Abstract
We report the growth of ZnO nanowires on nonwoven polyethylene fibers using a simple hydrothermal method at a temperature below the boiling point of water. The ZnO nanowires were grown from seed ZnO nanoparticles affixed onto the fibers. The seed ZnO nanoparticles, with diameters of about 6–7 nm, were synthesized in isopropanol by reducing zinc acetate hydrate with sodium hydroxide. The growth process was carried out in a sealed chemical bath containing an equimolar solution of zinc nitrate hexahydrate and hexamethylene tetramine at a temperature of 95 °C over a period of up to 20 h. The thickness and length of the nanowires can be controlled by using different concentrations of the starting reactants and growth durations. A 0.5 mM chemical bath yielded nanowires with an average diameter of around 50 nm, while a 25 mM bath resulted in wires with a thickness of up to about 1 μm. The length of the wires depends both on the concentration of the precursor solution as well as the growth duration, and in 20 h, nanowires as long as 10 μm can be grown. The nonwoven mesh of polyethylene fibers covered with ZnO nanowires can be used for novel applications such as water treatment by degrading pollutants by photocatalysis. Photocatalysis tests carried out on standard test contaminants revealed that the polyethylene fibers with ZnO nanowires grown on them could accelerate the photocatalytic degradation process by a factor of 3.
Keywords: nanowires, ZnO, hydrothermal, polyethylene, photocatalysis
Introduction
Metal oxides are essential for the development of smart and efficient materials, devices and systems. Nanostructured ZnO materials have received attention in a wide range of fields due to their interesting properties, which render them suitable for potential applications in microelectronic and optoelectronic devices [1]. Compared with bulk materials, nanocrystalline materials exhibit completely different properties due to their enhanced surface-to-volume ratio and quantum confinement effects. Bulk ZnO is a wide band-gap compound semiconductor (band gap ∼3.37 eV) that is suitable for short-wavelength optoelectronic applications. The exciton binding energy (60 meV) in bulk ZnO crystal is much larger than that of GaN (25 meV), which ensures efficient excitonic emission at room temperature. ZnO is transparent to visible light and can be made highly conductive by doping. ZnO exhibits a photocatalytic property whereby it can break up complex organic molecules into smaller organic or inorganic fragments in the presence of UV light [2]. Another feature of ZnO that makes it an attractive material is that its nanostructures are independent of the substrates on which they are grown; thus, they can be grown on a variety of substrates.
Several reports on the growth and characterization of one-dimensional nanowires of elemental and compound semiconductors such as Si [3], Ge [4], InP [5], GaAs [6] and ZnO [7–9] are available in the literature. A wide variety of nanostructures have been reported for ZnO, such as nanowires, nanocombs, nanorings, nanohelices, nanobows, nanobelts and nanocages, which have been successfully synthesized under specific growth conditions [10, 11]. The synthesis methods of zinc oxide nanostructures can broadly be classified as gas-phase synthesis and solution-phase synthesis. Gas-phase synthesis uses a gaseous environment in a closed chamber for the growth of nanostructures and is normally carried out at high temperatures from 500 to 1500 °C. Some commonly used gas-phase methods are vapor phase transport, which includes vapor solid (VS) and vapor liquid solid (VLS) growth, physical vapor deposition, chemical vapor deposition, metal vapor deposition and thermal oxidation of pure Zn followed by condensation. In the solution phase-synthesis, the growth process is carried out in a liquid. Normally aqueous solutions are used, and the process is then referred to as a hydrothermal growth process. Some of the solution-phase synthesis processes include a zinc acetate hydrate (ZAH)- derived nanocolloidal sol-gel route [12], ZAH in an alcoholic solution with sodium hydroxide (NaOH) or tetra methyl ammonium hydroxide (TMAH) [13–15], template-assisted growth [16], spray pyrolysis [17, 18] and electrophoresis [19] for fabricating nanostructured thin films.
One of the most energy-efficient and inexpensive strategies for synthesizing ZnO nanowires is the hydrothermal process, which does not require a high temperature or a complex vacuum environment. The hydrothermal process induces epitaxial, anisotropic crystal growth in a solution [20, 21]. The hydrothermal process is usually independent of the surface [22] and provides good control over the morphology of the nanowires grown [23]. The successful growths of ZnO nanowires on flat substrates such as Si [19], glass [19, 24], TCO [25], carbon cloth [26] and Al foil [27] have been reported. However, there have been no reports of ZnO nanowires grown on nonwoven polymeric fibrous substrates.
The hydrothermal process, first reported by Vayssieres et al [20] uses an equimolar solution of zinc nitrate hexahydrate and hexamethylene tetramine, popularly called hexamine, to epitaxially grow ZnO rods on various substrates by fixing presynthesized ZnO nanoparticles on the substrate as seeds. The growth technique relies on the innate anisotropy in the crystal structure of ZnO. The ZnO crystal is hexagonal wurtzite and exhibits partial polar characteristics [9] with lattice parameters a=0.3296 nm and c=0.52065 nm. The structure of ZnO can be simply described as a series of alternating planes composed of tetrahedrally coordinated O2− and Zn2+ arranged along the c-axis. The most common polar surface is the basal plane (001). One end of the basal polar plane terminates in partially positive Zn lattice points and the other end terminates in partially negative oxygen lattice points.
It was reported that in a chemical bath containing zinc nitrate and hexamine used in ZnO nanowire synthesis by the hydrothermal process, hexamine, a nonionic tertiary amine derivative and a nonpolar chelating agent, preferentially attaches to the nonpolar facets of the ZnO crystal, thereby exposing only the (002) plane for epitaxial growth [23]. Thus preferential growth along the [0002] direction is possible. The seeding of the substrate with ZnO nanoparticles was found to lower the thermodynamic barrier by providing nucleation sites, further improving the aspect ratio of the synthesized nanowires [20].
The seeding of the substrate is thus an important factor in the hydrothermal growth of ZnO. Seeding can be carried out by processes such as dip coating and spin coating [20] using a colloidal solution of ZnO nanoparticles or by sputtering, whereby a thin layer of ZnO particles is deposited on the substrate [28]. Growing ZnO nanowires and nanorods over a large area using the hydrothermal method is a difficult task as the seed particles tend to become dislodged from the substrate, and normally nanowire growth is observed in small patches across the substrate.
In this work, we have used the hydrothermal method to synthesize ZnO nanowires on polyethylene fibers, which are one of the toughest fibers available and are about 10–15 times stronger than steel [29]. What makes this work very interesting is the fact that the ZnO nanowires grown on a mesh of nonwoven fibers can be used as a membrane that can filter out harmful organic compounds from gases or solvents passing through it using photocatalysis.
Photocatalysis is a change in the reaction rate of a chemical reaction under the action of light in the presence of a catalyst often called a photocatalyst. Exposing a semiconductor surface to an energy greater than its band gap excites electrons from the valence band to the conduction band, generating electron-hole pairs on its surface. The electron-hole pairs generated by irradiation has very short life spans [30], and undergoes volume and surface recombination, surface trapping or catalyzes redox reactions through interfacial charge transfer. Volatile organic compounds can be removed through photocatalysis upon exposure to UV light using ZnO as a photocatalyst.
|
{\rm Band\,gap}}^{\kern-2.5pc\rm ZnO}} {\rm CO}_2 + \mbox{H}_2 \mbox{O + Mineral acid.}\end{eqnarray*}?>
|
ZnO nanoparticles as well as nanowires can be used for the degradation of organic pollutants and are more effective than bulk ZnO as their effective surface to volume ratio is much higher than that of the bulk.
Experimental
Synthesis of ZnO nanoparticles
The synthesis of ZnO nanoparticles was carried out in an alcoholic medium following the procedure reported by Bahnemann et al [31]. A 1 mM zinc acetate (Zn(CH3COO)2, Merck, 99% purity) solution was prepared in 20 ml of 2-propanol ((CH3)2CHOH, Carlo Erba, 99.7% purity) under vigorous stirring at 50 °C. The solution was then diluted to 230 ml with 2-propanol and cooled. Then 20 ml of 20 mM sodium hydroxide in 2-propanol was added dropwise to the cooled solution under continuous stirring. The mixture was then kept in a temperature controlled water bath at 60 °C for 2 h. The transparent colloidal solution of ZnO nanoparticles with diameters of approximately 6 nm was found to be stable over a period of several months. A high resolution transmission electron microscope (HRTEM) image of the nanoparticles was taken using a JEOL/JEM 2010 transmission electron microscope operated at 200 KV. Lattice spacing measurements based on the HRTEM image and fast Fourier transforms (FFT) were carried out using Scion image processing software. The polyethylene sheet was observed by atomic force microscopy (AFM) using Seiko AFM SP14000 in dynamic force microscope (DFM) mode.
Hydrothermal growth of ZnO nanowires
The substrate chosen for ZnO nanowire growth consisted of multilayered nonwoven polyethylene fibers procured from a commercially available source. The substrate was treated with 1% dodecane thiol solution in ethanol and then heated at 100 °C for 15 min prior to the ZnO nanowire growth [32]. The substrates were seeded by dipping the thiolated substrates into a concentrated colloidal solution of ZnO nanoparticles in isopropanol for 15 min. Three dippings were made and the substrates were heated at 150 °C for 15 min after each dipping to ensure that the seeds were securely attached. The wires were grown in a sealed chemical bath containing an equimolar solution of zinc nitrate hexahydrate (Zn(NO3)26H2O, Aldrich, 99% purity) and hexamethylene tetramine (C6H12N4, Carlo Erba, 99.5%) at 90 °C. Different concentrations of the precursor solution were used to study the growth of the ZnO nanowires. As the growth rate was observed to decrease after about 5 h, the precursor solution was changed every 5 h and growth was continued up to 20 h. The samples were then heated at 150 °C for 30 min to vaporize any organic deposits. The characterization of the nanowires was performed using scanning electron microscopy (SEM, IE350FSG FESEM) images through image processing software. X-ray diffraction (XRD) was performed using a Siemens D5000 x-ray diffractometer.
Photocatalysis
Photocatalysis tests were carried out on polyethylene fibers with the ZnO nanowires and on reference sample without any ZnO nanowires. 0.01 mM methylene blue (MB) solution was placed in two quartz cuvettes, and a small piece of the filter medium containing ZnO nanowire was placed in one cuvette and a small piece of the filter medium without ZnO nanowires was placed in the other. Both the cuvettes were placed under two Sylvania G6W UV source tubes placed adjacent to each other. Optical absorption spectra were obtained using an Elico SL 164 double beam UV-Vis spectrophotometer to observe the degradation of MB through photocatalysis.
Results and discussion
The colloidal solution of synthesized ZnO consisted of uniform nanoparticles with diameters of approximately 6–7 nm, as observed in the SEM images shown in figures 1(a) and (b). The images were analyzed to determine the particle size distribution, which shows the dominance of particles in the above size range (inset of figure 1(a)). The reason for synthesizing the nanoparticles in the alcoholic medium is that Zn(OH)2 more readily forms than ZnO in water, and as the dielectric constant of water (78.4 at 25 °C) is much higher than that of alcohol (approximately 18.3 at 25 °C), the nucleation and growth of the ZnO nanoparticles is much faster in alcohol, resulting in finer, well-defined crystallite growth.
Figure 1.
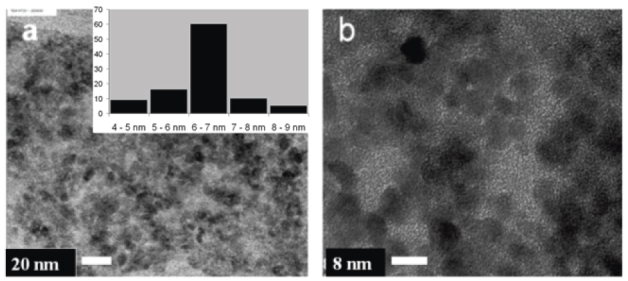
(a) ZnO nanoparticles synthesized by Bahnemann's method; inset: particle size distribution. (b) Close up showing the nearly spherical particles.
The HRTEM image (figure 2(a)) shows the lattice fringes on the ZnO nanoparticles with a fringe spacing of approximately 0.32 nm, conforming to the atomic spacing on the a- and b-axes of ZnO with the wurtzite structure. A fast Fourier transform (FFT) carried out on the square area in figure 2(a) confirmed the presence of crystalline particles. It was reported by Sugunan et al [23] that there is a fairly equal dominance of (100), (002), (101) and (102) crystallographic planes in the nanoparticles synthesized by this method. An inverse FFT carried out on the FFT indicates the presence of all the crystallographic planes of wurtzite ZnO [23].
Figure 2.
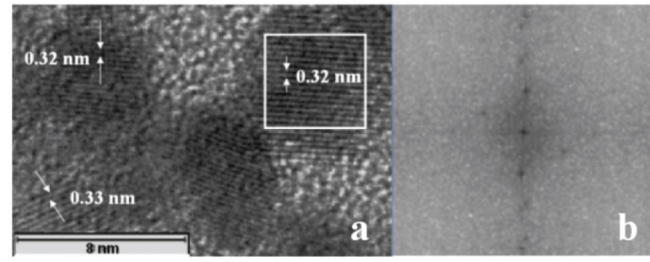
(a) HRTEM image of ZnO nanoparticles showing lattice fringes comparable to the wurtzite structure of ZnO. (b) FFT carried out on the square area in (a).
Seeding the substrate with nanoparticles is a vital issue in obtaining evenly grown ZnO nanowires all over the substrate. The seeding was carried out by dipping the substrate in a concentrated colloidal solution of ZnO nanoparticles using a custom-built dip coater. The loosely attached nanoparticles were removed after each dipping by washing with deionized water so that particles were attached securely in successive dippings. A layer of dodecane thiol was first formed on the substrate to enhance particle attachment. Claesson and Philipse [32] have reported that the surface functionalization of silica using thiol allows the irreversible binding of metal oxide particles from a solution. A similar procedure was followed for the surface functionalization of the polyethylene substrate. Thiol is adsorbed on the hydrolyzed PEO surface, similar to the functionalization of silica surfaces. It is clear from the AFM images (figure A.1 in the appendix) that functionalization improves the density of nanoparticles attached to the substrate.
Figure A.1.
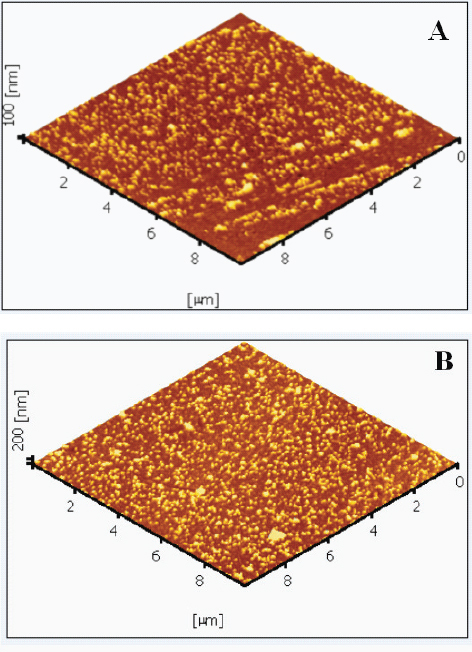
(A) ZnO seeds attached on a flat polyethylene substrate. (B) ZnO seeds on a thiolated flat polyethylene substrate.
In figure 3 (not to scale) we have schematically represented a possible mechanism for the binding of ZnO nanoparticles to the surface of a polyethylene fiber after it was thiolated.
Figure 3.
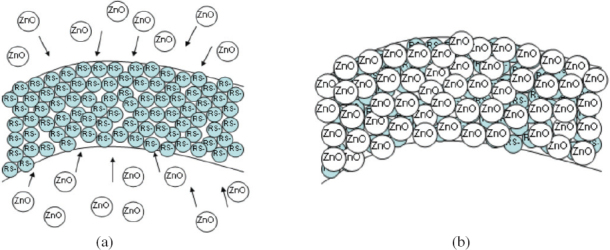
(a) ZnO nanoparticles are attracted to the surface of the thiolated polyethylene fiber. (b) ZnO nanoparticles become attached to the fiber surface. The organic compounds are removed by heating at 250 °C.
To verify the improvement in seeding due to thiolation, two samples of flat polyethylene sheets of dimensions 2×2 cm were used, and one of them was dipped in a 1% solution of dodecane thiol in ethanol for 2 h followed by drying by heating at 100 °C for 15 min. The two samples were then seeded with ZnO nanoparticles by dip coating for equal durations. AFM images revealed that the thiolated substrate had a more even coating of nanoparticles than the unthiolated substrate. AFM images taken over a 10×10 μm area of the samples are shown in the appendix.
The growth of the ZnO nanowires was carried out in a chemical bath containing an equimolar concentration of zinc nitrate hexahydrate and hexamine at 90 °C. The nanowires were grown in 0.5, 1, 5 and 10 mM solutions for durations of 10 and 20 h. The XRD pattern shown in figure 4 shows the preferential growth of the nanowires along the [0002] direction. A comparison of the XRD patterns for different growth durations shows an increase in the (002) peak intensity with time indicating the anisotropic growth of the crystal in the [0002] direction. The higher the concentration of Zn(NO3)2 and hexamine in the chemical bath, the thicker the rods formed. Over a 20 h growth period, 0.5 mM Zn(NO3)2 in a chemical bath yielded nanowires with an average diameter of approximately 50 nm, while the diameter was found to increase to 800 nm when the concentration of Zn(NO3)2 in the starting solution was increased to 20 mM. Zn2 ions, being lighter than hexamine, are more agile and diffuse faster than the hexamine molecules. The Zn2+ ions lead to the rapid isotropic growth of the ZnO crystals until the long chain hexamine molecules encapsulate the nonpolar facets of ZnO, thereby cutting off the access to further Zn2+ ions and preventing their attachment to the nonpolar facets. The ZnO nanorods are thus restricted to growth in only one direction along the uncovered c-axis.
Figure 4.
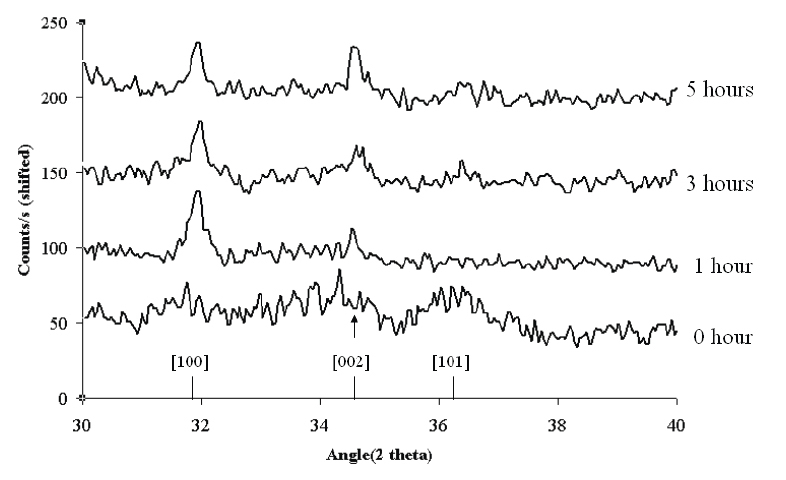
XRD patterns showing the preferential growth of the ZnO crystal in the [0002] direction using 0.5 mM zinc nitrate and hexamine for 0, 1, 3 and 5 h of growth.
The growth of ZnO nanowires/nanorods on the polyethylene fibers was found to be evenly spread with good coverage on the substrate surface. However, as the fibers normally have a mesh form with multiple layers, seeding needs to be carried out with care as the ZnO nanoparticles should be attached to all the layers of the fibers. Care also needs to be taken during nanowire growth so that all the layers of the polyethylene fiber mesh are exposed to Zn ions.
From the SEM images shown in figures 5 and 6 it can be observed that the growth of the ZnO nanowires was more prominent on the top layers of the polyethylene fibers and sparse on the underlying fiber layers. This is because the polyethylene fibers were fixed on a glass slide and the diffusion of Zn ions into the mesh of fibers was thus restricted.
Figure 5.
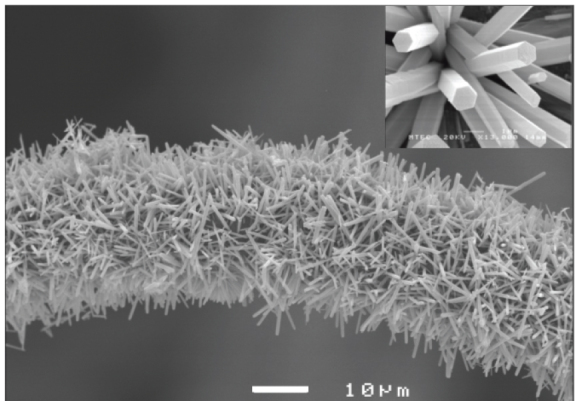
SEM image of the ZnO nanorods grown on polyethylene fibers using 5 mM solution for 10 h; inset: close up of the nanorods.
Figure 6.
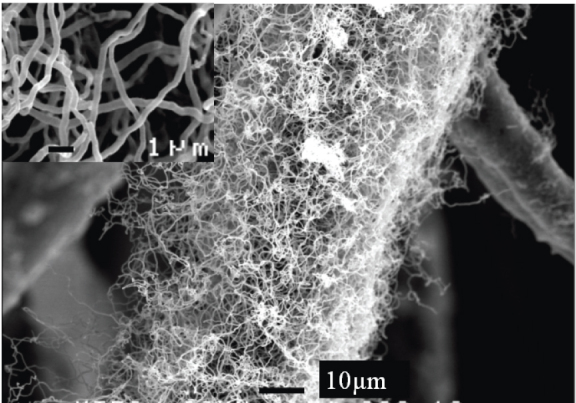
SEM image of the ZnO nanowires grown on polyethylene fibers using 0.5 mM solution for 20 h; inset: close up of the wires.
In an attempt to grow the ZnO nanowires on a multilayer assembly of polyethylene fibers, the assembly was fixed on a rectangular frame and was exposed to the precursor solution from both above and below. It was observed that the nanowires grew on all the fibers in different layers due to the diffusion of the ions through the fiber mesh. Figure 7 shows SEM images of ZnO nanowires grown on different layers of the multilayered polyethylene fibers.
Figure 7.
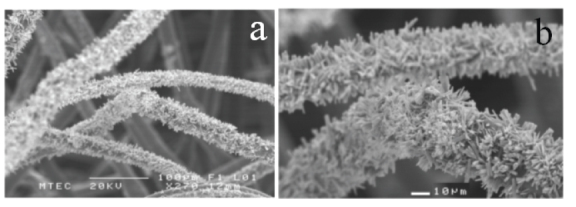
(a) and (b) SEM micrographs showing the growth of ZnO nanowires on different layers of the polyethylene fibers.
The results of the photocatalytic tests conducted using MB are shown in figures 8(a) and (b). After exposure to UV light for 2.5 h, the polyethylene fibers with ZnO nanowires were able to degrade 83% of the MB, whereas the fibers without ZnO nanowires could degrade only 32%. 24% of MB was found undergo self-degradation under the same UV light without using polyethylene fibers. The enhancement of the degradation of MB due to the ZnO nanowires suggests that the growth of nanowires on fibrous materials can be applied to the treatment of polluted water sources. Further experiments are underway to study the photocatalytic degradation of water pollutants, which will be reported later.
Figure 8.
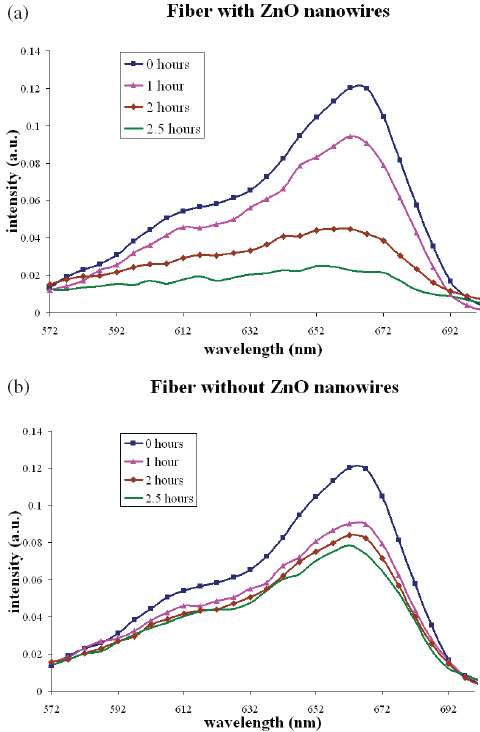
(a) Degradation of MB using polyethylene fibers with ZnO nanowires under UV light. (b) Degradation of MB using polyethylene fibers without ZnO nanowires under UV light.
Conclusion
We have successfully grown ZnO nanowires and nanorods on the surface of polyethylene fibers using a simple hydrothermal method at temperatures suitable for growth on polymeric substrates (<100 °C). The seeding of the substrate with ZnO nanoparticles was greatly improved by first treating the substrate with dodecane thiol. Experiments carried out to evaluate the photocatalytic properties of the ZnO nanowires grown on the polyethylene fibers showed that the degradation of MB is accelerated by a factor of 3. This property has strong potential for applications in the purification of water contaminated with organic compounds.
Appendix
Acknowledgments
The authors would like to acknowledge partial financial support from the National Nanotechnology Center (NANOTEC) and the National Metal and Materials Technology Center (MTEC), both of NSTDA, Ministry of Science and Technology, Royal Thai Government.
References
- Zhang J, Sun L, Pan H, Liao C. and Yan C. New J. Chem. 2002;26:33. doi: 10.1039/b108172a. [DOI] [Google Scholar]
- Pall B. and Sharon M. Mater. Chem. Phys. 2002;76:82. doi: 10.1016/S0254-0584(01)00514-4. [DOI] [Google Scholar]
- Cui Y, Lauhon L J. and Gudiksen M S. Appl. Phys. Lett. 2001;78:2214. doi: 10.1063/1.1363692. [DOI] [Google Scholar]
- Burghard G M, Kim G T, Dusberg G S, Chiu P W, Krstic V, Roth S. and Han W Q. J. Appl. Phys. 2001;90:5747. doi: 10.1063/1.1413495. [DOI] [Google Scholar]
- Duan X, Huang Y, Cui Y, Wang J. and Lieber C M. Nature. 2001;409:66. doi: 10.1038/35051047. [DOI] [PubMed] [Google Scholar]
- Bai Z G, Yu D P, Zhang H Z, Ding Y, Gai S Q, Hang X Z, Hiong Q L. and Feng G C. Chem. Phys. Lett. 1999;303:311. doi: 10.1016/S0009-2614(99)00066-4. [DOI] [Google Scholar]
- Huang M H, Wu Y, Feick H, Tran N, Webe E. and Yang P. Adv. Mater. 2001;13:113. doi: 10.1002/1521-4095(200101)13:2<113::AID-ADMA113>3.0.CO;2-H. [DOI] [Google Scholar]
- Huang M H, Mao S, Feick H, Yan H, Wu Y, Kind H, Weber E, Russo R. and Yang P. Science. 2001;292:1897. doi: 10.1126/science.1060367. [DOI] [PubMed] [Google Scholar]
- Shi G, Mo C M, Cai W L. and Zhang L D. Solid State Commun. 2005;115:253. doi: 10.1016/S0038-1098(00)00169-1. [DOI] [Google Scholar]
- Lee C Y, Tseng T Y, Li S Y. and Lin P. Tamkang J. Sci. Eng. 2003;6:127. [Google Scholar]
- Wang Z L. J. Phys.: Condens. Matter. 2004;16:R829. doi: 10.1088/0953-8984/16/25/R01. [DOI] [Google Scholar]
- Spanhel L. J. Sol-Gel Sci. Technol. 2006;39:7. doi: 10.1007/s10971-006-7302-5. [DOI] [Google Scholar]
- Ma X, Zhang H, Ji Y, Xu J. and Yang D. Mater. Lett. 2005;59:3393. doi: 10.1016/j.matlet.2005.05.076. [DOI] [Google Scholar]
- Kohls M, Bonnani M. and Spanhel L. Appl. Phys. Lett. 2002;81:3858. doi: 10.1063/1.1518774. [DOI] [Google Scholar]
- Xu H Y, Wang H, Zhang Y C, Wang S, Zhu M. and Yan H. Cryst. Res. Technol. 2003;38:429. doi: 10.1002/crat.200310053. [DOI] [Google Scholar]
- Shingubara S. J. Nanoparticle Res. 2003;5:17. doi: 10.1023/A:1024479827507. [DOI] [Google Scholar]
- Krunks M. and Mellikov E. Thin Solid Films. 1995;270:33. doi: 10.1016/0040-6090(95)06893-7. [DOI] [Google Scholar]
- Ayouchi R, Martin F, Leinen D. and Ramos-Barrado J R. J. Cryst. Growth. 2003;247:497. doi: 10.1016/S0022-0248(02)01917-6. [DOI] [Google Scholar]
- Wang Y C, Leu I C, Chung Y W. and Hon M H. Nanotechnol. 2006;17:4445. doi: 10.1088/0957-4484/17/17/027. [DOI] [Google Scholar]
- Vayssieres L, Keis K, Lindquist S E and Hagfeldt A. 2001. J. Phys. Chem. B 105 3350 10.1021/jp010026s [DOI] [Google Scholar]
- Hossain M K, Ghosh S C, Boontongkong Y, Thanachayanont C. and Dutta J. J. Metastable Nanocryst. Mater. 2005;23:27. [Google Scholar]
- Greene L E, Law M, Goldberger J, Kim F, Johnson J C, Zhang Y, Saykally R J. and Yang P. Angew. Chem. Int. Edn. 2003;42:3031. doi: 10.1002/anie.200351461. [DOI] [PubMed] [Google Scholar]
- Sugunan A, Warad H C, Boman M. and Dutta J. J. Sol-Gel Sci. Technol. 2006;39:49. doi: 10.1007/s10971-006-6969-y. [DOI] [Google Scholar]
- Wei M, Zhi D. and MacManus-Driscoll J L. Nanotechnol. 2005;16:1364. doi: 10.1088/0957-4484/16/8/064. [DOI] [Google Scholar]
- Tang Y, Luo L, Chen Z, Jiang Y, Li B, Jia Z. and Xu L. Electrochem. Commun. 2007;9:289. doi: 10.1016/j.elecom.2006.09.026. [DOI] [Google Scholar]
- Jo S H, Banerjee D. and Ren Z F. Appl. Phys. Lett. 2004;85:1407. doi: 10.1063/1.1784543. [DOI] [Google Scholar]
- Umar A, Kim B-K, Kim J-J. and Hahn Y B. Nanotechnol. 2007;18:175606. doi: 10.1088/0957-4484/18/17/175606. [DOI] [Google Scholar]
- Cross R B M, De Souza M M. and Narayanan E M S. Nanotechnol. 2005;16:2188. doi: 10.1088/0957-4484/16/10/035. [DOI] [PubMed] [Google Scholar]
- http://www51.honeywell.com/sm/afc/products-details/fiber.html downloaded on 2.10.07 [Google Scholar]
- Shah S I, Li W, Huang C-P, Jung O. and Ni C. Proc. Natl Acad. Sci. USA. 2002;99:6482. doi: 10.1073/pnas.052518299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahnemann D W, Kormann C. and Hofmann R. J. Phys. Chem. 1987;91:3789. doi: 10.1021/j100298a015. [DOI] [Google Scholar]
- Claesson E M and Philipse A P. 2007. Colloids Surf. A 297 46 10.1016/j.colsurfa.2006.10.019 [DOI] [Google Scholar]


