Abstract
We have investigated the effectiveness and safety of a newly developed biological adhesive for repair of meniscal tear. The adhesive was composed of disuccinimidyl tartrate (DST) as a crosslinker and human serum albumin (HSA) as a hardener. To determine adequate concentration, bonding strength was measured using a tensiometer 5 min after applying the adhesive on the avascular zone tear of porcine meniscus; it was compared with the strengths of commercially available cyanoacrylate-based and fibrin-based adhesives. In vivo examination was performed using Japanese white rabbits, creating longitudinal tears on the avascular zone of meniscus and applying DST–HSA adhesive. Three months after operation the rabbits were sacrificed and tension test and histological evaluation were performed. Bonding strength was measured in three porcine meniscus groups: (i) only suturing, (ii) suturing after applying the adhesive on surface and (iii) suturing using an adhesive-soaked suture. The optimum concentrations were 0.1 mmol of DST and 42 w/v% of HAS. Bonding strength was greatest with cyanoacrylate-based adhesive, followed by DST–HSA adhesive, and fibrin-based adhesive. No inflammation was observed in the synovium or surrounding tissues 3 months after using the DST–HSA adhesive. Bonding strength was greatest with DST–HSA adhesive-soaked suturing group (77 ± 6 N), followed by suturing only group (61 ± 5 N) and surface adhesive application group (60 ± 8 N). The newly developed DST-HSA adhesive is considered safe and may be effective in enforcement of bonding of avascular zone tear of the meniscus.
Keywords: biological adhesive, meniscal tear, disuccinimidyl tartrate, human serum albumin
1. Introduction
The meniscus is categorized as fibrocartilage and intervenes between the femoral condyle and tibial plateau. Meniscus not only absorbs shocks, but also facilitates gliding and stabilizes the knee joint, while assisting the rotation and transmitting loads. The meniscus is composed of more than 70% water at its wet weight, and the remainder is 60–90% type-I collagen at its dry weight. The peripheral of the meniscus is attached to the joint capsule, and is vascularized in 10–25% of the region from the periphery by arborizing vessels. The remaining two-thirds of the meniscus is a free edge scanty of vessels, which is supplied from the synovium [1]. Therefore, in case of meniscus injury, it is known that the region two-thirds from the free edge is difficult to heal spontaneously.
Surgical treatment methods for meniscal tear include rasping, suturing, partial menisectomy, allograft implantation, autograft implantation and scaffold implantation [2]. Of these, arthroscopic meniscal suturing is a generally known technique that preserves the meniscal function as much as possible. Some authors claimed a favorable treatment outcome after meniscal suture in more than 80% of cases [3–5]. However, Kurosaka et al [6] reported that re-rupture occurred in about 15% of sutured cases within 4 years after operation, although they were recognized as healed 1 year after suturing the meniscus tear. Therefore, suturing of the meniscus tear has not been proven to provide a complete recovery.
Currently, typical commercially available chemosynthetic biomedical adhesive are cyanoacrylate adhesive (Dermabond®, Ethicon, Inc., New Jersey, USA), aldehyde-based adhesive (GRF glue®, Cardial, Saint-Ethienne, France) and fibrin-based adhesive (Bolheal®, Kaketsuken, Kumamoto, Japan). Cyanoacrylate and aldehyde-based adhesives provide strong bonding, though cytotoxicity is relatively high. Fibrin-based adhesive is known to have a relatively low cytotoxicity, but also an inferior bonding strength.
Taguchi et al developed biodegradable adhesives derived from citrate, malate and tartrate as organic acid-based crosslinkers. They reported excellent bonding strength, reactivity and bonding time when using disuccinimidyl tartrate (DST) as a crosslinker and human serum albumin (HSA) as a hardener for the muscle of porcine, with the bonding strength being equivalent to that of cyanoacrylate-based adhesive [7, 8].
We contrived to use the novel DST–HSA biological adhesive for repairing longitudinal tear of the avascular zone of the meniscus, investigate the optimum usage, and evaluate the effectiveness and safety using animal models.
2. Materials and methods
2.1. Materials
HSA was purchased from Sigma Chem. Corp. (Tokyo, Japan). Tartaric acid (TA), N-hydroxysuccinimide (NHS) and tetrahydrofuran (THF), were purchased from Wako Pure Chemical Industries, Ltd (Osaka, Japan). Dicyclohexylcarbodiimide (DCC) was purchased from Kokusan Chemical Co., Ltd (Tokyo, Japan). All chemicals were used without further purification.
2.2. Synthesis of DST
DST was synthesized by the previously reported method [7]. In brief, CA, MA, or TA was first dissolved in THF, and then NHS and DCC were added. After mixing for 30 min, the mixture was concentrated via rotary evaporation under reduced pressure to remove THF. The resulting mixture was recrystallized to yield pure DST.
The DST was analyzed by proton nuclear magnetic resonance (1H-NMR) spectroscopy (JEOL EX-300) and elemental analysis with the following results:
DST: 1H-NMR (DMSO-d6) δ = 2.8 (s, 8H, succinimidyl ester's CH2 × 4), 4.8–4.9 (d, 2H, CH × 2), 6.73 (d, 2H, OH × 2), C12H12N2O10: calculated: C 41.90, H 3.52, N 8.14, measured: C 42.22, H 3.47, N 8.33.
2.3. Animal experiments
The protocol used in the present study was approved by the Animal Research and Use Committee of Shimane University. Menisci in our in vitro study were collected from Japanese swine (24 months old). The quality of these menisci was strictly controlled (Shimane Meat Wholesale Co., Shimane, Japan). We used menisci of Japanese white rabbits (2 months old, body weight 2.2–2.4 kg; KBT Oriental Co., Ltd, Saga, Japan) for the in vivo study.
2.4. Optimization of HSA/DST concentration
To determine adequate concentrations of the DST–HSA adhesive, bonding strength was measured by setting the concentrations of DST at 0.05, 0.075 and 0.10 mmol, and 0.8 mg of HSA at 38, 40 and 42 w/v% (0.1 M phosphate buffer solution (PBS, pH 6)). Using porcine models, a longitudinal tear was made with a scalpel in the avascular zone of the meniscus at 5 mm from the joint capsule. Five minutes after applying the adhesive to the artificially torn region, bonding strength was measured using a tension tester (Instron 5565; Instron, Canton, Massachusetts, USA) providing the tensile load normal to the surface of repaired region.
2.5. Comparison of the bonding strength with other adhesives
We compared the bonding strengths of the DST–HAS, cyanoacrylate-based and fibrin-based adhesives. We applied these adhesive to the surface of the artificially torn porcine meniscus and measured the bonding strength as described above.
2.6. Bonding strength of the adhesive in vivo
A total of ten knees of five Japanese white rabbits were used, and longitudinal tears were made in the avascular zone of the lateral meniscus in both knees. The left knees were left untreated as control, whereas the right knees were treated applying the DST–HSA adhesive (DST 0.1 mmol, HSA 42 w/v%). Three months after the operation, the bonding strength was measured and histological findings were analyzed.
2.7. Bonding strength when sutured using a adhesive-soaked thread in vivo
We used 2–0 braided polyester suture (Ethibond Excel®, Ethicon, Inc., Somerville, New Jersey, USA) for suturing the torn meniscus of the avascular zone of matured porcine. The bonding strength was compared among three groups: (i) sutured only (Suture Group), (ii) sutured after applying the adhesive on the surface of torn region (Adhesive + Suture Group) and (iii) sutured using the adhesive-soaked suture thread (Adhesive-soaked Suture Group). We set the soaking time as 5 min at 25 °C (room temperature) and used concentrations of 0.1 mmol of DST and 42 w/v% of HSA in the adhesive-soaked suture thread.
2.8. Statistical analysis
Statistical analysis was performed with StatView 5.0 software (SAS Institute, Cary, NC, USA). Analysis of variance (ANOVA) was used to compare the bonding strength in each experiment. A p-value of less than 0.05 was considered to indicate a significant difference.
3. Results
3.1. Optimization of HSA and DST concentrations
The bonding strength of porcine meniscus increases with concentrations of DST and HSA. Taguchi et al reported a similar increase for HSA content up to 42 w/v%, above which no significant difference in bonding strength was observed. A similar behavior was observed when DST content was 0.1 mmol: bonding strength increased with HSA concentration up to 42 w/v%, and then reached a plateau [7]. Therefore, we decided to use the concentrations of 0.1 mmol DST and 42 w/v% HSA for the following experiments (figure 1).
Figure 1.
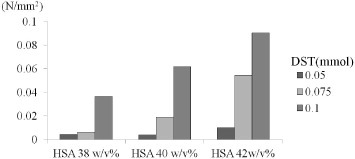
Bonding strength of porcine meniscus for different concentrations of HSA and DST.
3.2. Comparison of the bonding strength with other adhesive
The bonding strength was compared among the DST–HSA adhesive (DST 0.1 mmol and HSA 42 w/v%) and two commercially available adhesive. The corresponding values were (767 ± 220) × 10–3 Nmm−2 for the cyanoacrylate-based adhesive (78 ± 22) ×10–3 Nmm−2 for the DST–HAS adhesive and (43 ± 15) ×10–3 Nmm−2 (p < 0.05) for the fibrin-based adhesive (figure 2).
Figure 2.
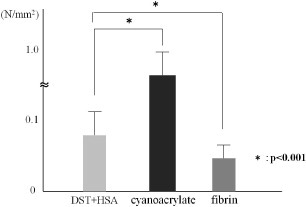
Bonding strength of each adhesive with porcine meniscus.
3.3. Bonding strength of the adhesive in vivo
At 3 months after operation, there were no noticeable complications in treated rabbits, such as inflammation or body weight loss. The bonding strength of the treated knee was 2.08 Nmm−2, approximately 1.5 times higher than that of the untreated control knee. Hematoxyline–eosin stained samples at 3 months after operation showed no inflammation in the synovium where the adhesive had been applied (figures 3(a) and (b)), and crosslinking was seen in the ruptured meniscus, suggesting biological repair (figures 3(c) and (d)).
Figure 3.
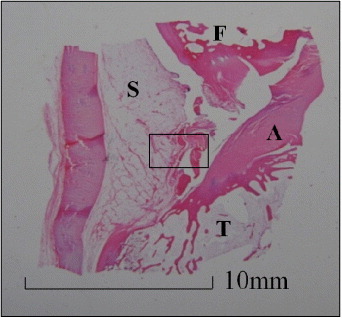
Histology of knee joint (hematoxyline and eosin stain). (a) Sagittal view of knee joint 3 months after adhesive was applied. F: femur, A: anterior cruciate ligament, T: tibia, S: synovium. (b) Magnification of (a), synovium (hematoxyline and eosin stain). There was no inflammation in the synovium. (c) Meniscus of applying DST + HSA, 3 months after operation (hematoxyline and eosin). Arrows show the region where longitudinal tear was induced. (d) Magnification of (c) (hematoxyline and eosin stain). Crosslinking was seen in the ruptured meniscus, suggesting biological repair. (e) Meniscus of untreated control group 3 months after operation (hematoxyline and eosin stain). (f) Magnification of (e). Torn region is not repaired, there was no crosslinking.
3.4. Bonding strength when sutured using a adhesive-soaked thread in vivo
There was no statistically significant difference in bonding strength between the Suture Group (61 ± 5 N) and the Adhesive + Suture Group (60 ± 8 N). There were statistically significant differences between the Adhesive-soaked Suture Group (77 ± 6 N) and the other two groups (p < 0.05; figure 4). In the ruptured samples all failures were suture rupture under the knot.
Figure 4.
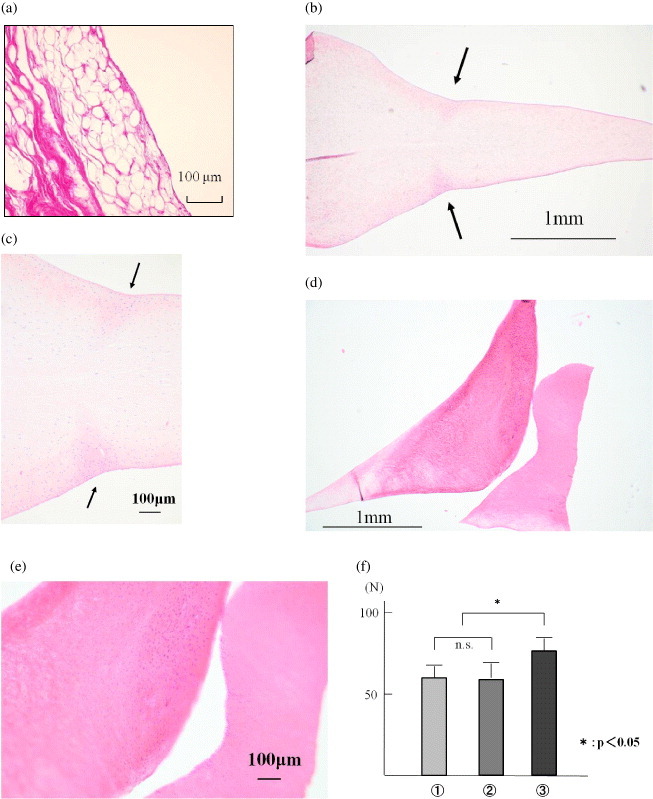
Bonding strength of soaking adhesive to suture strand. (i) Suture Group, (ii) Adhesive + Suture Group, (iii) Adhesive-soaked Suture Group. There were statistically significant differences between the adhesive-soaked Suture Group (77 ± 6 N) and the other two groups (p < 0.05).
4. Discussion
Bonding strength of the DST–HSA adhesive was approximately two times greater than that of the fibrin-based adhesive in our study, and healing was histologically confirmed. Meniscal injury is a common disease recognized as degenerative disease or sports injury of the knee, not only in adults but also in young people including teenagers. Blood flow is present in only one-third of the meniscus, and the remaining two-thirds of the meniscus is the avascular zone, in which spontaneous healing is difficult biologically.
In cases of radial tear or horizontal tear of the meniscus, sometimes menisectomy may be the only choice of treatment, and there has been no established repairing method. Menisectomy may eventuate the loss of original function of the meniscus as a shock absorber, glide and stabilize the knee joint, while assisting the rotation and transmitting loads, and it may cause secondary lesions of the bone and cartilage, or degenerative changes [6, 9–13]. Generally, to preserve the meniscus as much as possible, arthroscopic suturing of the meniscus is done for its longitudinal tear; however, the tensile load of the repaired site depends on the strength of the suture strands. Although the short-term outcome of meniscal suturing repair is reported to be relatively good in about 80% of the cases, it does not necessarily mean biological healing, but rather may be the fact that the lesion is held together by the suture strands. Therefore, we proposed to improve treatment of the meniscus by application of a newly developed biological adhesive.
Taguchi et al developed a biodegradable adhesive that was derived from tartrate as an organic acid-based crosslinker and human serum albumin as a hardener, and reported its bonding strength and safety in use. This adhesive is composed of DST as a crosslinker and HSA as the hardener, which combined active ester group with carboxyl group of tartrate. When DST and HSA are blended, the amino group of HSA and carboxylate ester of DST make an amide bond, and gelation occurs. When this blended adhesive is placed in collagen, amide bonding occurs with the amine in collagen, and results in crosslinking of collagens and biological bonding [7, 8, 14].
In our study, the combination of 42 w/v% of HSA and 0.1 mmol of DST yielded the highest bonding strength. Taguchi et al reported that even when the concentration of HSA was greater than 42 w/v%, bonding strength modulated plateau. Also, the bonding strength modulated plateau when the concentration of DST was increased from 0.1 to 0.3 mmol [7]. Therefore we selected the combination of 42 w/v% of HSA and 0.1 mmol of DST as the optimum concentration of the adhesive for this study and did not experiment with adhesives of higher concentration.
Currently, many commercially available biochemical adhesives are based on cyanoacrylate, fibrin, or aldehyde, though they exhibit cytotoxicity. For example, while the bonding strength is high for cyanoacrylate-based and aldehyde-based adhesives, they may cause inflammation to surrounding tissues [15–18]. Fibrin-based adhesives are less cytotoxic, but have lower bonding strength. In our experiments, the bonding strength of the DST–HSA adhesive was inferior to that of cyanoacrylate-based adhesive, but superior to that of fibrin-based adhesive. Taguchi et al applied all these adhesives to subcutaneous tissue of rats, and observed inflammation for aldehyde-based and fibrin-based adhesives, but not for the DST–HSA adhesive. Also, the DST–HSA adhesive was biologically fully absorbed within 6 weeks after use [7, 8].
The DST–HSA adhesive provides biochemical bonding (amide bonding) between collagens, which does not necessitate blood flow and synovial induction. As it is a derivative of citrate, which exists in the human body, it provides high bonding strength, biocompatibility and low toxicity [7, 8]. In our in vivo experiment using Japanese white rabbits, no inflammation was observed in the knee joint at 3 months after operation. Therefore, we believe our DST–HSA adhesive may be useful for clinical application to meniscus injury to preserve the meniscus in cases of horizontal tear or degenerative tear in the avascular zone.
In our study, the bonding strength of suturing using an adhesive-soaked suture strand was significantly greater than suturing after applying the adhesive on the surface of the lesion. We surmise that the DST–HSA adhesive remained in meniscal tissues and enforced bonding strength between the suture strand and collagen of meniscal tissues.
While further investigation is necessary for application in arthroscopic surgery in humans, our results demonstrate that the DST–HSA combination results in an effective adhesive that has biological characteristics of adhering collagen.
5. Conclusion
The newly developed DST–HSA adhesive is considered safe and may be effective in enforcement of bonding of avascular zone tear of the meniscus. If we can use this adhesive clinically, we may be able to keep the function of joints and avoid the procession of osteoarthritis in future.
References
- Arnoczky S P. Am. J. Sports Med. 1982;10:90. doi: 10.1177/036354658201000205. [DOI] [PubMed] [Google Scholar]
- Okuda K, Ochi M, Shu N. and Uchio Y. Arthroscopy. 1999;15:281. doi: 10.1016/S0749-8063(99)70035-6. [DOI] [PubMed] [Google Scholar]
- Rubman M H, Noyes F R. and Barber-Westin S D. Am. J. Sports Med. 1998;26:87. doi: 10.1177/03635465980260013301. [DOI] [PubMed] [Google Scholar]
- Brucker P U, Favre P, Puskas G J, von Campe A, Meyer D C. and Koch P P. Am. J. Sport Med. 2010;38:1838. doi: 10.1177/0363546510368131. [DOI] [PubMed] [Google Scholar]
- Konan S. and Haddad F S. Clin. Orthop. Relat. Res. 2010;468:1209. doi: 10.1007/s11999-009-1184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaka M, Yoshiya S, Kuroda R, Matsui N, Yamamoto T. and Tanaka J. J. Bone Joint Surg. Br. 2002;84:34. doi: 10.1302/0301-620X.84B1.11254. [DOI] [PubMed] [Google Scholar]
- Taguchi T, Saitoh H, Iwasashi M, Sakane M. and Ochiai N. Bioact. Compat. Polym. 2009;24:546. doi: 10.1177/0883911509349688. [DOI] [Google Scholar]
- Taguchi T, Saito H, Uchida Y, Sakane M, Kobayashi H, Kataoka K. and Tanaka J. Mater. Sci. Eng. C. 2004;24:775. doi: 10.1016/j.msec.2004.08.037. [DOI] [Google Scholar]
- Roos H, Lauren M, Adalberth T, Roos E M, Jonsson K. and Lohmander L S. Arthritis Rheum. 1998;41:687. doi: 10.1002/1529-0131(199804)41:4%3C687::AID-ART16%3E3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Stein T, Mehling A P, Welsh F, bon Eisenhart-Rothe R. and Jager A. Am. J. Sports Med. 2010;38:1542. doi: 10.1177/0363546510364052. [DOI] [PubMed] [Google Scholar]
- Haemer J M, Wang M J, Carter D R. and Giori N J. Clin. Orthop. Relat. Res. 2007;457:194. doi: 10.1097/BLO.0b013e3180303b5c. [DOI] [PubMed] [Google Scholar]
- Steenbrugge F, Verdonk R. and Verstraete K. Knee. 2002;9:181. doi: 10.1016/S0968-0160(02)00017-0. [DOI] [PubMed] [Google Scholar]
- McDermott I. Br. J. Sports Med. 2011;45:292. doi: 10.1136/bjsm.2010.081257. [DOI] [PubMed] [Google Scholar]
- Iwasashi M, Sakane M, Saito H, Taguchi T, Tateishi T. and Ochiai N. J. Biomed. Mater. Res. A. 2008;543 doi: 10.1002/jbm.a.31905. [DOI] [PubMed] [Google Scholar]
- Knox Cartwright N E. and Tole D M. J. Cataract. Refract. Surg. 2008;34:881. doi: 10.1016/j.jcrs.2008.02.026. [DOI] [PubMed] [Google Scholar]
- Tseng Y C, Tabata Y, Hyon S H. and Ikada Y. J. Biomed. Mater. Res. A. 1990;24:1355. doi: 10.1002/jbm.820241007. [DOI] [PubMed] [Google Scholar]
- Ciapetti G, Stea S, Cenni E, Sudanese A, Marraro D, Toni A. and Pizzoferrato A. Biomaterials. 1994;15:92. doi: 10.1016/0142-9612(94)90256-9. [DOI] [PubMed] [Google Scholar]
- Leggat P A, Smith D R. and Kedjarune U. ANZ J. Surg. 2007;77:209. doi: 10.1111/j.1445-2197.2007.04020.x. [DOI] [PubMed] [Google Scholar]


