Abstract
Hydroxylapatite (or hydroxyapatite, HAp) exhibits excellent biocompatibility with various kinds of cells and tissues, making it an ideal candidate for tissue engineering, orthopedic and dental applications. Nanosized materials offer improved performances compared with conventional materials due to their large surface-to-volume ratios. This review summarizes existing knowledge and recent progress in fabrication methods of nanosized (or nanostructured) HAp particles, as well as their recent applications in medical and dental fields. In section 1, we provide a brief overview of HAp and nanoparticles. In section 2, fabrication methods of HAp nanoparticles are described based on the particle formation mechanisms. Recent applications of HAp nanoparticles are summarized in section 3. The future perspectives in this active research area are given in section 4.
Keywords: hydroxyapatite, nanoparticle, composite, coating, medical device
1. Introduction
Apatite is a general term for the crystalline minerals that can be represented by the formula M10(ZO4)6X2. Each component (M, ZO4 and X) in the formula can be replaced by a large number of different ions as listed in table 1. The most common apatite found in the nature is calcium phosphate apatite, where M and ZO4 are Ca2+ and PO43−, respectively. When X is OH− (i.e. Ca10(PO4)6(OH)2, stoichiometric Ca/P molar ratio 1.67), the mineral is called hydroxylapatite [1, 2] or hydroxyapatite (HAp), which is the main subject of this review. HAp is the inorganic component of the hard tissues of vertebrates. Biological apatite is a non-stoichiometric Ca2+-deficient form of HAp containing trace ions. The trace ions include cations (such as Mg2+, Na+ and K+) and anions (such as CO32−, Cl− and F−). The most common substituting ion is carbonate (CO32−) that can replace OH− and PO43− ions via the so-called A-type and B-type replacements, respectively. Detailed chemical structures of HAp and other ion-substituted HAp can be found in recent reviews [3–9] and books [10–13].
Table 1.
Major ions that can be part of apatite, M10(ZO4)6X2.
| Component | Ions |
|---|---|
| M | Ca2+, Mg2+, Sr2+, Ba2+, Mn2+, Fe2+, Zn2+, Cd2+, Pb2+, H+, Na+, K+, Al3+, etc |
| ZO4 | PO43−, AsO43−, VO43−, SO43−, CO32−, SiO43−, etc |
| X | OH−, F−, Cl−, Br−, O2−, CO32−, etc |
Traditional HAp sintered ceramics (in dense, porous and powder forms) have been used in medical and dental fields [13–16], and the applications include alveolar ridge reconstruction and augmentation [17, 18], fillers for bone defects [19–22], middle ear implant [23], etc. HAp is bioactive (osteoconductive), that is, HAp can encourage bone growth along its surface when placed in the vicinity of viable bone or differentiated bone-forming cells [11]. HAp composites have been developed to overcome the inferior mechanical properties (brittleness and low stiffness) of traditional HAp sintered ceramics (or to improve bioactivity of the composite matrix). The original concept of bioceramics/polymer composite was introduced by Bonfield et al [24], and the idea was based on the concept that cortical bone itself comprises an organic matrix reinforced with a mineral component. The bone analogue HAp/polymer composite (commercialized under the trade name HAPEX) consists of HAp (∼50 vol%) mixed in a polyethylene matrix, and provides the stiffness and bioactivity of HAp and the toughness of polyethylene.
Nanoparticle (nanopowder, nanocrystal or nanostructured particle) is a microscopic particle with at least one dimension in the nanometer scale (usually, 100 nm or less). In general, nanoparticles offer improved performances compared with materials of conventional size due to their large surface-to-volume ratios (specific surface areas). For instance, HAp nanoparticles exhibit improved sinterability (densification) [25–29]. Recently, a transparent HAp block having nanosized pores was prepared simply by drying an aqueous dispersion of HAp nanoparticles (particle size 32 nm) at a low temperature (∼60 °C) without binder molecules [30]. Contrary to natural bone minerals, conventional HAp sintered ceramics are hardly resorbed after implantation [31, 32]. On the other hand, artificially precipitated carbonate-substituted HAp nanoparticles (also called as poorly crystalline apatites) exhibit similar physico-chemical characteristics to those of bone minerals [33, 34]. Moreover, nanostructured HAp ceramics are expected to have better bioactivity than conventional ones [35–39]. For example, Webster and co-workers showed an enhanced osteoclast-like cell adhesion and function on HAp ceramic surfaces with nanometer-sized surface topography [35, 36]. Sun et al [37] reported that nanophase HAp can better promote the proliferation and osteogenic differentiation of periodontal ligament cells compared to dense HAp. Nanoscale HAp/polymer composites also revealed improved bioactivity (and mechanical properties) compared to microscale HAp composites [40–43].
This review summarizes existing knowledge and recent progress in fabrication methods of HAp nanoparticles, specifically highlighting the techniques allowing to control the nanoparticle morphology. The morphology control is very important because HAp belongs to a hexagonal crystal system and possesses different properties on its a and c planes. Hence the morphology of HAp strongly affects the adsorption of biopolymers and the ion-exchange property [44]. In this review, the fabrication methods of HAp nanoparticles are classified on the basis of particle formation mechanisms. HAp is one of many types of calcium orthophosphates listed in table 2. While other calcium phosphates have also been used as biomaterials in their nanoparticle forms, they are summarized in other recent reviews [3–7, 45–47]. Recent applications of HAp nanoparticles are one of the topics of this paper. A major application of HAp is filling materials to impart bioactivity to various composite biomaterials, which can be found in recent reviews [47–52].
Table 2.
| Ca/P molar ratio | Chemical formula | Name | Abbreviation | Water solubility at 25 °C(g l−1) | pH stability range at 25 °C |
|---|---|---|---|---|---|
| 0.5 |  |
Monocalcium phosphate monohydrate | MCPM | ∼18 | 0–2 |
| 0.5 | Ca(H2PO4) | Monocalcium phosphate anhydrous | MCPA | ∼17 | a |
| 1.0 | CaHPO4 ⋅ 2H2 O | Dicalcium phosphate dihydrate (brushite) | DCPD | ∼0.088 | 2–6 |
| 1.0 | CaHPO4 | Dicalcium phosphate anhydrous (monetite) | DCPA | ∼0.0048 | a |
| 1.2–2.2 | CaxHy(PO4)z·nH2O (n=3–4.5) | Amorphous calcium phosphate | ACP | b | ∼5–12c |
| 1.33 | Ca8(HPO4)2(PO4)4·5H2O | Octacalcium phosphate | OCP | ∼0.0081 | 5.5–7 |
| 1.5 | α-Ca3(PO4)2 | α-Tricalcium phosphate | α-TCP | ∼0.0025 | d |
| 1.5 | β-Ca3(PO4)2 | β-Tricalcium phosphate | β-TCP | ∼0.0005 | d |
| 1.5–1.67 | Ca10−x(HPO4)x(PO4)6−x(OH)2 (0 < x < 1) | Calcium-deficient hydroxyapatite | CDHA | ∼0.0094 | 6.5–9.5 |
| 1.67 | Ca10(PO4)6(OH)2 | Hydroxyapatite | HAp | ∼0.0003 | 9.5–12 |
| 2.0 | Ca4(PO4)2O | Tetracalcium phosphate (hilgenstockite) | TTCP | ∼0.0007 | d |
Stable above 100 °C.
Cannot be measured precisely.
Always metastable.
These compounds cannot be precipitated from aqueous solutions.
2. Fabrication methods for HAp nanoparticles
HAp nanoparticles can be obtained via a variety of methods including dry-synthesis (solid-state) and wet-synthesis methods (table 3). Other attempts at classification of HAp nanoparticle fabrication techniques can be found elsewhere [3–7, 53]. Dry synthesis usually yields stoichiometric and well-crystallized products, but they require relatively high temperatures (typically above 700 °C) and long treatment time. Besides, HAp is generally obtained in a bulk form by dry synthesis, and therefore grinding or milling is necessary to obtain nanoparticles. Wet-synthesis methods require relatively low temperatures and easily produce nanoparticles, but their crystallinity and Ca/P ratio are relatively low.
Table 3.
Fabrication methods of HAp particles.
| Section | Ref. | |
|---|---|---|
| Synthesis method | ||
| Dry | ||
| Solid-state reaction | 2.1 | [54–69] |
| Mechanochemical reaction | 2.1 | [71–76] |
| Plasma spraying | 2.1 | [77] |
| Wet | ||
| Wet chemical precipitation | 2.2.1 | [6, 46, 81–123] |
| Hydrothermal conversion | 2.2.2 | [124, 128–146] |
| Homogeneous precipitation | ||
| Thermal pH change | 2.2.3 | [168–176] |
| Thermal dissociation of Ca ions | 2.2.3 | [177, 178, 180–182] |
| Sol–gel method | 2.2.4 | [191–193] |
| Emulsion method | 2.2.5 | [149, 194–210] |
| Post-treatment | ||
| Dry | ||
| Grinding/milling | 2.1 | [70] |
| Calcination with anti-sintering agent | 2.2.6 | [216–218] |
| Wet | ||
| Hydrothermal crystal growth | 2.2.2 | [26, 125–127, 147–167] |
2.1. Dry synthesis
Stoichiometric and well-crystallized HAp can be prepared by solid-state reactions of calcium orthophosphates with calcium oxide or related salts such as Ca(OH)2 (e.g. CaHPO4 + CaO [54], Ca2P2O7 + CaO [55, 56], β-Ca3(PO4)2 + CaO [57, 58]) at a stoichiometric Ca/P mixing ratio for HAp formation at high temperatures (typically above 700 °C). Calcination of chlorapatite in a steam also leads to the formation of a stoichiometric HAp [59]. Non-stoichiometric HAp can be derived from natural materials such as bovine bones [60–62], fish bones [63], coral [64–66] and eggshell [67, 68] after thermal treatments. By controlling the parameters of the solid-state reactions, the morphological control of the products in the nanoscale can be achieved. For example, nanostructured biphasic calcium phosphate (HAp and β-Ca3(PO4)2) particles in the form of plates with nanosized hollows have been prepared by a solid-state reaction [69].
These HAp materials are generally obtained in the bulk form, and therefore grinding or milling is necessary to obtain HAp as nanoparticles. Trakhtenberg et al [70] recently reported the effects of grinding in planetary mills on the phase composition, morphology and water content of HAp powder.
Mechanochemical processing (or mechanical alloying) through direct ball milling for the above mixtures (that is, calcium orthophosphates and calcium oxide) is another way to fabricate HAp nanoparticles in a dry state [71–76]. Otsuka et al [71] reported the effect of environmental conditions on the crystalline transformation of metastable calcium phosphates during grinding, and found that a mixture of CaHPO4 ⋅ 2H2 O and Ca(OH)2 transformed into HAp after grinding in air. The crystallinity of the products was low and hence post-synthesis annealing at temperatures between 500 and 1000 °C was required. Yeong and Wang [72] used CaHPO4 and CaO as raw materials and fabricated well-crystallized HAp nanoparticles (particle size 25 nm, specific surface area 75 m2 g−1) after 20 h of mechanical activation. They proposed a mechanism for the dry mechanochemical reaction: the initial stage of the mechanical activation resulted in a refinement in apatitic crystallite and particle sizes after amorphization (fragmentation and fracture) of the starting CaHPO4 and CaO. This was followed by the steady formation and subsequent growth of HAp crystals with increasing degree of mechanical activation.
Another physical method to fabricate nanoparticles in a dry state is the thermal plasma technique. During plasma processing, the raw material is partly or completely melted, or even instantaneously evaporated in a high-temperature flame. The melted or vaporized particles will quench or condense into ultrafine particles by subsequent rapid cooling. Xu et al [77] conducted a radiofrequency plasma spray process to fabricate HAp nanoparticles (particle size 10–100 nm), which contained both amorphous and crystalline phases, using 15 μm sized HAp powders as a raw material.
2.2. Wet synthesis
HAp nanoparticles can be fabricated under milder conditions in solutions via a number of ways, such as wet chemical precipitation, sol–gel method, emulsion method, etc. The morphology (shape and size) of HAp nanoparticles can be widely varied by adjusting the reaction conditions [78–80]. However, highly crystallized HAp can be obtained only at elevated temperatures, by hydrothermal treatment in a wet condition or calcination in a dry state.
2.2.1. Wet chemical precipitation.
Among the wet-synthesis methods, the precipitation process (wet chemical precipitation) has been widely used [6, 46, 81] because of its simplicity. Mixing of two aqueous solutions of calcium and orthophosphate (at pH > 7) results in the formation of highly supersaturated solutions for HAp, which induces a fast precipitation of nanoparticles [81] according to the following reactions:
Some researchers conducted this precipitation process in a simulated body fluid (SBF [82]) to obtain biomimetic apatite nanoparticles [83, 84].
The aqueous precipitation process yields calcium-deficient HAp (CDHA) via intermediate phases (precursors): amorphous calcium phosphate (ACP) is formed over a broad range of precipitation conditions [85–95] and octacalcium phosphate (OCP) was formed in some cases [46, 85, 96, 97]. Alternatively CDHA may precipitate without going through an ACP precursor [98]. Recently, Mossaad et al [99] reported the formation of 5-nm-sized HAp particles from a Ca(C2H3O2)2–K3PO4–H2O system at room temperature: the obtained nanoparticles were very unstable and, interestingly, a crystalline to amorphous phase transformation was observed when the nanoparticles were aged for 5 months in 30% relative humidity.
The morphology (shape and size) of HAp nanoparticles depends on precipitation conditions such as concentration of reactants, ionic strength, pH and temperature [100]. The mixing condition for two (calcium and orthophosphate) aqueous solutions is also important. A microreactor is a device that has microchannels on the order of micrometers and that enables chemical reactions to be performed in a space several orders of magnitude smaller than conventional batch reactors [101]. The potential advantages of using a microreactor, rather than a conventional batch reactor, include high-speed mixing, better control of reaction conditions and improved yield. Yang et al [102] prepared rod-like HAp nanoparticles (particle size 58 nm) using a tube-in-tube microchannel reactor. They reported that the particle size decreased sharply with increasing the continuous-phase flow rate, and a narrow size distribution was achieved at a high flow rate. Kandori et al [103] recently prepared smaller HAp nanoparticles (2 nm in width, 15 nm in length) by using a multichannel microreactor.
The morphology of HAp nanoparticles also depends on additives, such as surfactant molecules [104, 105] (cf micelle-templated method described in the next paragraph, and emulsion methods described in section 2.2.5), alcohol [106], amino acids [107–109], citrates [110, 111], poly(acrylic acid) [112, 113], poly(ethylene imine) [114, 115], poly(ethylene oxide) (poly(ethylene glycol)) [116, 117], poly(vinyl alcohol) [118, 119] and biopolymers [119], mainly due to the inhibition of crystal growth by preferentially adsorbing the additives onto a HAp surface (a or c plane).
Micelles form when the concentration of surfactant molecules is greater than the critical micelle concentration (CMC). The shape (sphere, cylinder or lamella) and size of a micelle can be controlled by the surfactant molecular geometry and solution conditions (surfactant concentration, temperature, pH and ionic strength). The addition of surfactant micelles as a template is beneficial for the fabrication of nanostructured HAp particles. Yao et al [120] utilized surfactant micelles for preparing mesoporous HAp nanoparticles. They prepared HAp nanoparticles with channels approximately 3 nm in diameter by precipitation of HAp on rod-like micelles of cationic surfactants (cetyltrimethylammonium bromide, CTAB) in an aqueous medium. Wu and Bose [121] have also reported the preparation of HAp nanoparticles by the precipitation process in the presence of anionic dodecyl phosphate micelles. Ye et al [122] reported micelle-templated synthesis of HAp hollow nanoparticles or nanotubes (outer diameter ∼35 nm, inner diameter 13 nm, length 50–250 nm) with nonionic surfactants (EO20PO70EO20; EO = ethylene oxide, PO = propylene oxide). Surfactant molecules can also stabilize oil droplets (or air bubbles) in aqueous solutions, and these droplets can be used for the fabrication of nanostructured HAp hollow or capsule particles having larger cavities as compared with the use of micelles alone. For example, He et al [123] reported the formation of HAp nanocapsules (size 50 nm–1 μm) by precipitation of HAp on hexane droplets stabilized with bis(2-ethylhexyl)sulfosuccinate.
2.2.2. Hydrothermal process.
Hydrothermal processes include several techniques for crystallizing materials in aqueous media at high temperatures (typically 100–250 °C, and hence under high vapor pressure when the temperature is above 100 °C). Microwave irradiation [124–127] can be used instead of conventional heating in pressure-resistant vessels. The hydrothermal process usually produces HAp particles with larger size (up to millimeter range), higher degree of crystallinity and a Ca/P ratio close to the stoichiometric value compared with those obtained by the precipitation processes at lower temperature (see section 2.2.1). However, the size distribution of the produced particles is usually broad. Hydrothermal processes can be further classified into two categories: (i) hydrothermal conversion and (ii) hydrothermal crystal growth techniques.
2.2.2.1. Hydrothermal conversion.
The hydrothermal conversion technique involves the hydrolysis of other calcium phosphates (CaHPO4, CaHPO4·2H2O, Ca8(HPO4)2(PO4)4·5H2O, Ca3(PO4)2, fluorapatite and chlorapatite [128]) into HAp in aqueous media, usually with the aid of calcium, phosphate and/or alkaline sources to control the Ca/P ratio of HAp particles [129–135]. Hydrothermal conversions of poorly soluble calcium salts such as CaCO3 (from coral [136–138], seashells [139, 140], aragonite or calcite crystals [141, 142]) and CaSO4·2H2O [124] into HAp have also been studied, and the conversion mechanism—ion exchange, dissolution and recrystallization—has been discussed. Although hydrothermal conversion techniques generally lead to the formation of large crystalline HAp particles due to the small number of nucleation sites, Shih et al [132] prepared stoichiometric HAp nanoparticles (50 nm in width and 100 nm in length) by hydrolysis of a mixture of CaHPO4·2H2O and CaCO3, performed with 2.5 M aqueous solution of NaOH at a relatively low temperature (75 °C). Rahaman and co-workers reported the fabrication of hollow HAp microspheres with nanosized porous shells (∼13 nm) via a conversion of Li2O–CaO–B2O3 glass microspheres in a K2HPO4 solution [143, 144].
As is the case in the wet chemical processes, the morphology of HAp particles can be varied by adding chemicals, such as carboxylic acids [145] and methanol [146], during the hydrothermal conversion.
2.2.2.2. Hydrothermal crystal growth.
The hydrothermal crystal growth technique involves post-hydrothermal treatment for as-prepared low-crystallinity HAp nanoparticles (prepared by wet chemical precipitation in many cases) in aqueous media. The dimensions of the precipitated HAp nanoparticles increase by the Ostwald ripening (maturation) under boiling or ambient aging in the mother liquid [147–150]. Pang and Bao [148] investigated the effects of temperature and ripening time on the crystallinity and morphology of the HAp nanoparticles, and found that the crystallinity and crystallite size increased with the increase in the treatment temperature and time. Wei and co-workers also reported the same tendency, but they observed that the length of the HAp decreased when the temperature was raised above 170 °C [26]. Pathi et al [151] recently reported a two-step hydrothermal method to obtain HAp nanoparticles with varying crystallinity and average length (32–103 nm). Although general hydrothermal treatment is a batch process, Darr and co-workers developed a continuous hydrothermal flow process [152–154] and prepared 8-g crystalline HAp nanoparticles (39 nm in width, 166 nm in length) within 1 h (yield >95%).
Many investigations have been carried out to understand the effect of organic additives. Additives such as surfactants [155–158], amino acid [159, 160], dicarboxylic acid [161] and citrates [162] as well as pH [162] affect the morphologies of the particles treated. Wu et al [163] recently revealed that the chirality of the additives plays an important role in the asymmetric crystal growth of apatite by using l- or d-form of glutamic acid or aspartic acid.
Some researchers succeeded in preparing nanostructured HAp hollow particles without templates. For examples, Nathanael et al [164] reported a template-free formation of HAp nanorings with an inner diameter of 70 nm by a combined high gravity and hydrothermal crystal growth approach. Jiang et al [165] reported a template-free route to fabricate nanostructured hollow HAp microspheres assembled with nanorods (50 nm in thickness, 0.5–1 μm in length) by hydrothermal approach using poly(aspartic acid) as both a chelating and a capping agent. Ma and Zhu [166] reported the fabrication of hierarchically nanostructured HAp hollow spheres (assembled from nanorods) using solvothermal method at 200 °C in water/N,N-dimethylformamide (DMF) mixed solvents. They proposed a self-assembly/base-erosion mechanism for the formation of the nanostructured hollow spheres [167].
2.2.3. Homogeneous precipitation.
The homogeneous precipitation starts with a homogeneous, acidic calcium phosphate solution, and nucleation/growth of HAp is induced by thermal decomposition (hydrolysis) of urea ((NH2)2CO + H2O → CO2 + 2NH3) [168–171] or acetamide (CH3CONH2 + H2O → CH3COOH + NH3) [172, 173]. The resultant NH3 raises the solution pH (and degree of supersaturation for HAp), leading to the formation (precipitation) of HAp particles. Slow hydrolysis of the molecules at a high temperature leads to the formation of large and well-crystallized HAp particles. The hydrolysis of urea can be accelerated by the addition of enzyme (urease), especially at a low temperature of 37 °C [174]. Zhuang et al [175] recently synthesized plate-shaped HAp particles with a preferred orientation to the c plane by a homogeneous precipitation method via an enzyme reaction of urea. Yu et al fabricated willow-leaf-like nanorods (diameter 25 nm, length 120 nm) in a water/ethanol mixed solvent using urea as additive at 80 °C [176].
Hydrothermal treatment of the homogeneous calcium phosphate solution, in which calcium ions are dissolved by a chelating agent ethylenediaminetetraacetic acid (EDTA), also leads to the formation of HAp particles due to thermal dissociation of calcium-EDTA chelates in phosphate solutions [177, 178]. From the Ca-EDTA chelate solutions, HAp particles are obtained in the micrometer-size range after hydrothermal treatments, because EDTA ions do not inhibit HAp crystal growth [179]. López-Macipe et al [180] used citrate ions, which inhibit HAp crystal growth, as a calcium-chelating agent, and rod-like HAp nanoparticles (30–60 nm) were obtained after microwave heating. These calcium-chelated homogeneous precipitation methods utilize the promotion of Ca2+ dissociation by the calcium chelates (and solubility decrease of HAp) at an elevated temperature above 100 °C. Recently, He et al [181] and Cheng et al [182] fabricated nanostructured HAp porous microspheres by employing homogeneous precipitation methods with calcium-chelated phosphate solution in the presence of CO2 bubbles as a hollow template.
2.2.4. Sol–gel method.
The sol–gel method has been used for fabricating fine ceramics in wet conditions for a long time [183]. A typical precursor is metal alkoxide, which undergoes hydrolysis and polycondensation reactions to form a solid phase. In this process, the sol (i.e. solution) dissolving precursors evolves gradually towards the formation of a gel-like network of the solid phase. The solid phase can also be deposited on a substrate to form a film. By controlling the reaction parameters, the solid phase can also be obtained as nanoparticles dispersed in media [184].
The sol–gel method is also an effective way to fabricate nanostructured HAp sintered ceramics [185–187] and coating layers on several substrates [188–190] with a number of precursor combinations (calcium alkoxides and phosphorus alkoxides). Some researchers reported the effect of the starting precursor structure on the product (such as size, structure and crystallinity) [191, 192].
In the sol-gel method for HAp preparation, the as-obtained solid phase is generally amorphous Ca–P intermediates (and/or the mixture of unreacted precursors) and hence a thermal treatment (typically at 400–500 °C, which is lower than the sintering temperature of HAp powder, ∼800–1000 °C) is necessary to obtain well-crystallized HAp. The products are obtained in a sintered polycrystalline form and hence grinding or milling is usually necessary to obtain HAp nanoparticles (see also section 2.1). Recently, Costa et al [193] prepared microspheres consisting of nanosized HAp wires (25–800 nm in thickness) by a combination of sol–gel and hydrothermal (conversion) processes.
2.2.5. Emulsion method (surfactant-based biphasic process).
This process involves the crystal nucleation/growth in a restricted space [194–202], which is generally prepared with inverse (water-in-oil type) emulsion droplets, inverse microemulsion droplets or inverse micelles (cf precipitation of HAp ‘on’ the normal micelles formed in aqueous media, described in section 2.2.1). The morphology control of HAp nanoparticles can be achieved by restricting the crystal growth, and this surfactant-based process can also inhibit excessive agglomeration of the particles. That is, this process has a lot of promise to synthesize nanocrystalline material without aggregation, but it has a drawback of contamination by the surfactants. For example, Lim et al [194] have prepared HAp powders by reacting CaCl2 and (NH4)2HPO4 in an inverse microemulsion formed with non-ionic C15H23O(C2H4O)xH (x = 5 or 9) in cyclohexane. They found that the microemulsion route led to a significant refinement in the particle size and the degree of particle agglomeration as compared with the particles obtained by a direct reaction in the absence of the emulsifiers and oil (that is, by a conventional wet chemical precipitation). The effect of reaction temperature (25–70 °C) on the morphology of HAp nanoparticles formed in non-ionic C12H25O(C2H4O)5H-stabilized inverse emulsion droplets in dodecane media was investigated in terms of the cloud point (the temperature at which dissolved molecules are no longer completely soluble) of the non-ionic emulsifier [203, 204]. Sun et al [205] demonstrated the morphology control of HAp nanoparticles using inverse microemulsion systems (aqueous microemulsion in n-butanol/cyclohexane media stabilized with nonionic C14H22O(C2H4O)9–10H and cationic CTAB) under hydrothermal conditions at 160 °C. Cationic CTAB micelles are also used to modulate the formation of HAp nanoparticles [206, 207]. Cao et al [208] reported the preparation of ultrahigh-aspect-ratio HAp nanofibers in inverse CTAB micelles under hydrothermal conditions. They showed that after the HAp nucleation in the inverse micelles, the surfactant headgroups preferentially adsorb on the surface planes parallel to the c-axis of HAp, resulting in the formation of anisometric nanofibers. Saha et al [209] compared the surfactant types (anionic dioctyl sulfosuccinate sodium salt (AOT) and dodecyl phosphate (DP); and nonionic C15H23O(C2H4O)xH (x = 5 or 12)) in terms of the morphology of the particles obtained in inverse microemulsions [209].
Cosurfactants are often used to increase the stability and the solubilizing capacity of microemulsions. An example of such a cosurfactant is a long-chain alcohol, which is also known to reduce the rigidity of water-in-oil interfaces [149]. García et al [210] studied the effect of cosurfactant (n-butanol) in a CTAB/toluene/water microemulsion system under hydrothermal conditions. They found that the cosurfactant to surfactant molar ratio also played an important role in controlling the morphology of HAp nanoparticles.
2.2.6. Calcined HAp nanoparticles.
When low-crystallinity HAp nanoparticles prepared by precipitation processes (see section 2.2.1) or precursor nanoparticles prepared by sol–gel methods (see section 2.2.4) are calcined to improve their crystallinity (and hence their thermal and chemical stabilities), the nanoparticles typically sinter into large polycrystals (figure 1(a)) [211–215]. Consequently, calcined HAp crystals dispersed in a liquid on the nanoscale have been difficult to obtain. Hydrothermal crystal growth (see section 2.2.2) of HAp particles in aqueous medium is effective for preparing well-crystallized single crystals, but generally leads to an increase in crystal size and is restricted to laboratory-scale products as it is a high-pressure process.
Figure 1.
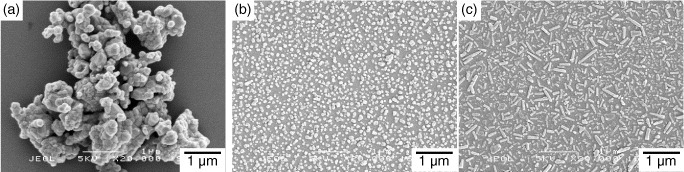
SEM images of HAp calcined (a) without additives and (b, c) with anti-sintering agents for (a, b) spherical and (c) rod-shaped nanoparticles.
A novel calcination method was developed to prepare well-crystallized HAp nanoparticles [216–218]. In this calcination method, an anti-sintering agent covering the nanoparticles is used to prevent the contact (and hence to prevent the calcination-induced sintering) between the nanoparticles; the agent is removed after calcination. Calcium salts (such as Ca(OH)2 and Ca(NO3)2) were selected as the anti-sintering agent, and poly(acrylic acid) (PAA) was used to cover the HAp nanoparticles with the anti-sintering agent. The morphology of the calcined nanoparticles corresponds to that of as-prepared low-crystallinity nanoparticles (prepared by a wet chemical process) before calcination. Spherical or rod-like single HAp nanocrystals were fabricated by the novel calcination methods (figures 1(b) and (c)). Calcination with an anti-sintering agent has potential application to a wide range of calcined nanoceramic powders, such as alumina, titania and magnesia.
3. Applications of HAp nanoparticles
HAp nanoparticles received considerable attention due to their large surface area, improved sinterability (densification) [25–29] and better bioactivity [35–39] compared to coarser crystals. A major application of HAp nanoparticles is filling materials to impart bioactivity to various composites [47–52]. In this section, other applications of HAp nanoparticles are summarized.
3.1. Carriers for drug, protein and gene delivery
HAp surfaces can bind various kinds of molecules (both acidic and alkaline proteins), because HAp belongs to a hexagonal crystal system and possesses different properties on its a and c planes [44]. HAp nanoparticles, therefore, have been investigated as a carrier for delivery of drugs such as growth factors [219, 220], antibiotics [5, 221–225] and anticancer drugs [5, 226, 227]. The adsorption/desorption characteristics [44, 220, 228–230] and conformation changes [230–235] of various kinds of proteins (or peptides) on HAp surfaces have been studied. Mukhopadhyay et al [236] recently reported that HAp nanoparticle supplementation increases thermal stability of pectate lyase from Bacillus megaterium, that is, this enzyme can retain high activity at elevated temperatures (up to 90 °C) in the presence of HAp nanoparticles. Recently, computational (in silico) studies have been gradually progressed to clarify the interaction between proteins (or peptides) and HAp surfaces [237–239]. Detailed information on the protein/peptide adsorptions can be found in the original papers or a recent review by Goobes et al [235].
Calcium phosphate (CaP)-deoxyribonucleic acid (DNA) co-precipitation method has been used for in vitro gene transfection because of the biocompatibility, biodegradability and ease of handling of CaP [240, 241]. To achieve effective gene transfection, the conditions for CaP-DNA co-precipitation method have been studied [242–244]. HAp nanoparticles can also be utilized as gene carriers because of their capability to absorb DNA molecules [245, 246].
Because of their small size, inhaled nanoparticles can have a high deposition rate in alveolar regions of the lung. Fan et al [247] investigated a time-dependent effect of spherical HAp nanoparticles (∼90 nm, prepared by a wet chemical process) on a pulmonary surfactant protein. They found that at a low concentration of 50 μg ml−1 HAp nanoparticles inhibited the biophysical function of the surfactant protein due to adsorption of surfactant proteins onto the nanoparticles, whereas the nanoparticle concentrations up to 100 μg ml−1 did not elicit any significant toxicological effects on human bronchial epithelial BEAS-2B cells in vitro. Fan et al claimed that the conventional cytotoxicological test alone may not be sufficient in evaluating the toxicological effect of inhaled nanoparticles, but also claimed that the interaction between HAp nanoparticles and pulmonary surfactant proteins shed light on the feasibility of HAp nanoparticle-based pulmonary drug delivery.
3.2. Reparative materials for damaged enamel
HAp nanoparticles have been investigated as fillers for dental composites [248–250] and glass-ionomer cements to improve their mechanical properties [251]. The application of HAp to repair the damaged enamel has recently attracted considerable attention in dental field because of the chemical and structural similarities of HAp to tooth minerals (20–40 nm particles of HAp [252, 253]). Li et al [254] reported a higher remineralization effect when using 20-nm-sized HAp compared with several hundred nanometer-sized HAp or 20-nm-sized ACP. Huang et al [255] demonstrated that HAp nanoparticles have a similar re-mineralization effect on an initial caries lesion to fluoride. Kim et al [256] examined the effect of carbonate HAp nanoparticles to prevent re-staining and the change of enamel surface after dental bleaching.
3.3. Particulate emulsifier (Pickering emulsion stabilizer)
General emulsions are stabilized by adsorption of surfactant molecules onto oil/water interfaces. Pickering emulsions are solid-particle-stabilized emulsions, in which solid particles are adsorbed onto oil/water interfaces [257, 258]. Inorganic particles (such as silica [259–263], clay [264, 265] and carbon black [266, 267]), organic particles (such as latex [268–270] and microgels [271, 272]) and Janus particles [273–276] have been used as particulate emulsifiers. These solid-stabilized emulsion droplets can be used as polymerization vessels; therefore, Pickering-type suspension polymerization [277, 278], inversed suspension polymerization [279, 280], and mini-emulsion polymerization [281–283] methods can be used to prepare nanocomposite particles.
HAp nanoparticles have been tested as a particulate emulsifier [284]. Stable oil-in-water type emulsions were readily obtained using oils containing an ester group (e.g. methyl myristate or methyl trimethyl acetate, see figure 2). Although no stable emulsion was obtained using oils without an ester group (e.g. dichloromethane), dichloromethane droplets could be stabilized by dissolving polymers having ester groups (or carboxyl terminal group) via the interaction between polymer and HAp nanoparticles at the oil/water interfaces [285, 286]. It was also demonstrated that emulsification–demulsification cycles could be repeatedly achieved by pH adjustment, because the HAp-adsorbed emulsion droplets became unstable at lower pH (<4) and the emulsion stability was recovered by raising pH. The HAp-nanoparticle-stabilized emulsions are pH-responsive emulsions, and can be used for preparing porous HAp ceramics and HAp-coated microspheres (see section 3.4.2).
Figure 2.
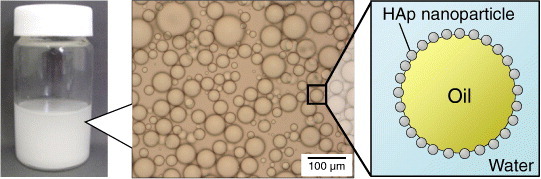
Photograph (left), optical microscopy image (center), and schematic (right) of oil droplets stabilized by adsorption of spherical HAp nanoparticles in an aqueous medium.
3.4. Coating agent
HAp composites are generally fabricated by mixing HAp particles with a matrix. However, the bioactivity of HAp is sometimes hindered because most of HAp is buried inside the composite matrix by the simple mixing methods. HAp coatings are employed to improve the bioactivity of the matrix (table 4).
Table 4.
HAp coating methods.
| Method | Substratea | Ref. |
|---|---|---|
| Dry | ||
| Thermal spraying | M | [287–290] |
| Physical vapor deposition | M | [291–305] |
| Chemical vapor deposition | M | [306–312] |
| Electrospray deposition | M | [313–315] |
| Co-blast deposition | M | [316, 317] |
| Wet | ||
| Biomimetic mineralization process | P, M, C | [82, 318–323] |
| Laser assisted biomimetic process | P, M, C | [324, 325] |
| Alternate soaking | P, M, C | [326–328] |
| Hydrothermal treatment | P, M, C | [329–331] |
| Thermal substrate method | M | [332–335] |
| Cathodic electrolysis method | M | [334, 336, 337] |
| Electrophoretic deposition | M | [338–345] |
| Nanocrystal coating | P, M | [346–358] |
| Pickering emulsion coating | P | [285, 359–362] |
P: polymer; M: metal; C: ceramic.
HAp coating in the dry state (especially by plasma spraying) is a well-accepted and widely used technique, but it has some intrinsic drawbacks related to the extremely high processing temperature (above 10 000 °C): coating on polymer substrates and onto intricate shapes [363, 364], as well as incorporation of biological molecules (such as growth factors that stimulate bone healing) in the coating layer, is impossible by the dry-coating methods.
Recent research has shown that the biomimetic process is one of the most promising techniques for producing a bioactive coating under nearly ambient conditions (e.g. body temperature and atmospheric pressure) [365], overcoming the drawbacks of dry processes. Other wet coating techniques (e.g. thermal substrate method, cathodic electrolysis method and electrophoretic method) have been proposed as approaches to forming HAp coatings on metallic substrates. However, these wet-synthesis methods have disadvantages such as poorly crystalized products (in the case of biomimetic approach) or restriction of electron-conducting substrates (in the cases of thermal substrate method, electrolysis and electrophoretic approaches).
3.4.1. Nanocrystal coating.
To coat well-crystallized HAp on a broad range of substrates, the nanocrystal coating has been developed [346–355]. This approach involves four steps: (i) preparation of calcined HAp nanocrystals (see section 2.2.6), (ii) surface modification of the substrate to react with HAp surfaces, (iii) adsorption of HAp nanocrystals onto the modified substrate, and (iv) reaction at the interface between the HAp nanoparticle and the substrate (figure 3).
Figure 3.
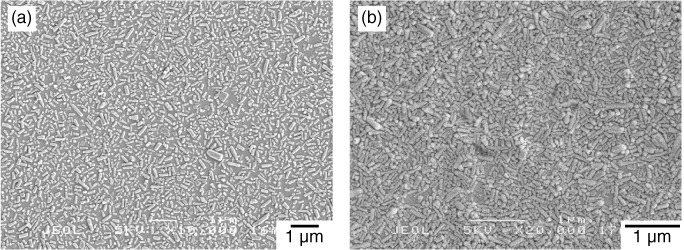
SEM images of HAp-nanocrystal-coated polymer substrates: (a) low- and (b) high-density coatings.
The surface modification of the substrate is essential to determine the bonding strength of HAp [354]. In the nanocrystal coating method, the substrates were modified by graft polymerization of functional monomers having alkoxysilyl, isocyanate or carboxyl groups. The HAp surface possesses hydroxyl groups and calcium and phosphate ionic sites that can react with several functional groups. For example, alkoxysilyl groups (silane coupling agents) [210, 366] and isocyanate groups [367] can covalently react with surface hydroxyl groups of HAp, and carboxyl groups can interact ionically with calcium ions on the HAp surfaces. Polymer and metal substrates have been used [346–355]. After adsorption of HAp nanocrystals and reaction at the interface between HAp nanocrystals and substrate surfaces, the HAp-nanocrystal-coated substrates retained the mechanical properties of the substrates and showed improved cell adhesion properties owing to the presence of HAp crystals on the surface [349–353, 355]. The HAp nanocrystal coating is applied to percutaneous devices [349,356], artificial blood vessels [357, 358] and stents [352].
3.4.2. Nanoparticle coating via Pickering emulsion route.
Solid-particle-stabilized emulsions (see section 3.3) have been utilized for the preparation of HAp-coated biodegradable polymer microspheres by evaporation of solvent (oil) from HAp-nanoparticle-stabilized oil droplets dissolving polymer (figure 4). By using a water-in-oil-in-water multiple emulsion, multiholow microspheres can also be prepared in the same manner [359]. The HAp-nanoparticle-coated microspheres showed improved cell adhesion and spreading compared with bare biodegradable microspheres [285]. Recently, Mima et al [368] showed the effectiveness of HAp-coated biodegradable polymer microspheres as an injectable cell scaffold for cell-based therapeutic angiogenesis.
Figure 4.
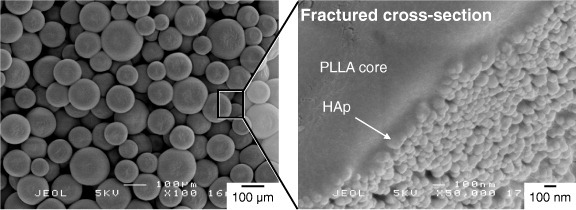
SEM images of HAp/PLLA microspheres prepared by evaporation of CH2Cl2 from a HAp-nanoparticle-stabilized emulsion.
4. Summary and future perspectives
This paper reviewed the fabrication methods of HAp nanoparticles, highlighting recent improvements for morphology control. Numerous methods for fabricating HAp nanoparticles have evolved in the past few decades to control biological effects of nanostructured HAp. However, the development of facile fabrication methods for monodispersed HAp nanoparticles (with very narrow size distribution) is still a challenging task. The monodispersity of nanoparticles is important to guarantee the homogeneity of the nanoparticles in a batch, and hence to define the biological effects of HAp nanoparticles. Monodispersed HAp nanoparticles are now fabricated in a limited size range [102] because of the fabrication strategy to eliminate the particle growth stage. The fabrication of monodispersed HAp nanoparticles in a broad size range will be realized by precise adjustment (separation) of the nucleation and growth stages [369], seeded crystal growth techniques [369] or by entirely different approaches [370]. Dispersion of HAp nanoparticles in liquid media (or in solid matrices) is also important for the applications of HAp nanoparticles and for the development of well-designed HAp-filled composites [53]. Although the dispersion is now improved by adding surface modifiers, another approach will be proposed because modifying pristine HAp surfaces generally hinders their bioactivity.
Recent applications of HAp nanoparticles are also summarized in this review. The greatest motivation behind the use of HAp is to accurately mirror the chemistry of natural minerals. The recent progress in computational analyses for the conformation changes, which alter structure and function at a distant active site, of the proteins interacting with HAp surfaces [235, 237–239] will provide a guide for designing nanostructured HAp surfaces to transcend the chemistry of natural minerals. Although nanostructured biomaterials have many potential advantages, it is important to remember that the effects of nanoparticle exposure on human health are not well understood. Understanding the biological effects of nanosized HAp is essential for the applications.
Acknowledgments
The authors thank Dr Shinya Fukumoto and Dr Yohei Mima of Osaka City University (Department of Metabolism, Endocrinology, and Molecular Medicine) and Dr Syuji Fujii of Osaka Institute of Technology (Department of Applied Chemistry, Faculty of Engineering) for fruitful discussions. This study was partially supported by a Grant-in-Aid for Young Scientists (B) (grant no. 23792301) of the Japan Society for the Promotion of Science, and Adaptable & Seamless Technology Transfer Program through Target-driven R&D (A-STEP) from Japan Science and Technology Agency (JST).
References
- Pasero M, Kampf A R, Ferraris C, Pekov I V, Rakovan J. and White T J. Eur. J. Mineral. 2010;22:163. doi: 10.1127/0935-1221/2010/0022-2022. [DOI] [Google Scholar]
- Pasero M, Kampf A R, Ferraris C, Pekov I V, Rakovan J. and White T J. Rocks Miner. 2010;85:204. doi: 10.1080/00357521003749078. [DOI] [Google Scholar]
- Evis Z. and Webster T. Adv. Appl. Ceram. 2011;110:311. doi: 10.1179/1743676110Y.0000000005. [DOI] [Google Scholar]
- Zhou H. and Lee J. Acta Biomater. 2011;7:2769. doi: 10.1016/j.actbio.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Uskoković V. and Uskoković D P. J. Biomed. Mater. Res. B. 2011;96:152. doi: 10.1002/jbm.b.31746. [DOI] [PubMed] [Google Scholar]
- Dorozhkin S V. Acta Biomater. 2010;6:715. doi: 10.1016/j.actbio.2009.10.031. [DOI] [PubMed] [Google Scholar]
- Meyers M A, Chen P-Y, Lin A Y-M. and Seki Y. Prog. Mater. Sci. 2008;53:1. doi: 10.1016/j.pmatsci.2007.05.002. [DOI] [Google Scholar]
- Pietak A M, Reid J W, Stott M J. and Sayer M. Biomaterials. 2007;28:4023. doi: 10.1016/j.biomaterials.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Koutsopoulos S. J. Biomed. Mater. Res. 2002;15:62. doi: 10.1002/jbm.10280. [DOI] [PubMed] [Google Scholar]
- Corno M, Chiatti F, Pedone A. and Ugliengo P. In: Biomaterials—Physics and Chemistry. Pignatello R, editor. Rijeka InTech Open Access Books; 2011. p. p 275. [Google Scholar]
- Hench L L. and Best S. In: Biomaterials Science: An Introduction to Materials in Medicine. 2nd edn. Ratner B D, , Hoffman A S, , Schoen F J, , Lemons J E, , editors. Amsterdam Elsevier, Academic; 2004. p. p 153. [Google Scholar]
- Elliott J C. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates. Amsterdam Elsevier; 1994. [Google Scholar]
- Aoki H. Medical Applications of Hydroxyapatite. Tokyo Ishiyaku EuroAmerica; 1994. [Google Scholar]
- Aoki H. and Kato K. Ceram. Japan. 1975;10:469. [Google Scholar]
- Jarcho M. Clin. Orthop. Relat. Res. 1981;157:259. [PubMed] [Google Scholar]
- Hing K A, Best S M, Tanner K E, Revell P A. and Bonfield W. Proc. Inst. Mech. Eng. H. 1998;212:437. doi: 10.1243/0954411981534204. [DOI] [PubMed] [Google Scholar]
- Quinn J H. and Kent J N. Oral Surg. Oral Med. Oral Pathol. 1984;58:511. doi: 10.1016/0030-4220(84)90071-9. [DOI] [PubMed] [Google Scholar]
- Cranin A N, Tobin G P. and Gelbman J. Compendium. 1987;8:334. [PubMed] [Google Scholar]
- Froum S J, Kushner L, Scopp I W. and Stahl S S. J. Periodontol. 1982;53:719. doi: 10.1902/jop.1982.53.12.719. [DOI] [PubMed] [Google Scholar]
- Galgut P N, Waite I M. and Tinkler S M. Clin. Mater. 1990;6:105. doi: 10.1016/0267-6605(90)90002-D. [DOI] [PubMed] [Google Scholar]
- Wilson J. and Low S B. J. Appl. Biomater. 1992;3:123. doi: 10.1002/jab.770030208. [DOI] [PubMed] [Google Scholar]
- Fujishiro Y, Hench L L. and Oonishi H. J. Mater. Sci. Mater. Med. 1997;8:649. doi: 10.1023/A:1018527621356. [DOI] [PubMed] [Google Scholar]
- van Blitterswijk C A, Hesseling S C, Grote J J, Koerten H K. and de Groot K. J. Biomed. Mater. Res. 1990;24:433. doi: 10.1002/jbm.820240403. [DOI] [PubMed] [Google Scholar]
- Bonfield W, Grynpas M D, Tully A E, Bowman J. and Abram J. Biomaterials. 1981;2:185. doi: 10.1016/0142-9612(81)90050-8. [DOI] [PubMed] [Google Scholar]
- Mostafa N Y. Mater. Chem. Phys. 2005;94:333. doi: 10.1016/j.matchemphys.2005.05.011. [DOI] [Google Scholar]
- Kothapalli C R, Wei M, Legeros R Z. and Shaw M T. J. Mater. Sci. Mater. Med. 2005;16:441. doi: 10.1007/s10856-005-6984-5. [DOI] [PubMed] [Google Scholar]
- Wang J. and Shaw L L. Adv. Mater. 2007;19:2364. doi: 10.1002/adma.200602333. [DOI] [Google Scholar]
- Kalita S J, Bhardwaj A. and Bhatt H A. Mater. Sci. Eng. C. 2007;27:441. doi: 10.1016/j.msec.2006.05.018. [DOI] [Google Scholar]
- Drouet C, Bosc F, Banu M, Largeot C, Combes C, Dechambre G, Estournès C, Raimbeaux G. and Rey C. Powder Technol. 2009;190:118. doi: 10.1016/j.powtec.2008.04.041. [DOI] [Google Scholar]
- Okada M. and Furuzono T. J. Colloid Interface Sci. 2011;360:457. doi: 10.1016/j.jcis.2011.04.068. [DOI] [PubMed] [Google Scholar]
- Klein C P, Driessen A A, de Groot K. and van den Hooff A. J. Biomed. Mater. Res. 1983;17:769. doi: 10.1002/jbm.820170505. [DOI] [PubMed] [Google Scholar]
- Cornell C N. Orthop. Clin. North Am. 1999;30:591. doi: 10.1016/S0030-5898(05)70112-7. [DOI] [PubMed] [Google Scholar]
- Rey C, Kim H M. and Glimcher M J. In: Hydroxyapatite and Related Materials. Brown P W, , Constantz B, , editors. Boca Raton, FL CRC Press; 1994. p. p 181. [Google Scholar]
- Cazalbou S, Combes C, Eichert D, Rey C. and Glimcher M J. J. Bone Miner. Metab. 2004;22:310. doi: 10.1007/s00774-004-0488-0. [DOI] [PubMed] [Google Scholar]
- Webster T J, Ergun C, Doremus R H, Siegel R W. and Bizios R. Biomaterials. 2001;22:1327. doi: 10.1016/S0142-9612(00)00285-4. [DOI] [PubMed] [Google Scholar]
- Balasundaram G, Sato M. and Webster T J. Biomaterials. 2006;27:2798. doi: 10.1016/j.biomaterials.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Sun W, Chu C, Wang J. and Zhao H. J. Mater. Sci. Mater. Med. 2007;18:677. doi: 10.1007/s10856-006-0019-8. [DOI] [PubMed] [Google Scholar]
- Huang J, Best S M, Bonfield W, Brooks R A, Rushton N, Jayasinghe S N. and Edirisinghe M J. J. Mater. Sci. Mater. Med. 2004;15:441. doi: 10.1023/B:JMSM.0000021117.67205.cf. [DOI] [PubMed] [Google Scholar]
- Appleford M R, Oh S, Oh N. and Ong J L. J. Biomed. Mater. Res. A. 2009;89:1019. doi: 10.1002/jbm.a.32049. [DOI] [PubMed] [Google Scholar]
- Lewandrowski K U, Bondre S P, Wise D L. and Trantolo D J. Biomed. Mater. Eng. 2003;13:115. [PubMed] [Google Scholar]
- Ramay H R, Li Z, Shum E. and Zhang M. J. Biomed. Nanotechnol. 2005;1:151. doi: 10.1166/jbn.2005.026. [DOI] [Google Scholar]
- Li J, Dou Y, Yang J, Yin Y, Zhang H, Yao F, Wang H. and Yao K. Mater. Sci. Eng. C. 2009;29:1207. doi: 10.1016/j.msec.2008.09.038. [DOI] [Google Scholar]
- Ai J, Rezaei-Tavirani M, Biazar E, Heidari K S. and Jahandideh R. J. Nanomater. 2011;2011:064103. doi: 10.1155/2011/391596. [DOI] [Google Scholar]
- Kawasaki T. J. Chromatogr. 1991;544:147. doi: 10.1016/S0021-9673(01)83984-4. [DOI] [Google Scholar]
- Carrodeguas R G. and De Aza S. Acta Biomater. 2011;7:3536. doi: 10.1016/j.actbio.2011.06.019. [DOI] [PubMed] [Google Scholar]
- Dorozhkin S V. Acta Biomater. 2010;6:4457. doi: 10.1016/j.actbio.2010.06.031. [DOI] [PubMed] [Google Scholar]
- Suzuki O. Acta Biomater. 2010;6:3379. doi: 10.1016/j.actbio.2010.04.002. [DOI] [PubMed] [Google Scholar]
- James R, Deng M, Laurencin C T. and Kumbar S G. Front. Mater. Sci. 2011;5:342. doi: 10.1007/s11706-011-0151-3. [DOI] [Google Scholar]
- Canal C. and Ginebra M P. J. Mech. Behav. Biomed. Mater. 2011;4:1658. doi: 10.1016/j.jmbbm.2011.06.023. [DOI] [PubMed] [Google Scholar]
- Dorozhkin S V. J. Mater. Sci. 2009;44:2343. doi: 10.1007/s10853-008-3124-x. [DOI] [Google Scholar]
- Rezwan K, Chen Q Z, Blaker J J. and Boccaccini A R. Biomaterials. 2006;27:3413. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- Murugan R. and Ramakrishna S. Compos. Sci. Technol. 2005;65:2385. doi: 10.1016/j.compscitech.2005.07.022. [DOI] [Google Scholar]
- Šupová M. J. Mater. Sci. Mater. Med. 2009;20:1201. doi: 10.1007/s10856-009-3696-2. [DOI] [PubMed] [Google Scholar]
- Arita I H, Wilkinson D S, Mondragón M A. and Castaño V M. Biomaterials. 1995;16:403. doi: 10.1016/0142-9612(95)98858-B. [DOI] [PubMed] [Google Scholar]
- Fowler B O. Inorg. Chem. 1974;13:207. doi: 10.1021/ic50131a040. [DOI] [Google Scholar]
- Fleet M E, Liu X. and King P L. Am. Mineral. 2004;89:1422. [Google Scholar]
- Rao R R, Roopa H N. and Kannan T S. J. Mater. Sci. Mater. Med. 1997;8:511. doi: 10.1023/A:1018586412270. [DOI] [PubMed] [Google Scholar]
- Ota Y, Iwashita T, Kasuga T. and Abe Y. J. Am. Ceram. Soc. 1998;81:1665. doi: 10.1111/j.1151-2916.1998.tb02529.x. [DOI] [Google Scholar]
- Elliott J C. and Young R A. Nature. 1967;214:904. doi: 10.1038/214904b0. [DOI] [Google Scholar]
- Ozyegina L S, Oktarb F N, Gollerc G, Kayalic E S. and Yazici T. Mater. Lett. 2004;58:2605. doi: 10.1016/j.matlet.2004.03.033. [DOI] [Google Scholar]
- Joschek S, Nies B, Krotz R. and Göpferich A. Biomaterials. 2000;21:1645. doi: 10.1016/S0142-9612(00)00036-3. [DOI] [PubMed] [Google Scholar]
- Lin F-H, Liao C-J, Chen K-S. and Sun J-S. Biomaterials. 1999;20:475. doi: 10.1016/S0142-9612(98)00193-8. [DOI] [PubMed] [Google Scholar]
- Ozawa M. and Suzuki S. J. Am. Ceram. Soc. 2002;85:1315. doi: 10.1111/j.1151-2916.2002.tb00268.x. [DOI] [Google Scholar]
- Roy D M. and Linnehan S K. Nature. 1974;247:220. doi: 10.1038/247220a0. [DOI] [PubMed] [Google Scholar]
- Sivakumar M, Kumar T S, Shantha K L. and Rao K P. Biomaterials. 1996;17:1709. doi: 10.1016/0142-9612(96)87651-4. [DOI] [PubMed] [Google Scholar]
- Toriyama M, Kawamura S, Ito Y. and Nagae H. J. Ceram. Soc. Japan. 1989;97:554. doi: 10.2109/jcersj.97.554. [DOI] [Google Scholar]
- Rivera E M, Araiza M, Brostow W, Victor M, Castaño J R, Díaz-Estrada R, Hernández J. and Rodríguez R. Mater. Lett. 1999;41:128. doi: 10.1016/S0167-577X(99)00118-4. [DOI] [Google Scholar]
- Gergely G, Wéber F, Lukács I, Tóth A L, Horváth Z E, Mihály J. and Balázsi C. Ceram. Int. 2010;36:803. doi: 10.1016/j.ceramint.2009.09.020. [DOI] [Google Scholar]
- Furuzono T, Walsh D, Yasuda S, Sato K, Tanaka J. and Kishida A. J. Mater. Sci. 2005;40:2595. doi: 10.1007/s10853-005-2083-8. [DOI] [Google Scholar]
- Trakhtenberg I S, Rubshtein A P, Volkova E G, Petrova S A, Fishman A Y, Zakharov R G, Vykhodets V B. and Kurennykh T E. Inorg. Mater. 2011;47:45. doi: 10.1134/S0020168510121052. [DOI] [Google Scholar]
- Otsuka M, Matsuda Y, Hsu J, Fox J L. and Higuchi W I. Biomed. Mater. Eng. 1994;4:357. [PubMed] [Google Scholar]
- Yeong K C B. and Wang J. Biomaterials. 2001;22:2705. doi: 10.1016/S0142-9612(00)00257-X. [DOI] [PubMed] [Google Scholar]
- Serraj S, Boudeville P, Pauvert B. and Terol A. J. Biomed. Mater. Res. 2001;55:566. doi: 10.1002/1097-4636(20010615)55:4%3C566::AID-JBM1050%3E3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- El Briak-BenAbdeslam H, Mochales C, Ginebra M P, Nurit J, Planell J A. and Boudeville P. J. Biomed. Mater. Res. A. 2003;67:927. doi: 10.1002/jbm.a.10025. [DOI] [PubMed] [Google Scholar]
- Nasiri-Tabrizi B, Honarmandi P, Ebrahimi-Kahrizsangi R. and Honarmandi P. Mater. Lett. 2009;63:543. doi: 10.1016/j.matlet.2008.11.030. [DOI] [Google Scholar]
- Honarmandi P, Honarmandi P, Shokuhfar A, Nasiri-Tabrizi B. and Ebrahimi-Kahrizsangi R. Adv. Appl. Ceram. 2010;109:117. doi: 10.1179/174367509X12447975734230. [DOI] [Google Scholar]
- Xu J L, Khor K A, Dong Z L, Gu Y W, Kumar R. and Cheang P. Mater. Sci. Eng. A. 2004;374:101. doi: 10.1016/j.msea.2003.12.040. [DOI] [Google Scholar]
- Jarcho M, Bolen C H, Thomas M B, Bobick J, Kay J F. and Doremus R H. J. Mater. Sci. 1976;11:2027. doi: 10.1007/BF02403350. [DOI] [Google Scholar]
- Schmidt H K, Geiter E, Mennig M, Krug H, Becker C. and Winkler R-P. J. Sol–Gel Sci. Techol. 1998;13:397. doi: 10.1023/A:1008660909108. [DOI] [Google Scholar]
- Cushing B L, Kolesnichenko V L. and O'Connor C J. Chem. Rev. 2004;104:3893. doi: 10.1021/cr030027b. [DOI] [PubMed] [Google Scholar]
- Ferraz M P, Monteiro F J. and Manuel C M. J. Appl. Biomater. Biomech. 2004;2:74. [PubMed] [Google Scholar]
- Kokubo T, Miyaji F. and Kim H M. J. Am. Ceram. Soc. 1999;79:1127. doi: 10.1111/j.1151-2916.1996.tb08561.x. [DOI] [Google Scholar]
- Tas A C. Biomaterials. 2000;21:1429. doi: 10.1016/S0142-9612(00)00019-3. [DOI] [PubMed] [Google Scholar]
- Cengiz B, Gokce Y, Yildiz N, Aktas Z. and Calimli A. Colloids Surf. A. 2008;322:29. doi: 10.1016/j.colsurfa.2008.02.011. [DOI] [Google Scholar]
- Wang L. and Nancollas G H. Chem. Rev. 2008;108:4628. doi: 10.1021/cr0782574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes C. and Rey C. Acta Biomater. 2010;6:3362. doi: 10.1016/j.actbio.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Nancollas G H. and Mohan M S. Arch. Oral. Biol. 1970;15:731. doi: 10.1016/0003-9969(70)90037-3. [DOI] [PubMed] [Google Scholar]
- Blumenthal N C, Betts F. and Posner A S. Calcif. Tiss. Int. 1981;33:111. doi: 10.1007/BF02409422. [DOI] [PubMed] [Google Scholar]
- Meyer J L. Croat. Chem. Acta. 1983;56:753. [Google Scholar]
- Harries J E, Hukins D W L, Holt C. and Hasnain S S. J. Cryst. Growth. 1987;84:563. doi: 10.1016/0022-0248(87)90046-7. [DOI] [Google Scholar]
- Lopez-Valero I, Gomez-Lorente C. and Boistelle R. J. Cryst. Growth. 1992;121:297. doi: 10.1016/0022-0248(92)90139-A. [DOI] [Google Scholar]
- Lazić S. J. Cryst. Growth. 1995;147:147. doi: 10.1016/0022-0248(94)00587-7. [DOI] [Google Scholar]
- Gadaleta S J, Paschalis E P, Betts F, Mendelson R. and Boskey A L. Calcif. Tiss. Int. 1996;58:9. doi: 10.1007/BF02509540. [DOI] [PubMed] [Google Scholar]
- Tarasevich B J, Chusuei C C. and Allara D L. J. Phys. Chem. B. 2003;107:10367. doi: 10.1021/jp027445p. [DOI] [Google Scholar]
- Kim S, Ryu H S, Shin H, Jung H S. and Hong K S. Chem. Lett. 2004;33:1292. doi: 10.1246/cl.2004.1292. [DOI] [Google Scholar]
- Brecević L. and Füredi-Milhofer H. Calcif. Tiss. Res. 1972;10:82. doi: 10.1007/BF02012538. [DOI] [PubMed] [Google Scholar]
- Liu C, Huang Y, Shen W. and Cui J. Biomaterials. 2001;22:301. doi: 10.1016/S0142-9612(00)00166-6. [DOI] [PubMed] [Google Scholar]
- Boskey A L. and Posner A S. J. Phys. Chem. 1976;80:40. doi: 10.1021/j100542a009. [DOI] [Google Scholar]
- Mossaad C, Tan M C, Starr M, Payzant E A, Howe J Y. and Riman R E. Cryst. Growth Des. 2011;11:45. doi: 10.1021/cg9015146. [DOI] [Google Scholar]
- Lee W H, Zavgorodniy A V, Loo C Y. and Rohanizadeh R. J. Biomed. Mater. Res. A. 2012;100:1539. doi: 10.1002/jbm.a.34093. [DOI] [PubMed] [Google Scholar]
- Hessel V, Löwe H, Müller A. and Kolb G. Chemical Micro-Process Engineering: Processing and Plant. New York Wiley-VCH; 2005. [Google Scholar]
- Yang Q, Wang J-X, Shao L, Wang Q-A, Guo F, Chen J-F, Gu L. and An Y-T. Ind. Eng. Chem. Res. 2010;49:140. doi: 10.1021/ie9005436. [DOI] [Google Scholar]
- Kandori K, Kuroda T, Togashi S. and Katayama E. J. Phys. Chem. B. 2011;115:653. doi: 10.1021/jp110441e. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hou D. and Wang G. Mater. Chem. Phys. 2004;86:69. doi: 10.1016/j.matchemphys.2004.02.009. [DOI] [Google Scholar]
- SL Shanthi P M, Ashok M, Balasubramanian T, Riyasdeen A. and Akbarsha M A. Mater. Lett. 2009;63:2123. doi: 10.1016/j.matlet.2009.07.008. [DOI] [Google Scholar]
- El Hammari L, Merroun H, Coradin T, Cassaignon S, Laghzizil A. and Saoiabi A. Mater. Chem. Phys. 2007;104:448. doi: 10.1016/j.matchemphys.2007.04.002. [DOI] [Google Scholar]
- Gonzalez-McQuire R, Chane-Ching J Y, Vignaud E, Lebugle A. and Mann S. J. Mater. Chem. 2004;14:2277. doi: 10.1039/b400317a. [DOI] [Google Scholar]
- Rosseeva E V, Golovanova O A. and Frank-Kamenetskaya O V. Glass Phys. Chem. 2007;33:283. doi: 10.1134/S1087659607030170. [DOI] [Google Scholar]
- Sharma R, Pandey R R, Gupta A A, Kar S. and Dhayal M. Mater. Chem. Phys. 2012;133:718. doi: 10.1016/j.matchemphys.2012.01.072. [DOI] [Google Scholar]
- López-Macipe A, Gómez-Morales J. and Rodríguez-Clemente R. Adv. Mater. 1998;10:49. doi: 10.1002/(SICI)1521-4095(199801)10:1%3C49::AID-ADMA49%3E3.0.CO;2-R. [DOI] [Google Scholar]
- Martins M A, Santos C, Almeida M M. and Costa M E V. J. Colloid Interface Sci. 2008;318:210. doi: 10.1016/j.jcis.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Bertoni E, Bigi A, Falini G, Panzavolta S. and Roveri N. J. Mater. Chem. 1999;9:779. doi: 10.1039/a807890d. [DOI] [Google Scholar]
- Liou S C, Chen S Y. and Liu D M. Biomaterials. 2003;24:3981. doi: 10.1016/S0142-9612(03)00303-X. [DOI] [PubMed] [Google Scholar]
- Shkilnyy A, Friedrich A, Tiersch B, Schöne S, Fechner M, Koetz J, Schläpfer C-W. and Taubert A. Langmuir. 2008;24:2102. doi: 10.1021/la702523p. [DOI] [PubMed] [Google Scholar]
- Shkilnyy A, Schöne S, Rumplasch C, Uhlmann A, Hedderich A, Günter C. and Taubert A. Colloid Polym. Sci. 2011;289:881. doi: 10.1007/s00396-011-2403-2. [DOI] [Google Scholar]
- Adzamli K, Dorshow R B, Hynes M R, Li D, Nosco D L. and Adams M D. J. Magn. Reson. Imag. 1997;7:204. doi: 10.1002/jmri.1880070131. [DOI] [PubMed] [Google Scholar]
- Xiao F, Ye J, Wang Y. and Rao P. J. Mater. Sci. 2005;40:5439. doi: 10.1007/s10853-005-1919-6. [DOI] [Google Scholar]
- Mollazadeh S, Javadpour J. and Khavandi A. Ceram. Int. 2007;33:1579. doi: 10.1016/j.ceramint.2006.06.006. [DOI] [Google Scholar]
- Sinha A, Nayar S. and Agrawak A C. J. Am. Ceram. Soc. 2003;86:357. doi: 10.1111/j.1151-2916.2003.tb00024.x. [DOI] [Google Scholar]
- Yao J, Tjandra W, Chen Y Z, Tam K C, Ma J. and Soh B. J. Mater. Chem. 2003;13:3053. doi: 10.1039/b308801d. [DOI] [Google Scholar]
- Wu Y. and Bose S. Langmuir. 2005;21:3232. doi: 10.1021/la046754z. [DOI] [PubMed] [Google Scholar]
- Ye F, Guo H, Zhang H. and He X. Acta Biomater. 2010;6:2212. doi: 10.1016/j.actbio.2009.12.014. [DOI] [PubMed] [Google Scholar]
- He W H, Tao J H, Pan H H, Xu X R. and Tang R K. Chem. Lett. 2010;39:674. doi: 10.1246/cl.2010.674. [DOI] [Google Scholar]
- Katsuki H, Furuta S. and Komarneni S. J. Am. Ceram. Soc. 1999;82:2257. doi: 10.1111/j.1151-2916.1999.tb02073.x. [DOI] [Google Scholar]
- Sarig S. and Kahana F. J. Cryst. Growth. 2002;237–239:55. doi: 10.1016/S0022-0248(01)01850-4. [DOI] [Google Scholar]
- Siddharthan A, Seshadri S K. and Kumar T S S. Trends Biomater. Artif. Organs. 2005;18:110. [Google Scholar]
- Han J K, Song H Y, Saito F. and Lee B T. Mater. Chem. Phys. 2006;99:235. doi: 10.1016/j.matchemphys.2005.10.017. [DOI] [Google Scholar]
- García-Tuñón E, Franco J, Dacuña B, Zaragoza G. and Guitián F. Mater. Sci. Forum. 2010;636–637:9. doi: 10.4028/www.scientific.net/MSF.636-637.9. [DOI] [Google Scholar]
- Onuma K. Prog. Cryst. Growth Charact. Mater. 2006;52:223. doi: 10.1016/j.pcrysgrow.2006.06.003. [DOI] [Google Scholar]
- Ishikawa K. and Eanes E D. J. Dent. Res. 1993;72:474. doi: 10.1177/00220345930720020101. [DOI] [PubMed] [Google Scholar]
- De Maeyer E A P, Verbeeck R M H. and Naessens D E. J. Cryst. Growth. 1994;135:539. doi: 10.1016/0022-0248(94)90145-7. [DOI] [Google Scholar]
- Shih W J, Chen Y F, Wang M C. and Hon M H. J. Cryst. Growth. 2004;270:211. doi: 10.1016/j.jcrysgro.2004.06.023. [DOI] [Google Scholar]
- Zhang X. and Vecchio K S. J. Cryst. Growth. 2007;308:133. doi: 10.1016/j.jcrysgro.2007.07.059. [DOI] [Google Scholar]
- Lee D K, Park J Y, Kim M R. and Jang D-J. Cryst. Eng. Commun. 2011;13:5455. [Google Scholar]
- Rendón-Angeles J C, Yanagisawa K, Ishizawa N. and Oishi S. J. Solid State Chem. 2000;151:65. doi: 10.1006/jssc.1999.8623. [DOI] [Google Scholar]
- Roy D M. and Linnehan S K. Nature. 1974;247:220. doi: 10.1038/247220a0. [DOI] [PubMed] [Google Scholar]
- Zeremba C M, Morse D E, Mann S, Hansma P K. and Stucky G D. Chem. Mater. 1998;10:3813. doi: 10.1021/cm970785g. [DOI] [Google Scholar]
- Zhang X. and Vecchio K S. Mater. Sci. Eng. C. 2006;26:1445. doi: 10.1016/j.msec.2005.08.007. [DOI] [Google Scholar]
- Lemos A F, Rocha J H G, Quaresma S S F, Kannan S, Oktar F N, Agathopoulos S. and Ferreira J M F. J. Eur. Ceram. Soc. 2006;26:3639. doi: 10.1016/j.jeurceramsoc.2005.12.011. [DOI] [Google Scholar]
- Vecchio K S, Zhang X, Massie J B, Wang M. and Kim C W. Acta Biomater. 2007;3:910. doi: 10.1016/j.actbio.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Jinawath S, Polchai D. and Yoshimura M. Mater. Sci. Eng. C. 2002;22:35. doi: 10.1016/S0928-4931(02)00110-8. [DOI] [Google Scholar]
- Yoshimura M, Sujaridworakun P, Koh F, Fujiwara T, Pongkao D. and Ahniyaz A. Mater. Sci. Eng. C. 2004;24:521. doi: 10.1016/j.msec.2004.01.005. [DOI] [Google Scholar]
- Huang W, Rahaman M N, Day D E. and Miller B A. J. Mater. Sci. Mater. Med. 2009;20:123. doi: 10.1007/s10856-008-3554-7. [DOI] [PubMed] [Google Scholar]
- Fu H, Rahaman M N, Day D E. and Brown R F. J. Mater. Sci. Mater. Med. 2011;22:579. doi: 10.1007/s10856-011-4250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama T, Oshima A. and Yasue T. J. Ceram. Soc. Japan. 2001;109:232. doi: 10.2109/jcersj.109.1267_232. [DOI] [Google Scholar]
- Nagata F, Yokogawa Y, Toriyama M, Kawamoto Y, Suzuki T. and Nishizawa K. J. Ceram. Soc. Japan Int. Ed. 1995;103:69. [Google Scholar]
- Wei M, Ruys A J, Milthorpe B K. and Sorrell C C. J. Biomed. Mater. Res. 1999;45:11. doi: 10.1002/(SICI)1097-4636(199904)45:1%3C11::AID-JBM2%3E3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Pang Y X. and Bao X. J. Eur. Ceram. Soc. 2003;23:1697. doi: 10.1016/S0955-2219(02)00413-2. [DOI] [Google Scholar]
- Wang Y J, Lai C, Wei K. and Tang S Q. Mater. Lett. 2005;59:1098. doi: 10.1016/j.matlet.2004.12.016. [DOI] [Google Scholar]
- Loo S C, Siew Y E, Ho S, Boey F Y. and Ma J. J. Mater. Sci. Mater. Med. 2008;19:1389. doi: 10.1007/s10856-007-3261-9. [DOI] [PubMed] [Google Scholar]
- Pathi S P, Lin D D, Dorvee J R, Estroff L A. and Fischbach C. Biomaterials. 2011;32:5112. doi: 10.1016/j.biomaterials.2011.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrin P, Chaudhry A A, Khalid F, Darr J A. and Rehman I U. Chem. Commun. 2006;21:2286. doi: 10.1039/b518102j. [DOI] [PubMed] [Google Scholar]
- Chen M, Ma C Y, Mahmud T, Darr J A. and Wang X Z. J. Supercrit. Fluids. 2011;59:131. doi: 10.1016/j.supflu.2011.07.002. [DOI] [Google Scholar]
- Chaudhry A A. Acta Biomater. 2011;7:791. doi: 10.1016/j.actbio.2010.09.029. [DOI] [PubMed] [Google Scholar]
- Yan L, Li Y, Deng Z X, Zhuang J. and Sun X. Int. J. Inorg. Mater. 2001;3:633. doi: 10.1016/S1466-6049(01)00164-7. [DOI] [Google Scholar]
- Wang Y, Zhang S, Wei K, Zhao N, Chen J. and Wang X. Mater. Lett. 2006;60:1484. doi: 10.1016/j.matlet.2005.11.053. [DOI] [Google Scholar]
- Wang Y, Chen J, Wei K, Zhao S. and Wang X. Mater. Lett. 2006;60:3227. doi: 10.1016/j.matlet.2006.02.077. [DOI] [Google Scholar]
- Chen J D, Wang Y J, Wei K, Zhang S H. and Shi X T. Biomaterials. 2007;28:2275. doi: 10.1016/j.biomaterials.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Gao W, Li Q, Ruan C. and Chen Y. Key Eng. Mater. 2005;280–283:1533. doi: 10.4028/www.scientific.net/KEM.280-283.1533. [DOI] [Google Scholar]
- Zhang G, Chen J, Yang S, Yu Q, Wang Z. and Zhang Q. Mater. Lett. 2011;65:572. doi: 10.1016/j.matlet.2010.10.078. [DOI] [Google Scholar]
- Prakash Parthiban S, Elayaraja K, Girija E K, Yokogawa Y, Kesavamoorthy R, Palanichamy M, Asokan K. and Narayana Kalkura S. J. Mater. Sci. Mater. Med. 2009;20:S77. doi: 10.1007/s10856-008-3484-4. [DOI] [PubMed] [Google Scholar]
- Zhang C, Yang J, Quan Z, Yang P, Li C, Hou Z. and Lin J. Cryst. Growth Des. 2009;9:2725. doi: 10.1021/cg801353n. [DOI] [Google Scholar]
- Wu Y-J, Tsai T W T. and Chan J C C. Cryst. Growth Des. 2012;12:547. doi: 10.1021/cg201246m. [DOI] [Google Scholar]
- Nathanael A J, Hong S I, Mangalaraj D, Ponpandian N. and Chen P C. Cryst. Growth Des. 2012 DOI: 10.1021/cg3003959. [Google Scholar]
- Jiang S-D, Yao Q-Z, Zhou G-T. and Fu S-Q. J. Phys. Chem. C. 2012;116:4484. doi: 10.1021/jp211648x. [DOI] [Google Scholar]
- Ma M-G. and Zhu J-F. Eur. J. Inorg. Chem. 2009;2009:5522. doi: 10.1002/ejic.200801134. [DOI] [Google Scholar]
- Chen Z T. and Gao L. Cryst. Growth Des. 2008;8:460. doi: 10.1021/cg070277b. [DOI] [Google Scholar]
- Kinoshita M, Kishioka A, Hayashi H. and Itatani K. Gypsum Lime. 1989;219:79. [Google Scholar]
- Aizawa M, Howell F S, Itatani K, Yokogawa Y, Nishizawa K, Toriyama M. and Kameyama T. J. Ceram. Soc. Japan. 2000;108:249. doi: 10.2109/jcersj.108.1255_249. [DOI] [Google Scholar]
- Aizawa M, Ueno H, Itatani K. and Okada I. J. Eur. Ceram. Soc. 2006;26:501. doi: 10.1016/j.jeurceramsoc.2005.07.007. [DOI] [Google Scholar]
- Neira I S, Kolen'ko Y V, Lebedev O I, Tendeloo G V, Gupta H S, Guitián F. and Yoshimura M. Cryst. Growth. Des. 2009;9:466. doi: 10.1021/cg800738a. [DOI] [Google Scholar]
- Yasukawa A, Takase H, Kandori K. and Ishikawa T. Polyhedron. 1994;13:3071. doi: 10.1016/S0277-5387(00)83673-6. [DOI] [Google Scholar]
- Yasukawa A, Yokoyama T. and Ishikawa T. Mater. Res. Bull. 2001;36:775. doi: 10.1016/S0025-5408(01)00536-0. [DOI] [Google Scholar]
- Bayraktar D. and Tas A C. Turk. J. Med. Sci. 2000;30:235. [Google Scholar]
- Zhuang Z, Yamamoto H. and Aizawa M. Powder Technol. 2012;222:193. doi: 10.1016/j.powtec.2012.02.046. [DOI] [Google Scholar]
- Xu Y, Jiang J, Lu Y, Sun R-J, Song J, Ren L. and Yu S-H. Cryst. Growth Des. 2008;8:3822. doi: 10.1021/cg800485t. [DOI] [Google Scholar]
- Fujishiro Y, Fujimoto A, Sato T. and Okuwaki A. J. Colloid Interface Sci. 1995;173:119. doi: 10.1006/jcis.1995.1304. [DOI] [Google Scholar]
- Toriyama M, Kawamoto Y, Suzuki T, Yokogawa Y, Nishizawa K, Nagata F. and Mucalo M R. J. Mater. Sci. Lett. 1996;15:179. doi: 10.1007/BF00291461. [DOI] [PubMed] [Google Scholar]
- Füredi-Milhofer H, Brecevic L J, Oljica E, Purgaric B, Grass Z. and Perovic G. In: Particle Growth in Suspension. Smith A L, editor. London Academic; 1973. p. p 109. [Google Scholar]
- López-Macipe A, Gómez-Morales J. and Rodríguez-Clemente R. Adv. Matter. 1998;10:49. doi: 10.1002/(SICI)1521-4095(199801)10:1%3C49::AID-ADMA49%3E3.0.CO;2-R. [DOI] [Google Scholar]
- He Q, Huang Z, Liu Y, Chen W. and Xu T. Mater. Lett. 2006;61:141. doi: 10.1016/j.matlet.2006.04.082. [DOI] [Google Scholar]
- Cheng X, Huang Z, Li J, Liu Y, Chen C, Chi R-A. and Hu Y. Cryst. Growth Des. 2010;10:1180. doi: 10.1021/cg901088c. [DOI] [Google Scholar]
- Hench L L. and West J K. Chem. Rev. 1990;90:33. doi: 10.1021/cr00099a003. [DOI] [Google Scholar]
- Matijevic E. Langmuir. 1986;2:12. doi: 10.1021/la00067a002. [DOI] [Google Scholar]
- Masuda Y, Matubara K. and Sakka S. J. Ceram. Soc. Japan. 1990;98:1266. [Google Scholar]
- Deptula A, Lada W, Olezak T, Borello A, Avani C. and di Bartolomea A. J. Non-Cryst. Solids. 1992;147:537. doi: 10.1016/S0022-3093(05)80672-6. [DOI] [Google Scholar]
- Layrolle P, Ito A. and Takishi T. J. Am. Ceram. Soc. 1998;81:1421. doi: 10.1111/j.1151-2916.1998.tb02499.x. [DOI] [Google Scholar]
- Piveteau L D, Girona M I, Schlapbach L, Barboux P, Boilot J P. and Gasser B. J. Mater. Sci. Mater. Med. 1990;10:161. doi: 10.1023/A:1008985423644. [DOI] [PubMed] [Google Scholar]
- Brendel T, Engel A. and Russel C. J. Mater. Sci. Mater. Med. 1992;3:175. doi: 10.1007/BF00713445. [DOI] [Google Scholar]
- Gross K A, Chai C S, Kannangara G S K, Bin-Nissan B. and Hanley L. J. Mater. Sci. Mater. Med. 1998;9:839. doi: 10.1023/A:1008948228880. [DOI] [PubMed] [Google Scholar]
- Cihlar J. and Castkova K. Monatsh. Chem. 2002;133:761. doi: 10.1007/s007060200048. [DOI] [Google Scholar]
- Vijayalakshmi U. and Rajeswari S. J. Sol–Gel Sci. Technol. 2012;63:45. doi: 10.1007/s10971-012-2762-2. [DOI] [Google Scholar]
- Costa D O, Dixon S J. and Rizkalla A S. ACS Appl. Mater. Interfaces. 2012;4:1490. doi: 10.1021/am201735k. [DOI] [PubMed] [Google Scholar]
- Lim G K, Wang J, Ng S C. and Gan L M. Mater. Lett. 1996;28:431. doi: 10.1016/0167-577X(96)00095-X. [DOI] [Google Scholar]
- Lim G K, Wang J, Ng S C. and Gan L M. Langmuir. 1999;15:7472. doi: 10.1021/la981659+. [DOI] [Google Scholar]
- Sun Y, Guo G, Wang Z. and Guo H. Ceram. Int. 2006;32:951. doi: 10.1016/j.ceramint.2005.07.023. [DOI] [Google Scholar]
- Bose S. and Saha S K. Chem. Mater. 2003;15:4464. doi: 10.1021/cm0303437. [DOI] [Google Scholar]
- Lai C, Tang S Q, Wang Y J. and Wei K. Mater. Lett. 2005;59:210. doi: 10.1016/j.matlet.2004.08.037. [DOI] [Google Scholar]
- Sato K, Hotta Y, Nagaoka T, Yasuoka M. and Watari K. J. Mater. Sci. 2006;41:5424. doi: 10.1007/s10853-006-0258-6. [DOI] [Google Scholar]
- Li H, Zhu M Y, Li L H. and Zhou C R. J. Mater. Sci. 2008;43:384. doi: 10.1007/s10853-007-2182-9. [DOI] [Google Scholar]
- Koetz J, Baier J. and Kosmella S. Colloid Polym. Sci. 2007;285:1719. doi: 10.1007/s00396-007-1757-y. [DOI] [Google Scholar]
- Lai C, Tang S Q, Wang Y J, Wei K. and Zhang S Y. Synth. React. Inorg. Met. 2005;35:717. doi: 10.1080/15533170500302034. [DOI] [Google Scholar]
- Sonoda K, Furuzono T, Walsh D, Sato K. and Tanaka J. Solid State Ionics. 2002;151:321. doi: 10.1016/S0167-2738(02)00730-0. [DOI] [Google Scholar]
- Furuzono T, Walsh D, Sato K, Sonoda K. and Tanaka J. J. Mater. Sci. Lett. 2001;20:111. doi: 10.1023/A:1006725931450. [DOI] [Google Scholar]
- Sun Y, Guo G, Tao D. and Wang Z. J. Phys. Chem. Solids. 2007;68:373. doi: 10.1016/j.jpcs.2006.11.026. [DOI] [Google Scholar]
- Koumoulidis G C. and Katsoulds A P. J. Colloid Interface Sci. 2003;259:254. doi: 10.1016/S0021-9797(02)00233-3. [DOI] [PubMed] [Google Scholar]
- Coelho J M, Moreira J A, Almeida A. and Monteiro F J. J. Mater. Sci. Mater. Med. 2010;21:254. doi: 10.1007/s10856-010-4122-5. [DOI] [PubMed] [Google Scholar]
- Cao M, Wang Y, Guo C, Qi Y. and Hu C. Langmuir. 2004;20:4784. doi: 10.1021/la0498197. [DOI] [PubMed] [Google Scholar]
- Saha S K, Banerjee A, Banerjee S. and Bose S. Mater. Sci. Eng. C. 2009;29:2294. doi: 10.1016/j.msec.2009.05.019. [DOI] [Google Scholar]
- García C, García C. and Paucar C. Inorg. Chem. Commun. 2012;20:90. doi: 10.1016/j.inoche.2012.02.024. [DOI] [Google Scholar]
- Frenkel J. J. Phys. USSR. 1945;9:385. [Google Scholar]
- Kuczynski G C. Trans. AIME. 1949;185:169. [Google Scholar]
- Barralet J E, Best S M. and Bonfield W. J. Mater. Sci. Mater. Med. 2000;11:719. doi: 10.1023/A:1008975812793. [DOI] [PubMed] [Google Scholar]
- Landi E, Tampieri A, Celotti G. and Sprio S. J. Eur. Ceram. Soc. 2000;20:2377. doi: 10.1016/S0955-2219(00)00154-0. [DOI] [Google Scholar]
- Bernache-Assollant D, Ababoua A, Championa E. and Heughebaertb M. J. Eur. Ceram. Soc. 2003;23:229. doi: 10.1016/S0955-2219(02)00186-3. [DOI] [Google Scholar]
- Okada M. and Furuzono T. J. Mater. Sci. 2006;41:6134. doi: 10.1007/s10853-006-0444-6. [DOI] [Google Scholar]
- Okada M. and Furuzono T. J. Nanosci. Nanotechnol. 2007;7:848. doi: 10.1166/jnn.2007.505. [DOI] [PubMed] [Google Scholar]
- Okada M. and Furuzono T. J. Nanopart. Res. 2007;9:807. doi: 10.1007/s11051-006-9126-1. [DOI] [Google Scholar]
- Gorbunoff M J. and Timasheff S N. Anal. Biochem. 1984;136:440. doi: 10.1016/0003-2697(84)90241-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Okazaki M, Inoue M, Yamaguchi S, Kusunose T, Toyonaga T, Hamada Y. and Takahashi J. Biomaterials. 2004;25:3807. doi: 10.1016/j.biomaterials.2003.10.081. [DOI] [PubMed] [Google Scholar]
- Kano S, Yamazaki A, Otsuka R, Ohgaki M, Akao M. and Aoki H. Biomed. Mater. Eng. 1994;4:283. [PubMed] [Google Scholar]
- Roy M E. and Nishimoto S K. Bone. 2002;31:296. doi: 10.1016/S8756-3282(02)00821-9. [DOI] [PubMed] [Google Scholar]
- Sundar M, Babu N R, Victor S P, Kumar K R. and Kumar T S S. Trends Biomater. Artif. Organs. 2005;18:213. [Google Scholar]
- Devanand Venkatasubbu G, Ramasamy S, Ramakrishnan V. and Kumar J. 3 Biotech. 2011;1:173. doi: 10.1007/s13205-011-0021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uskoković V. and Desai T A. J. Biomed. Mater. Res. A. 2012 DOI: 2012:00A:000–000. [Google Scholar]
- Barroug A. and Glimcher M J. J. Orthop. Res. 2002;20:274. doi: 10.1016/S0736-0266(01)00105-X. [DOI] [PubMed] [Google Scholar]
- Dorozhkin S V. and Epple M. Angew. Chem. Int. Ed. 2002;41:3130. doi: 10.1002/1521-3773(20020902)41:17%3C3130::AID-ANIE3130%3E3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Paul W. and Sharma C P. J. Mater. Sci. Mater. Med. 1999;10:383. doi: 10.1023/A:1008918412198. [DOI] [PubMed] [Google Scholar]
- Ikoma T, Tagaya M, Hanagata N, Yoshioka T, Chakarov D, Kasemo B. and Tanaka J. J. Am. Ceram. Soc. 2009;92:1125. doi: 10.1111/j.1551-2916.2009.02957.x. [DOI] [Google Scholar]
- Iafisco M, Palazzo B, Falini G, Foggia M D, Bonora S, Nicolis S, Casella L. and Roveri N. Langmuir. 2008;24:4924. doi: 10.1021/la703381h. [DOI] [PubMed] [Google Scholar]
- Yongli C, Xiufang Z, Yandao G, Nanming Z, Tingying Z. and Xinqi S. J. Colloid Interface Sci. 1999;214:38. doi: 10.1006/jcis.1999.6159. [DOI] [PubMed] [Google Scholar]
- Monkawa A, Ikoma T, Yunoki S, Yoshioka T, Tanaka J, Chakarov D. and Kasemo B. Biomaterials. 2006;27:5748. doi: 10.1016/j.biomaterials.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Capriotti L A, Beebe T P., Jr and Schneider J P. J. Am. Chem. Soc. 2007;129:5281. doi: 10.1021/ja070356b. [DOI] [PubMed] [Google Scholar]
- Goobes G, Goobes R, Schueler-Furman O, Baker D, Stayton P S. and Drobny G P. Proc. Natl Acad. Sci. 2006;103:16083. doi: 10.1073/pnas.0607193103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goobes G. Magn. Reson. Chem. 2007;45:s32. doi: 10.1002/mrc.2123. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A, Dasgupta A K, Chattopadhyay D. and Chakrabarti K. Bioresour. Technol. 2012;16:348. doi: 10.1016/j.biortech.2012.03.094. [DOI] [PubMed] [Google Scholar]
- Rimola A, Sakhno Y, Bertinetti L, Lelli M, Martra G. and Ugliengo P. J. Phys. Chem. Lett. 2011;2:1390. doi: 10.1021/jz200457x. [DOI] [Google Scholar]
- Jimenez-Izal E, Chiatti F, Corno M, Rimola A. and Ugliengo P. Langmuir. 2012;116:14561. [Google Scholar]
- Rimola A, Aschi M, Orlando R. and Ugliengo P. J. Am. Chem. Soc. 2012;134:10899. doi: 10.1021/ja302262y. [DOI] [PubMed] [Google Scholar]
- Graham F L. and Van Der Eb A J. Virology. 1973;52:456. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Chen C. and Okayama H. Mol. Cell. Biol. 1987;7:2745. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y W. and Yang J C. Biomaterials. 1997;18:213. doi: 10.1016/S0142-9612(96)00120-2. [DOI] [PubMed] [Google Scholar]
- Chowdhury E H, Sasagawa T, Nagaoka M, Kundu A K. and Akaike T. Anal. Biochem. 2003;314:316. doi: 10.1016/S0003-2697(02)00648-6. [DOI] [PubMed] [Google Scholar]
- Jiang M. and Chen G. Nature Protoc. 2006;1:695. doi: 10.1038/nprot.2006.86. [DOI] [PubMed] [Google Scholar]
- Kumta P N, Sfeir C, Lee D H, Olton D. and Choi D. Acta Biomater. 2005;1:65. doi: 10.1016/j.actbio.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Kimura T, Nibe Y, Funamoto S, Okada M, Furuzono T, Ono T, Yoshizawa H, Fujisato T, Nam K. and Kishida A. J. Drug Deliv. 2011;2011:064103. doi: 10.1155/2011/962743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q, Wang Y E, Zhao X, Loo J S. and Zuo Y Y. ACS Nano. 2011;5:6410. doi: 10.1021/nn2015997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labella R, Braden M. and Deb S. Biomaterials. 1994;15:1197. doi: 10.1016/0142-9612(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Domingo C, Arcís R W, Osorio E, Toledano M. and Saurina J. Analyst. 2000;125:2044. doi: 10.1039/b004662k. [DOI] [PubMed] [Google Scholar]
- Arcís R W, López-Macipe A, Toledano M, Osorio E, Rodríguez-Clemente R, Murtra J, Fanovich M A. and Pascual C D. Dent. Mater. 2002;18:49. doi: 10.1016/S0109-5641(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Arita K, Lucas M E. and Nishino M. Dent. Mater. J. 2003;22:126. doi: 10.4012/dmj.22.126. [DOI] [PubMed] [Google Scholar]
- Robinson C, Connell S, Kirkham J, Shorea R. and Smith A. J. Mater. Chem. 2004;14:2242. doi: 10.1039/b401154f. [DOI] [Google Scholar]
- Tang R, Wang L, Orme C A, Bonstein T, Bush P J. and Nancollas G H. Angew. Chem., Int. Ed. 2004;43:2697. doi: 10.1002/anie.200353652. [DOI] [PubMed] [Google Scholar]
- Li L, Pan H H, Tao J H, Xu X R, Mao C Y, Gu X H. and Tang R K. J. Mater. Chem. 2008;18:4079. doi: 10.1039/b806090h. [DOI] [Google Scholar]
- Huang S B, Gao S S. and Yu H Y. Biomed. Mater. 2009;4:064103. doi: 10.1088/1748-6041/4/3/034104. [DOI] [Google Scholar]
- Kim Y S, Kwon H K. and Kim B I. J. Dent. 2011;39:636. doi: 10.1016/j.jdent.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Ramsden W. Proc. R. Soc. Lond. 1903;72:156. doi: 10.1098/rspl.1903.0034. [DOI] [Google Scholar]
- Pickering S U. J. Chem. Soc. 1907;91:2001. doi: 10.1039/ct9079102001. [DOI] [Google Scholar]
- Binks B P. and Lumsdon S O. Phys. Chem. Chem. Phys. 1999;1:3007. doi: 10.1039/a902209k. [DOI] [Google Scholar]
- Binks B P. and Lumsdon S O. Langmuir. 2000;16:8622. doi: 10.1021/la000189s. [DOI] [Google Scholar]
- Yan N, Gray M R. and Masliyah J H. Colloids Surf. A. 2001;193:97. doi: 10.1016/S0927-7757(01)00748-8. [DOI] [Google Scholar]
- Hong L, Jiang S. and Granick S. Langmuir. 2006;22:9495. doi: 10.1021/la062716z. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang X, Wu D, Liu L. and Zhao H. Chem. Mater. 2009;21:4012. doi: 10.1021/cm901437n. [DOI] [Google Scholar]
- Choi Y S, Xu M. and Chung I J. Polymer. 2005;46:531. doi: 10.1016/j.polymer.2004.09.036. [DOI] [Google Scholar]
- Hirsemann D, Shylesh S, De Souza R A, Diar-Bakerly B, Biersack B, Mueller D N, Martin M, Schobert R. and Breu J. Angew. Chem. Int. Ed. 2012;51:1348. doi: 10.1002/anie.201106710. [DOI] [PubMed] [Google Scholar]
- Golomb D, Barry E, Ryan D, Swett P. and Duan H. Ind. Eng. Chem. Res. 2006;45:2728. doi: 10.1021/ie051085l. [DOI] [Google Scholar]
- Zaragoza-Contreras E A, Hernández-Escobar C A, Navarrete-Fontes A. and Flores-Gallardo S G. Micron. 2011;42:263. doi: 10.1016/j.micron.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Velev O D, Furusawa K. and Nagayama K. Langmuir. 1996;12:2374. doi: 10.1021/la9506786. [DOI] [Google Scholar]
- Dinsmore A D, Hsu M F, Nikolaides M G, Marquez M, Bausch A R. and Weitz D A. Science. 2002;298:1006. doi: 10.1126/science.1074868. [DOI] [PubMed] [Google Scholar]
- Wu C-H, Chiu W-Y. and Don T-M. Polymer. 2012;53:1086. doi: 10.1016/j.polymer.2012.01.005. [DOI] [Google Scholar]
- Fujii S, Read E S, Armes S P. and Binks B P. Adv. Mater. 2005;17:1014. doi: 10.1002/adma.200401641. [DOI] [Google Scholar]
- Destribats M, Lapeyre V, Sellier E, Leal-Calderon F, Ravaine V. and Schmitt V. Langmuir. 2012;28:3744. doi: 10.1021/la2043763. [DOI] [PubMed] [Google Scholar]
- Binks B P. and Fletcher P D I. Langmuir. 2001;17:4708. doi: 10.1021/la0103315. [DOI] [Google Scholar]
- Nonomura Y, Komura S. and Tsujii K. Langmuir. 2004;20:11821. doi: 10.1021/la0480540. [DOI] [PubMed] [Google Scholar]
- Ruhland T M, Gröschel A H, Walther A. and Müller A H E. Langmuir. 2011;27:9807. doi: 10.1021/la201863x. [DOI] [PubMed] [Google Scholar]
- Aveyard R. Soft Matter. 2012;8:5233. doi: 10.1039/c2sm07230k. [DOI] [Google Scholar]
- Hohenstein W P. Polym. Bull. 1945;1:13. [Google Scholar]
- Hohenstein W P. and Mark H. J. Polym. Sci. 1946;1:127. doi: 10.1002/pol.1946.120010207. [DOI] [Google Scholar]
- Gao Q, Wang C, Liu H, Wang C, Liu X. and Tong Z. Polymer. 2009;50:2587. doi: 10.1016/j.polymer.2009.03.049. [DOI] [Google Scholar]
- Duan L, Chen M, Zhou S. and Wu L. Langmuir. 2009;25:3467. doi: 10.1021/la8041617. [DOI] [PubMed] [Google Scholar]
- Tiarks F, Landfester K. and Antonietti M. Langmuir. 2001;17:5775. doi: 10.1021/la010445g. [DOI] [Google Scholar]
- Bon S A F. and Colver P J. Langmuir. 2007;23:8316. doi: 10.1021/la701150q. [DOI] [PubMed] [Google Scholar]
- Cao Z, Schrade A, Landfester K. and Ziener U. J. Polym. Sci. A. 2011;49:2382. doi: 10.1002/pola.24668. [DOI] [Google Scholar]
- Fujii S, Okada M. and Furuzono T. J. Colloid Int. Sci. 2007;315:287. doi: 10.1016/j.jcis.2007.06.071. [DOI] [PubMed] [Google Scholar]
- Fujii S, Okada M, Sawa H, Furuzono T. and Nakamura Y. Langmuir. 2009;25:9759. doi: 10.1021/la901100z. [DOI] [PubMed] [Google Scholar]
- Okada M, Maeda H, Fujii S, Nakamura Y. and Furuzono Y. Langmuir. 2012;28:9405. doi: 10.1021/la3015964. [DOI] [PubMed] [Google Scholar]
- Heimann R B. Plasma-Spray Coating: Principles and Applications. Cambridge VCH; 1996. [Google Scholar]
- Ciccotti M G, Rothman R H, Hozack W J. and Moriarty L. J. Arthroplasty. 1994;9:631. doi: 10.1016/0883-5403(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Gledhill H C, Turner I G. and Doyle C. Biomaterials. 1999;20:315. doi: 10.1016/S0142-9612(98)00166-5. [DOI] [PubMed] [Google Scholar]
- Gledhill H C, Turner I G. and Doyle C. Biomaterials. 2001;22:1233. doi: 10.1016/S0142-9612(00)00273-8. [DOI] [PubMed] [Google Scholar]
- Reichelt K. and Jiang X. Thin Solid Films. 1990;191:91. doi: 10.1016/0040-6090(90)90277-K. [DOI] [Google Scholar]
- Burt R J, Meyer S F. and Hsieh E J. J. Vac. Sci. Technol. 1979;17:407. doi: 10.1116/1.570467. [DOI] [Google Scholar]
- Kim K H, Park K C. and Ma D Y. J. Appl. Phys. 1997;81:7764. doi: 10.1063/1.365556. [DOI] [Google Scholar]
- van Dijk K, Schaeken H G, Wolke J G C. and Jansen J A. Biomaterials. 1996;17:405. doi: 10.1016/0142-9612(96)89656-6. [DOI] [PubMed] [Google Scholar]
- Cotell C M, Chrisey D B, Grabowski K S. and Spregue J A. J. Appl. Biomater. 1992;3:87. doi: 10.1002/jab.770030204. [DOI] [Google Scholar]
- Singh R K, Qian F, Nagabushnam V, Damodaran R. and Moudgil B M. Biomaterials. 1994;15:522. doi: 10.1016/0142-9612(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Koch C F, Johnson S, Kumar D, Jelinek M, Chrisey D B, Doraiswamy A, Jine C, Narayan R J. and Mihailescu I N. Mater. Sci. Eng. C. 2007;27:484. doi: 10.1016/j.msec.2006.05.025. [DOI] [Google Scholar]
- KenKnight C E. and Wehner G K. J. Appl. Phys. 1964;35:322. doi: 10.1063/1.1713308. [DOI] [Google Scholar]
- Ong J L, Lucas L C, Lacefield W R. and Rigney E D. Biomaterials. 1992;13:249. doi: 10.1016/0142-9612(92)90192-Q. [DOI] [PubMed] [Google Scholar]
- Chen T S. and Lacefield W R. J. Mater. Res. 1994;9:1284. doi: 10.1557/JMR.1994.1284. [DOI] [Google Scholar]
- Martin P J, Macleod H A, Netterfield R P, Pacey C G. and Sainty W G. Appl. Opt. 1983;22:178. doi: 10.1364/AO.22.000178. [DOI] [PubMed] [Google Scholar]
- Choi J-M, Kim H-E. and Lee I-S. Biomaterials. 2000;21:469. doi: 10.1016/S0142-9612(99)00186-6. [DOI] [PubMed] [Google Scholar]
- Yoshinari M, Ohtsuka Y. and Derand T. Biomaterials. 1994;15:529. doi: 10.1016/0142-9612(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Hontsu S, Matsumoto T, Ishii J, Nakamori M, Tabata H. and Kawai T. Thin Solid Films. 1997;295:214. doi: 10.1016/S0040-6090(96)09146-8. [DOI] [Google Scholar]
- Hontsu S, Yoshikawa K, Kato N, Kawakami Y, Hayami T, Nishikawa H, Kusunoki M. and Yamamoto K. J. Aust. Ceram. Soc. 2011;47:11. [Google Scholar]
- Bryant W A. J. Mater. Sci. 1977;12:1285. doi: 10.1007/BF00540843. [DOI] [Google Scholar]
- Jones A C. and Chalker P R. J. Phys. D: Appl. Phys. 2003;36:R80. doi: 10.1088/0022-3727/36/6/202. [DOI] [Google Scholar]
- Allen G C, Ciliberto E, Fragalà I. and Spoto G. Nucl. Instrum. Methods Phys. Res. B. 1996;116:457. doi: 10.1016/0168-583X(96)00088-2. [DOI] [Google Scholar]
- Darr J A, Guo Z X, Raman V, Bououdina M. and Rehman I U. Chem. Commun. 2004;696 doi: 10.1039/b312855p. [DOI] [PubMed] [Google Scholar]
- Putkonen M, Sajavaara T, Rahkila P, Xu L, Cheng S, Niinistö L. and Whitlow H J. Thin Solid Films. 2009;517:5819. doi: 10.1016/j.tsf.2009.03.013. [DOI] [Google Scholar]
- Sato M, Tu R, Goto T, Ueda K. and Narushima T. Mater. Sci. Forum. 2010;631–632:193. doi: 10.4028/www.scientific.net/MSF.631-632.193. [DOI] [Google Scholar]
- Cabañas M V. and Vallet-Regí M. J. Mater. Chem. 2003;13:1104. doi: 10.1039/b301435e. [DOI] [Google Scholar]
- Leeuwenburgh S, Wolke J, Schoonman J. and Jansen J. J. Biomed. Mater. Res. A. 2003;66:330. doi: 10.1002/jbm.a.10590. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh S, Wolke J, Schoonman J. and Jansen J. J. Biomed. Mater. Res. A. 2005;74:275. doi: 10.1002/jbm.a.30420. [DOI] [PubMed] [Google Scholar]
- Thian E S, Li X, Huang J, Edirisinghe M J, Bonfield W. and Best S M. Thin Solid Films. 2011;519:2328. doi: 10.1016/j.tsf.2010.11.035. [DOI] [Google Scholar]
- Tan F, Naciri M, Dowling D. and Al-Rubeai M. Biotechnol. Adv. 2012;30:352. doi: 10.1016/j.biotechadv.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Keady F. and Murphy B P. J. Mater. Sci. Mater. Med. 2012 doi: 10.1007/s10856-012-4775-3. DOI: 10.1007/s10856-012-4775-3. [DOI] [PubMed] [Google Scholar]
- Ohtsuki C, Kamitakahara M. and Miyazaki T. J. Tissue Eng. Regen. Med. 2007;1:33. doi: 10.1002/term.3. [DOI] [PubMed] [Google Scholar]
- Kamitakahara M, Ohtsuki C. and Miyazaki T. Biomed. Mater. 2007;2:R17. doi: 10.1088/1748-6041/2/4/R01. [DOI] [PubMed] [Google Scholar]
- Nagata F, Miyajima T. and Yokogawa Y. J. Eur. Ceram. Soc. 2006;26:533. doi: 10.1016/j.jeurceramsoc.2005.06.006. [DOI] [Google Scholar]
- Oyane A, Uchida M. and Ito A. J. Biomed. Mater. Res. A. 2005;72:168. doi: 10.1002/jbm.a.30205. [DOI] [PubMed] [Google Scholar]
- Leonor I B, Azevedo H S, Alves C M. and Reis R L. Key Eng. Mater. 2003;240–242:97. doi: 10.4028/www.scientific.net/KEM.240-242.97. [DOI] [Google Scholar]
- Barrere F, van Blitterswijk C A, de Groot K. and Layrolle P. Biomaterials. 2002;23:2211. doi: 10.1016/S0142-9612(01)00354-4. [DOI] [PubMed] [Google Scholar]
- Oyane A, Sakamaki I, Shimizu Y, Kawaguchi K. and Koshizaki N. J. Biomed. Mater. Res. A. 2012;100:2573. doi: 10.1002/jbm.a.34192. [DOI] [PubMed] [Google Scholar]
- Oyane A, Sakamaki I, Shimizu Y, Kawaguchi K, Sogo Y, Ito A. and Koshizaki N. Key Eng. Mater. 2013;529–530:217. doi: 10.4028/www.scientific.net/KEM.529-530.217. [DOI] [Google Scholar]
- Taguchi T, Kishida A. and Akashi M. Chem. Lett. 1998;27:711. doi: 10.1246/cl.1998.711. [DOI] [Google Scholar]
- Taguchi T, Muraoka Y, Matsuyama H, Kishida A. and Akashi M. Biomaterials. 2001;22:53. doi: 10.1016/S0142-9612(00)00162-9. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Oyane A. and Ito A. J. Biomed. Mater. Res. B. 2008;86:341. doi: 10.1002/jbm.b.31025. [DOI] [PubMed] [Google Scholar]
- Fujishiro Y, Sato T. and Okuwaki A. J. Mater. Sci., Mater. Med. 1995;6:172. doi: 10.1007/BF00120295. [DOI] [PubMed] [Google Scholar]
- Liu D X, Savino K. and Yates M Z. Adv. Funct. Mater. 2009;19:3941. doi: 10.1002/adfm.200900318. [DOI] [Google Scholar]
- Wei X, Fu C, Savino K. and Yates M Z. Cryst. Growth Des. 2012;12:217. doi: 10.1021/cg200943s. [DOI] [Google Scholar]
- Ziani-Cherif H, Abe Y, Imachi K. and Matsuda T. J. Biomed. Mater. Res. 2002;59:390. doi: 10.1002/jbm.10002. [DOI] [PubMed] [Google Scholar]
- Kuroda K, Ichino R, Okido M. and Takai O. J. Biomed. Mater. Res. 2002;61:354. doi: 10.1002/jbm.10197. [DOI] [PubMed] [Google Scholar]
- Kuroda K. and Okido M. Bioinorg. Chem. Appl. 2012;2012:064103. doi: 10.1155/2012/730693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K, Moriyama M, Ichino R, Okido M. and Seki A. Mater. Trans. 2009;50:1190. doi: 10.2320/matertrans.MRA2008459. [DOI] [Google Scholar]
- Ishizawa H. and Ogino M. J. Mater. Sci. 1996;31:6279. doi: 10.1007/BF00354450. [DOI] [Google Scholar]
- Okido M, Nishikawa K, Kuroda K, Ichino R, Zhao Z. and Takai O. Mater. Trans. 43:3010. doi: 10.2320/matertrans.43.3010. [DOI] [Google Scholar]
- Zhitomirsky L. and Gal-Or L. J. Mater. Sci. Mater. Med. 1997;8:213. doi: 10.1023/A:1018587623231. [DOI] [PubMed] [Google Scholar]
- Nie X, Leyland A. and Matthews A. Surf. Coat. Technol. 2000;125:407. doi: 10.1016/S0257-8972(99)00612-X. [DOI] [Google Scholar]
- Agata De Sena L, Calixto De Andrade M, Malta Rossi A. and de Almeida Soares G. J. Biomed. Mater. Res. 2002;60:1. doi: 10.1002/jbm.10003. [DOI] [PubMed] [Google Scholar]
- Stoch A, Brozek A, Kmita G, Stoch J, Jastrzebski W. and Rakowska A. J. Mol. Struct. 2001;596:191. doi: 10.1016/S0022-2860(01)00716-5. [DOI] [Google Scholar]
- Xiao X F. and Liu R F. Mater. Lett. 2006;60:2627. doi: 10.1016/j.matlet.2006.01.048. [DOI] [Google Scholar]
- Wang C, Ma J, Cheng W. and Zhang R. Mater. Lett. 2002;57:99. doi: 10.1016/S0167-577X(02)00706-1. [DOI] [Google Scholar]
- Monkawa A, Ikoma T, Yunoki S, Yoshioka T, Tanaka J, Chakarov D. and Kasemo B. Biomaterials. 2006;27:5748. doi: 10.1016/j.biomaterials.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Bai Y, Park I S, Park H H, Bae T S. and Lee M H. J. Biomed. Mater. Res. B. 2010;95:365. doi: 10.1002/jbm.b.31724. [DOI] [PubMed] [Google Scholar]
- Furuzono T, Kishida A. and Tanaka J. J. Mater. Sci. Mater. Med. 2004;15:19. doi: 10.1023/B:JMSM.0000010093.39298.5a. [DOI] [PubMed] [Google Scholar]
- Korematsu A, Furuzono T, Yasuda S, Tanaka J. and Kishida A. J. Mater. Sci. 2004;39:3221. doi: 10.1023/B:JMSC.0000025865.44900.74. [DOI] [Google Scholar]
- Korematsu A, Furuzono T, Yasuda S, Tanaka J. and Kishida A. J. Mater. Sci., Mater. Med. 2005;16:67. doi: 10.1007/s10856-005-6448-y. [DOI] [PubMed] [Google Scholar]
- Furuzono T, Yasuda S, Kimura T, Kyotani S, Tanaka J. and Kishida A. J. Artif. Organs. 2004;7:137. doi: 10.1007/s10047-004-0264-x. [DOI] [PubMed] [Google Scholar]
- Furuzno T, Masuda M, Okada M, Yasuda S, Kadono H, Tanaka R. and Miyatake K. ASAIO J. 2006;52:315. doi: 10.1097/01.mat.0000214860.08820.f9. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Hirose M, Kotobuki N, Ohguchi H, Furuzono T. and Sato J. Mater. Sci. Eng. C. 2007;27:817. doi: 10.1016/j.msec.2006.09.019. [DOI] [Google Scholar]
- Okada M, Masuda M, Tanaka R, Miyatake K, Kuroda D. and Furuzono T. J. Biomed. Mater. Res. A. 2008;86:589. doi: 10.1002/jbm.a.31668. [DOI] [PubMed] [Google Scholar]
- Yanagida H, Okada M, Masuda M, Ueki M, Narama I, Kitao S, Koyama Y, Takakuda K. and Furuzono T. J. Biosci. Bioeng. 2009;108:235. doi: 10.1016/j.jbiosc.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Okada M, Furukawa K, Serizawa T, Yanagisawa Y, Tanaka H, Kawai T. and Furuzono T. Langmuir. 2009;25:6300. doi: 10.1021/la804274q. [DOI] [PubMed] [Google Scholar]
- Yanagida H, Okada M, Masuda M, Narama I, Nakano S, Kitao S, Takakuda K. and Furuzono T. J. Artif. Organs. 2011;14:331. doi: 10.1007/s10047-011-0594-4. [DOI] [PubMed] [Google Scholar]
- Furuzono T, Ueki M, Kitamura H, Oka K. and Imai E. J. Biomed. Mater. Res. B. 2009;89:77. doi: 10.1002/jbm.b.31189. [DOI] [PubMed] [Google Scholar]
- Kadono H, Furuzono T, Masuda M, Okada M, Ueki M, Takamizawa K, Tanaka R, Miyatake K, Koyama Y. and Takakuda K. ASAIO J. 2010;56:61. doi: 10.1097/MAT.0b013e3181c945ae. [DOI] [PubMed] [Google Scholar]
- Sonoda H, Urayama S, Takamizawa K, Nakayama Y, Uyama C, Yasui H. and Matsuda T. J. Biomed. Mater. Res. 2002;60:191. doi: 10.1002/jbm.10055. [DOI] [PubMed] [Google Scholar]
- Maeda H, Okada M, Fujii S, Nakamura Y. and Furuzono T. Langmuir. 2010;26:13727. doi: 10.1021/la102529d. [DOI] [PubMed] [Google Scholar]
- Fujii S, Okada M, Nishimura T, Maeda H, Sugimoto T, Hamasaki H, Furuzono T. and Nakamura Y. J. Colloid Interface Sci. 2012;374:1. doi: 10.1016/j.jcis.2012.01.058. [DOI] [PubMed] [Google Scholar]
- Liu X, Okada M, Maeda H, Fujii S. and Furuzono T. Acta Biomater. 2011;7:821. doi: 10.1016/j.actbio.2010.08.023. [DOI] [PubMed] [Google Scholar]
- Okada M, Fujii S, Nishimura T, Nakamura Y, Takeda S. and Furuzono T. Appl. Surf. Sci. 2012 at press (DOI: 10.1016/j.apsusc.2012.01.016) [Google Scholar]
- Duchyene P, van Raemdonk W, Heughbaert J C. and Heughbaert M. Biomaterials. 1986;7:97. doi: 10.1016/0142-9612(86)90063-3. [DOI] [PubMed] [Google Scholar]
- Thomas K A, Kay J F, Cook S D. and Jarcho M. J. Biomed. Mater. Res. 1987;21:1395. doi: 10.1002/jbm.820211205. [DOI] [PubMed] [Google Scholar]
- Costa N. and Maquis P M. Med. Eng. Phys. 1998;20:602. doi: 10.1016/S1350-4533(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Dupraz A M, de Wijn J R, van der Meer S A. and de Groot K. J. Biomed. Mater. Res. 1996;30:231. doi: 10.1002/(SICI)1097-4636(199602)30:2%3C231::AID-JBM13%3E3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Kiu Q, de Wijn J R, de Groot K. and van Blitterswijk C A. Biomaterials. 1998;19:1067. doi: 10.1016/S0142-9612(98)00033-7. [DOI] [PubMed] [Google Scholar]
- Mima Y, Fukumoto S, Koyama H, Okada M, Tanaka S, Shoji T, Emoto M, Furuzono T, Nishizawa Y. and Inaba M. PLoS One. 2012;7:e35199. doi: 10.1371/journal.pone.0035199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T-D. and Do T-O. In: Nanocrystal. Masuda Y, editor. Rijeka InTech Open Access Books; 2011. p. p 55. [Google Scholar]
- Pan D, Wang Q. and An L. J. Mater. Chem. 2009;19:1063. doi: 10.1039/b810972a. [DOI] [Google Scholar]


