Abstract
The current research and development of metallic materials used for medicine and dentistry is reviewed. First, the general properties required of metals used in medical devices are summarized, followed by the needs for the development of α + β type Ti alloys with large elongation and β type Ti alloys with a low Young's modulus. In addition, nickel-free Ni–Ti alloys and austenitic stainless steels are described. As new topics, we review metals that are bioabsorbable and compatible with magnetic resonance imaging. Surface treatment and modification techniques to improve biofunctions and biocompatibility are categorized, and the related problems are presented at the end of this review. The metal surface may be biofunctionalized by various techniques, such as dry and wet processes. These techniques make it possible to apply metals to scaffolds in tissue engineering.
Keywords: Metal, Alloy, Biomaterial, Implant, Medical device, Biofunction, Biocompatibility
1. Introduction
The use of metals has a long history, integral to materials science and engineering. However, metals are sometimes thought as ‘unfavorable materials’ for medical purposes due to the environmental and health concerns over heavy metals. With the emphasis on safety of metals for medical use, considerable effort is given to the improvement of corrosion resistance and mechanical durability. On the other hand, the technological evolution of ceramics and polymers during the last three decades has made it possible to apply these materials to medical devices; as a result, many metal devices have been replaced with those made of ceramics and polymers. In spite of this development, over 80% of implant devices are made of metals because of their strength, toughness and durability. The advantages of metals in medical devices are the following:
-
(a)
high strength,
-
(b)
high elasticity,
-
(c)
high fracture toughness,
-
(d)
a combination of high elasticity and stiffness and
-
(e)
high electrical conductivity.
When considering the above properties, metals are generally superior to ceramics and polymers for medical devices. Therefore, it is difficult to replace metals in medical devices with ceramics or polymers.
Research and development of metals continue with the purpose of improving mechanical and surface properties, which govern their mechanical and tissue compatibility. In this paper, we review developments in the research on metallic materials used for medicine, including dentistry.
2. General properties required of metals used in medical devices
Metals are essential for orthopedic implants, bone fixators, artificial joints and external fixators since they substitute for the functions of hard tissues in orthopedics. Stents and stent grafts are placed in blood vessels for dilatation. Therefore, elasticity or plasticity for expansion and rigidity for maintaining dilatation are required in the devices. In dentistry, metals are used for restorations, orthodontic wire and dental implants.
The most important property of these biomaterials is safety. Metals implanted in tissues do not show any toxicity without metal ion dissolution by corrosion and/or generation of debris by wear. Therefore, corrosion-resistant materials, such as stainless steel, the Co–Cr–Mo alloy, commercially pure Ti and Ti alloys, are employed. Noble-metal-based alloys, such as Au alloys and Ag alloys, are also used in dentistry.
A disadvantage of using metals as biomaterials is that they are typically artificial materials and have no biofunction. Therefore, metals require additional properties before they can be used as biomaterials. Requirements for metals in medical devices are summarized in table 1. To respond to these requirements, new alloy designs and many techniques for the surface modification of metals have been researched and even commercialized.
Table 1.
Requirements of metals for medical devices.
| Required property | Target medical devices | Effect |
|---|---|---|
| Elongation to fracture | Spinal fixation; maxillofacial plate | Improvement of durability |
| Elastic modulus | Bone fixation; spinal fixation | Prevention of bone absorption by stress shielding |
| Superelasticity Shape memory effect | Multi-purpose | Improvement of mechanical compatibility |
| Wear resistance | Artificial joint | Prevention of generation of wear debris; improvement of durability |
| Bioderadability | Stent; artificial bone; bone fixation | Elimination of materials after healing; no need of retrieval |
| Bone formation Bone bonding | Stem and cup of artificial hip joint; dental implant | Fixation of devices in bone |
| Prevention of bone formation | Bone screw; bone nail | Prevention of assimilation |
| Adhesion of soft tissue | Dental implant; trans skin device; external fixation; pacemaker housing | Fixation in soft tissue; prevention of inflectional disease |
| Inhibition of platelet adhesion | Devices contacting blood | Prevention of thrombus |
| Inhibition of biofilm formation | All implant devices; treatment tools and apparatus | Prevention of infectious disease |
| Low magnetic susceptibility | All implant devices; treatment tools and apparatus | No artifact in MRI |
3. Categories of research and development
Research and development of metals for medical devices or metallic biomaterials are categorized as follows:
-
(1)
Design of new alloys
-
(2)
Development of working processes and heat treatments
-
(3)
Development of new surface treatment and modification techniques
-
(i)
Ceramic coating and growth of surface oxide
-
(ii)
Immobilization of functional molecules and biomolecules
-
(iii)
Composite with polymers
-
(i)
-
(4)
Control of surface morphology
-
(5)
Evaluation of mechanical properties
-
(6)
Evaluation of corrosion resistance
-
(7)
Evaluation of safety and toxicity
-
(8)
Evaluation of biocompatibility and biofunctions
-
(9)
Development of in vitro evaluation techniques
Development of new materials is performed by (1) design of alloys, (2) development of working processes, (3) surface treatment techniques and (4) control of surface morphology. In addition, (5) the evaluation of mechanical properties is necessary because good mechanical properties are essential for metals. For medical devices, (6) the evaluation of the corrosion resistance is significant. If these properties are confirmed in new materials, (7) safety and toxicity and (8) biocompatibility and biofunctions may be evaluated. Evaluation of mechanical properties and corrosion resistance is essential for metallic materials. In addition, (9) in vitro evaluation techniques of materials are necessary to assess the biofunction and biocompatibility of the materials without animal testing.
4. Titanium alloys
4.1. α + β type titanium alloys with large elongation
Ti and Ti alloys were developed for aerospace applications and have also been used for medical and dental implants because they show high corrosion resistance and specific strength. Their Young's modulus is about one half that of stainless steel and Co–Cr alloys, and this low Young's modulus makes them the preferred material for use in bone fixators. Ti materials form stable titanium oxide films on their surface, and their corrosion resistance is better than that of stainless steel and Co–Cr alloys as a result. Ti and most of the Ti alloys are safe for use in the human body; moreover, they exhibit good tissue compatibility, especially for hard tissue. On the other hand, they are not suitable for bone fixation wires and sternal wires that require ligatures because of their low torsion strength (torque) and torsion angle to fracture.
Ti alloys at ambient temperatures are categorized as α-type, α + β-type, and β-type alloys, according to the quantities and types of their alloying elements. Many kinds of alloys have been developed (table 2), but the Ti–6Al–4V alloy, an α + β-type alloy, is the most conventional one for medical use. This alloy exhibits good workability, heat treatment stability, weldability, corrosion resistance, strength and biocompatibility. The extra low interstitial (ELI) grade alloy containing small amounts of interstitial impurities, oxygen, carbon, nitrogen and hydrogen is used for biomaterials. The Ti–6Al–4V ELI alloy shows significant toughness because the impurities decrease the fatigue strength via the notch effect. The ELI alloy is used for bone fixation plates and the stems of artificial hip joints. The Ti–6Al–4V alloy has an extremely high 0.2% offset yield strength of 895 MPa, which is much higher than that of stainless steel and Co–Cr–Mo alloys, hindering plastic deformation even under high load.
Table 2.
Specified titanium alloys.
| Composition (mass%) | Type | UNS | ASTM | ISO |
|---|---|---|---|---|
| Ti–3Al–2.5V | α+β | R56320 | ASTM B 348 | – |
| Ti–5Al–2.5Fe | α+β | – | – | ISO 5832–10 |
| Ti–6Al–4V | α+β | R56400 | ASTM F 1472 | ISO 5832–3 |
| Ti–6Al–4V ELI | α+β | R56401 | ASTM F 136 | ISO 5832–3 |
| Ti–6Al–7Nb | α+β | R56700 | ASTM F 1295 | ISO 5832–11 |
| Ti–15Mo | β | R58150 | ASTM F 2066 | – |
| Ti–13Nb–13Zr | β | R58130 | ASTM F 1713 | – |
| Ti–12Mo–6Zr–2Fe | β | R58120 | ASTM F 1813 | – |
| Ti–45Nb | β | R58450 | AMS 4982 | – |
| Ti–35Nb–7Zr–5Ta | β | R58350 | – | – |
| Ti-55.8Ni | Metallic compound | – | ASTM F 2063 | – |
The Ti–6Al–7Nb alloy [1] has been developed as a replacement for the Ti–6Al–4V alloy. This alloy was developed and is mainly used in Europe because one of its components, vanadium, shows strong cytotoxicity, although there has been no report of accidents when using Ti–6Al–4V alloy implants. The Ti–6Al–7Nb alloy has been created by substituting V with Nb at the same atomic concentration. Its corrosion resistance and safety are greater than those of Ti–6Al–4V. Elsewhere, the Ti–6Al–2.5Fe alloy developed in Europe, the Ti–13Zr–13Ta alloy (nearly β) developed in the United States, and the Ti–6Al–2Nb–1Ta and Ti–15 Zr–4Nb–4Ta alloys developed in Japan have been standardized. These are α + β type alloys.
Materials scientists and engineers understand that the fracture of metallic materials can be avoided when the materials have sufficient fatigue strength and corrosion resistance. However, recently, the lower elongation of α + β type Ti alloys than that of stainless steel has caused an ‘artificial fracture’ or ‘human error’ in spinal fixators and maxillofacial plates because the elongation to fracture of α + β type Ti alloys is only about 10–17%. The following four causes of fracture of metals are feasible in medicine: (i) larger plastic deformation than the elongation to fracture is applied by medical doctor at the operation site, (ii) multiple plastic deformation is applied if the first bending by the doctor at the operation site is unsuccessful, (iii) alloy fatigue and (iv) large crevices as a result of corrosion initiate fracture.
Causes (i)–(iv) are illustrated in figure 1. The phenomena described in (i) and (ii) may occur as a result of mismanaging the material when its elongation and hardening are miscalculated, whereas those described in (iii) and (iv) can be prevented by improving the mechanical and electrochemical properties of the material. To prevent fractures such as those described in (i) and (ii), specific training of medical professionals is required to understand the relationship between the fracture and elongation and work hardening. In addition, the development of α + β type Ti alloys having large elongation and sufficient strength is required. However, no optimal Ti alloy has been developed so far.
Figure 1.
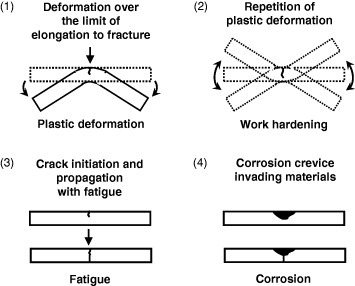
Causes of fracture of metals in medicine: (i) larger plastic deformation than the elongation to fracture is applied by medical doctor at the operation site; (ii) multiple plastic deformation is applied if the first bending by the medical doctor at the operation site is unsuccessful; (iii) alloy fatigue; (iv) large crevices as a result of corrosion work initiate fracture.
4.2. β type titanium alloys and porous titanium with small Young's modulus
The Young's modulus of metallic materials is higher than that of cortical bone: it is about 200 GPa in stainless steels and Co–Cr–Mo alloys, about 100 GPa in Ti and Ti alloys, and 10–20 GPa in cortical bone. When fractured bone is fixed with a metallic bone fixator, such as bone plate and screws and bone nail, during healing, a load to the fixation part is mainly received by metallic fixators because of the difference in their Young's modulus, as shown in figure 2, inducing device-related osteoporosis or osteopenia. This phenomenon is well known as ‘stress shielding’ in orthopedics. This high Young's modulus generates other problems. When a metal is used as a metallic spacer in spinal fixation, the spacer is mounted in bone. In the case of a dental implant, occlusal pressure is not absorbed by the implant and directly conducted to the jaw bone.
Figure 2.
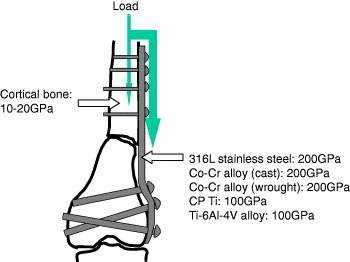
When fractured bone is fixed with a metallic bone fixator, such as a bone plate and screws and bone nail, the load to the fixation part during healing is mainly received by the metallic fixators because of the difference in the Young's moduli.
To solve these problems, metals with low Young's modulus are required. Two approaches are feasible: decrease Young's modulus of the metal itself or decrease the apparent Young's modulus by forming a porous body.
The requirement for a low Young's modulus to prevent stress shielding in bone fixation is fulfilled by β-type alloys, in which the Young's modulus may decrease to about 60 GPa. Various β-type alloys, Ti–12Mo–6Zr–2Fe [2] and T–15Mo [3], have been developed in the United States, and the Ti–15Mo–5Zr–2Al alloy has been specified. The mechanical properties of β-type alloys are shown in figure 3 [4]. Ti alloys consisting of elements with low toxicity have been developed. The basic design of the alloys is the substitution of V and Al with Nb, Ta, Zr and Hf, which belong to the groups 4 and 5 of the periodic table; for this reason, Ti–29Nb–13Ta–4.6Zr has been developed as a β-type alloy [5]. This alloy is transformed to the β phase by heat treatment and forging and shows the lowest Young's modulus among β-type alloys. Elsewhere, the Ti–Nb–Sn alloy system is in the process of development [6].
Figure 3.
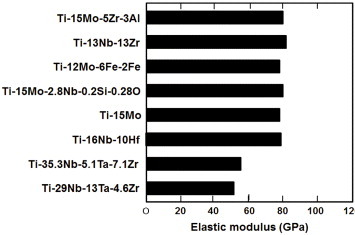
Elastic moduli of β-type alloys [4].
Figure 4 shows the dependence of the elastic modulus of Ti porous body on porosity [7]. The pores are sometimes filled by polymers to adjust the apparent Young's modulus during bone healing. Ultrahigh-molecular-weight polyethylene (UHMWPE) [8] and poly(methyl methacrylate) (PMMA) [9] are used to fill the pores in porous Ti. Figure 5 shows porous Ti with pores filled by UHMWPE.
Figure 4.
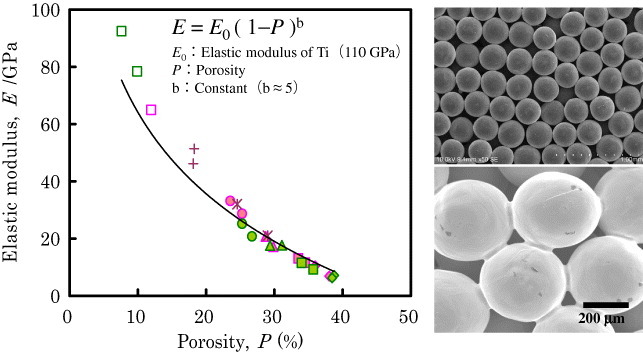
Dependence of Young's modulus of Ti porous body on porosity (left) and scanning electron photographs of porous Ti (right).
Figure 5.
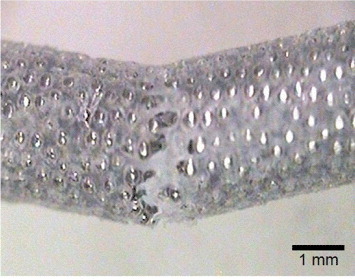
Porous Ti body in which the pores are filled with ultrahigh-molecular-weight polyethylene.
4.3. Nickel-free titanium-based superelastic alloy
Ti–Ni alloys consisting of equal atomic amounts of Ti and Ni (49–51 mol% Ni) show unique mechanical properties, such as shape memory, superelasticity and efficient damping. Because of the superelasticity, the Ti–Ni alloy is used for guide wires, stents, orthodontic arch wires, endodontic reamers and files.
Recently, early-stage fractures of stents in service have been reported. Among self-expanding Ti–Ni femoral stents, 37.2% (45 of 121) fractured within 10.7 months of implantation, as detected by x-ray examination (figure 6) [10]. In drug-eluting stents, all fractures occurred around areas of increased rigidity due to the overlapping of metals, which may have formed a fulcrum for metal deformation due to vessel movement [11]. Ti–Ni stents implanted for suboptimal angioplasty in the superficial femoral artery tend to fracture [12]. Coronary stent fractures with shear bonding forces result from cardiac contractions [13]. In addition, stent fractures after percutaneous coronary intervention with sirolimus-eluting stents have been reported 2 days after stent implantation [14]. On the other hand, the tensile strength and fatigue strength of stainless steels are sufficient; thus, the fractures might originate from improper stent design. Stents consisting of Ti–Ni alloys must be carefully designed to avoid fractures during service. In particular, during expansion, unexpectedly high local deformation may generate fractures. The fatigue and fracture mechanisms of the superelastic Ti–Ni alloy have been only partly elucidated [15–17].
Figure 6.
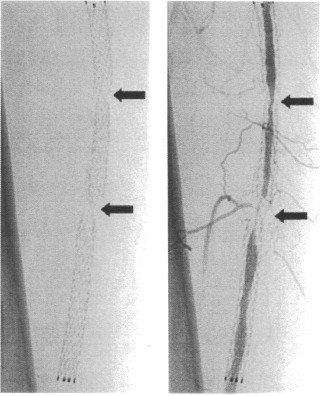
Early-stage fractures of self-expanding Ti–Ni femoral stents in service observed by x-ray examination [10].
On the other hand, severe pitting and crevice corrosion are observed in Ti–Ni alloys in stent grafts [18–20], as shown in figure 7 [18]. These problems may occur in Ti–Ni alloys and stainless steel at crevices between an artificial blood vessel and a metallic stent because of the electrochemical properties of the alloys. Fracture may then initiate at corroded sites. Alloys that are resistant to pitting and corrosion must be developed and used. The safety and durability of Ti–Ni alloys as stent and stent graft materials are not guaranteed because of their severe corrosion and unknown fatigue properties.
Figure 7.
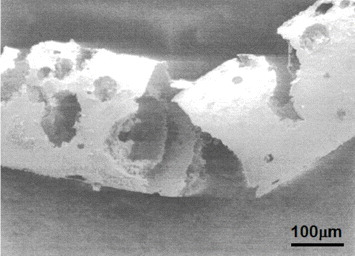
Severe pitting and crevice corrosion observed in Ti–Ni alloys in stent grafts [18].
The use of Ti–Ni alloy with about 50 mol% Ni in medicine is limited by safety. Therefore, there is a great demand for Ni-free shape-memory and superelastic alloys to solve such problems. Ti–Sn–Nb [21], Ti–Nb–Al [22], Ti–Mo–Ga [23], Ti–Mo–Sn [24], Ti–Nb–O [25], and Ti–Nb–Zr [26] alloy systems exhibit shape-memory effect and superelasticity, while the recovery strain and superelastic deformation stress of these alloys are still lower than those of the Ti–Ni alloy. It is possible to improve the shape-memory effect and superelasticity by adjusting the alloy composition and working heat treatment.
5. Bioabsorbable metals
Common metals used for stents are not bioabsorbable but remain in the vessel semipermanently. To eliminate a stent material after service, it is sometimes preferable to use bioabsorbable materials; however, only polymeric bioabsorbable materials are currently available. Another application of absorbable metals is as bone fixators that should also be eliminated after bone healing. Currently, there are two candidates for bioabsorbable metallic materials: magnesium alloy [27, 28] and pure iron [29].
Stents made of biodegradable Mg alloy are safe [28] but associated with a modest degree of neointima formation and late recoil. Mg is a bioessential element, even though it is corrosive in an aqueous environment. Because the control of absorbance, i.e. corrosion, of Mg in the human body, is difficult due to the extreme activity of Mg, alloying is absolutely necessary to control the corrosion rate. In addition, hydrogen evolution occurs when Mg is dissolved and a gas cavity is formed during the implantation of Mg alloys [30]. Control of the corrosion rate for safety in the human body is a key factor in the utilization of Mg alloys for medical devices. Efforts to achieve this purpose are currently underway [27].
6. Nickel-free austenitic stainless steel
Nitrogen (N), which is one of the austenitizing elements, is an important alloying element in austenitic stainless steels in terms of corrosion resistance and strength [31]. Nitrogen dissolved in austenitic stainless steel improves its strength resistance to pitting corrosion and crevice corrosion in solutions containing chloride ions. The related mechanisms are summarized as follows [32]:
-
(1)
N in solid solution produces NH4+, hindering oxidation inside a pit;
-
(2)
Concentrated N at the passive film/alloy surface stabilizes the film and prevents the attack by anions (Cl-);
-
(3)
Nitrate ions are produced to improve resistance to pitting corrosion;
-
(4)
N addition stabilizes the austenitic phase; and
-
(5)
N blocks kinks and controls the increase of electric current for pit production.
Therefore, austenitic high-nitrogen stainless steels containing over 0.3 wt% N have been developed. On the other hand, Ni-free austenitic stainless steels have been designed where Ni is replaced by N and Mn; such steels show high strength and corrosion resistance [33]. The Fe–(19–23)Cr–(21–24)Mn–(0.5–1.5)Mo–(0.85–1.1)N alloy (BioDur® 108) in the United States [34], the Fe–18Cr–18Mn–2Mo–0.9N alloy in Germany [35] and the Fe–(15–18)Cr–(10–12)Mn–(3–6)Mo–0.9N alloy in Switzerland [36] have been developed for medical use. These alloys are fabricated with an electrical slug-remelting process whereby N is absorbed in the alloy during melting under a high-pressure N2 atmosphere. A new manufacturing process has been developed, in which N is absorbed into ferritic stainless steel after forming at 1200 °C [37].
7. Low magnetic susceptibility alloys for MRI
Magnetic resonance imaging (MRI) is widely used as an important diagnostic tool, especially for orthopedic and brain surgery. This method has remarkable advantages for obtaining various cross-sectional views and for diagnosis of the human body with no invasion and no exposure of the human body to x-ray radiation. However, MRI diagnosis is inhibited when metals are implanted in the body, since metallic implants, such as stainless steels, Co–Cr alloys and Ti alloys become magnetized in the intense magnetic field of the MRI instrument, and artifacts occur in the image [38, 39]. Such artifacts can disturb the images of organs and tissues around the implant, preventing exact diagnosing. The area affected by the artifacts is related to the magnetic susceptibility of the implants [40–42] and decreases with decreasing magnetic susceptibility. Operations under open MRI conditions require devices with low magnetic susceptibility. Therefore, metals with low magnetic susceptibility should be developed as MRI continues to increase in popularity.
A concept for the development of alloys with low magnetic susceptibility is shown in figure 8. Figure 8(a) presents a method of alloying a paramagnetic metal with diamagnetic metal. In this sense, the magnetic susceptibility of a Au–Pt–Nb alloy is similar to that of water [43]. Figure 8(b) outlines a technique to precipitate a diamagnetic or low magnetic susceptibility phase in a paramagnetic matrix phase. This technique is explained below. Finally, figure 8(c) illustrates a technique to form a composite of paramagnetic metal and diamagnetic material that is proven both theoretically and empirically [44].
Figure 8.
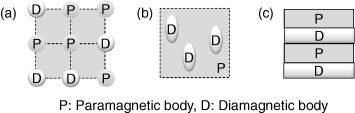
Concept of the development of alloys with low magnetic susceptibility. Alloying paramagnetic metal with diamagnetic metal (a), precipitation of a diamagnetic or low magnetic susceptibility phase in a paramagnetic matrix phase (b), and formation of composite of paramagnetic metal and diamagnetic material (c).
To reduce artifacts in the second technique mentioned above, Ti–Zr, Zr–Nb, and Zr–Mo alloys with low magnetic susceptibility have been developed. The magnetic susceptibility of the Zr–Nb alloy is shown in figure 9; it exhibits minimum values where the ω phase is formed. The magnetic susceptibility was reduced in Zr–Nb and Zr–Mo alloys up to about one-seventh of that of the Co–Cr–Mo alloy and one-third of that of Ti and Ti alloys [45–47], as shown in figure 10. Among Zr-based alloys, Zr–9Nb and Zr–3Mo possess low magnetic susceptibility owing to the contribution of the ω phase in their structure, as shown in figure 11. The magnetic susceptibilities of the α, β, and ω phases in Zr-based alloys obey the relation 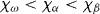 [46, 47]. Although Zr–3Mo and Zr–9Nb alloys have low magnetic susceptibilities, it is difficult to apply plastic deformation to these alloys during processing for medical devices because their tensile strength and elongation are limited by the ω phase [48]. The ω phase is categorized into isothermal ω, athermal ω and strain-induced ω phases depending on the formation processes [49]. The isothermal ω phase is formed during heat treatment; it causes hardening and embrittlement [50–52] and decreases the magnetic susceptibility of the alloy. The athermal ω phase is martensitically formed on quenching from the β phase region to room temperature and also contributes to lowering the magnetic susceptibility [46, 47]. The strain-induced ω phase is formed by applying plastic deformation and appeared in the series of Ti-based alloys consisting of the metastable β phase, such as Ti–Cr, Ti–Mo, and Ti–V alloys [53–55]. The phase constitutions of Zr–Nb alloys are similar to those of the above-mentioned alloys, and the formation of the strain-induced ω phase can be expected in the Zr–14Nb alloy consisting of the metastable β phase [48]. Therefore, the magnetic susceptibility of Zr–Nb alloys can be reduced by applying plastic deformation because of the formation of the strain-induced ω phase.
[46, 47]. Although Zr–3Mo and Zr–9Nb alloys have low magnetic susceptibilities, it is difficult to apply plastic deformation to these alloys during processing for medical devices because their tensile strength and elongation are limited by the ω phase [48]. The ω phase is categorized into isothermal ω, athermal ω and strain-induced ω phases depending on the formation processes [49]. The isothermal ω phase is formed during heat treatment; it causes hardening and embrittlement [50–52] and decreases the magnetic susceptibility of the alloy. The athermal ω phase is martensitically formed on quenching from the β phase region to room temperature and also contributes to lowering the magnetic susceptibility [46, 47]. The strain-induced ω phase is formed by applying plastic deformation and appeared in the series of Ti-based alloys consisting of the metastable β phase, such as Ti–Cr, Ti–Mo, and Ti–V alloys [53–55]. The phase constitutions of Zr–Nb alloys are similar to those of the above-mentioned alloys, and the formation of the strain-induced ω phase can be expected in the Zr–14Nb alloy consisting of the metastable β phase [48]. Therefore, the magnetic susceptibility of Zr–Nb alloys can be reduced by applying plastic deformation because of the formation of the strain-induced ω phase.
Figure 9.
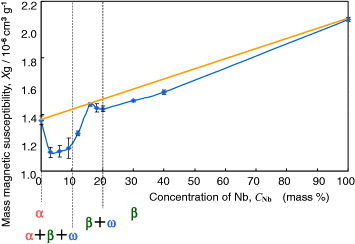
Effects of Nb content and constituent phases on the magnetic susceptibility of Zr–Nb alloy.
Figure 10.
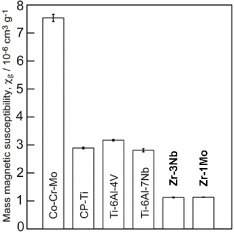
Magnetic susceptibilities of metals used for medical devices and Zr–Nb and Zr–Mo alloys.
Figure 11.
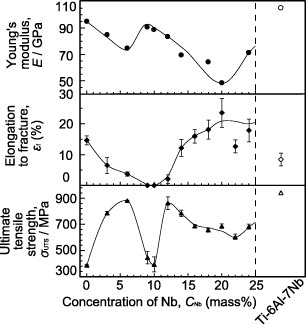
Mechanical properties of Zr–Nb alloys versus Nb content.
8. Surface modification of metallic biomaterials
A disadvantage of using metals as biomaterials is that they are typically artificial materials and have no biofunction. To add biofunction to metals, surface modification is necessary because a biofunction cannot be added during manufacturing processes such as melting, casting, forging and heat treatment. Surface modification is a process that changes a material's surface composition, structure and morphology, leaving the bulk mechanical properties intact. With surface modification, the tissue compatibility of the surface layer can be improved. Dry processes and wet processes are conventional and predominant surface modification techniques. Figure 12 summarizes the surface modification techniques by both dry and wet processes used in research and industry. Surface modification techniques are reviewed elsewhere [56, 57].
Figure 12.
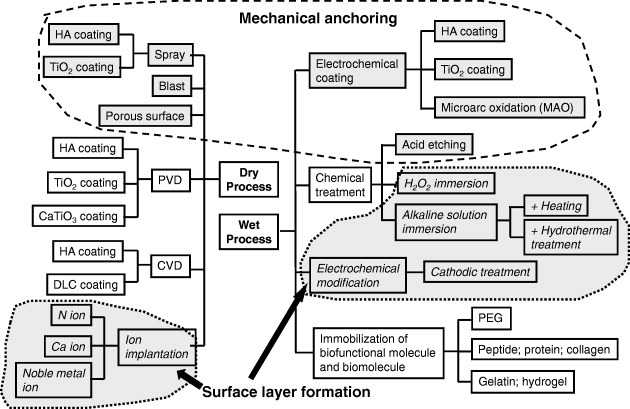
Surface modification techniques by both dry and wet processes used in research and industry.
8.1. Surface treatment for bone formation
8.1.1. Current techniques.
Ti and its alloys, which show good hard tissue compatibility, are used for dental implants and artificial hip joints. However, the hard-tissue compatibility of these materials is lower than that of bioactive ceramics, such as hydroxyapatite and bioactive glasses. Therefore, numerous surface modification techniques to improve the hard tissue compatibility of Ti have been developed, and some have been commercialized. Research to improve hard-tissue compatibility involves two approaches based on the resultant surface layer: a calcium phosphate layer with the thickness in the micrometer scale and a surface-modified layer with the thickness in the nanometer scale, as shown in figure 13.
Figure 13.
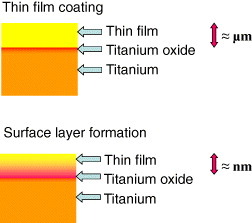
Research to improve hard-tissue compatibility involves two approaches based on the resultant surface layer: a calcium phosphate layer with the thickness in the micrometer scale and a surface-modified layer with the thickness in the nanometer scale.
In the former case, currently, plasma spraying of apatite on metallic materials is widely used to form the apatite layer. In the case of plasma-sprayed apatite, however, the apatite–Ti interface or the apatite itself may fracture under relatively low stress because of low interface bonding strength and low toughness of the sprayed layer. To overcome this weakness, dynamic ion mixing is applied to form an apatite with high interface bonding strength.
In the latter case, hard-tissue compatibility can be improved by modifying the Ti surface instead of the apatite coating. Many surface modification techniques have been developed that involve neither a hydroxyapatite coating nor a calcium phosphate coating.
8.1.2. Mechanical anchoring and chemical bonding.
In the stems of artificial hip joints and dental implants, the chemical bonding of metal surfaces with bone is not expected. In other words, it is impossible for metals as typical artificial materials to chemically and naturally bond with bone as living tissue, especially in the human body with body fluid. Therefore, the surface morphology is sometimes controlled, and rough and porous surface is formed in biomaterials. Living tissue, such as bone, is expected to grow into the rough porous surface, and the materials and bone are strongly connected as a result of the so-called anchoring effect. Figure 14 shows chemical bonding and a mechanical anchoring connection between bone and material. The current surface modification techniques for the improvement of hard tissue compatibility focus on the effect of chemical bonding and/or mechanical anchoring.
Figure 14.
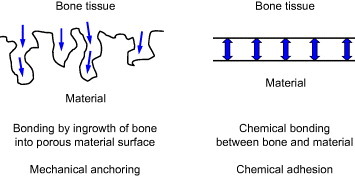
Schematics of chemical bonding and mechanical anchoring connection between bone and implanted material.
8.1.3. Gap in surface modification techniques between research and commercialization.
Figure 15 shows the evolution of surface modification techniques to improve hard tissue compatibility at the research level, with reference to the stems of artificial hip joints and the fixtures of dental implants:
First generation: grind machining of the surface.
Second generation: grooving, blast, acid etching, anodic oxidation and laser abrasion. Nanostructured titanium oxide and titanium oxide nanotube are also categorized in this group.
Third generation: chemical treatment and hydroxyapatite coating.
Fourth generation: Immobilization of biofunctional molecules (collagen, bone morphogenetic protein and peptide).
The bone formation of the materials surface is accelerated when biomolecules concerning bone formation are immobilized on the material surface, such as in the fourth generation above. Therefore, many studies have achieved good results in this direction. However, to increase the popularity of the immobilization of biofunctional molecules, it is necessary to ensure the safety, quality maintenance during storage and dry-conditioned durability of the immobilized layer. Therefore, it is difficult for manufacturers to commercialize those research results.
Figure 15.
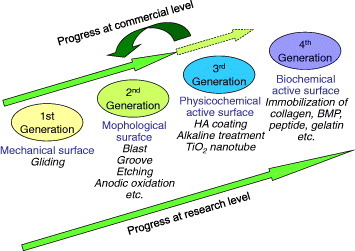
History of surface modification techniques to improve hard-tissue compatibility at the research level and estrangement in surface modification techniques between research and commercialization.
Most of commercialized goods are categorized into the second generation, a few belong to the third generation, and there is no prospect for the commercialization of the fourth generation, at present. The commercialization went faster for the second than third generation possibly because materials employing mechanical anchoring are more practical than materials employing chemical bonding with bone.
8.2. Inhibition of assimilation of titanium alloy
When Ti alloys are used for bone fixators, such as bone screws and bone nails implanted in bone marrow, Ti alloys form callus on their surfaces and sometimes assimilate with bone. Therefore, bone may be refractured when the fixators are retrieved after bone healing because Ti easily forms calcium phosphate on itself [58, 59]. Stainless steel is used for complete retrieval after healing. Therefore, surface treatments that do not cause callus formation are necessary for the safe utilization of Ti alloy devices. It has been reported that Zr forms zirconium phosphate but not calcium phosphate [60, 61].
The coating of Zr inhibits the formation of calcium phosphate on Ti [62]. Figure 16 shows the relative concentration of elements detected in Zr-coated Ti, Zr and Ti. Calcium was not detected in Zr-coated Ti and Zr. According to the binding energies of the Ca 2p3/2 and P 2p electrons, Zr-coated Ti and Zr surfaces precipitate zirconium phosphate but not calcium phosphate. Therefore, Zr coating is a useful technique to inhibit the assimilation of Ti alloys with bone. Another approach is the development of a Zr-based alloy, which is being actively studied in Japan [63, 64].
Figure 16.
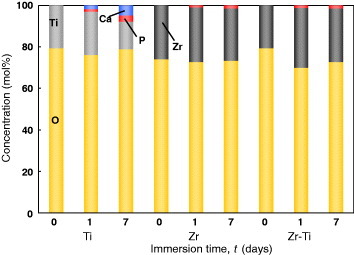
Relative concentration of elements in Zr-coated Ti, Zr and Ti determined by x-ray photoelectron spectroscopy [62].
9. Biofunctionalization—immobilization of biofunctional molecules
9.1. Purpose
Immobilization of biofunctional molecules on metal surfaces is effective for the biofunctionalization of the surfaces. Bone formation and soft tissue compatibility of metallic materials are sometimes required where the materials are used as parts of implant devices. Stents are placed in stenotic blood vessels for dilatation, and blood compatibility or prevention of adhesion of platelets is necessary. In addition, lubrication of the blood vessels is necessary for proper sliding and insertion of guide wires and catheters. When metals are used as sensing devices, cell adhesion must be controlled. The major cause of the retrieval of implants is infection due to biofilm formation. What is required is a biofilm-inhibiting surface, the fundamental property of which is to control the adsorption of proteins, cells, platelets and bacteria. This functional surface may be created by the immobilization of biofunctional molecules. These techniques make it possible to apply metals to scaffolds in tissue engineering. Some metal-polymer composites are reviewed in textbooks [65–67].
9.2. Poly(ethylene glycol)
Poly(ethylene glycol) (PEG) is an oligomer or polymer of ethylene oxide, but historically PEG referred to oligomers and polymers with a molecular weight below 20 000. PEG has the structure shown in figure 17. It is soluble in water, methanol, benzene and dichloromethane and is insoluble in diethyl ether and hexane. It is coupled to hydrophobic molecules to produce non-ionic surfactants. This property, combined with the availability of PEGs with a wide range of end-functions, contributes to the wide use of PEGs in biomedical research, particularly in drug delivery, tissue engineering scaffolds, surface functionalization and many other applications [68]. On the other hand, PEG is a biofunctional molecule on which adsorption of proteins is inhibited. Therefore, immobilization of PEG on metal surface is important for biofunctionalization of the metal.
Figure 17.
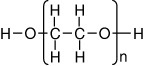
Chemical structure of poly(ethylene glycol).
9.2.1. Chemical immobilization.
Biofunctional polymers are usually immobilized on noble metals, such as Au, via the –SH or –SS– group; however, this technique can only be used for noble metals. The adhesion of platelets and adsorption of proteins, peptides, antibodies and DNA are controlled by modifications of the above technique. A class of copolymers based on poly(l-lysine)-g-poly(ethylene glycol), PLL-g-PEG, has been found to spontaneously adsorb from aqueous solutions onto TiO2, Si0.4Ti0.6O2 and Nb2O5 to develop blood-contacting materials and biosensors [69]. In another case, TiO2 and Au surfaces were functionalized by the attachment of poly(ethylene glycol)-poly(dl-lactic acid) (PEG-PLA) copolymeric micelles. The micelle layer can enhance the resistance to protein adsorption to the surfaces up to 70% [70]. Stainless steel (SS) surface was modified by a silane-coupling agent (SCA), (3-mercaptopropyl)trimethoxysilane. This surface was subsequently activated by argon plasma and subjected to UV-induced graft polymerization of poly(ethylene glycol)methacrylate, PEGMA. The PEGMA graft-polymerized stainless-steel coupon, PEGMA-g-SCA-SS, with a high graft concentration and, thus, a high PEG content was found to be very effective in preventing the absorption of bovine serum albumin and γ-globulin [71]. These processes require several steps, but are effective for immobilization; however, no promising technique for the immobilization of PEG to a metal surface has been developed so far. Photoreactive PEG was photoimmobilized on Ti [72].
9.2.2. Electrodeposition.
Electrodeposition is useful for immobilizing PEG on a metal surface, because metals are usually electrically conductive. In one immobilization method both ends of PEG molecule (MW = 1000) are terminated with –NH2 (NH2–PEG–NH2). The cathodic potential is applied to Ti; during the charging, the terminated PEGs electrically migrate to and are deposited on the Ti cathode, as shown in figure 18. Terminated amines combine with Ti oxide as an NH–O bond by electrodeposition [73, 74]. The amount of the PEG immobilized onto the metals is governed by the concentrations of the active hydroxyl groups on each surface oxide [75]. The PEG-immobilized surface inhibits the adsorption of proteins and cells as well as the adhesion of platelets [76] and bacteria [77] (figure 19), indicating that this electrodeposition technique is useful for the biofunctionalization of metal surfaces. It can be applied to all electrically conductive materials and materials having complex surface topography.
Figure 18.
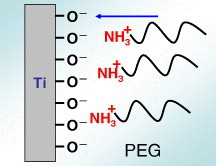
The cathodic potential was applied to Ti; during charging, the terminated PEGs electrically migrate to and are deposited on the Ti cathode.
Figure 19.
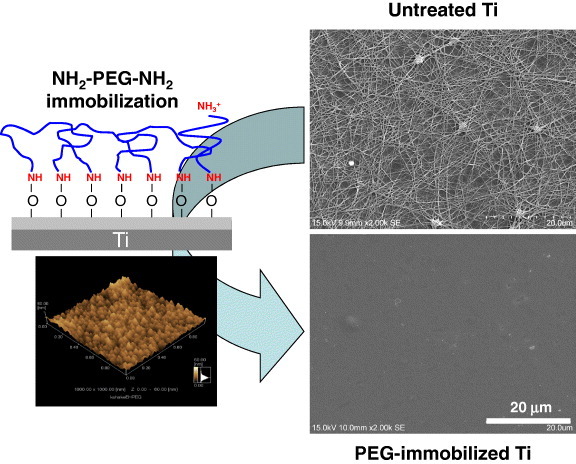
PEG immobilization by electrodeposition on the Ti surface and inhibition of platelet adhesion by immobilization [100].
9.3. Biomolecules
9.3.1. Concept.
Since the natural environment around the implant is aqueous, while the surface of the implant is either bare or oxidized metal, specific demands are imposed on the coating to mediate between these different structural entities. The purpose of these demands is to obtain the native conformation of all proteins and cells that are in contact with the coating and to avoid all forms of aggregation and other conformational changes that might lead to protein denaturalization or cell death.
One approach is the immobilization of biological molecules (growth factors, adhesive proteins) on the implant surface to induce a specific cellular response and promote osseointegration. The application of large extracellular matrix proteins, however, can be impractical due to their low chemical stability, solubility in biological fluids, and high cost. In addition, entire extracellular matrix molecules are usually of allogenetic or xenogenetic origin and, thus, are associated with the risk of immune reaction and pathogen transfer.
Self-assembled monolayers provide chemically and structurally well-defined surfaces that can often be manipulated using standard synthetic methodologies [78]. Thiols on self-assembled monolayers [79, 80] and siloxane-anchored self-assembled monolayers [81] have been thoroughly studied. A problem related to the application of immobilized biomolecules via silanization techniques is the hydrolysis of siloxane films when exposed to aqueous (physiological) conditions [82]. More recently, alkyl phosphate films that remain robust under physiological conditions [83] have been used to provide an ordered monolayer on tantalum oxide surfaces [84, 85], and alkaliphosphonic acids have been applied to coat the native oxide surfaces of metals and their alloys including iron [86], steel [87] and Ti [88].
9.3.2. Peptides.
In a living tissue, the most important role played by the extracellular matrix has been highlighted to favor cell adhesion [89]. Interactions occur between cell membrane receptors and adhesion proteins (or synthetic peptides) derived from the bone matrix, such as type I collagen or fibronectin [90]. These proteins contain the RGD (Arg–Gly–Asp) motif which specially connects transmembrane between the actin cytoskeleton and the RGD motif, and the whole system can activate several intracellular signaling pathways modulating cell behavior (e.g. proliferation, apoptosis, shape, mobility, gene expression and differentiation) [91].
Owing to the main role of the RGD sequence in cell adhesion, several research groups developed biofunctionalized surfaces by immobilization of RGD peptides. Grafting of RGD peptides has been performed on different biomaterials, such as Ti [92–94], and has been shown to improve osteoconduction in vitro. Methodologies differ by the conformation of RGD (cyclic or linear) and by the technique used for the immobilization [89, 90, 93–95]. Since the graft of an RGD peptide is known to be efficient in bone reconstruction [96], the challenge is to develop simple and cheap methods to favor cell attachment to biomaterial surfaces [94, 95].
Self-assembled molecular monolayers bearing RGD moieties have been grafted to various surfaces using either silanes [97], phosphonates on oxidized surfaces [94], or thiols on Au [95], but have revealed some application problems for large-scale production. Phosphonates are known to adsorb on Ti. To be mechanically and physiologically stable, phosphonate layers have to be covalently bound to the material surface by using drastic conditions [88, 98], such as anhydrous organic solvents or high temperature, which are not compatible with bimolecular stability. Monolayers of RGD phosphonates have been formed using a complex multistep process that requires tethering a primer onto a Ti surface, then a linker, and finally the peptide [99]. To immobilize RGD to the electrodeposited PEG on Ti, PEG with an –NH2 group and a –COOH group (NH2–PEG–COOH) must be employed. One terminal group, –NH2, is required to bind stably with a surface oxide on a metal. The other terminal group, –COOH, is useful to bind biofunctional molecules such as RGD, as shown in figure 20 [100]. This RGD/PEG/Ti surface accelerates calcification by the MC3t0003-E1 cell [101]. The calcification and bone formation are most pronounced on the RGD/PEG/Ti surface (figure 21) [102].
Figure 20.
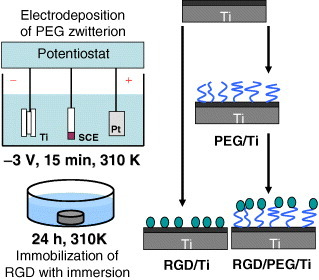
Schematic of the immobilization of RGD on PEG electrodeposited on Ti surface. To immobilize RGD, PEG with an –NH2 group and a –COOH group (NH2–PEG–COOH) must be employed. The –NH2 group is required to bind with the metal oxide on the metal surface, whereas the –COOH group binds RGD.
Figure 21.
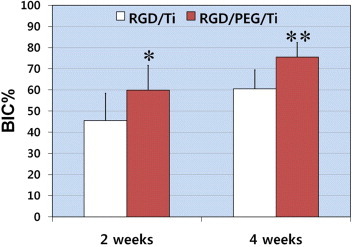
Mean percentage of the bone-to-implant contact (BIC%) over all threads of implants 2 and 4 weeks after implantation (∗p < 0.05, ∗∗p < 0.01) [102]. RGD/PEG/Ti implants displayed significantly higher BIC% values in all threads and in the total lateral length compared with RGD/Ti implants at 2 and 4 weeks of healing.
The glycine (G)-arginine (R)-glycine (G)-asparaginic acid (D)-serine (S) sequence peptide, the GRGDS peptide, is coated using a chloride activation technique to enhance the adhesion and migration of osteoblastic cells [103]. The expression levels of many genes in MC3t0003-E1 cells are altered.
9.3.3. Protein and collagen.
Among the relevant molecules involved in biochemical modification of bone-contacting surfaces, growth factors, such as bone morphogenetic protein-2 (BMP-2), are of primary interest. BMP-2 has been known to play an important role in bone healing processes and to enhance therapeutic efficiency. Ectopic bone formation by BMP-2 in animals has been well established following the first reports of BMP-2 [104–106]. A synthetic receptor-binding motif mimicking BMP-2 is covalently linked to Ti surfaces through a chemical conjunction process [107]. A complete and homogeneous peptide layer covers the Ti surfaces; the content is further measured by gamma counting. Biological evaluations show that the biochemically modified Ti was active in terms of cell attachment behavior. The rate of bone healing is higher on treated than untreated Ti surfaces. BMP-4 is immobilized on a Ti–6Al–4V alloy through lysozyme to improve the hard tissue response [108]. Proteins are silane-coupled to the oxidized surfaces of the Co–Cr–Mo alloy, the Ti–6Al–4V alloy, Ti and the Ni–Ti alloy to improve tissue compatibility [109].
Type I collagen is immobilized by immersion in a collagen solution [110]. The production of type I collagen increases with modification by ethane-1,1,2-triphosphonic acid and methylenediphosphonic acid grafted onto Ti [111]. Type I collagen is grafted through glutaraldehyde as a crosslinking agent [112]. For electrodeposition, the current alternating at 1 Hz between −1 V and + 1 V versus SCE is effective to immobilize type I collagen on Ti, and the durability in water is high [113].
Fibronectin was immobilized directly on Ti using the tresyl chloride activation technique [114]. l-threonine and O-phospho-l-threonine were immobilized on an acid-etched Ti surface [115].
9.3.4. Hydrogel.
Immobilization or coating of hydrogel on the metal surface is currently used in an attempt to add a drug delivery ability to orthopedic implants and stents or fluorescent sensing ability to microchips. Currently, synthetic polymeric hydrogels, such as poly(hydroxyethylmethacrylate) (pHEMA) and poly(hydroxyethylacrylate) (pHEA), are widely used as compliant materials, particularly in the case of contact with blood or other biological fluids [116]. Moreover, when the adhesion between the hydrogel coating and the metal surface is inadequate, a breakage at the coating-steel interface may occur [117]. A spray-coating method was developed to control the coating by pHEMA of complex surfaces of a 316L steel stent for percutaneous coronary intervention [118]. A promising synthetic route is represented by electrochemical polymerization, which produces thin coatings directly on metal substrates with interesting applications in corrosion protection and development of bioactive films [119–122]. As far as the field of orthopedics is concerned, in recent years, many procedures based on surface modification have been suggested to improve the biocompatibility and biofunctionality of Ti-based implants [123]. HEMA, a macromer poly(ethylene-glycol diacrylate) (PEGDE), and PEGDE copolymerized with acrylic acid were used to obtain hydrogels. A model protein and a model drug were entrapped in the hydrogel and released according to the pH change [124].
10. Conclusions
Metallic materials are widely used in medicine for not only orthopedic implants but also cardiovascular devices and other purposes. Their physical properties, such as mechanical, biodegradable and magnetic properties, have been improved by redesigning alloys and manufacturing processes. On the other hand, the metal surface may be biofunctionalized by various techniques, such as dry and wet processes, the immobilization of biofunctional molecules, and the creation of metal-polymer composites. In particular, the electrodeposition technique is useful for all electroconductive and morphological materials. These techniques make it possible to apply metals to scaffolds in tissue engineering.
References
- Semlitsch M, Staub F. and Weber H. Biomed. Tech. 1985;30:334. doi: 10.1515/bmte.1985.30.12.334. [DOI] [PubMed] [Google Scholar]
- Wang K, Gustavson L. and Dumbleton J. Titanium ‘92: Science and Technology. Warrenda TMS; 1993. p. 2697. [Google Scholar]
- Zardiackas L D, Mitchell D W. and Disegi J A. Medical Applications of Titanium and its Alloys. West Conshohoken, PA ASTM; 1996. p. 60. [Google Scholar]
- Hanawa T. In: Metals for Biomedical Devices. Niinomi M, editor. Oxford Woodhead Publishing; 2010. p. p 3. [Google Scholar]
- Kuroda D, Niinomi M, Morinaga M, Kato Y. and Yashiro T. Mater. Sci. Eng. A. 1998;243:244. doi: 10.1016/S0921-5093(97)00808-3. [DOI] [Google Scholar]
- Ozaki T, Matsumoto H, Watanabe S. and Hanada S. Mater. Trans. 2004;45:2776. doi: 10.2320/matertrans.45.2776. [DOI] [Google Scholar]
- Nomura N, Sakamoto K, Takahashi K, Kato S, Abe Y, Doi H, Tsutsumi Y, Kobayashi M, Kobayashi E, Kim W J, Kim K H. and Hanawa T. Mater. Trans. 2010;51:1449. doi: 10.2320/matertrans.M2010092. [DOI] [Google Scholar]
- Nomura N, Baba Y, Kawamura A, Fujinuma S, Chiba A, Masahashi N. and Hanada S. Mater. Sci. Forum. 2007;539–543:1033. doi: 10.4028/www.scientific.net/MSF.539-543.1033. [DOI] [Google Scholar]
- Nakai M, Niinomi M, Akahori T, Yamanoi H, Itsuno S, Haraguchi N, Itoh Y, Ogasawara T, Onishi T. and Shindo T. J. Japan. Inst. Metal. 2008;72:839. doi: 10.2320/jinstmet.72.839. [DOI] [Google Scholar]
- Scheinert D, Scheinert S, Sax J, Piorkowski C, Braunlich S, Ulrich M, Biamino G. and Schmidt A. J. Am. Coll. Cardiol. 2005;45:312. doi: 10.1016/j.jacc.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Sianos G, Hofma S, Lighthart J M R, Saia F, Hoye A, Lemos P A. and Serruys P W. Catheter. Cardiovasc. Interv. 2004;61:111. doi: 10.1002/ccd.10709. [DOI] [PubMed] [Google Scholar]
- Schlager O, Dick P, Sabeti S, Amighi J, Mlekusch W, Minar E. and Schillinger M. J. Endovasc. Ther. 2005;12:676. doi: 10.1583/05-1672.1. [DOI] [PubMed] [Google Scholar]
- Chowdhury P. and Ramos R G. N. Engl. J. Med. 2002;347:581. doi: 10.1056/NEJMicm020259. [DOI] [PubMed] [Google Scholar]
- Kang W Y, Kim W, Kim H G. and Kim W. Int. J. Cardiol. 2007;120:273. doi: 10.1016/j.ijcard.2006.07.192. [DOI] [PubMed] [Google Scholar]
- Robertson S W. and Ritchie R O. J. Biomed. Mater. Res. Appl. Biomater. B. 2008;84:26. doi: 10.1002/jbm.b.30840. [DOI] [PubMed] [Google Scholar]
- Gall K, Tyber J, Wilkesanders G, Robertson S W, Ritchie R O. and Maier H J. Mater. Sci. Eng. A. 2008;486:389. doi: 10.1016/j.msea.2007.11.033. [DOI] [Google Scholar]
- Robertson S W. and Ritchie R O. Biomaterials. 2007;28:700. doi: 10.1016/j.biomaterials.2006.09.034. [DOI] [PubMed] [Google Scholar]
- Heintz C, Riepe G, Birken L, Kaiser E, Chakfe N, Morlock M, Delling G. and Imig H. J. Endovasc. Ther. 2001;8:248. doi: 10.1583/1545-1550(2001)008%3C0248:CNWIEA%3E2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Guidoin R. J. Endovasc. Ther. 2000;7:105. doi: 10.1583/1545-1550(2000)007%3C0105:FGAEAO%3E2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- Kong H, Wilkinson J L, Coe J Y, Gu X, Urness M, Kim T H. and Bass J L. Cardiol. Young. 2002;12:260. doi: 10.1017/S1047951102000562. [DOI] [PubMed] [Google Scholar]
- Takahashi E, Sakurai T, Watanabe S, Masahashi N. and Hanada S. Mater. Trans. 2002;43:2978. doi: 10.2320/matertrans.43.2978. [DOI] [Google Scholar]
- Hosoda H, Fukui Y, Inamura T, Wakashima K, Miyazaki S. and Inoue K. Mater. Sci. Forum. 2003;426–432:3121. doi: 10.4028/www.scientific.net/MSF.426-432.3121. [DOI] [Google Scholar]
- Kim H Y, Ohmatsu Y, Kim J I, Hosoda H. and Miyazaki S. Mater. Trans. 2004;45:1090. doi: 10.2320/matertrans.45.1090. [DOI] [Google Scholar]
- Maeshima T. and Nishida M. Mater. Trans. 2004;45:1096. doi: 10.2320/matertrans.45.1096. [DOI] [Google Scholar]
- Kim J I, Kim H Y, Hosoda H. and Miyazaki S. Mater. Trans. 2004;45:852. [Google Scholar]
- Kim J I, Kim H Y, Inamura T, Hosoda H. and Miyazaki S. Mater. Sci. Eng. A. 2005;403:334. doi: 10.1016/j.msea.2005.05.050. [DOI] [Google Scholar]
- Heublein B, Rohde R, Kaese V, Niemeyer M, Hartung W. and Haverich A. Heart. 2003;89:651. doi: 10.1136/heart.89.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksman R. Catheter. Cardiovasc. Interv. 2006;68:607. doi: 10.1002/ccd.20727. [DOI] [PubMed] [Google Scholar]
- Peuster M, Wohlsein P, Brugmann M, Ehlerding Seidler K, Fink C, Brauer H, Fischer A. and Hausdorf G. Heart. 2001;86:563. doi: 10.1136/heart.86.5.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte F, Kaese V, Haferkamp H, Switzer E, Meyer-Lindenberg A, Wirth C J. and Windhagen H. Biomaterials. 2005;26:3557. doi: 10.1016/j.biomaterials.2004.09.049. [DOI] [PubMed] [Google Scholar]
- Ornhagen C, Nilsson J O. and Vannevik H. J. Biomed. Mater. Res. 1996;31:97. doi: 10.1002/(SICI)1097-4636(199605)31:1%3C97::AID-JBM12%3E3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Baba H, Kodama T. and Katada Y. Corros. Sci. 2002;44:2393. doi: 10.1016/S0010-938X(02)00040-9. [DOI] [Google Scholar]
- Sumita M, Hanawa T. and Teoh S H. Mater. Sci. Eng. C. 2004;24:753. doi: 10.1016/j.msec.2004.08.030. [DOI] [Google Scholar]
- Gebau R C. and Brown R S. Adv. Mater. Process. 2001;159:46. [Google Scholar]
- Menzel J, Kirschner W. and Stein G. ISIJ Int. 1996;36:893. doi: 10.2355/isijinternational.36.893. [DOI] [Google Scholar]
- Uggowitzer P J, Magdowski R. and Speidel M O. ISIJ Int. 1996;36:893. doi: 10.2355/isijinternational.36.893. [DOI] [Google Scholar]
- Kuroda D, Hanawa D, Hibaru T, Kuroda S, Kobayashi M. and Kobayashi T. Mater. Trans. 2003;44:414. doi: 10.2320/matertrans.44.414. [DOI] [Google Scholar]
- Olsrud J, Lätt J, Brockstedt S, Romner B. and Björkman-Burtscher I. J. Magn. Reson. Imaging. 2005;22:433. doi: 10.1002/jmri.20391. [DOI] [PubMed] [Google Scholar]
- Shafiei F, Honda E, Takahashi H. and Sasaki T. J. Dent. Res. 2003;82:602. doi: 10.1177/154405910308200806. [DOI] [PubMed] [Google Scholar]
- Matsuura H, Inoue T, Konno H, Sasaki M, Ogasawara K. and Ogawa A. J. Neurosurg. 2002;97:1472. doi: 10.3171/jns.2002.97.6.1472. [DOI] [PubMed] [Google Scholar]
- Matsuura H, Inoue T, Ogasawara K, Sasaki M, Konno H, Kuzu Y, Nishimoto H. and Ogawa A. Neurol. Med. Chir. 2005;45:395. doi: 10.2176/nmc.45.395. [DOI] [PubMed] [Google Scholar]
- Ernstberger T, Heidrich G, Bruening T, Krefft S, Buchhorn G. and Klinger H M. Eur. Spine J. 2007;16:179. doi: 10.1007/s00586-006-0064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami E, Hamada K. and Asaoka K. J. Japan. Soc. Dent. Mater. Dev. 2010;29:415. [Google Scholar]
- Chauvel B, Cathelineau G, Balac S, Lecerf J. and de Certaines J D. J. Magn. Reson. Imaging. 1996;6:936. doi: 10.1002/jmri.1880060615. [DOI] [PubMed] [Google Scholar]
- Popowski Y, Hiltbrand E, Joliat D. and Rouzaud M. Int. J. Radiat. Oncol. Biol. Phys. 2000;47:759. doi: 10.1016/S0360-3016(00)00488-0. [DOI] [PubMed] [Google Scholar]
- Nomura N, Tanaka Y, Suyalatu S, Kondo R, Doi H, Tsutsumi Y. and Hanawa T. Mater. Trans. 2009;50:2466. doi: 10.2320/matertrans.M2009187. [DOI] [Google Scholar]
- Suyalatu S, Nomura N, Oya K, Tanaka Y, Kondo R, Doi H, Tsutsumi Y. and Hanawa T. Acta Biomater. 2010;6:1033. doi: 10.1016/j.actbio.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Kondo R, Nomura N, Suyalatu S, Tsutsumi Y, Doi H. and Hanawa T. Acta Biomater. 2011;7:4278. doi: 10.1016/j.actbio.2011.07.020. [DOI] [PubMed] [Google Scholar]
- Fontaine D D. Metall. Mater. Trans. A. 1988;19:169. doi: 10.1007/BF02652523. [DOI] [Google Scholar]
- Sikka S K, Vohra Y K. and Chidambaram R. Prog. Mater. Sci. 1982;27:245. doi: 10.1016/0079-6425(82)90002-0. [DOI] [Google Scholar]
- Buenconsejo P J S, Kim H Y. and Miyazaki S. Scr. Mater. 2011;64:1114. doi: 10.1016/j.scriptamat.2011.03.004. [DOI] [Google Scholar]
- Balazic M, Kopac J, Jackson M J. and Ahmed W. Int. J. Nano Biomater. 2007;1:3. doi: 10.1504/IJNBM.2007.016517. [DOI] [Google Scholar]
- Hanada S. and Izumi O. J. Mater. Sci. 1986;21:4131. doi: 10.1007/BF01106518. [DOI] [Google Scholar]
- Hanada S, Takemura A. and Izumi O. Trans. Japan. Inst. Met. 1982;23:507. [Google Scholar]
- Kuan T S, Ahrens R R. and Sass S L. Metall. Mater. Trans. A. 1975;6:1767. doi: 10.1007/BF02642306. [DOI] [Google Scholar]
- Hanawa T. J. R. Soc. Interface. 2009;6:S361. doi: 10.1098/rsif.2008.0427.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawa T. Japan. J. Dent. Sci. Rev. 2010;46:93. doi: 10.1016/j.jdsr.2009.11.001. [DOI] [Google Scholar]
- Hanawa T. and Ota M. Biomaterials. 1991;12:767. doi: 10.1016/0142-9612(91)90028-9. [DOI] [PubMed] [Google Scholar]
- Hiromoto S, Hanawa T. and Asami K. Biomaterials. 2004;25:979. doi: 10.1016/S0142-9612(03)00620-3. [DOI] [PubMed] [Google Scholar]
- Hanawa T, Okuno O. and Hamanaka H. J. Japan. Inst. Met. 1992;56:1168. (in Japanese) [Google Scholar]
- Hanawa T, Hiromoto S, Asami K, Okuno O. and Asaoka K. Mater. Trans. 2002;43:3000. doi: 10.2320/matertrans.43.3000. [DOI] [Google Scholar]
- Kobayashi E, Ando M, Tsutsumi Y, Doi H, Yoneyama T, Kobayashi M. and Hanawa T. Mater. Trans. 2007;48:301. doi: 10.2320/matertrans.48.301. [DOI] [Google Scholar]
- Ikarashi Y, Toyoda K, Kobayashi E, Doi H, Yoneyama T, Hamanaka H. and Tsuchiya T. Mater. Trans. 2005;46:2260. doi: 10.2320/matertrans.46.2260. [DOI] [Google Scholar]
- Kobayashi E, Matsumoto S, Doi H, Yoneyama T. and Hamanaka H. J. Biomed. Mater. Res. 1995;29:943. doi: 10.1002/jbm.820290805. [DOI] [PubMed] [Google Scholar]
- Possart W. Metals as Biomaterials. New York Wiley; 1998. p. 197. [Google Scholar]
- Worch H. Metals as Biomaterials. New York Wiley; 1998. p. 177. [Google Scholar]
- Xiao S J, Kenausis G. and Textor M. Titanium in Medicine. Amsterdam , the Netherlands Springer; 2001. p. 417. [Google Scholar]
- Mahato R I. Biomaterials for Delivery and Targeting of Proteins and Nucleic Acids. Warrenda , NY CRC Press; 2005. [Google Scholar]
- Kenausis G L, Voros J, Elbert D L, Huang N, Hofer R, Ruiz-Taylor L, Textor M, Hubbell J A. and Spencer N D. J. Phys. Chem. B. 2000;104:3298. doi: 10.1021/jp993359m. [DOI] [Google Scholar]
- Huang N P, Csucs G, Emoto K, Nagasaki Y, Kataoka K, Textor M. and Spencer N D. Langmuir. 2002;18:252. doi: 10.1021/la0109563. [DOI] [Google Scholar]
- Zhang F, Kang E T, Neoh K G, Wang P. and Tan K L. Biomaterials. 2001;22:1541. doi: 10.1016/S0142-9612(00)00310-0. [DOI] [PubMed] [Google Scholar]
- To Y, Hasuda H, Sakuragi M. and Tsuzuki S. Acta Biomater. 2007;3:1024. doi: 10.1016/j.actbio.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Doi H, Iwasaki Y, Hiromoto S, Yoneyama T, Asami K, Imai H. and Hanawa H. Mater. Sci. Eng. C. 2007;27:206. doi: 10.1016/j.msec.2006.03.007. [DOI] [Google Scholar]
- Tanaka Y, Doi H, Kobayashi E, Yoneyama T. and Hanawa T. Mater. Trans. 2007;48:287. doi: 10.2320/matertrans.48.287. [DOI] [Google Scholar]
- Tanaka Y, Saito H, Tsutsumi Y, Doi H, Imai H. and Hanawa T. Mater. Trans. 2008;49:805. doi: 10.2320/matertrans.MRA2007317. [DOI] [Google Scholar]
- Tanaka Y, Matsuo Y, Komiya T, Tsutsumi Y, Doi H, Yoneyama T. and Hanawa T. J. Biomed. Mater. Res. A. 2010;92:350. doi: 10.1002/jbm.a.32375. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Matin K, Gyo M, Okada A, Tsutsumi Y, Doi H, Nomura N, Tagami J. and Hanawa T. J. Biomed. Mater. Res. A. 2010;95:1105. doi: 10.1002/jbm.a.32932. [DOI] [PubMed] [Google Scholar]
- Balachander N. and Sukenik C N. Langmuir. 1990;6:1621. doi: 10.1021/la00101a001. [DOI] [Google Scholar]
- Bain C D, Troughton Y, Tao Y T, Evall J, Whitesides G M. and Nuzzo R G. J. Am. Chem. Soc. 1989;111:437. [Google Scholar]
- Dubois L H. and Nuzzo R G. Annu. Rev. Phys. Chem. 1992;43:437. doi: 10.1146/annurev.pc.43.100192.002253. [DOI] [Google Scholar]
- Ulman A. Chem. Rev. 1996;96:1533. doi: 10.1021/cr9502357. [DOI] [PubMed] [Google Scholar]
- Xiao S J, Textor M. and Spencer N D. Langmuir. 1998;14:5507. doi: 10.1021/la980257z. [DOI] [Google Scholar]
- Gawalt E S, Avaltroni M J, Danahy M P, Silverman B M, Hanson E L, Midwood K S, Schwarzbauer J E. and Schwartz J. Langmuir. 2003;19:200. doi: 10.1021/la0203436. [DOI] [Google Scholar]
- Brovelli D, Hahner G, Ruis L, Hofer R, Kraus G, Waldner A, Schlosser J, Oroszlan P, Ehart M. and Spencer N D. Langmuir. 1999;15:4324. doi: 10.1021/la981758n. [DOI] [Google Scholar]
- Textor M, Ruiz L, Hofer R, Rossi K, Feldman K, Hahner G. and Spencer N D. Langmuir. 2000;16:3257. doi: 10.1021/la990941t. [DOI] [Google Scholar]
- Fang J L, Wu N J, Wang Z W. and Li Y. Corrosion. 1991;47:169. doi: 10.5006/1.3585238. [DOI] [Google Scholar]
- Van Alsten J G. Langmuir. 1999;15:7605. doi: 10.1021/la981694g. [DOI] [Google Scholar]
- Gawalt E S, Avaltroni M J, Koch N. and Schwartz J. Langmuir. 2001;17:5736. doi: 10.1021/la010649x. [DOI] [Google Scholar]
- Verrier S, Pallu S, Bareille R, Jonczyk A, Meyer J, Dard M. and Amedee J. Biomaterials. 2002;23:585. doi: 10.1016/S0142-9612(01)00145-4. [DOI] [PubMed] [Google Scholar]
- Reyes C D, Petrie T A, Burns K L, Schwartz Z. and Garcia A J. Biomaterials. 2007;28:3228. doi: 10.1016/j.biomaterials.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R O. Cell. 2002;110:673. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Bagno A, Piovan A, Dettin M, Chiarion A, Brun P, Gambaretto R, Fontana G, Di Bello C, Palu G. and Castagliuolo I. Bone. 2007;40:693. doi: 10.1016/j.bone.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Elmengaard B, Bechtold J E. and Soballe K. Biomaterials. 2005;26:3521. doi: 10.1016/j.biomaterials.2004.09.039. [DOI] [PubMed] [Google Scholar]
- Rammelt S, Illert T, Bierbaum S, Scharnweber D, Zwipp H. and Schneiders W. Biomaterials. 2006;27:5561. doi: 10.1016/j.biomaterials.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Auernheimer J, Zukowski D, Dahmen C, Kantlehner M, Enderle A, Goodman S L. and Kessker H. Chem. Biochem. 2005;6:2034. doi: 10.1002/cbic.200500031. [DOI] [PubMed] [Google Scholar]
- Ferris D M, Moodie G D, Dimond P M, Gioranni C W, Ehrlich M G. and Valentini R F. Biomaterials. 1999;20:2323. doi: 10.1016/S0142-9612(99)00161-1. [DOI] [PubMed] [Google Scholar]
- Xiao S J, Textor M, Spencer N D, Wieland M, Keller B. and Sigrist H. J. Mater. Sci. Mater. Med. 1997;8:867. doi: 10.1023/A:1018501804943. [DOI] [PubMed] [Google Scholar]
- Silverman B M, Wieghaus K A. and Schwartz J. Langmuir. 2005;21:225. doi: 10.1021/la048227l. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Avaltroni M J, Danahy M P, Silverman B M, Hanson E L, Schwarzbauer J E, Midwood K S. and Gawalt E S. Mater. Sci. Eng. C. 2003;23:395. doi: 10.1016/S0928-4931(02)00310-7. [DOI] [Google Scholar]
- Tanaka Y, Saito H, Tsutsumi Y, Doi H, Nomura N, Imai H. and Hanawa T. J. Colloid Interface Sci. 2009;330:138. doi: 10.1016/j.jcis.2008.10.042. [DOI] [PubMed] [Google Scholar]
- Oya K, Tanaka Y, Saito H, Kurashima K, Nogi K, Tsutsumi H, Tsutsumi Y, Doi H, Nomura N. and Hanawa T. Biomaterials. 2009;30:1281. doi: 10.1016/j.biomaterials.2008.11.030. [DOI] [PubMed] [Google Scholar]
- Park J W, Kurashima K, Tustusmi Y, An C H, Suh J Y, Doi H, Nomura N, Noda K. and Hanawa T. Acta Biomater. 2011;7:3222. doi: 10.1016/j.actbio.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Yamanouchi N, Pugdee K, Chang W J, Lee S Y, Yoshinari M, Hayakawa T. and Abiko Y. Dent. Mater. J. 2008;27:744. doi: 10.4012/dmj.27.744. [DOI] [PubMed] [Google Scholar]
- Urist M R. Science. 1965;150:893. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- Lee Y M, Nam S H, Seol Y J, Kim T I, Lee S J, Ku Y, Rhyu I C, Chung C P, Han S B. and Choi S M. J. Periodontol. 2003;74:865. doi: 10.1902/jop.2003.74.6.865. [DOI] [PubMed] [Google Scholar]
- Wikesjo U M, Lim W H, Thomson R C, Cook A D, Wozney J M. and Hardwick W R. J. Periodontol. 2003;74:635. doi: 10.1902/jop.2003.74.5.635. [DOI] [PubMed] [Google Scholar]
- Seol Y J. J. Biomed. Mater. Res. A. 2006;77:599. doi: 10.1002/jbm.a.30639. [DOI] [PubMed] [Google Scholar]
- Puleo D A, Kissling R A. and Sheu M S. Biomaterials. 2002;23:2079. doi: 10.1016/S0142-9612(01)00339-8. [DOI] [PubMed] [Google Scholar]
- Nanci A, Wuest J D, Peru L, Brunet P, Sharma V, Zalzal S. and McKee M D. J. Biomed. Mater. Res. 1998;40:324. doi: 10.1002/(SICI)1097-4636(199805)40:2%3C324::AID-JBM18%3E3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Nagai M, Hayakawa T, Fukatsu A, Yamamoto M, Fukumoto M, Nagahama F, Mishima H, Yoshinari M, Nemoto K. and Kato T. Dent. Mater. J. 2002;21:250. doi: 10.4012/dmj.21.250. [DOI] [PubMed] [Google Scholar]
- Viornery C, Guenther H L, Aronsson B O, Pechy P, Descouts P. and Gratzel M. J. Biomed. Mater. Res. 2002;62:149. doi: 10.1002/jbm.10205. [DOI] [PubMed] [Google Scholar]
- Chang W J, Qu K L, Lee S Y, Chen J Y, Abiko Y, Lin C T. and Huang H M. Dent. Mater. J. 2008;27:340. doi: 10.4012/dmj.27.340. [DOI] [PubMed] [Google Scholar]
- Kamata H, Suzuki S, Tanaka Y, Tsutsumi Y, Doi H, Nomura N, Hanawa T. and Moriyama K. Mater. Trans. 2011;52:81. doi: 10.2320/matertrans.M2010311. [DOI] [Google Scholar]
- Pugdee K, Shibata Y, Yamamichi N, Tsutsumi H, Yoshinari M, Abiko Y. and Hayakawa T. Dent. Mater. J. 2007;26:647. doi: 10.4012/dmj.26.647. [DOI] [PubMed] [Google Scholar]
- Abe Y, Hiasa K, Takeuchi M, Yoshida Y, Suzuki K. and Akagawa Y. Dent. Mater. J. 2005;24:536. doi: 10.4012/dmj.24.536. [DOI] [PubMed] [Google Scholar]
- Cadotte A J. and DeMarse T B. J. Neural. Eng. 2005;2:114. doi: 10.1088/1741-2560/2/4/007. [DOI] [PubMed] [Google Scholar]
- Belkasm J S, Munro C A, Shoichet M S, Johnston M. and Midha R. Biomaterials. 2005;26:1741. doi: 10.1016/j.biomaterials.2004.05.031. [DOI] [PubMed] [Google Scholar]
- Indolfi L, Causa F. and Netti P A. J. Mater. Sci. Mater. Med. 2009;20:1541. doi: 10.1007/s10856-009-3699-z. [DOI] [PubMed] [Google Scholar]
- Fenelon A M. and Breslin C B. Corros. Sci. 2003;45:2837. doi: 10.1016/S0010-938X(03)00104-5. [DOI] [Google Scholar]
- Mengoli G. Adv. Polym. Sci. 1979;33:1. doi: 10.1016/S0010-938X(03)00104-5. [DOI] [Google Scholar]
- De Giglio E, Guascito M R, Sabbatini L. and Zambonin G. Biomaterials. 2001;22:2609. doi: 10.1016/S0142-9612(00)00449-X. [DOI] [PubMed] [Google Scholar]
- Rammelt U, Nguyen P T. and Plieth W. Electrochem. Acta. 2003;48:1257. doi: 10.1016/S0013-4686(02)00833-2. [DOI] [Google Scholar]
- De Giglio E, Gennaro I, Sabbatini L. and Zambonin G. J. Biomater. Sci. Polym. Ed. 2001;12:63. doi: 10.1163/156856201744452. [DOI] [PubMed] [Google Scholar]
- De Giglio E, Cometa S, Satriano C, Sabbatini L. and Zambonin G. J. Biomed. Mater. Res. A. 2009;88:1048. doi: 10.1002/jbm.a.31908. [DOI] [PubMed] [Google Scholar]


