a half-life of about only 20 min and will degrade back into oxygen, and must be used within the first 5 to 10 min after production to assure its potency.
In order to overcome problems associated with the short half-life of ozonated water, Chiba and Takahashi [23] developed a patented procedure to produce ozone nano-bubble water (NBW3). A nano-bubble is less than 100 nm in diameter, and is produced by the collapse of a micro-bubble (≰50 μm in diameter) in an electrolyte solution by means of a physical stimulus (such as a shock wave caused by electrical discharge in the solution). The physical properties of nano-bubble are inferred from those of micro-bubble [24]. The zeta potential (the electrical potential at the slipping plane as measured by electrophoresis) of micro-bubble is negatively charged under a wide range of pH conditions [25], and observations of collapsing micro-bubbles over time have shown that the zeta potential increases according to the rate of shrinkage, which is inversely proportional to the bubble size [26]. From these properties of micro-bubble, the gas-water interface of nano-bubble is thought to be distributed with OH− ions predominantly over H+ ions. The positive ions in electrolyte solution become concentrated around the gas nucleus due to its negatively charged surface and act as shells that prevent the gas from dispersing (the salting-out phenomenon). Due to this characteristic of ion behavior, nano-bubbles remain stable for more than 6 months in electrolyte solution. It is confirmed that NBW3 retains the oxidation ability as aqueous ozone for more than 6 months if it is protected against exposure to ultraviolet rays. The high stability of NBW3 allows for bottling and use as a disinfectant solution.
Owing to its bactericidal efficacy and usability, the use of NBW3 as an adjunctive antiseptic in periodontal treatment would be promising. Indeed, in a randomized controlled trial, we demonstrated that subgingival irrigation with NBW3 might be a valuable adjunct to periodontal treatment [27]. However, to date there are no reports regarding the fundamental properties of NBW3, such as the bactericidal activity against periodontal pathogens or the cytotoxicity against human oral tissues. Therefore, in the present study we evaluated the bactericidal activity of NBW3 against the representative periodontopathic bacteria P. gingivalis and A. actinomycetemcomitans using in vitro time-kill studies. Moreover, we investigated the cytotoxicity of NBW3 to the cells of human oral tissues using three-dimensional human buccal and gingival tissue models.
2. Material and methods
2.1. Production of NBW3
We used a commercially available NBW3 (Nikken Rentacom Corporation, Tokyo, Japan) for the present tests. According to the patent of NBW3 electrolyte aqueous solution is needed for the preparation of NBW3. To prepare the test samples ozone microbubbles were dispersed in groundwater collected near a beach in Miyagi Prefecture, Japan. The concentration of ozone gas dispersed as microbubbles was about 50 g Nm−3. The salt concentration of the original water was about 0.9%, and it contained trace inorganic minerals such as iron and manganese ions, derived from both sea water and underground layers. During the ozone microbubble dispersion for more than 3 h, the total organic content of the water decreased to less than 3 mg L−1 and iron levels were less than 0.03 mg L−1 because of the strong oxidation ability of ozonation. The water changed to a pink color because of the oxidation of manganese ions, other trace inorganic minerals of the NBW3, per 1 L, were as follows: sodium, 16 mg; chloride ion, 14.6 mg; calcium, 27 mg; magnesium, 14 mg. The pH of the water was 7.5. It was then stored in a cool dark place for at least one month before the tests. The aqueous ozone concentration evaluated by indigo method was about 1.5 mg L−1 at the tests.
2.2. Time-kill study
2.2.1. Microorganisms
The P. gingivalis JCM12257 and A. actinomycetemcomitans JCM8577 isolates used in this study were obtained from the Japan Collection of Microorganisms (RIKEN BioResource Center, Tsukuba, Ibaraki, Japan). P. gingivalis was grown anaerobically on modified GAM agar (Nissui Pharmaceutical Co., Ltd, Ueno, Tokyo, Japan) for 3 days at 37 °C. A. actinomycetemcomitans was grown on modified GAM agar for 24 h at 37 °C in an atmosphere of 5% CO2 in air. The initial inocula were adjusted to 1 × 107 colony forming units (CFUs)/mL.
2.2.2. Sample preparation
A 0.1 mL volume of culture medium containing approximately 1 × 107 CFUs mL−1 of test bacterium was added to either 10 mL of NBW3, 0.2% CHX (Sigma-Aldrich Co. LLC., St. Louis, Missouri, USA) or 0.9% NaCl (as a control) that had been pre-warmed to 25 °C. Following the incubation (for 0.5, 1, 1.5 and 5 min, at 37 °C), aliquots (1 mL) of the test bacterial suspensions were added to tubes containing 9 mL of SCDLP bouillon culture medium (Nissui Pharmaceutical Co., Ltd, Ueno, Tokyo, Japan) for inactivation of the test solutions. The 0.9% NaCl control bacterial suspensions were incubated 0 and 5 min at 37 °C and treated in the same manner as test ones.
2.2.3. Enumeration of bacterial colonies following exposure to NBW3
Bacteria were enumerated using a Petri-plate method. Briefly, aliquots were removed from each tube at each time point and serial 10-fold dilutions were made in sterile physiological saline. Next, 1 mL aliquots of each dilution were aseptically transferred to Petri dishes, mixed with approximately 20 mL of modified GAM agar medium, and incubated at 37 °C for 5 days under anaerobic conditions (P. gingivalis) and for 2 days in an atmosphere of 5% CO2 in air (A. actinomycetemcomitans). Colonies were then counted and the number of CFUs/mL was plotted against time for each bacterium tested. The lower limit of detection using this method was 10 CFUs mL−1.
2.3. Cytotoxicity study
2.3.1. Exposure of organotypic human oral tissue models to the test materials
The potential for NBW3 to irritate oral tissues was tested in vitro using human buccal (EpiORL-200™) and gingival (EpiGIN-100™) tissue models developed by MatTek Corporation (Ashland, Massachusetts, USA), which consist of normal, human-derived epithelial cells. On the day of arrival, the tissues were placed in 6-well plates in which each well contained 1.0 mL of pre-warmed ORL-200-MM or GIN-100-MM (MatTek Corporation, Ashland, Massachusetts, USA), supplemented with 1 M HEPES (Dojindo Laboratories, Shibadaimon, Tokyo, Japan). Following overnight incubation at 37 °C in a 5% CO2 atmosphere, the tissues were moved to new 6-well plates containing fresh ORL-200-MM or GIN-100-MM supplemented with 1 M HEPES. Next, 0.1 mL of NBW3, 0.2% CHX (Sigma-Aldrich Co. LLC., St. Louis, Missouri, USA), sterile, deionized water (Quality Biological, Inc., Gaithersburg, Maryland, USA) (as a negative control), or Triton™ X-100 (Fisher Scientific Inc., Pittsburgh, Pennsylvania, USA) (as a positive control) was applied to the top (apical) surface of the tissues. Tissues were exposed to NBW3 for 1 and 30 min, and 1, 4, 12 and 24 h. Tissues were exposed to 0.2% CHX for 4, 8, 16 and 24 h. Tissues were exposed to sterile, deionized water for 30 min, and 12, and 24 h. Tissues were exposed to Triton™ X-100 for 4, 8, and 12 h (EpiGIN-100™) and 20, 60, and 120 min (EpiORL-200™). After the exposure period was complete, the tissues were rinsed with Ca2+/Mg2+-free Dulbecco’s phosphate buffered saline (DPBS, Wako Pure Chemical Industries, Ltd, Osaka, Osaka, Japan) and tissue cell viability was determined using the MTT assay (described below).
2.3.2. MTT viability assay
Tissue cell viability was determined using the MTT tissue viability assay, which is a quantitative, colorimetric assay that measures the reduction of a yellow tetrazolium component (MTT: 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide) to an insoluble purple formazan product by the mitochondria of viable cells. The extent to which MTT is reduced to the purple formazan product is correlated with cell viability [28]. For this assay, we used MTT Cell Proliferation Kit I (Roche Applied Science, Penzberg, Bavaria, Germany). Following the Ca2+/Mg2+-free DPBS rinse step, tissues were transferred into 24-well plates in which each well contained 100 μL of MTT labeling reagent (final concentration 0.5 mg mL−1) in culture medium. Tissues were incubated with the MTT labeling reagent for 4 h in a humidified 6.5% CO2 atmosphere at 37 °C. After the MTT reaction, tissues were transferred to a second 24-well plate containing 1.0 mL of solubilization solution (10% sodium dodecyl sulfate in 0.01 M HCl) and incubated overnight in a humidified 6.5% CO2 atmosphere at 37 °C to solubilize the formazan salt crystals. The soluble formazan product was spectrophotometrically quantified using a Vmax™ plate reader (Molecular Devices, LLC., Sunnyvale, California, USA). The wavelength to measure absorbance of the formazan product was 550 nm. The amount of color produced is directly proportional to the number of viable cells. The raw Sabsorbance values were corrected by subtracting the blank optical density at 550 nm (OD550) from the OD550 for the treated tissues as in equation (1). The tissue viability was determined by normalizing the corrected OD550 for the test tissues as a percent of corrected OD550 for the negative control tissues as in equation (2).
2.3.3. Calculation of ET50 value
The exposure time resulting in a 50% reduction in cell viability (ET50) was determined mathematically as in equation (3).
3. Results
3.1. Time-kill study
The results of time-kill experiments to determine the rate at which NBW3 kills P. gingivalis JCM12257 and A. actinomycetemcomitans JCM8577 cells are shown in figure 1. Time-kill curves for P. gingivalis and A. actinomycetemcomitans treated with 0.9% NaCl showed that there were no significant changes in the number of CFUs/mL after 5 min of exposure, suggesting that the test system was valid. The number of CFUs/mL of P. gingivalis exposed to 0.2% CHX did not drop to below the lower limit of detection (<10 CFUs mL−1) until 5 min of exposure. In contrast, the number of CFUs/mL of P. gingivalis exposed to NBW3 dropped to below the lower limit of detection (<10 CFUs mL−1) after only 0.5 min of exposure. Also, the number of CFUs/mL of A. actinomycetemcomitans exposed to NBW3 and 0.2% CHX dropped to below the lower limit of detection (<10 CFUs mL−1) after 0.5 min of exposure.
Figure 1.
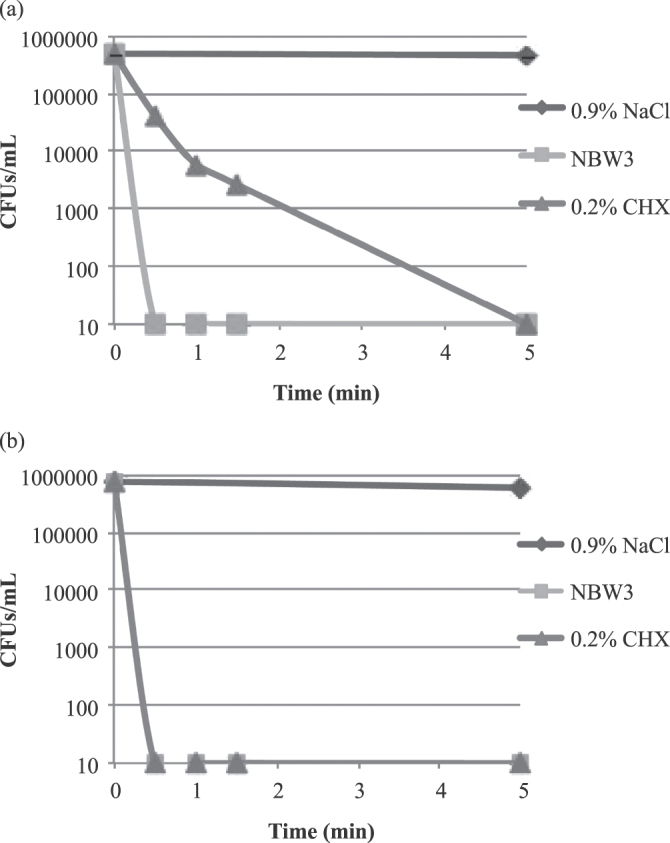
Time-kill curves for P. gingivalis JCM12257 (a) and A. actinomycetemcomitans JCM8577 (b) following exposure to 0.9% NaCl (control), 0.2% CHX, and NBW3. The number of CFUs/mL of P. gingivalis exposed to 0.2% CHX did not drop to below the lower limit of detection (<10 CFUs mL−1) until 5 min of exposure. In contrast, the number of CFUs/mL of P. gingivalis exposed to NBW3 dropped to below the lower limit of detection (<10 CFUs mL−1) after only 0.5 min of exposure. Also, the number of CFUs/mL of A. actinomycetemcomitans exposed to NBW3 and 0.2% CHX dropped to below the lower limit of detection (<10 CFUs mL−1) after 0.5 min of exposure.
3.2. Cytotoxicity study
Time response curves for the EpiGIN-100™ and EpiORL-200™ tissues exposed to the positive control, 1% Triton™ X-100, showed that the viability of cells in the EpiGIN-100™ tissue deteriorated to 8.9% after 12 h of exposure, while the viability of cells in the EpiORL-200™ tissue deteriorated to 14.4% after 120 min of exposure (figure 2). The ET50 values for the EpiGIN-100™ (6.80 h) and EpiORL-200™ (47.1 min) tissues treated with 1% Triton™ X-100 matched the quality control acceptance criteria established by Klausner et al [29] (6.77 h < ET50 < 9.16 h for the EpiGIN-100™ and 31 min < ET50 < 92 min for the EpiORL-200™ tissue models), suggesting that the test system was valid.
Figure 2.
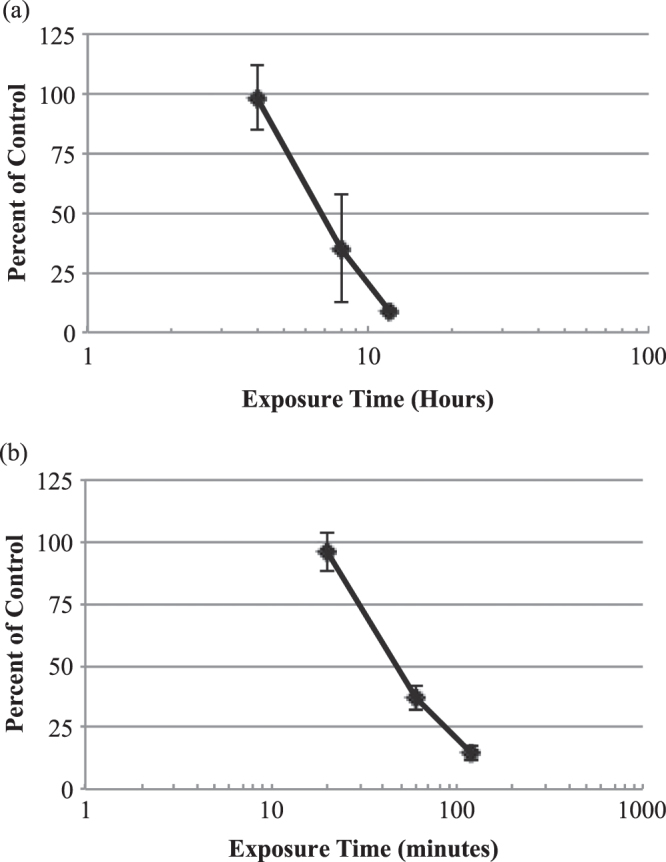
In vitro time-response curves for EpiGIN-100™ (a) and EpiORL-200™ (b) tissues exposed to 1% Triton™ X-100. The exposure time necessary to reduce the viability of tissue cells by 50% (ET50) was 6.8 h for EpiGIN-100™ and 47.1 min for EpiORL-200™ tissues. Data are expressed as the mean ± standard deviation of triplicate determinations. The experiment was performed three times and similar results were obtained in each experiment.
Regarding 0.2% CHX, the viability of EpiGIN-100™ and EpiORL-200™ cells deteriorated to less than 10% after 24 h of exposure (figure 3). The ET50 value was 10.8 h for EpiGIN-100™ cells and 8.4 h for EpiORL-200™ cells.
Figure 3.
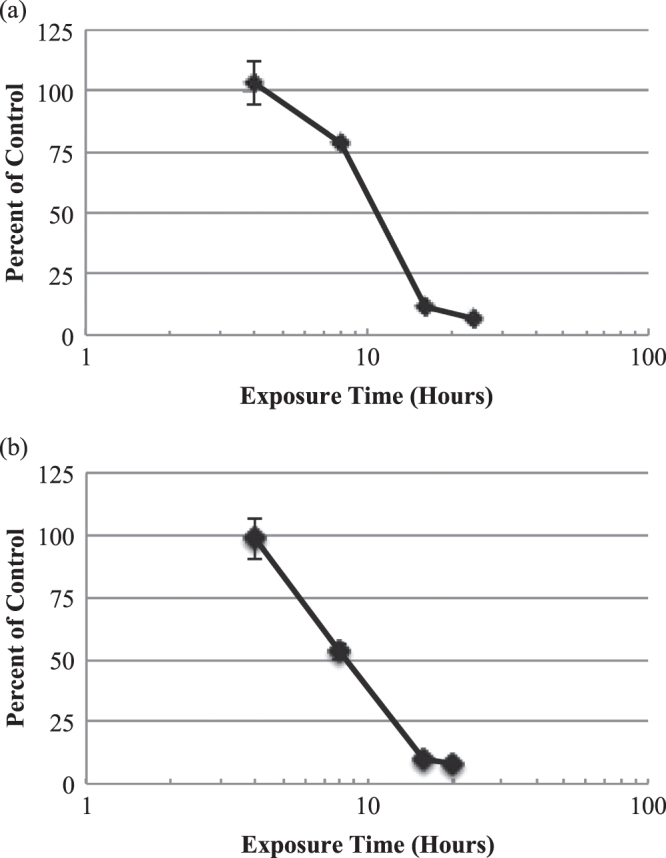
In vitro time-response curves for EpiGIN-100™ (a) and EpiORL-200™ (b) tissues exposed to 0.2% CHX. The exposure time necessary to reduce the viability of tissue cells by 50% (ET50) was 10.8 h for EpiGIN-100™ and 8.4 h for EpiORL-200™ tissues. Data are expressed as the mean ± standard deviation of triplicate determinations. The experiment was performed three times and similar results were obtained in each experiment.
Time-response curves for these tissues exposed to NBW3 indicated that there were only minor decreases in the viability of cells after 24 h of exposure (figure 4). The ET50 values for both tissue cells were over 24 h.
Figure 4.
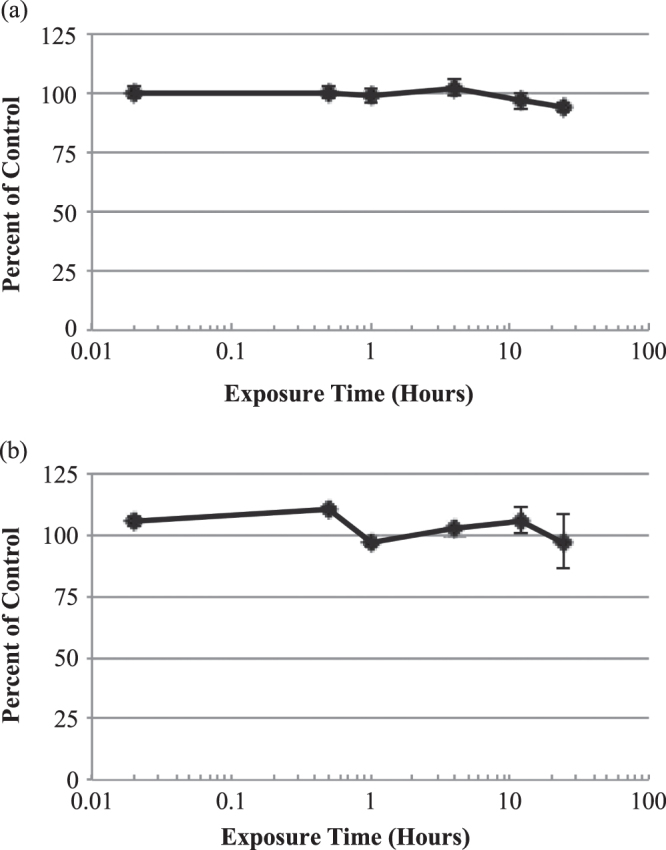
In vitro time-response curves for EpiGIN-100™ (a) and EpiORL-200™ (b) tissues exposed to NBW3. The exposure time necessary to reduce the viability of tissue cells by 50% (ET50) was over 24 h for both EpiGIN-100™ and EpiORL-200™ tissues. Data are expressed as the mean ± standard deviation of triplicate determinations. The experiment was performed three times and similar results were obtained in each experiment.
4. Discussion
Recently, the novel antiseptic NBW3 was developed using nano-bubble generating technology, and there were expectations that NBW3 would show a fast-acting and potent microbicidal efficacy against specific periodontal pathogens and that it would be safe for use in humans as an adjunctive antiseptic in periodontal treatment. In order to understand the mode of action of NBW3 against periodontopathic bacteria, we conducted in vitro time-kill studies. We also investigated whether NBW3 was cytotoxic to cells of human oral tissues using 3D human buccal and gingival tissue models.
The time-kill study is a basic and accurate microbiological method for assessing antimicrobial activity of an antimicrobial material or disinfectant [30]. In this study, the number of CFUs/mL of P. gingivalis and A. actinomycetemcomitans exposed to NBW3 dropped to below the lower limit of detection (<10 CFUs mL−1) after only 0.5 min of exposure. These results suggest that NBW3 possesses equivalent to or more potent bactericidal activity against these periodontopathic bacteria than established oral antiseptic, 0.2% CHX. The rapidity of the bactericidal effect is one of the most important characteristics of a disinfectant intended for use in periodontal treatment. However, translating our findings to the clinic is not straightforward. It has been recognized that dental plaque is a bacterial biofilm [31], and that biofilm microorganisms show a remarkable resistance to antibiotics and antiseptics that are effective in controlling their free-living counterparts [32]. In addition, in the case of ozone, it decomposes after contact with organic substances, like the extracellular matrix of biofilms [19]. Although in this study the efficacy of NBW3 on biofilms was not tested, a limited effect could be assumed. Therefore, in clinical situations, we recommend mechanical disruption of subgingival biofilms (scaling and root planing) to be performed concurrently with NBW3 irrigation. This combined approach enables NBW3 to react directly with the planktonic bacteria and allows NBW3 to exert optimal bactericidal efficacy.
Previous study by Huth et al [33] found that aqueous ozone at 1.25 mg L−1 reduced the levels of P. gingivalis and A. actinomycetemcomitans by approximately 60% after 1 min of exposure. Although it is impossible to compare rigorously the bactericidal activity of aqueous ozone (at 1.25 mg L−1) used in that study [33] and NBW3 (at 1.5 mg L−1) used in our study due to the differences of the study design, NBW3 might be more effective against these two bacteria than aqueous ozone at comparable concentration. The bactericidal mechanisms of NBW3 are considered to be basically similar to those of the existing ozonated water. Briefly, ozone is a potent oxidizing agent [34] and free radical-mediated oxidation reactions might play a role in the destruction of bacteria by NBW3. Besides, ozone nano-bubbles per se might play an important role in the bactericidal mechanisms of NBW3. Since there is no investigation regarding the detailed bactericidal mechanisms of NBW3, close examinations must be progressed.
In this study, we also investigated whether NBW3 was cytotoxic to the cells of human oral tissues using 3D buccal and gingival tissue models. Recently, in vitro organotypic human oral tissue models have been brought into wide use for a broad variety of oral toxicology and biology studies [35, 36]. These tissues used in this study are composed of normal, human-derived epithelial cells and histologically and phenotypically resemble native buccal and gingival tissues [29]. Moreover, standardized quality control tests show the tissues to be highly reproducible [29]. Utilizing these highly differentiated organotypic tissue culture models to test the toxicity and irritation potential of new dental materials and oral care products is more predictive of human responses and more clinically relevant than are animal and monolayer cell culture test systems [29]. We used the MTT assay to assess the viability of tissue model cells. The MTT assay is the most widely used cell-viability assay available for examining cytotoxicity. The MTT cell viability data were used to determine the ET50, which is the exposure time resulting in a 50% reduction in viability. In general, the lower the ET50, the more irritating/damaging is the test material, while the higher the ET50 is, the milder and less damaging is test material. The MTT assay and ET50 have been successfully used to correlate in vitro and in vivo irritancy scores for other mucosal tissue models [37–39].
First, we confirmed that the ET50 values for the EpiGIN-100™ and EpiORL-200™ tissues treated with 1% Triton™ X-100 matched the quality control acceptance criteria established by Klausner et al [29], suggesting that the test system was valid. Next, established oral antiseptic, 0.2% CHX, was tested to compare its possible toxic effects on resident oral cells with those of NBW3. The viability of EpiGIN-100™ and EpiORL-200™ cells deteriorated to less than 10% after 24 h of exposure to 0.2% CHX. The ET50 value was 10.8 h for EpiGIN-100™ cells and 8.4 h for EpiORL-200™ cells. In contrast, time-response curves for both tissues exposed to NBW3 indicated only minor decreases in the viability of cells after 24 h of exposure. The ET50 values for both tissue cells were over 24 h. These results suggest that 0.2% CHX can cause damage to human oral tissues, and NBW3 treatment would be safer for use as an oral antiseptic. However, in the light of clinically relevant short exposure times, both 0.2% CHX and NBW3 do not seem to cause significant cytotoxic damage and can be tolerated for use as an adjunctive oral antiseptic. Further studies are projected in order to investigate more detailed impact of NBW3 on human oral tissue cells, for example, to determine whether and to what extent NBW3 stimulates cytokine expression known as toxicity biomarkers, specifically IL-1α and IL-1β, by human oral cells.
According to the results of this study, NBW3 possesses fast-acting and potent bactericidal activity against representative periodontopathic bacteria and does not exhibit significant cytotoxicity against the cells of human oral tissues. These results suggest that the use of NBW3 as an adjunctive antiseptic in periodontal treatment would be promising. However, these in vitro models cannot be directly compared to the clinical situation in which oral antiseptics are diluted with saliva, and oral mucosa is constantly bathed with saliva. Moreover, in the subgingival environment, the potency of oral antiseptics should be reduced because of the rapid clearance of oral antiseptics from the pocket by continuous flow of gingival crevicular fluid and partial inactivation of applied oral antiseptics via binding to serum proteins. The extent to which the potency of NBW3 might be reduced in the actual clinical situation should be determined.
Acknowledgments
The authors thank Drs. M Umeda and Y Takeuchi for supplying the bacteria used in this study. The authors also acknowledge Olympus Corporation for technical project support. This work was supported by a Grant-in-aid for scientific research (B) #18390561 to Y I and a Grant-in-aid for scientific research (C) (2) #21592620 to S A from the Ministry of Education, Culture, Sports, Science, and Technology.
Appendices.
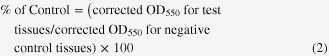 |
V = a + b × lg(t), where V = % viability, t = time in minutes, and ‘a’ and ‘b’ are constants.
 |
References
- Ali R W, Velcescu C, Jivanescu M C, Lofthus B. and Skaug N. J. Clin. Periodontol. 1996;23:133–139. doi: 10.1111/j.1600-051X.1996.tb00546.x. [DOI] [PubMed] [Google Scholar]
- Ashimoto A, Chen C, Bakker I. and Slots J. Oral Microbiol. Immunol. 1996;11:266–273. doi: 10.1111/j.1399-302X.1996.tb00180.x. [DOI] [PubMed] [Google Scholar]
- Dzink J L, Socransky S S. and Haffajee A D. J. Clin. Periodontol. 1988;15:316–323. doi: 10.1111/j.1600-051X.1988.tb01590.x. [DOI] [PubMed] [Google Scholar]
- Haffajee A D, Socransky S S, Patel M R. and Song X. Oral Microbiol. Immunol. 2008;23:196–205. doi: 10.1111/j.1399-302X.2007.00411.x. [DOI] [PubMed] [Google Scholar]
- Drisko C H. Ann. Periodontol. 1996;1:491–566. doi: 10.1902/annals.1996.1.1.491. [DOI] [PubMed] [Google Scholar]
- Greenstein G. J. Periodontol. 1999;70:1397–1405. doi: 10.1902/jop.1999.70.11.1397. [DOI] [PubMed] [Google Scholar]
- Mariotti A J. and Rumpf D A. J. Periodontol. 1999;70:1443–1448. doi: 10.1902/jop.1999.70.12.1443. [DOI] [PubMed] [Google Scholar]
- Krautheim A B, Jermann T H. and Bircher A J. Contact Dermatitis. 2004;50:113–116. doi: 10.1111/j.0105-1873.2004.00308.x. [DOI] [PubMed] [Google Scholar]
- Kim J G, Yousef A E. and Dave S. J. Food Prot. 1999;62:1071–1087. doi: 10.4315/0362-028x-62.9.1071. [DOI] [PubMed] [Google Scholar]
- Restaino L, Frampton E W, Hemphill J B. and Palnikar P. Appl. Environ. Microbiol. 1995;61:3471–3475. doi: 10.1128/aem.61.9.3471-3475.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal R, Kim J G. and Yousef A E. J. Food Prot. 2001;64:777–782. doi: 10.4315/0362-028x-64.6.777. [DOI] [PubMed] [Google Scholar]
- Paraskeva P. and Graham N J. Water Environ. Res. 2002;74:569–581. doi: 10.2175/106143002X140387. [DOI] [PubMed] [Google Scholar]
- Baysan A, Whiley R A. and Lynch E. Caries Res. 2000;34:498–501. doi: 10.1159/000016630. [DOI] [PubMed] [Google Scholar]
- Baysan A. and Lynch E. Am. J. Dent. 2004;17:56–60. [PubMed] [Google Scholar]
- Nagayoshi M, Kitamura C, Fukuizumi T, Nishihara T. and Terashita M. J. Endod. 2004;30:778–781. doi: 10.1097/00004770-200411000-00007. [DOI] [PubMed] [Google Scholar]
- Arita M, Nagayoshi M, Fukuizumi T, Okinaga T, Masumi S, Morikawa M, Kakinoki Y. and Nishihara T. Oral Microbiol. Immunol. 2005;20:206–210. doi: 10.1111/j.1399-302X.2005.00213.x. [DOI] [PubMed] [Google Scholar]
- Hems R S, Gulabivala K, Ng Y L, Ready D. and Spratt D A. Int. Endod. J. 2005;38:22–29. doi: 10.1111/j.1365-2591.2004.00891.x. [DOI] [PubMed] [Google Scholar]
- Huth K C. Int. Endod. J. 2009;42:3–13. doi: 10.1111/j.1365-2591.2008.01460.x. [DOI] [PubMed] [Google Scholar]
- Huth K C, Quirling M, Lenzke S, Paschos E, Kamereck K, Brand K, Hickel R. and Ilie N. Eur. J. Oral Sci. 2011;119:204–210. doi: 10.1111/j.1600-0722.2011.00825.x. [DOI] [PubMed] [Google Scholar]
- Huth K C, Jakob F M, Saugel B, Cappello C, Paschos E, Hollweck R, Hickel R. and Brand K. Eur. J. Oral Sci. 2006;114:435–440. doi: 10.1111/j.1600-0722.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- Ebensberger U, Pohl Y. and Filippi A. Dent. Traumatol. 2002;18:262–266. doi: 10.1034/j.1600-9657.2002.00087.x. [DOI] [PubMed] [Google Scholar]
- Nagayoshi M, Fukuizumi T, Kitamura C, Yano J, Terashita M. and Nishihara T. Oral Microbiol. Immunol. 2004;19:240–246. doi: 10.1111/j.1399-302X.2004.00146.x. [DOI] [PubMed] [Google Scholar]
- Chiba K. and Takahashi M. Nano-bubble production method. P4144669. JAPAN PATENT. 2008 2008-09-03.
- Hirose Y. Urolithiasis. 2013;41:279–294. doi: 10.1007/s00240-013-0576-5. [DOI] [PubMed] [Google Scholar]
- Takahashi M. J. Phys. Chem. B. 2005;109:21858–21864. doi: 10.1021/jp0445270. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Chiba K. and Li P. J. Phys. Chem. B. 2007;111:1343–1347. doi: 10.1021/jp0669254. [DOI] [PubMed] [Google Scholar]
- Hayakumo S, Arakawa S, Mano Y. and Izumi Y. Clin. Oral Invest. 2013;17:379–388. doi: 10.1007/s00784-012-0711-7. [DOI] [PubMed] [Google Scholar]
- Mosmann T. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Klausner M, Ayehunie S, Breyfogle B A, Wertz P W, Bacca L. and Kubilus J. Toxicol. In Vitro. 2007;21:938–949. doi: 10.1016/j.tiv.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Pankuch G A, Jacobs M R. and Appelbaum P C. Antimicrob. Agents Chemother. 1994;38:2065–2072. doi: 10.1128/AAC.38.9.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh P D. and Bradshaw D J. J. Ind. Microbiol. Biotechnol. 1995;15:169–175. doi: 10.1007/bf01569822. [DOI] [PubMed] [Google Scholar]
- Slots J. and Jorgensen M G. Periodontology 2000. 2002;28:298–312. doi: 10.1034/j.1600-0757.2002.2801123.x. [DOI] [PubMed] [Google Scholar]
- Huth K C, Quirling M, Lenzke S, Paschos E, Kamereck K, Brand K, Hickel R. and Llie N. Eur. J. Oral Sci. 2011;119:204–210. doi: 10.1111/j.1600-0722.2011.00825.x. [DOI] [PubMed] [Google Scholar]
- Broadwater W T, Hoehn R C. and King P H. Appl. Microbiol. 1973;26:391–393. doi: 10.1128/am.26.3.391-393.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moharamzadeh K, Franklin K L, Brook I M, van Noort R. J. Periodontol. 2009;80:769–775. doi: 10.1902/jop.2009.080610. [DOI] [PubMed] [Google Scholar]
- Pianigiani E, Andreassi A, Lorenzini G, Alessandrini C, Fimiani M, Atrei A, Fonzi L, Giorgetti R. and Sestini S. Bull Group Int. Rech. Sci. Stomatol. Odontol. 2004;46:63–71. [PubMed] [Google Scholar]
- Ayehunie S, Cannon C, Lamore S, Kubilus J, Anderson D J, Pudney J. and Klausner M. Toxicol. In Vitro. 2006;20:689–698. doi: 10.1016/j.tiv.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Faller C, Bracher M, Dami N. and Roguet R. Toxicol. In Vitro. 2002;16:557–572. doi: 10.1016/S0887-2333(02)00053-X. [DOI] [PubMed] [Google Scholar]
- Stern M, Klausner M, Alvarado R, Renskers K. and Dickens M. Toxicol. In Vitro. 1998;12:455–459. doi: 10.1016/S0887-2333(98)00017-4. [DOI] [PubMed] [Google Scholar]


