Abstract
In skin tissue engineering, a three-dimensional porous scaffold is necessary to support cell adhesion and proliferation and to guide cells moving into the repair area in the wound healing process. Structurally, the porous scaffold should have an open and interconnected porous architecture to facilitate homogenous cell distribution. Moreover, the scaffolds should be mechanically strong to protect deformation during the formation of new skin. In this study, the hybrid scaffolds were prepared by forming funnel-like collagen or gelatin sponge on a woven poly(l-lactic acid) (PLLA) mesh. The hybrid scaffolds combined the advantages of both collagen or gelatin (good cell-interactions) and PLLA mesh (high mechanical strength). The hybrid scaffolds were used to culture dermal fibroblasts for dermal tissue engineering. The funnel-like porous structure promoted homogeneous cell distribution and extracellular matrix production. The PLLA mesh reinforced the scaffold to avoid deformation. Subcutaneous implantation showed that the PLLA–collagen and PLLA–gelatin scaffolds promoted the regeneration of dermal tissue and epidermis and reduced contraction during the formation of new tissue. These results indicate that funnel-like hybrid scaffolds can be used for skin tissue regeneration.
Keywords: funnel-like pore structure, hybrid porous scaffold, skin tissue engineering
1. Introduction
Patients with serious skin injuries such as burns or chronic wounds require a prompt closure of full-thickness wounds, which is critical for survival [1]. Limited donor sites, donor site morbidity and the reduced surgical procedures are the major impetus for the development of skin substitute. Skin tissue engineering by using cultured fibroblasts and keratinocyte to treat skin defects has been developed as a challenging method for wound healing [2, 3]. In this approach, a temporary scaffold serves as support for culture of fibroblasts and keratinocytes to construct three-dimensional (3D) skin architecture [4].
Various types of scaffolds have been developed for research and clinical applications of skin tissue engineering [5]. In both clinical and preclinical models, collagen and gelatin are the most commonly used scaffolding materials owing to their advantageous properties, including low antigenicity and promotion of cell adhesion and proliferation [4]. However, the problem that compromises the effect of collagen- and gelatin-based scaffolds is low mechanical strength. To maintain the scaffold structure and shape after seeding the cells and during implantation, the scaffolds should be mechanically strong, because the dermis needs to provide physical support and flexibility to the skin. Biodegradable synthetic polymers such as poly(l-lactic acid) (PLLA), poly(glycolic acid) (PGA), poly(dl-lactic-co-glycolic acid) (PLGA) and poly (ε-caprolactone) (PCL) have also been used to prepare porous scaffolds for skin tissue engineering [6, 7]. The scaffolds prepared from biodegradable synthetic polymers have favorable mechanical properties. Owing to the respective advantages of naturally derived collagen or gelatin and biodegradable synthetic polymers, hybridization of these two types of biodegradable polymers has been reported to combine their advantages. Hybrid scaffolds composed of collagen and synthetic polymer retain the advantages of both biodegradable synthetic polymers and collagen with high mechanical strength and good cell-interactions [8, 9]. The ideal scaffold also should have an open and interconnected porous architecture, which helps cells easily penetrate into the inner part of the scaffold and results in homogenous cell distribution and tissue formation. We have developed a method using embossing ice particulate templates to prepare funnel-like porous scaffolds that have open surface pores and interconnected pore structures [10, 11]. The funnel-like scaffolds facilitate cell penetration into the inner parts of the scaffolds and spatially homogeneous cell distribution.
In this study, a PLLA woven mesh was hybridized with funnel-like collagen or gelatin sponges to construct PLLA–collagen or PLLA–gelatin hybrid scaffold to combine the advantages of PLLA mesh, collagen (gelatin) sponge and funnel-like structure for skin tissue engineering. The hybrid scaffold was used for in vitro dermal fibroblast culture and in vivo wound healing assessment.
2. Materials and methods
2.1. Fabrication of funnel-like hybrid scaffold
2.1.1. Fabrication of funnel-like PLLA–collagen hybrid scaffold.
The funnel-like PLLA–collagen hybrid scaffolds were prepared by forming funnel-like collagen sponges on one side of a PLLA woven mesh (figure 1(a)). The PLLA mesh was woven with the warp and weft yarns (120 denier, 30 filaments). The densities of warp and weft PLLA yarns were 42 and 40 counts per inch, respectively. The opening in the PLLA mesh was 430 μm in warp direction and 450 μm in weft direction (figures 1(b) and (c)). The thickness of PLLA mesh was 160 μm.
Figure 1.
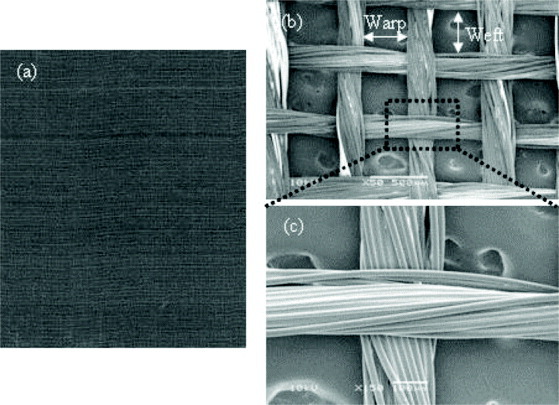
PLLA woven mesh: (a) appearance and (b,c) SEM images of the warp and weft yarns (scale bars = 500 and 100 μm, respectively).
Embossing ice particulates were used as templates to prepare funnel-like collagen sponges using the previously reported method [10, 12, 13]. First, micron-sized hemispheric water droplets were formed by application of moisture to a perfluoroalkoxy (PFA) film-wrapped copper plate in a plastic chamber using an ultrasonic humidifier. The PFA-wrapped copper plate was attached with a 1 mm-thick silicone frame to control the thickness of the loaded solution, and then placed in the moisture chamber for 11 min to form water droplets. The water droplets formed on the PFA-wrapped copper plate were frozen in a freezer at −30 °C for 5 h to prepare the embossing ice particulates. The frozen ice particulate templates were stabilized at −3 °C in a low-temperature chamber (WT-201, ESPEC Corp.) for 1 h before use. The average diameter of the ice particulates was about 400 μm. Subsequently, a 1% aqueous solution of porcine type I collagen was poured onto the embossing ice template with a 1 mm-thick silicone frame to maintain the collagen thickness. The aqueous collagen solution was stored at −1 °C for 24 h for cooling before being poured onto the template. The collagen aqueous solution was poured over the embossing ice particulates, and the solution surface was flattened with a plastic stick. The PLLA woven mesh was pre-wetted with the aqueous collagen solution, attached to a glass plate and carefully placed on the above collagen solution. The construct was frozen at −3 °C for 1 h. The frozen collagen solution and the template were further frozen at −80 °C for 5 h and freeze-dried for 24 h in an AdVantage 2.0 freeze-drier (VirTis, Gardiner, NY). Finally, the freeze-dried scaffolds were cross-linked by glutaraldehyde vapor for 4 h at 37 °C. The non-reacted aldehyde groups were blocked with a 0.1 M glycine solution. Funnel-like PLGA–collagen hybrid porous scaffolds were obtained after washing and freeze-drying.
2.1.2. Fabrication of funnel-like PLLA–gelatin hybrid scaffold.
Bovine bone gelatin with an isoelectric point of 5.7 was obtained from Nitta Gelatin (Osaka, Japan). Gelatin powder was put into Milli-Q water in a 50 ml conical tube and kept at 60 °C until it was completely swollen. The pH of aqueous gelatin solution was adjusted to pH 3.2 with acetic acid, and the final concentration of gelatin was 1 % w/v. The gelatin solution was incubated at −3 °C for 1.5 h to reach −1 °C. The pre-cooled gelatin solution was then immediately used to prepare the hybrid PLLA–gelatin scaffold with the method described above by replacing collagen aqueous solution by gelatin aqueous solution. Control collagen porous sponges were prepared by the same procedure but without the use of ice template and PLLA mesh.
2.1.3. Evaluation of hybrid scaffold.
The microstructures of the hybrid scaffolds were observed using a JSM-5610 scanning electron microscope (SEM; JEOL, Japan). Samples were coated with platinum before observation. The mechanical properties of the PLLA mesh, hybrid scaffolds and collagen sponge were evaluated by uniaxial tensile testing. Rectangular samples (15 × 5 mm2) were tested in triplicate by a static tensile mechanical test machine (TMI UTM-10T; Toyo Baldwin Co., Ltd, Tokyo, Japan) at a crosshead speed of 1.0 mm min−1. The specimen thickness was measured with calipers at three locations. The elastic modulus and ultimate tensile strength were calculated from the stress–strain curve. The results are reported as a mean ± standard deviation (SD).
2.2. In vitro cell culture
Neonatal human dermal fibroblasts (NHDF, Cascade Biologics Incorporation) were used for the cell culture experiment. The cells were cultured in 75 cm2 tissue culture flasks in serum Medium 106 supplemented with low serum growth supplement kit (LSGS kit) under an atmosphere of 5% CO2 at 37 °C. The serum Medium 106 contained 2% fetal bovine serum, 1 μg ml−1 hydrocortisone, 10 ng ml−1 human epidermal growth factor, 3 ng ml−1 basic fibroblast growth factor and 10 μg ml−1 heparin. The subcultured fibroblasts were seeded onto the collagen side of the hybrid scaffolds and control collagen scaffold. For cell culture use, the scaffolds were punched into discs with a diameter of 10 mm. The discs were sterilized with 70% ethanol, washed with Milli-Q water and conditioned with serum Medium 106. The seeded cell number was 3.9 × 106 cells for each hybrid scaffold disc (5 × 106 cell cm−2). The seeded scaffolds were then placed in the wells of 24-well cell culture plates and cultured in the serum medium described above under an atmosphere of 5% CO2 at 37 °C. The medium was exchanged twice per week. Fibroblasts adhesion in the scaffolds after culture for 1 h was investigated by SEM. The scaffolds were washed with phosphate buffered saline (PBS), rinsed with Milli-Q water and crosslinked with 0.1% glutaraldehyde aqueous solution. Then the scaffolds were freeze-dried for the SEM observation.
Live/dead staining was performed to evaluate cell viability using calcein-AM (live) and propidium iodide (dead; Cellstain Double Staining Kit, Dojindo Laboratories). Fibroblasts were cultured in the scaffolds for 7 days, washed with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer and incubated in 2 μM calcein-AM and 4 μM propidium iodide solution in HEPES buffer for 30 min. After being rinsed with HEPES buffer, the submerged specimens were observed with a fluorescence microscope. After culture for 1 week, the cell/scaffold constructs were also fixed in 10% formalin, embedded in paraffin and cross-sectioned at 10 μm intervals. The cross-sections were stained by hematoxylin and eosin (HE) for histological evaluation.
The cell proliferation in the scaffolds was evaluated by quantifying the DNA amount. After being cultured for 7 days, the cell/scaffold constructs were washed with pure water, freeze-dried and then digested with papain solution. Papain (Sigma-Aldrich, St Louis, Missouri, USA) was dissolved at 400 μg ml−1 in 0.1 M phosphate buffer (pH 6.0), with 5 mM cysteine hydrochloride and 5 mM ethylenediaminetetraacetic acid (EDTA). An aliquot of papain digests was used to measure the DNA content with Hoechst 33258 dye (Sigma-Aldrich) and an FP-6500 spectrofluorometer (JASCO, Tokyo, Japan) at an excitation wavelength of 360 nm and emission wavelength of 460 nm. Three samples were used to calculate the average and SD (n = 3).
2.3. Implantation of hybrid scaffolds in athymic nude mice
The hybrid scaffolds and control collagen sponges were cut into squares (20 × 20 mm2) and sterilized with 70% ethanol. The fibroblasts were seeded in the scaffold pieces (2 × 107 cells per scaffold). After in vitro culture for 1 week, the cell/scaffold constructs were implanted in the dermal defects prepared by removing the full skin in the dorsa of athymic nude mice. Athymic female mice were obtained at 4 weeks and acclimated for 2 weeks before use. Surgical plane anesthesia in each animal was induced and maintained with 2% isoflurane in oxygen. After disinfection of the dorsolateral surface of the mouse with 70% ethanol, a 20 × 20 mm2 full-thickness skin section was excised, sparing the panniculus carnosus. The scaffold pieces were placed on the wound with the collagen layer facing panniculus carnosus. The graft was sutured to the edges of the wound with 4–0 nylon and covered with one layer of ADAPTIC® (Systagenix, Gargrave, UK). ADAPTIC® is a non-adhesive dressing made of knitted cellulose acetate fabric. Then cotton gauze was covered on the wound to protect the graft. The animals were given daily injections of 3 mg ceftazidime intraperitoneally for 7 days after surgery. The implants were harvested 4 weeks after transplantation. The mice were sacrificed by an overdose of ethyl ether. Besides the cell-populated scaffolds, the cell-free scaffolds were also implanted on the nude mouse skin. After implantation for 4 weeks, the mice were sacrificed and the implants were harvested for histological evaluation. The in vivo specimens were fixed in 10% formalin, embedded in paraffin and cross-sectioned. The cross-sections were stained by HE and observed under an optical microscope. The animal experiment was conducted according to the committee guidelines of the National Institute for Materials Science for Animal Experiments.
2.4. Statistical analysis
All data are reported as mean ± standard deviation (SD). One-way analysis of variance was performed to reveal statistical differences followed by Tukey’s post hoc test for pairwise comparison. A p < 0.05 was considered statistically significant.
3. Results
3.1. Characterization of funnel-like hybrid scaffolds
The PLLA–collagen hybrid scaffold was prepared by forming funnel-like collagen sponge on one side of a PLLA mesh (figure 2(a)). The microstructure of the hybrid scaffold was observed by SEM. Open surface pores were observed on the top surface of the hybrid scaffolds (figure 2(b)). The open surface pores were interconnected with the small bulk pores under the surface pores, which structure was similar to that of a Büchner funnel. The funnel-like collagen sponge layer was well integrated with the PLLA mesh through the physical linkage between the PLLA filament bundles and collagen fibers (figures 2(c) and (d)). The funnel-like PLLA-gelatin hybrid scaffold and PLLA–collagen scaffold showed similar gross appearance and bilayer pore structure (figures 2 (e)–(h)). The results indicate that funnel-like pore structure of both collagen sponge and gelatin sponge could be prepared by using ice particulates as a template and the funnel-like sponges could be introduced in PLLA mesh skeleton to form a hybrid structure.
Figure 2.
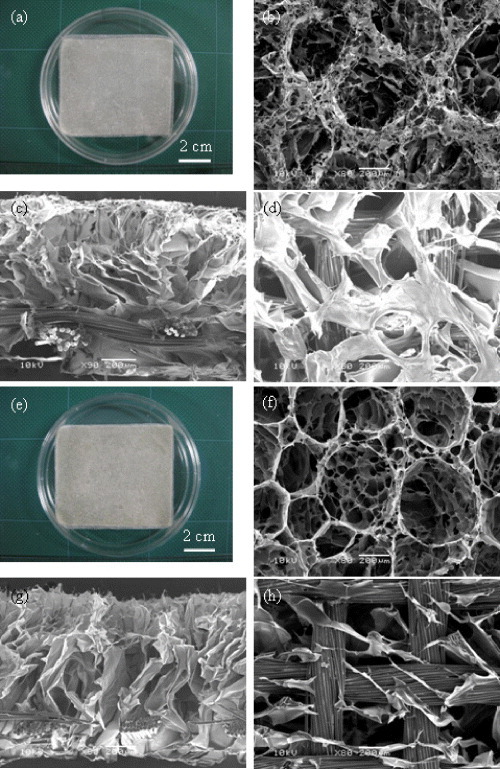
Funnel-like PLLA–collagen (a–d) and PLLA–gelatin (e–h) hybrid scaffolds: gross appearance (a, e) and SEM images of the top surfaces (b, f), cross-section (c, g), and bottom surface (d, i). Scale bars: (a), (e): 2 cm; b–d, f–h: 200 μm.
The tensile strengths of PLLA mesh, hybrid scaffolds and collagen sponge are listed in table 1. The PLLA mesh showed the highest ultimate tensile strength and elastic modulus while the collagen sponge had the lowest. The hybrid scaffolds exhibited much higher mechanical strength than did the collagen sponge, whereas the difference between PLLA–collagen and PLLA–gelatin scaffolds was not evident. There was no significant warp and weft direction preference in the PLLA mesh and hybrid scaffolds. These results demonstrate that collagen sponge or gelatin sponge was reinforced by PLLA mesh after hybridization. The values of ultimate tensile strength and elastic modulus of hybrid scaffolds were lower than those of PLLA mesh, which was due to increase of the scaffold thickness from 160 μm (PLLA mesh) to 1 mm (hybrid scaffold).
Table 1.
The ultimate tensile strength and elastic modulus of PLLA mesh, PLGA–collagen hybrid mesh, PLLA–gelatin hybrid mesh and control collagen sponge.
| Ultimate tensile strength (MPa) | Elastic modulus (MPa) | |||
|---|---|---|---|---|
| Warp | Weft | Warp | Weft | |
| PLLA mesh | 33.8 ± 0.7 | 32.3 ± 0.1 | 212 ± 11 | 177 ± 28 |
| PLLA–collagen | 5.0 ± 0.4 | 4.9 ± 0.8 | 55 ± 12 | 43 ± 4 |
| PLLA–gelatin | 5.8 ± 0.4 | 5.7 ± 1.7 | 43 ± 5 | 41 ± 3 |
| Collagen sponge | 0.01 ± 0.00 | □ | 0.11 ± 0.05 | □ |
Data represent mean ± SD, n = 3.
□ Data not available. There was no warp and weft differentiation for the collagen sponge.
3.2. Cell seeding and proliferation in hybrid scaffolds
Adhesion of fibroblasts in the scaffolds after culture for 1 h was observed by SEM (figure 3). Fibroblasts adhered to the scaffolds 1 h after seeding. Cells penetrated through the large surface pores more easily, and more cells were observed in the bottom layer of the funnel-like scaffolds (both PLLA–collagen and PLLA–gelatin) compared to the control collagen sponge. Cells were more evenly distributed in the funnel-like hybrid scaffolds than in the control collagen sponge. The cell seeding efficiency of the hybrid scaffolds was higher than that of the control collagen sponge (figure 4(a)). To investigate cell proliferation, DNA contents in the cell/scaffold constructs were quantified (figure 4(b)). The amount of DNA increased during the in vitro culture. Statistically, more cells were found in the PLLA–collagen hybrid scaffold than in the PLLA–gelatin hybrid scaffold and control collagen sponge. The PLLA–gelatin hybrid scaffold had more cells than control collagen sponge although there was no statistical difference. These results indicate that both PLLA–collagen and PLLA–gelatin hybrid scaffold facilitated cell seeding and promoted fibroblast proliferation more efficiently than did the collagen sponge. The effect of PLLA–collagen hybrid scaffold was stronger than that of the PLLA–gelatin hybrid scaffold.
Figure 3.
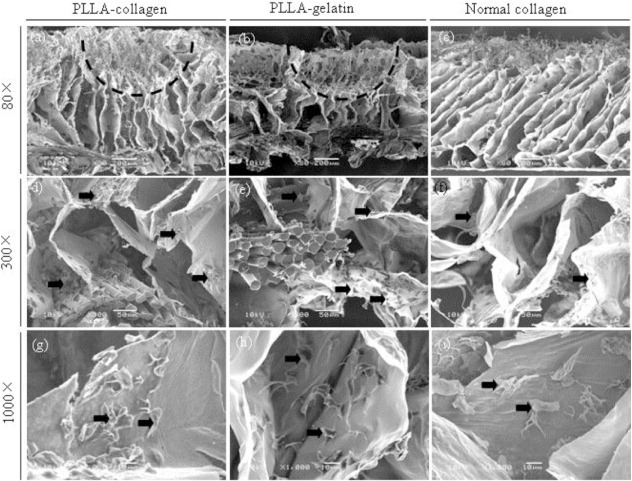
SEM images of fibroblast adhesion in the PLLA–collagen hybrid scaffold (a, d, g), PLLA–gelatin hybrid scaffold (b, e, h) and control collagen sponge (c, f, i) 1 hour after cell seeding. The vertical cross sections of scaffolds were used for the SEM observation. Arrows indicate adhered fibroblasts. Dashed lines indicate a large surface pore. Scale bars: 200 μm (a–c), 50 μm (d–f), and 10 μm (g–i).
Figure 4.
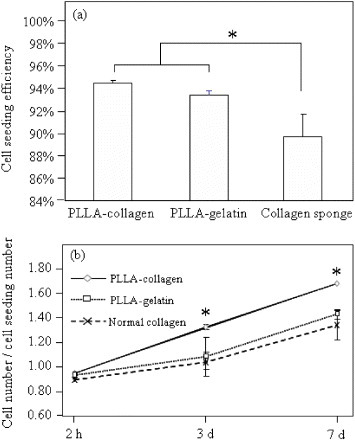
Cell seeding efficiency (a) and cell proliferation rate (b) in the PLLA–collagen hybrid scaffold, PLLA–gelatin hybrid scaffold and control collagen sponge. Data represent means ± SD, n = 3, significant difference p < 0.05.
No obvious deformation of the PLLA–collagen and PLLA–gelatin hybrid scaffolds was observed after 1 week of culture (figures 5(a) and (b)). However, without hybridization with the PLLA mesh, the control collagen sponge was deformed by cell contraction and twisted during cell culture (figure 5(c)). The PLLA woven mesh provided appropriate mechanical strength to protect deformation of the hybrid scaffolds during cell culture. The live/dead staining revealed that most of the cells were stained green, which indicated high viability of fibroblasts figure 5(d)–(f). However, there were more dead cells (red dots) observed in the control collagen sponge than in the hybrid scaffolds. After being cultured for 1 week in vitro, the cross-sections of the cell/scaffold constructs were stained with HE (figures 5(g)–(i). Cells and extracellular matrix were homogeneously distributed within the hybrid scaffolds (figures 5(g) and (h)). The cell distribution of cells was not homogenous in the control collagen sponge. Cell-free areas were observed in the inner part of control collagen sponge (figure 5(i)).
Figure 5.
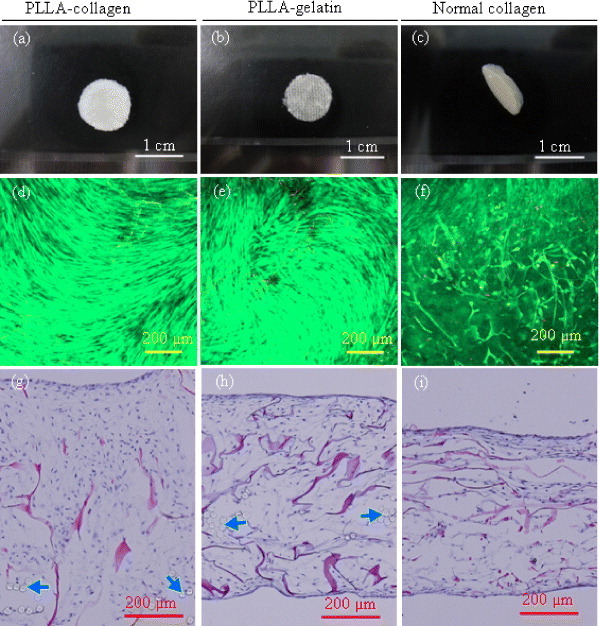
Gross appearance (a–c), cell viability (d–f) and HE staining photomicrographs (g–i) of cell/scaffold constructs after 1 week culture in vitro. Cell viability was evaluated via live/dead staining and observed by fluorescence microscopy. Arrows indicate PLLA fibers. Scale bars: 1 cm in (a–c) and 200 μm in (d–i).
3.3. Wound closure in athymic nude mice
Both the cell-free scaffolds and cell-populated scaffolds were subcutaneously implanted on the backs of nude mice. The cell-populated scaffolds were prepared by culturing fibroblasts in vitro for 1 week on 20 × 20 mm2 cuts of PLLA-collagen hybrid scaffold, PLLA-gelatin scaffold and control collagen sponge. Figures 6(a)–(c) and (g)–(i) show representative images of animals from each group 4 weeks after implantation. Four weeks after surgery, the skin defects of all the groups were entirely closed. The surface areas of regenerated skin were larger in hybrid scaffold groups (both PLLA–collagen and PLLA–gelatin) than in the collagen sponge group. The regenerated skins were smoother and glossier in the funnel-like hybrid scaffold groups than in the control collagen sponge group. Four weeks after implantation, histological evaluation of a skin biopsy collected from each animal showed good wound closure in all groups (6(d)–(f) and (j)–(l). The regenerated tissues displayed a thicker epidermis in the fibroblast-populated than in cell-free scaffolds, and the keratin layers on the epidermis were thicker in the funnel-like hybrid scaffold groups than in the control collagen group. This was consistent with the gross appearances of the regenerated skins, indicating that the funnel-like hybrid scaffolds accelerated the wound healing more efficiently than the control collagen sponge. The dermis contained fibroblasts and dense collagen matrices. The cells and matrices were more homogeneously distributed in the fibroblast-populated scaffolds than those in the cell-free scaffolds.
Figure 6.
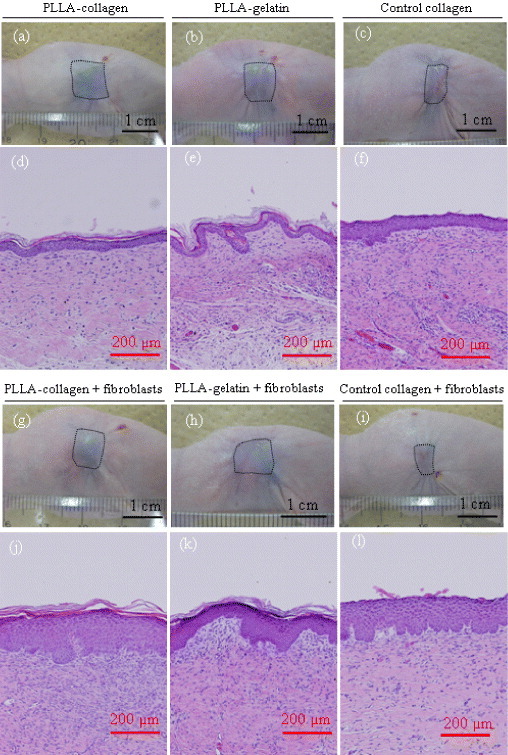
Gross appearance of the wound closure in athymic nude mice (a–c and g–i) and histological photomicrographs of skin biopsies (d–f and j–l) 4 weeks after implantation. Scale bars represent 1 cm in (a–c, g–i) and 200 μm (d–f, j–l). Wound areas are traced with the dotted rectangles.
4. Discussion
Skin tissue engineering addresses the need for early, permanent coverage of extensive skin injury in patients with an insufficient source of autologous skin for grafting [14]. Bioengineered skin in the form of cultured keratinocytes or in combination with fibroblasts to form human skin equivalents has allowed a promising therapy approach for such patients [15]. Porous 3D scaffolds have been used extensively for skin tissue engineering [16]. Ideal scaffolds should have high cell seeding efficiency, promote fast cell attachment to the scaffolds and facilitate uniform spatial distribution of cells and extracellular matrix throughout the scaffolds [17]. It has been reported that a homogeneous spatial distribution of cells influences cell density in the engineered tissue constructs, which in turn impacts the kinetics of cell proliferation and extracellular matrix deposition and affects the functionality of engineered tissue [18].
In this study, the mechanically strong PLLA mesh was hybridized with funnel-like collagen or gelatin sponge to meet the requirements of scaffold. Owing to the unique funnel-like porous structure, the hybrid scaffolds showed high cell seeding efficiency and improved fibroblast adhesion and proliferation. Cell distribution was more homogeneous in the funnel-like hybrid scaffolds (both PLLA–collagen and PLLA–gelatin) than in the control collagen sponge. The pore structure of control collagen sponge partially inhibited cell penetration. During cell culture in porous scaffolds, the shrinkage always happens when the scaffold is not strong enough to withstand the contractile forces exerted by cells. PLLA woven mesh served as a skeleton to reduce the shrinkage of scaffold during cells culture. As a result, the cell proliferation was improved.
During in vitro culture of fibroblasts, the PLLA–collagen scaffold promoted cell proliferation more efficiently than did the PLLA–gelatin scaffold. Gelatin is the partially denatured product of collagen. The bioactivity of gelatin is lower than that of collagen because of the denaturation [19–21]. Therefore, the different proliferation rates can be attributed to the different bioactivity of collagen and gelatin.
Both the control collagen sponge and hybrid scaffolds improved the wound closure of the full-thickness skin defects in the athymic nude mice. The dermis and epidermis layer were fully regenerated 4 weeks after implantation, as judged from the histological images. However, the area of regenerated skin was smaller in the control collagen sponge than in the hybrid scaffolds. Uniform distribution of cells and extracellular matrices in the PLLA–collagen and PLLA–gelatin hybrids should also reduce tissue contraction during the formation of new tissue. This, the strong PLLA mesh reduced the contraction of newly formed dermal tissue. Four weeks after implantation no PLLA fibers were detected in the histological micrographs. Because of the relatively low degradation speed of PLLA in vivo, the absence of PLLA fibers may be related to the detachment of PLLA mesh from the wound bed. The PLLA mesh gradually detached from the wound bed accompanied with infiltration of inflammatory cells and maturation of epidermis. Some researchers also reported the inflammatory responses and separation of implanted materials from regenerated skin, which is a common phenomenon in the wound healing [22–26].
It has been reported that the fibroblast-repopulated scaffolds rather than the cell-free scaffolds improved the re-epithelization on the full-thickness skin defect [23, 27]. The cultured fibroblasts in the meshes should produce extracellular matrix proteins and release growth factors and cytokines in physiological concentration, which are essential for wound healing and epithelization [8, 27]. In this study, the fibroblast-repopulated scaffolds resulted in thicker epidermis regeneration than did the cell-free scaffolds. The fibroblasts in the scaffolds facilitated migration of keratinocytes and promoted re-epithelization. The homogeneous distribution of cells and matrices in the fibroblast-populated scaffolds can also be explained by the promotive effect of the populated fibroblasts. The porosity, mean pore size and orientation affect the migration and distribution of the cells in the scaffolds containing open-pore structures.
5. Conclusions
Hybrid scaffolds were constructed by forming funnel-like collagen or gelatin sponges on one side of a PLLA woven mesh. The funnel-like hybrid scaffolds improved cell adhesion and proliferation, compared to the control collagen sponges. The PLLA–collagen scaffolds showed more promotive effect than did the PLLA–gelatin scaffolds. The hybrid scaffolds improved the wound healing of full-thickness skin defects in athymic nude mice. The funnel-like structure facilitated the cell penetration and the PLLA mesh improved the mechanical strength of the scaffolds. The funnel-like PLLA–collagen or PLLA–gelatin hybrid scaffold will be a useful tool for skin tissue engineering.
Acknowledgments
This work was supported in part by the JST Innovation Satellite Ibaraki (Practical Application Research Program) of Japan and in part by the World Premier International Research Center Initiative (WPI) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- Yannas I V. and Burke J F. J. Biomed. Mater. Res. 1980;14:65. doi: 10.1002/jbm.820140108. [DOI] [PubMed] [Google Scholar]
- Boyce S T. Burns. 2001;27:523. doi: 10.1016/S0305-4179(01)00019-5. [DOI] [PubMed] [Google Scholar]
- Ng K W, Khor H L. and Hutmacher D W. Biomaterials. 2004;25:2807. doi: 10.1016/j.biomaterials.2003.09.058. [DOI] [PubMed] [Google Scholar]
- Trasciatti S, Podesta A, Bonaretti S, Mazzoncini V. and Rosini S. Biomaterials. 1998;19:897. doi: 10.1016/S0142-9612(97)00175-0. [DOI] [PubMed] [Google Scholar]
- Bello Y M, Falabella A F. and Eaglstein W H. Am. J. Clin. Dermatol. 2001;2:305. doi: 10.2165/00128071-200102050-00005. [DOI] [PubMed] [Google Scholar]
- Cooper M L, Hansbrough J F, Spielvogel R L, Cohen R, Bartel R L. and Naughton G. Biomaterials. 1991;12:243. doi: 10.1016/0142-9612(91)90207-Q. [DOI] [PubMed] [Google Scholar]
- Yang W S, Roh H W, Lee W K. and Ryu G H. J. Biomater. Sci., Polym. Ed. 2006;17:151. doi: 10.1163/156856206774879108. [DOI] [PubMed] [Google Scholar]
- Chen G, Sato T, Ohgushi H, Ushida T, Tateishi T. and Tanaka J. Biomaterials. 2005;26:2559. doi: 10.1016/j.biomaterials.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Hokugo A, Takamoto T. and Tabata Y. Biomaterials. 2006;27:61. doi: 10.1016/j.biomaterials.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Lu H, Ko Y G, Kawazoe N. and Chen G. Biomaterials. 2010;31:5825. doi: 10.1016/j.biomaterials.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Ko Y G, Kawazoe N, Tateishi T. and Chen G. J. Bioact. Compat. Polym. 2010;25:360. doi: 10.1177/0883911510370002. [DOI] [Google Scholar]
- Ko Y G, Grice S, Kawazoe N, Tateishi T. and Chen G. Macromol. Biosci. 2010;10:860. doi: 10.1002/mabi.200900468. [DOI] [PubMed] [Google Scholar]
- Lu H, Ko Y G, Kawazoe N. and Chen G. Biomed. Mater. 2011;6:45011. doi: 10.1088/1748-6041/6/4/045011. [DOI] [PubMed] [Google Scholar]
- Schulz J T, Tompkins R G. and Burke J F. Annu. Rev. Med. 2000;51:231. doi: 10.1146/annurev.med.51.1.231. [DOI] [PubMed] [Google Scholar]
- Ishikawa O, Kondo A, Okada K, Miyachi Y. and Furumura M. Br. J. Dermatol. 1997;136:6. doi: 10.1111/j.1365-2133.1997.tb08738.x. [DOI] [PubMed] [Google Scholar]
- Bannasch H, Fo hn M, Unterberg T, Bach A D, Weyand B. and Stark G B. Clinics Plast. Surg. 2003;30:573. doi: 10.1016/S0094-1298(03)00075-0. [DOI] [PubMed] [Google Scholar]
- Chen G, Ushida T. and Ushida T. Macromol. Biosci. 2002;2:67. doi: 10.1002/1616-5195(20020201)2:2%3C67::AID-MABI67%3E3.0.CO;2-F. [DOI] [Google Scholar]
- Cheng G, Youssef B B, Markenscoff P. and Zygourakis K. Biophys. J. 2006;90:713. doi: 10.1529/biophysj.105.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H W, Tabata Y. and Ikada Y. Biomaterials. 1999;20:1339. doi: 10.1016/S0142-9612(99)00036-8. [DOI] [PubMed] [Google Scholar]
- Lee S B, Kim Y H, Chong M S, Hong S H. and Lee Y M. Biomaterials. 2005;26:1961. doi: 10.1016/j.biomaterials.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Mao J, Zhao L, De Y, ao K, Shang Q, Yang G. and Cao Y. J. Biomed. Mater. Res. A. 2003;64:301. doi: 10.1002/jbm.a.10223. [DOI] [PubMed] [Google Scholar]
- Ananta M, Brown R A. and Mudera V. Tissue Eng. A. 2012;18:353. doi: 10.1089/ten.tea.2011.0208. [DOI] [PubMed] [Google Scholar]
- Choi Y S, Hong S R, Lee Y M, Song K W, Park M H. and Nam Y S. J. Biomed. Mater. Res. 1999;48:631. doi: 10.1002/(SICI)1097-4636(1999)48:5%3C631::AID-JBM6%3E3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Choi Y S, Lee S B, Hong S R, Lee Y M, Song K W. and Park M H. J. Mater. Sci. Mater. Med. 2001;12:67. doi: 10.1023/A:1026765321117. [DOI] [PubMed] [Google Scholar]
- Tchemtchoua V T. Biomacromolecules. 2011;12:3194. doi: 10.1021/bm200680q. [DOI] [PubMed] [Google Scholar]
- Rho K S, Jeong L, Lee G, Seo B M, Park Y J, Hong S D, Roh S, Cho J J, Park W H. and Min B M. Biomaterials. 2006;27:1452. doi: 10.1016/j.biomaterials.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Lee S B, Jeon H W, Lee Y W, Lee Y M, Song K W. and Park M H. Biomaterials. 2003;24:2503. doi: 10.1016/S0142-9612(03)00003-6. [DOI] [PubMed] [Google Scholar]


