Abstract
Spintronic is a multidisciplinary field and a new research area. New materials must be found for satisfying the different types of demands. The search for stable half-metallic ferromagnets and ferromagnetic semiconductors with Curie temperatures higher than room temperature is still a challenge for solid state scientists. A general understanding of how structures are related to properties is a necessary prerequisite for material design. Computational simulations are an important tool for a rational design of new materials. The new developments in this new field are reported from the point of view of material scientists. The development of magnetic Heusler compounds specifically designed as material for spintronic applications has made tremendous progress in the very recent past. Heusler compounds can be made as half-metals, showing a high spin polarization of the conduction electrons of up to 100% in magnetic tunnel junctions. High Curie temperatures were found in Co2-based Heusler compounds with values up to 1120 K in Co2FeSi. The latest results at the time of writing are a tunnelling magnet resistance (TMR) device made from the Co2FeAl0.5Si0.5 Heusler compound and working at room temperature with a (TMR) effect higher than 200%. Good interfaces and a well-ordered compound are the precondition to realize the predicted half-metallic properties. The series Co2FeAl1- xSix is found to exhibit half-metallic ferromagnetism over a broad range, and it is shown that electron doping stabilizes the gap in the minority states for x=0.5. This might be a reason for the exceptional temperature behaviour of Co2FeAl0.5Si0.5 TMR devices. Using x-ray diffraction (XRD), it was shown conclusively that Co2FeAl crystallizes in the B2 structure whereas Co2FeSi crystallizes in the L21 structure. For the compounds Co2FeGa or Co2FeGe, with Curie temperatures expected higher than 1000 K, the standard XRD technique using laboratory sources cannot be used to easily distinguish between the two structures. For this reason, the EXAFS technique was used to elucidate the structure of these two compounds. Analysis of the data indicated that both compounds crystallize in the L21 structure which makes these two compounds suitable new candidates as materials in magnetic tunnel junctions.
Keywords: Heusler compounds, electronic structure, intermetallics, half-metallic ferromagnets
Introduction
Spintronic is a multidisciplinary field and a new research area. New materials must be found that satisfy different types of demands like high spin polarization or high Curie temperature. One important group of materials with suitable candidates for spintronic applications are the Heusler compounds [1] as found from reviews about spintronic and half-metals in general in the recent past [2–4]. Heusler's compounds [5] are ternary intermetallics with a 2 : 1 : 1 stoichiometry and the chemical formula X2YZ. They usually consist of two transition metals (X2, Y) and a main group element (Z). They first attracted the interest of the magnetism community when Heusler et al had shown that the compound CuxMnyAl becomes ferromagnetic in the 2 : 1 : 1 form (x = 2 and y = 1), even none of its constituents is ferromagnetic by itself [6]. However, it took three decades before their structure was explained to be that of an ordered compound with a face centred cubic structure [7, 8].
The main interest during the first decades after the discovery of the Heusler compounds was concentrated on Cu and Mn containing compounds. Co2-based compounds were synthesized and investigated in the 1970s [9]. Kübler et al [10] recognized that the minority spin densities at the Fermi energy (∊F) nearly vanish for Co2MnAl and Co2MnSn. The authors concluded that this should lead to peculiar transport properties in these Heusler compounds because only the majority density contributes to the states at ∊F. At the same time, de Groot et al [11] proposed the concept of the so-called half-metallic ferromagnets (HMFs) that are materials predicted to exhibit 100% spin polarization at ∊F. This exceptional property would make the HMF ideal candidates for spin injection devices to be used in spin electronics [12].
The calculation of the electronic structure plays an important role in determining the magnetic properties of Heusler compounds and, in particular, for predicting half-metallic ferromagnetism. Therefore, the band structure calculations must be performed very carefully. The first attempt to calculate the band structure of some Co2 based compounds (Co2MnSn, Co2TiSi and Co2TiAl) did not indicate half-metallic ferromagnetism [13]. These calculations displayed a minimum of the minority density of states (DOS) at ∊F but not a gap. At that time, the calculations were based on spherical potentials, and the exchange-correlation potential of the local spin density approximation (LSDA) was used in a rather simple form [14–17]. The first clear indication of half-metallic ferromagnetism in Co2 based Heusler compounds was reported by Ishida et al [18, 19] for Co2MnZ and Ru2MnZ (Z=Al, Si, Sn and Sb). Using full symmetry potentials, Mohn et al [20] found the magnetic ground state of Co2TiZ (Z=Al and Sn), but not a half-metallic state. Galanakis et al [21] reported half-metallic behaviour in various X2YZ compounds, but not for the Co2 compounds with Ti or Fe. The results were compatible with those found for the Mn compounds as calculated by Picozzi et al [22] using the generalized gradient approximation (GGA) instead of the pure LSDA. The GGA, as introduced by Perdew et al [23–26], accounts for gradients of the density that are absent in the pure LSDA parameterization of the exchange-correlation functional [14–17]. Using spherical potentials and the GGA, a half-metallic state could not be verified for Co2FeAl [27, 28]. A half-metallic ferromagnetic ground state was also found for the complete series Co2Cr1−xFexAl, when the full symmetry potentials were used along with the GGA in the calculations [29]. This clearly indicates that one principally needs both the full symmetry potentials and the GGA to find the correct electronic structure and ground state for the Heusler compounds. In some cases it is sufficient to use LSDA with spherical potentials. Parts of the results shown in section 8 and the work of Kübler et al [10] are calculated using this method.
Theory and motivation
For both scientific and technological reasons it is useful to define the electron spin polarization at the Fermi energy of a material, although it is difficult to measure [30]. The spin polarization at ∊F is given by:
where ρ↑(∊F) and ρ↓(∊F) are the spin projected DOS at the ∊F. The arrows ↑ and ↓ assign states of opposite spin that are majority and minority states, respectively. P vanishes for paramagnetic or in anti-ferromagnetic materials even below the magnetic transition temperature. However, it has a finite value in ferromagnetic or ferrimagnetic materials below the Curie temperature. The electrons at ∊F are fully spin polarized (P=100%) when either  or
or  equals zero. In the present work, the classification scheme for half-metals as proposed by Coey et al is used [1, 12].
equals zero. In the present work, the classification scheme for half-metals as proposed by Coey et al is used [1, 12].
The Slater–Pauling curve [31, 32] is a simple way to study for ferromagnetic alloys the interrelation between the valence electron concentration and the magnetic moments (see figure 1). It is well known that Heusler compounds based on Co2 follow the Slater–Pauling rule for predicting their total spin magnetic moment [21, 33, 34] that scales linearly with the number of valence electrons. The Co2- based compounds are found on the so called localized part of the Slater–Pauling curve [33, 34] where the magnetic moment increases with an increasing number of valence electrons. In this part of the curve one has predominantly materials with bcc or bcc-derived structures. Like iron, for the example, the electronic structure of these alloys exhibits a minimum in the minority DOS and the Fermi energy is pinned in this minimum. The minimum in the minority spin density constrains the number of occupied electrons in the minority bands to be approximately three such that the number of majority electrons increases proportional to the total number of electrons and so does the magnetic moment as a direct consequence (for more details see for example: [33, 34]).
Figure 1.

Slater–Pauling curve for 3d transition metals and their alloys. Experimental values for selected Co2-based Heusler compounds are given for comparison. (Note: the A1- xBx alloys are given as AB in the legend, for short.)
Half-metallic ferromagnets, like the Co2 based Heusler compounds exhibit not only a minimum but a real gap in the minority DOS and the Fermi energy is pinned inside of that gap. From this point of view, the Slater–Pauling rule is strictly fulfilled with
for the mean magnetic moment per atom (mHMF). nV is the mean number of valence electrons per atom found by averaging over all atoms and 6 is two times the mean number of occupied minority states. The advantage of this equation is that it neither depends on the number of atoms in the compound nor relies on integer site occupancies.
For ordered compounds with different kinds of atoms it might be more convenient to work with all atoms of the unit cell. In the case of four atoms per unit cell, as in Heusler compounds, one has to subtract 24 (6 multiplied by the number of atoms) from the accumulated number of valence electrons in the unit cell NV (s, d electrons for the transition metals and s, p electrons for the main group element) to find the magnetic moment per unit cell (m):
with NV denoting the accumulated number of valence electrons in the unit cell containing four atoms. In the case of Heusler compounds, the number 24 arises from the number of completely occupied minority bands that has to be 12 in the half-metallic state. In particular, these are one s (a1g), three p (t1u), and eight d bands [35, 36]. The latter consist of two triply degenerate bands with t2g symmetry and one with eg symmetry (note that the given assignments of the irreducible representations are only valid at the Γ-point and neglect the spin of the electrons.).
A similar rule was first noted by Kübler [35] for C1b compounds with three atoms per unit cell (mC1b=NV−18). In both cases the magnetic moment per unit cell becomes strictly integer (in multiples of Bohr magnetons μB) for type I or II half-metals, what may be seen as an advantage of the valence electron rule (equation (3)) compared to the original Slater–Pauling approach (equation (2)) even so it suggests the existence of different laws. It is worthwhile to note that equation (3) leads only for ternary 2 : 1 : 1 compounds to integer magnetic moments but not for quarternary derivatives like X2Y1−xYxZ or X2YZ1−xZx as reported in [37] or [38], respectively. In those cases, one observes from (3) non-integer values of the magnetic moment even in the half-metallic case due to the non-integer site occupancy.
The Slater–Pauling curve is shown in figure 1. Experimental values of the magnetic moments in selected Co2-based Heusler compounds are compared to 3d transition metals and their alloys. This comparison is only possible if the Slater–Pauling rule in the formulation of (2) is used. The itinerant part of the curve is included for clarity about the behaviour of the Heusler compounds in comparison to other ferromagnetic alloys. For a detailed discussion of that part see [1, 33, 34].
Inspecting the magnetic data of the known Heusler compounds in more detail (see data and references in [39, 40]), one finds a very interesting aspect. A linear dependence is obtained approximately for Co2-based Heusler compounds when plotting the Curie temperature (TC) of the known, 3d metal based Heusler compounds as function of their magnetic moment (see figure 2, left panel). According to this plot, TC is highest for those half-metallic compounds that exhibit a large magnetic moment, or equivalently for those with a high valence electron concentration as derived from the Slater–Pauling rule. TC is estimated to be above 1000 K for compounds with 6 μB by an extrapolation from the linear dependence.
Figure 2.

Curie-temperatures of X2YZ Heusler compounds. The line is found from a linear fit of the measured TC for Co2-based compounds (red cross). The elemental metals Fe, Co and Ni are given for comparison (left panel). Calculated versus measured Curie temperatures for Co2-based compounds. The calculation done with GGA are denoted with a plus sign (right panel).
Recently, Kübler [41] developed an ab initio estimate for calculating the Curie temperature of an itinerant-electron ferromagnet in the spherical approximation using the LDA scheme. With this approach, Kübler et al [42] calculated the Curie temperatures of Co2-based Heusler compounds. Figure 2 (right panel) compares the calculated Curie temperatures with the measured ones for several Co2-based compounds. A very good agreement between calculation and experiment is obvious. A Curie temperature of 1185 K was calculated for Co2FeSi that fits quite well the experimental value of 1100 K [43].
From this finding one expects Co2FeZ (with Z=Al, Ga, Si or Ge) compounds to have a large magnetic moment and high Curie temperatures that makes these compounds suitable candidates for spintronic applications.
Crystal structure
Besides the high Curie temperature the crystallographic order of the sample is highly important for the use of these compounds in any kind of spintronic application.
Generally, the X2YZ Heusler compounds crystallize in the cubic L21 structure (space group no 225:  ), the prototype is AlCu2Mn. The structure was first explained by Heusler [7] as well as Bradley and Rodgers [8]. In general, the X and Y atoms are transition metals and Z is a main group element. In some cases, Y is replaced by a rare earth element. The X atoms are placed on the Wyckoff position 8c
), the prototype is AlCu2Mn. The structure was first explained by Heusler [7] as well as Bradley and Rodgers [8]. In general, the X and Y atoms are transition metals and Z is a main group element. In some cases, Y is replaced by a rare earth element. The X atoms are placed on the Wyckoff position 8c  . The Y and Z atoms are located on 4a (0, 0, 0) and 4b
. The Y and Z atoms are located on 4a (0, 0, 0) and 4b  positions, respectively. The cubic L21 structure consists of four interpenetrating fcc sub-lattices, two of which are equally occupied by X. The two X-site fcc sub-lattices combine to form a simple cubic sub-lattice. The Y and Z atoms occupy alternatingly the centre of the simple cubic sub-lattice resulting in a CsCl-type super structure. This results in Oh symmetry for the 4a and 4b positions, whereas the 8c position has Td symmetry.
positions, respectively. The cubic L21 structure consists of four interpenetrating fcc sub-lattices, two of which are equally occupied by X. The two X-site fcc sub-lattices combine to form a simple cubic sub-lattice. The Y and Z atoms occupy alternatingly the centre of the simple cubic sub-lattice resulting in a CsCl-type super structure. This results in Oh symmetry for the 4a and 4b positions, whereas the 8c position has Td symmetry.
The cubic X2YZ compounds are not only found with the AlCu2Mn type structure but also with the CuHg2Ti type structure (note that the classification of the cubic X2YZ compounds with those two structures is sometimes not uniquely given in Pearson's Handbook [44]). The CuHg2Ti type structure exhibits Td symmetry (space group no 216:  ). In that structure the X2 atoms occupy the non equivalent 4a, 4c Wyckoff positions at (0, 0, 0) and
). In that structure the X2 atoms occupy the non equivalent 4a, 4c Wyckoff positions at (0, 0, 0) and  . The Y and Z are located on 4b
. The Y and Z are located on 4b  and 4d
and 4d  positions, respectively. All four positions adopt Td symmetry and there is no position with Oh symmetry. This structure is similar to the XYZ compounds with C1b structure, but with the vacancy filled by an additional X atom. This structure is frequently observed if the nuclear charge of the Y element is larger than the one of the X element from the same period, that is Z(Y)>Z(X) for two 3d transition metals. The structure may also appear in compounds with transition metals from different periods. However, the two structures may be hardly distinguishable by XRD and much care has to be taken in the structural analysis, as both have the general fcc-like symmetry.
positions, respectively. All four positions adopt Td symmetry and there is no position with Oh symmetry. This structure is similar to the XYZ compounds with C1b structure, but with the vacancy filled by an additional X atom. This structure is frequently observed if the nuclear charge of the Y element is larger than the one of the X element from the same period, that is Z(Y)>Z(X) for two 3d transition metals. The structure may also appear in compounds with transition metals from different periods. However, the two structures may be hardly distinguishable by XRD and much care has to be taken in the structural analysis, as both have the general fcc-like symmetry.
The reviewing articles of Neumann and Ziebeck [39, 40] summarize a possible disorder in Heusler (X2YZ) and C1b (XYZ) compounds. A detailed description of the disorder in L21 and C1b compounds with half and quarter occupancies of the sites is given by Bacon and Plant [45]. The simplest types of disorder are the B2 and the A2 structure. In the B2 structure the Y and Z atoms are mixing which results in a CsCl-like structure (see figure 3, middle) while in the A2 structure all the atoms are mixing resulting in a complete alloyed structure (see figure 3, right). Almost all properties of the compound react very sensitive to any kind of disorder even if there is only a small amount of B2-type disorder.
Figure 3.
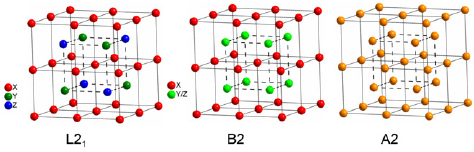
The ordered L21 Heusler structure (left) and the simplest types of disorder: The B2 (middle) and the A2 (right) structure. Note that all positions are shifted by  with respect to the standard
with respect to the standard  cell to make the CsCl superstructure visible.
cell to make the CsCl superstructure visible.
For example, Wurmehl et al calculated the magnetic moments for different kinds of disorder of Co2FeAl and reported a value of 5.46μB for the A2 structure [46]. The increase of the magnetic moment in the disordered A2 structure emerges mainly from an increase of the magnetic moment of the Co atoms. At the same time the orbital moment is enhanced. The latter effect can be attributed to the loss of the local cubic environment in the disordered structure. It is worthwhile to note that the structural disorder destroys the half-metallic character and results in a dramatic decrease of the spin polarization [27].
Co2FeSi
From the findings above that one expects for Co2FeZ (with Z=Al, Ga, Si or Ge) compounds to have a large magnetic moment and high Curie temperatures Co2FeSi was revisited as a practical test for the findings above [43, 47].
Theory
As starting point, self consistent first principle calculations were performed using the linearized muffin tin orbital (LMTO) method [48], as this method is very fast. Using the experimental lattice parameter (aexp=5.64 Å), the results predicted Co2FeSi to be a regular ferromagnet with a magnetic moment of 5.08μB per formula unit. The latter value is much too small compared to the experimental one of 6μB.
More detailed calculations were performed to check if the low value of the moment is the result of a particular method or the parameterization of the energy functional. The Korringa–Kohn–Rostocker method as provided by Akai and Dederichs [49] was chosen as this program allows calculations in the muffin tin (MT) and the atomic sphere (ASA) approximations. Additionally, it provides the coherent potential approximation (CPA) to be used for disordered systems. This method was used to estimate the influence of disorder on the magnetic structure.
The calculations were started with the most common parameterizations of the exchange-correlation functional as given by Moruzzi, Janak and Williams (MJW) [50], von Barth and Hedin (vBH) [16], and Vosco, Wilk, and Nussair (VWN) [17]. The GGA was used in the form given by Perdew et al [51]. To include non-local effects, the VWN parameterization with additions by Perdew et al [23, 52] was used (PYVWN). The so-called exact exchange is supposed to give the correct values for the gap in semiconductors using local density approximation. Here it was used in the form given by Engel and Vosko (EV) [53].
The calculated total magnetic moments range from ≈4.9μB to ≈5.7μB, thus they are throughout too low compared to the experiment. They include, however, the value of 5.27μB found in [21] by means of the full potential KKR method.
In the next step, the full potential linear augmented plane wave (FLAPW) method as provided by Wien2k [54] was used to exclude that the observed deviations are due to the spherical approximation for the potential (MT or ASA) as used in the above KKR methods.
First, the exchange-correlation energy functional being parameterized within the GGA was used. It turned out, however, that the magnetic moment of Co2FeSi is still too small compared to the experimental value. Comparing the result for Co2FeSi, the magnetic moments found by the different calculational schemes are very similar, implying that Co and Fe atoms are aligned parallel independent of the method used. The small, induced moment at the Si atom is aligned anti-parallel to that at the transition metal sites. As for KKR, the use of the EV parameterization of the energy functional did not improve the magnetic moment, the result was only 5.72μB.
In contrast to the LMTO or KKR methods (spherical potentials), the FLAPW (full symmetry potential) calculations revealed a very small gap in the minority states, but being located below the Fermi energy. The fact that the Fermi energy cuts the minority bands above the gap causes the magnetic moment to be too low and not integer, as would be expected for a HMF.
A structural refinement was performed to check if the experimental lattice parameter minimizes the total energy. The dependence of the energy with lattice parameter a revealed that the minimum occurs at the experimentally observed lattice parameter aexp. From the lattice parameter-dependent calculations it is seen that the experimentally found magnetic moment appears at larger values of a. At the same time the size of the gap increased. Inspecting the band structure, one finds that the Fermi energy is inside of the gap for lattice parameters being enlarged by about 2.4–4%.
The magnetic moment is integer (6μB) in the region were ∊F falls into the gap, that is the region of half-metallic ferromagnetism. The reason for the integer value is clear: the number of filled minority states is integer and thus the magnetic moment, too (see equations (2) and (3)).
Usually, Heusler compounds are attributed to exhibit localized moments. In that case, electron–electron correlation may play an important rule. The LDA+U scheme [55] was used for calculation of the electronic structure to find out whether the inclusion of correlation resolves the discrepancy between the theoretical and measured magnetic moment. In Wien2k, the effective Coulomb-exchange interaction (Ueff=U−J, where U and J are the Coulomb and exchange parameter) is used. The use of Ueff neglects multipole terms in the expansion of the Coulomb interaction. The self interaction correction (SIC) scheme was used to account for double-counting corrections. It turned out that values of Ueff from 2.5 to 5.0 eV for Co and simultaneously 2.4 to 4.8 eV for Fe result in a magnetic moment of 6μB and a gap in the minority states.
Figure 4 shows the band structure and DOS calculated using the LDA+U method. The effective Coulomb-exchange parameters were set to Ueff, Co=4.8 eV and Ueff, Fe=4.5 eV at the Co and Fe sites, respectively. These values are comparable to those found in [56] for bcc Fe (4.5 eV) and fcc Ni (5 eV).
Figure 4.

LDA+U band structure and DOS of Co2FeSi. The calculation was performed by Wien2k using the experimental lattice parameter.
The minority DOS (figure 4) exhibits a clear gap around ∊F, confirming the half-metallic character of the material. The high density below ∊F is dominated by d-states being located at Co and Fe sites. Inspecting the majority DOS one finds a small DOS near ∊F. This density is mainly derived from states located at Co and Si sites.
It is found that in average three minority states per atom are occupied (2n↓ = 6) as required by the Slater–Pauling rule for the range of increasing magnetic moments with an increasing number of valence electrons. It is worthwhile to note that the same is true for the other Heusler compounds shown in figure 1 exhibiting half-metallic ferromagnetism. However, the electrons are distributed in a different way across the X, Y and Z sites.
Experiments
The correct L21 structure of the Co2FeSi compound (prepared by arc-melting in an argon atmosphere) was verified by XRD. The lattice constant was determined to be 5.64 Å. A disorder between Co and Fe atoms (DO3 type disorder) can be excluded from the Rietveld refinement of the XRD data, as well as from neutron scattering data. A small (<10%) disorder between Fe and Si atoms (B2 type disorder) can not be excluded by either of these methods, particularly due to the low intensities of the (111) and (200) diffraction peaks in XRD.
For further site specific structural information, extended x-ray absorption fine structure (EXAFS) measurements were carried out. A powder sample, as used for XRD, was investigated in transmission mode. The best fitting of the Fourier transform modulus consider the L21 structure. It was not possible to fit the experimental data to a structural model including DO3 type disorder, as expected. Thus, the EXAFS measurements corroborate the XRD results even at the short range order for both Co and Fe.
Low temperature magnetometry was performed by means of SQUID to proof the estimated saturation moment. The measured magnetic moment in saturation is (5.97±0.05)μB at 5 K corresponding to 1.49μB per atom. An extrapolation to 6μB per unit cell at 0 K fits perfectly to the one estimated from the Slater–Pauling rule. The measurement of the magnetic moment reveals, as expected for a HMF, an integer within the experimental uncertainty. Regarding the result of the measurement (an integer) and the valence electron rule, it all sums up to an evidence for half-metallic ferromagnetism in Co2FeSi.
X-ray magnetic circular dichroism (XMCD) in photo absorption (XAS) was measured to investigate the site specific magnetic properties. The XAS and XMCD spectra taken at the L2, 3 absorption edges of Fe and Co are shown in figure 5. The feature seen at 3 eV below the L3 absorption edge of Co is related to the L21 structure and points at the high structural order of the sample (it vanishes for B2 like disorder). The magnetic moments per atom derived from a sum rule analysis [57, 58] are (2.6±0.1)μB for Fe and (1.2±0.1)μB for Co, at T=300 K and μ0H=0.4 T. The error arises mainly from the unknown number of holes in the 3d shell and the disregard of the magnetic dipole term in the sum rule analysis. A pronounced enhancement of the orbital magnetic moments (ml) (as in Co2Cr1−xFexAl [59]) or a field dependence of ml [60] was not observed for Co2FeSi.
Figure 5.

Site resolved magnetic properties of Co2FeSi. Shown are the XAS and XMCD (IMCD) spectra taken at the L2, 3 absorption edges of Fe (a) and Co (b) after subtracting a constant background.
To proof the estimated Curie temperature high temperature magnetization of Co2FeSi was measured by means of VSM. The ferromagnetic Curie temperature is found to be TC=(1100±20) K. This value fits very well the linear behaviour shown in figure 2 for Co2-based Heusler compounds. The highest known Curie temperature is reported for elemental Co to be 1388 K [61]. Only few materials exhibit a TC above 1000 K, for example the Fe–Co binary alloys. With a value of ≈1100K, Co2FeSi has a higher Curie temperature than Fe and the highest of all HMF and Heusler compounds being measured up to now.
The role of correlation in the Heusler compounds Co2MnSi and Co2FeSi
Besides Co2FeSi the Heusler alloy Co2MnSi has attracted particular interest because it is predicted to have a large minority spin band gap of 0.4 eV and, at 985 K, has one of the highest Curie temperature, among the known Heusler compounds [62, 63]. Structural and magnetic properties of Co2MnSi have been reported for films and single crystals [64–70]. In accordance with theoretical predictions, bulk Co2MnSi has been stabilized in the L21 structure with a magnetization of 5 μB per formula unit. From TMR data with one electrode consisting of a Co2MnSi film Schmalhorst et al [71, 72] implied a spin polarization of 61% at the barrier interface. Although the desired spin polarization of 100% was not reached, the experimental value of the spin polarisation is larger than the maximum 55% effective spin polarization of a variety of 3d-transition metal alloys in combination with Al2O3 barriers [73]. However, the spin polarization of photoelectrons emerging from single crystalline Co2MnSi films grown on GaAs by pulsed laser deposition indicate a quite low spin polarization at the Fermi level of only 12% at the free surface [69]. Wang et al [68, 69] assumed that partial chemical disorder was responsible for this discrepancy with the theoretical predictions.
In order to explain this quite low spin polarization of Co2MnSi and the assumption that on-site correlation will also play an important role in the Co2MnSi compound if it plays an important role in the Co2FeSi compound Kandpal et al [74] presented a comprehensive investigation of the equilibrium structural, electronic and magnetic properties of Co2FeSi and compared it to the properties of Co2MnSi.
The correct magnetic moment at the experimental value of the lattice parameter was only found if the +U functional was used. Using the LDA+U scheme improves the total magnetic moment considerably. It was found that the gap in the minority DOS stays up to a Ueff=2.3 eV for Mn, and a Ueff=2.5 eV for Co. For larger values of Ueff, which means stronger correlation, the system loses its HMF character because ∊F is shifted outside the minority gap.
In the following, the Ueff values will be denoted by Ux, where the subscript x stands for the values (in %) relative to the atomic values (neutral atoms). The atomic values for the neutral atoms are 22.71, 24.13 and 25.53 eV for Mn, Fe and Co, respectively (see e.g. figure 5 in [74]).
It was found for Co2MnSi that even a moderate correlation will destroy the gap. If U10 is applied, then the spin polarization at ∊F is 75%, a value that is compatible to the approximately 55% found in [67]. This indicates that on-site correlation might be one more reason why no complete spin polarization was found in this compound (for others, see [75]).
The dependence of the behaviour of the Co2FeSi minority gap and the Co2MnSi minority gap on the effective Coulomb exchange parameter was examined and compared. The extremal energies of the gap enveloping bands are shown for both compounds in figure 6.
Figure 6.

Dependence of the minority gap on the effective Coulomb exchange parameter. The extremal energies of the gap involving states for Co2MnSi (a) and Co2FeSi (b) are shown. The shaded areas indicate the region of half-metallic ferromagnetism. Lines are drawn for clarity. The atomic values for the neutral atoms are 22.71, 24.13 and 25.53 eV for Mn, Fe and Co, respectively.
In both compounds, the size of the gap increases with increasing Ueff. From figure 6(a), it is found that Co2MnSi stays in an HMF state up to approximately U8, which is the range of the integer magnetic moment. Larger values shift ∊F outside the gap. In Co2FeSi (figure 6(b)), the minority gap includes ∊F from approximately U8 to U20. This means that the integer value of the magnetic moment is related to the minority gap in both compounds and is therefore a direct consequence of the HMF state.
In both compounds, the gap is completely destroyed at very large values of Ueff (≽8 eV). The effect of the Coulomb exchange interaction on single atoms (Co, Mn or Fe) on the minority band gap was also investigated. As expected, it was found that the minority gap is destroyed in both materials if Ueff is added to only one of the 3d elements.
The gap in the minority states stays only within a certain range of the effective Coulomb exchange interaction. Outside of that limit, the Fermi energy no longer falls inside the gap, and the material loses its HMF character. It is also obvious that the same mechanism that leads to the half-metallic ferromagnetism in Co2FeSi serves to destroy it in Co2MnSi. The worst case for both materials would appear if Ueff is approximately 7,…,8% of the atomic values. That is the case where the LDA+U still predicts values very close to the measured magnetic moments but which is also the borderline for the loss of the HMF character. This indicates that nearly integer magnetic moments alone do not verify the half-metallic ferromagnetism and that it may be necessary to search for alternative materials.
Details of the Hubbard U for all 3d-elements in Heusler compounds are published in [74] and [76]. Some additional details about the values of U for Co, Mn, Fe are given in [37].
Co2Mn1−xFexSi
Co2MnSi and Co2FeSi are unstable HMF as a consequence of the fact that on-site correlation may destroy the half-metallic properties. Therefore, Balke et al [37] focused on the investigation of mixed compounds Co2Mn1−xFexSi to search for a stable half-metallic character.
Theory
After inspecting and comparing the electronic structure of Co2MnSi and Co2FeSi in more detail, some particular changes are found. The most striking effect, however, is the shift of the Fermi energy from the top of the minority valence band to the bottom of the minority conduction band. These particular positions of the minority gap with respect to the Fermi energy make both systems rather unstable with respect to their electronic and magnetic properties. Any small change of a physically relevant quantity may serve to destroy the HMF character by shifting the Fermi energy completely outside of the minority gap. As long as the shift is assumed to be small, the magnetic moment may still be similar to the one expected from a Slater–Pauling behaviour, even so, the minority gap is destroyed. For this reason, the magnetic moment may not provide evidence for a half-metallic state. It is to be expected immediately that the situation improves in the mixed compounds containing both Mn and Fe.
Band structure calculations using the LDA+U scheme were performed to prove this prediction (for more calculation details see [37]). The minority DOS is shifted with respect to the Fermi energy such that ∊F moves from the top of the minority valence bands at low x to the bottom of the minority conduction bands at high x (see figure 7). In general, it can be concluded that the additional electrons affect both majority and minority states.
Figure 7.

Dependence of the minority gap on the Fe concentration. The extremal energies of the gap involving states are shown. The shaded areas indicate the region of half-metallic ferromagnetism. Lines are drawn for clarity.
The largest gap in the minority states is found for Co2MnSi. The size of the gap decreases with increasing Fe content x. At the same time, the position of the Fermi energy is moved from the top of the valence band to the bottom of the conduction band. It is also seen that the compounds with x=0 and 1 are on the borderline to half-metallic ferromagnetism, as the Fermi energy just touches the top of the valence band or the bottom of the conduction band. In both cases, a slight change of Ueff in the calculation is able to shift ∊F outside of the gap in the minority states.
For intermediate Fe concentration, the Fermi energy falls close to the middle of the gap in the minority states (see also figure 7). This situation makes the magnetic and electronic properties of the compound very stable against external influences that will not be able to change the number of minority electrons. This applies both to the parameters in the theoretical calculations as well as the actual experimental situation. From this observation it can be concluded that Co2Mn1/2Fe1/2Si exhibits a very stable half-metallic character in this series of compounds. Indeed, this is also true for all concentration close to x=0.5.
Experiments
The substitutional series of the quaternary Heusler compound Co2Mn1−xFexSi was synthesized and investigated experimentally [37]. All samples of the substitutional series exhibit the L21 structure independent of the Fe concentration x. Mößbauer measurements show only a negligible paramagnetic contribution confirming the high degree of order over the whole substitutional series. In agreement with the expectation from the Slater–Pauling curve, the magnetic moment increases linearly with x from 5 to 6 μB (see figure 8).
Figure 8.

Concentration dependence of the magnetic moment in Co2Mn1- xFexSi. All measurements were performed at T=5 K.
Photo emission spectroscopy is the method of choice to study the occupied electronic structure of materials. But, low kinetic energies result in a low electron mean free path λ. It is only 5.2 Å at kinetic energies of 100 eV (calculated for Co2FeSi using the TPP2M equations [77]). Using low energies, only a depth of less than one cubic Heusler cell will contribute to the observed intensity. The situation becomes much better at high energies. In the hard x-ray region of about 8 keV one will reach a high bulk sensitivity with an escape depth being larger than 115 Å (corresponding to 20 cubic cells). High energy photo emission (at about 15 keV excitation energy) was first performed as early as 1989 [78] using a 57Co Mößbauer γ-source for excitation, however, with very low resolution only. Nowadays, high energy excitation and analysis of the electrons become easily feasible due to the development of high intense sources (insertion devices at synchrotron facilities) and multi-channel electron detection. Thus, high resolution—hard x-ray photo emission spectroscopy (HAXPES) was recently introduced by several groups [79–84] as a bulk sensitive probe of the electronic structure in complex materials. Balke et al [37] used HAXPES at hν≈8 keV to study the DOS of Co2Mn1−xFexSi with x=0, 1/2, 1.
Overall, the measured photoelectron spectra agree well with the calculated DOS and principally verify the use of the LDA+U scheme (see figure 9).
Figure 9.

Valence density of Co2Mn1- xFexSi (x=0, 1/2, 1). (a)–(c) Compare the calculated total DOS with photoelectron spectra excited by hν=7.939 keV. The calculated DOS is convoluted by a Fermi–Dirac distribution using T=20 K. (e)–(g) Show high resolution spectra close to the Fermi energy. The range of the calculated minority gap is marked by areas.
Most interesting is the behaviour of the calculated DOS and the measured spectra close to ∊F as this might give an advice about the gap in the minority states. The majority band structure contributes only few states to the density at ∊F emerging from strongly dispersing bands. This region of low density is enclosed by a high DOS arising from flat bands at the upper and lower limits of the minority band gap. The onset of the minority valence band is clearly seen in the total DOS as well as the low majority density at the Fermi energy. The same behaviour is observed in the measured valence band spectra. From the spectra, it can be estimated that the Fermi energy is in all three cases about 0.5 eV above the minority valence band. This gives strong evidence that all compounds of the Co2Mn1−xFexSi series exhibit really half-metallic ferromagnetism.
For clarity about the gap, spin resolved photo emission spectroscopy at high energies would be highly desirable. However, this will make another step of improvement of the instrumentation necessary, for both photon sources as well as electron energy and spin analyzers. In particular, the spin detection will need a factor of at least three to four orders of magnitude more intensity for a single energy channel at the same resolution as used here for the intensity spectra.
True bulk sensitive, high energy photo emission bearded out the inclusion of electron–electron correlation in the calculation of the electronic structure and gave an indirect advise on the gap in the minority states. Both, valence band spectra and hyperfine fields indicate an increase of the effective Coulomb exchange parameters with increasing Fe concentration.
From both the experimental and computational results it is concluded that a compound with an intermediate Fe concentration of about 50% should be most stable and best suited for spintronic applications.
Co2FeAl1−xSix
The end members of this series Co2Mn1−xFexSi (x=0 and 1) have been used for fabrication of magnetic tunnel junctions [85–87]. The TMR ratios of 159% (with AlOx barrier at 2 K [70]) or 195% (with MgO barrier at 4.2 K [88]) in the Mn compound at low temperature compared to about 70% (for both barrier materials) at room temperature (41% in the Fe compound) suggest that still an improvement with respect to the temperature stability of the TMR is necessary.
In Co2Mn1−xFexSi the transition metal carrying the localized moment is exchanged. This might lead to unexpected effects on the magnetic properties if the samples are not completely homogeneous. The situation is different in the iso-electronic series Co2FeAl1−xSix where the main group element is substituted. Tezuka et al [89, 90] reported about TMR junctions build from Co2FeAl0.5Si0.5. The junctions exhibited TMR ratios of 76% at 300 K and 106% at 5 K for the B2 structure while the junctions with L21 structure showed 51 and 78% at 300 and 5 K, respectively. The TMR ratio is 175% at 300 K for optimized junctions with L21 structure [90]. This value is pronouncedly larger than the ones found for pure Co2FeAl or Co2FeSi electrodes.
Theory
The electronic structure of the substitutional series of the quaternary Heusler compounds Co2FeAl1−xSix was investigated by means of FLAPW band structure calculations using the LDA and LDA+U approximations [38]. It was found that the Co2FeAl1−xSix series of compounds exhibits half-metallic ferromagnetism if using the LDA+U scheme. Moderate Coulomb-interaction parameters of less than 2 eV were used. For the two end-members, Co2FeAl and Co2FeSi, the Fermi energy is close to the band edges of the minority states. The high densities at those band edges make the half-metallic character of both compounds rather unstable at finite temperatures above 0 K. This might be one reason explaining the low TMR ratio found in those compounds at room temperature.
Figure 10 shows the spin resolved DOS of Co2FeAl1−xSix for x=0, 0.25, 0.5, 0.75 and 1. For x≈0.5, the calculations predict that the Fermi energy is located in the middle of the gap of the minority states. This behaviour will make Co2FeAl0.5Si0.5 stable against temperature variations as discussed in the introduction.
Figure 10.

Spin resolved DOS of Co2FeAl1- xSix. The panels (a, … , e) show—from top to button—the DOS with increasing amount of Si for x=0, 0.25, 0.5, 0.75 and 1. The DOS is calculated using LDA+U.
Experiments
Experiments were started to verify over what range of compositions the series Co2FeAl1−xSix crystallizes in the required L21 structure and to find the most stable HMF in this series [91]. The L21 structure is essentially required for a high spin polarization resulting in high magneto-resistive effects.
It was found that the samples exhibiting the L21 structure for x≽0.4 and the B2 structure for x<0.4. Differential scanning calorimetry (DSC) was used to find the high temperature phase transitions in the substitutional series. Figure 11 displays the dependence of the order–disorder transition temperature as a function of the Si concentration x. Overall, figure 11 demonstrates that the structural transition temperature of the L21 to the B2 phase  decreases almost linearly with increasing Si content at least for x>0.4. The results from both, XRD and DSC, demonstrate the better structural stability of the compounds with high Si content. This is expected from the stronger hybridization between Co and Si in these compounds [38].
decreases almost linearly with increasing Si content at least for x>0.4. The results from both, XRD and DSC, demonstrate the better structural stability of the compounds with high Si content. This is expected from the stronger hybridization between Co and Si in these compounds [38].
Figure 11.

Order–disorder phase transitions in Co2FeAl1- xSix. Shown is the composition dependence of the phase transition temperature. The length of the vertical bars corresponds to the experimental hysteresis.
From the combination of experimental (better order for high Si content) and theoretical findings (robust gap at x≈0.5±0.25) it is concluded that a compound with an intermediate Si concentration close to x=0.5…0.7 would be best suited for spintronic applications. It was shown that the variation of the main group element in Heusler compounds is a strong tool in order to tune their physical properties.
Co2FeGa and Co2FeGe
Studies of the Co2FeZ compounds with Ga or Ge on the Z position are mostly reported for bulk samples rather than for thin films. Bulk Co2FeSi has been reported by Niculescu et al [92] and was investigated in detail by Wurmehl et al [43, 47]. Co2FeGa and Co2FeGe have been reported by Bushow et al [93] to exist in the L21 structure. In many cases, when the main group element is from the same period of the periodic system as the transition metals, x-ray or Neutron diffraction does not provide enough information to determine the correct structure unambiguously. The correct L21 structure, however, is a necessary requirement for a high spin polarization of the materials as base for a high TMR ratio [1]. Therefore, additional methods are needed to explore the correct structure. Particularly, EXAFS and Mößbauer spectroscopy can provide additional information about the short range order of the structure [94].
Theory
The half-metallic ferromagnetism manifests itself in a band gap in one of the spin densities and the Fermi energy is inside of the gap. However, there exist also cases where such a gap exists in the calculations but the Fermi energy may fall outside of that gap. Both compounds Co2FeGa and Co2FeGe show a gap along Γ−X that is in the Δ-direction of the paramagnetic state. The Δ-direction is perpendicular to the Co2 (100)-planes. As was shown earlier [29], just the Δ-direction plays the important role for understanding of the HMF character and magnetic properties of Heusler compounds. This fact was also pointed out by Öĝüt and Rabe [95].
Co2FeGe shows the properties of the Type I half-metals where ∊F is located inside of the gap of the minority band structure, even it is very close to a Type III half-metal. As a direct consequence, the minority density ρ↓(∊F) at the Fermi energy vanishes and the spin polarization is 100%. On the other hand, Co2FeGa is a Type III half-metal, which means that the compound exhibits a band gap in the minority states but with ∊F outside of the gap. In this class of compounds the minority band gap does not include ∊F. For a detailed investigation and discussion about the calculated electronic and magnetic properties of the half-metallic, transition metal based Heusler compounds see [76]. Figures 12 and 13 show the LDA+U band structure and DOS of Co2FeGa and Co2FeGe, respectively.
Figure 12.

LDA+U band structure and DOS of Co2FeGa. The calculation was performed by Wien2k using the experimental lattice parameter.
Figure 13.

LDA+U band structure and DOS of Co2FeGe. The calculation was performed by Wien2k using the experimental lattice parameter.
As already mentioned above, Kübler [41] developed an ab initio estimate for calculating the Curie temperature of an itinerant-electron ferromagnet in the spherical approximation using the LDA scheme. With this approach the Curie temperatures for Co2FeGa and Co2FeGe were calculated. Depending on the approximation—LSDA or full-potential GGA—the calculations result in Curie temperatures between 1252 and 1369 K for Co2FeGa. For Co2FeGe the range is between 972 and 1141 K.
Experiments
Using XRD, it was shown conclusively that Co2FeAl crystallizes in the B2 structure whereas Co2FeSi crystallizes in the L21 structure. For the compounds Co2FeGa or Co2FeGe the XRD technique cannot be used to easily distinguish between the two structures. For this reason, the EXAFS technique was used to elucidate the structure of these two compounds [96].
The EXAFS measurements have been performed at the XAFS1 beamline of the LNLS (Brazilian Synchrotron Light Laboratory). The spectra have been collected at the Fe (7112 eV) and Co (7709 eV) K-edges at room temperature in the transmission mode using three ionization chambers. The EXAFS signals at the Fe and Co (figure 14 (a) and (c)) K-edges display the characteristic pattern of a cubic structure. At both edges, the EXAFS signals for the alloys containing the lighter Z elements (Al and Si) are more attenuated due to lower back scattering amplitudes of these elements, if compared to the Ga and Ge ones. The fitting of the Fourier transforms are displayed in part (b) and (d) of figure 14. The imaginary part is displayed for the Co2FeGe in order to exemplarily demonstrate the typical fine quality of the fittings achieved.
Figure 14.

EXAFS at the Fe K-edges (left panel) and Co K-edges (right panel) of Co2FeZ with Z=Al, Si, Ga, Ge. EXAFS oscillations extracted from the x-ray absorption measurements at the Fe K-edge (a) and Co K-edge (c). (b) and (d) Corresponding Fourier transforms (symbols) and best fitting results (grey line). The imaginary part of the Fourier transform is displayed for the Co2FeGe compound (open circles).
Taking the results from the Fe- and the Co-K-edges together, EXAFS gives a clear indication for the L21 structure in the compounds with Z = Ga and Ge which makes these two compounds suitable new candidates as materials in magnetic tunnel junctions.
This results demonstrate that EXAFS of Heusler compounds is a suitable method for structural investigations. It is particularly useful if XRD gives ambiguous results about the correct structure. It is expected that EXAFS may also help for a better understanding of the structure of thin films in order to improve the quality of TMR—junctions.
Summary and conclusions
This review reported about the design of materials for spintronics. For the search of new materials for spintronic applications one should focus one Heusler compounds with Curie temperature at least around 1000 K or even higher. Additionally, the compounds should exhibit the required L21 structure to show half-metallicity. It has been shown that the combination of synthesis and electronic structure calculations is a powerful tool to pick out suitable materials. It was shown that the variation of the main group element in Heusler compounds is a strong tool in order to tune their physical properties. The HAXPES results of the Co2Mn1−xFexSi series gave strong evidence that all compounds of the series exhibit really half-metallic ferromagnetism. For clarity about the gap, spin resolved photo emission spectroscopy at high energies would be highly desirable. Due to the high bulk sensitivity spin polarized high resolution HAXPES will be a powerful and necessary tool to investigate different materials for tunnelling barriers and spacer layers to optimize the structure, configuration and composition of magnetic tunnel junctions and GMR devices to achieve sophisticated properties and results.
Acknowledgments
We thank K Kobayashi, E Ikenaga, J-J Kim and S Ueda (all Spring-8, Japan) for support with the photo emission experiments at Spring-8; G Azevedo and F F Ferreira (both LNLS, Campinas) for their great help with the EXAFS and XRD experiments; F Schäfers and the staff of BESSY (Berlin) for help during the beamtimes; M C M Alves and J Morais (both from UFRGS, Porto Alegre, Brazil) for their support during lots of LNLS beamtimes and discussions; Y Hwu (Academia Sinica, Taipei, Taiwan) and H-J Lin (NSRRC, Hsinchu, Taiwan) for help with the XMCD experiments. We are very grateful to P Blaha (Wien2k) and H Ebert (Munich-SPRKKR) and their groups for development and providing the computer codes. Further we thank our colleagues in Mainz who provided samples and characterized the materials: J Barth, L Basit, S Berinskat, Ch Blum, F Caspar, A Gloskovskii, V Jung, H C Kandpal, V Ksenofontov, S Ouardi, G Schönhense, H Spiering, U Stumm and J Winterlik. This work is financially supported by the Deutsche Forschungs Gemeinschaft (projects P1 and P7 in research group FG 559), BMBF (05KS7UMI) and DAAD (D06/33952).
Footnotes
Invited paper.
References
- Felser C, Fecher G H. and Balke B. Angew. Chem. Int. Edn. Engl. 2007;46:668. doi: 10.1002/anie.200601815. [DOI] [PubMed] [Google Scholar]
- Galanakis I, , Dederichs P H, , editors. Berlin: Springer; 2005. Half-Metallic Alloys (Lecture Notes in Physics vol 676) [Google Scholar]
- Ivanov V A, Aminov T G, Novotortsev V M. and Kalinnikov V T. Russ. Chem. Bull. Int. Edn. 2004;53:2357. doi: 10.1007/s11172-005-0135-5. [DOI] [Google Scholar]
- Zutic I, Fabian J. and Das Sarma S. Rev. Mod. Phys. 2004;76:323. doi: 10.1103/RevModPhys.76.323. [DOI] [Google Scholar]
- Heusler Fr. Verh. d. DPG. 1903;5:219. [Google Scholar]
- Heusler Fr, Starck W. and Haupt E. Verh. d. DPG. 1903;5:220. [Google Scholar]
- Heusler O. Ann. Phys., Lpz. 1934;19:155. doi: 10.1002/andp.19344110205. [DOI] [Google Scholar]
- Bradley A J and Rodgers J W. 1934. Proc. R. Soc.A 144 340 10.1098/rspa.1934.0053 [DOI] [Google Scholar]
- Webster P J. J. Phys. Chem. Solids. 1971;32:1221. doi: 10.1016/S0022-3697(71)80180-4. [DOI] [Google Scholar]
- Kübler J, Williams A R and Sommers C B. 1983. Phys. Rev.B 28 1745 10.1103/PhysRevB.28.1745 [DOI] [Google Scholar]
- de Groot R A, Müller F M, van Engen P G. and Buschow K H J. Phys. Rev. Lett. 1983;50:2024. doi: 10.1103/PhysRevLett.50.2024. [DOI] [Google Scholar]
- Coey J M D, Venkatesan M. and Bari M A. vol 595. Heidelberg: Springer; 2002. Lecture Notes in Physics. [Google Scholar]
- Ishida S, Akazawa S, Kubo Y. and Ishida J. J. Phys. F: Met. Phys. 1982;12:1111. doi: 10.1088/0305-4608/12/6/012. [DOI] [Google Scholar]
- Kohn W. and Sham L J. Phys. Rev. 1965;140:1133. doi: 10.1103/PhysRev.140.A1133. [DOI] [Google Scholar]
- Hedin L. and Lundquist B I. J. Phys. C: Solid State Phys. 1971;4:2064. doi: 10.1088/0022-3719/4/14/022. [DOI] [Google Scholar]
- von Barth U. and Hedin L. J. Phys. C: Solid State Phys. 1972;5:1629. doi: 10.1088/0022-3719/5/13/012. [DOI] [Google Scholar]
- Vosko S H, Wilk L. and Nusair M. Can. J. Phys. 1980;58:1200. [Google Scholar]
- Ishida S, Fujii S, Kashiwagi S. and Asano S. J. Phys. Soc. Japan. 1995;64:2152. doi: 10.1143/JPSJ.64.2152. [DOI] [Google Scholar]
- Ishida S, Kashiwagi S, Fujii S and Asano S. 1995. PhysicaB 210 140 10.1016/0921-4526(94)00920-Q [DOI] [Google Scholar]
- Mohn P, Blaha P. and Schwarz K. J. Magn. Magn. Mater. 1995;140–144:183. doi: 10.1016/0304-8853(94)00941-4. [DOI] [Google Scholar]
- Galanakis I, Dederichs P H and Papanikolaou N. 2002. Phys. Rev.B 66 174429 10.1103/PhysRevB.66.174429 [DOI] [Google Scholar]
- Picozzi S, Continenza A and Freeman A J. 2002. Phys. Rev.B 66 094421 10.1103/PhysRevB.66.094421 [DOI] [Google Scholar]
- Perdew J P and Wang Y. 1986. Phys. Rev.B 33 8800 10.1103/PhysRevB.33.8800 [DOI] [PubMed] [Google Scholar]
- Perdew J P and Wang Y. 1992. Phys. Rev.B 45 13244 10.1103/PhysRevB.45.13244 [DOI] [PubMed] [Google Scholar]
- Perdew J P, Burke K. and Ernzerhof M. Phys. Rev. Lett. 1996;77:3865. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- Perdew J P, Burke K. and Ernzerhof M. Phys. Rev. Lett. 1997;78:1396. doi: 10.1103/PhysRevLett.78.1396. [DOI] [PubMed] [Google Scholar]
- Miura Y, Nagao K and Shirai M. 2004. Phys. Rev.B 69 144413 10.1103/PhysRevB.69.144413 [DOI] [Google Scholar]
- Antonov V N, Drr H A, Kucherenko Y, Bekenov L V and Yaresko A N. 2005. Phys. Rev.B 72 054441 10.1103/PhysRevB.72.054441 [DOI] [Google Scholar]
- Fecher G H, Kandpal H C, Wurmehl S, Morais J, Lin H-J, Elmers H-J, Schönhense G. and Felser C. J. Phys.: Condens. Matter. 2005;17:7237. doi: 10.1088/0953-8984/17/46/008. [DOI] [Google Scholar]
- Soulen Jr R J. et al Science. 1998;282:85. doi: 10.1126/science.282.5386.85. [DOI] [PubMed] [Google Scholar]
- Slater J C. Phys. Rev. 1936;49:931. doi: 10.1103/PhysRev.49.931. [DOI] [Google Scholar]
- Pauling L. Phys. Rev. 1938;54:899. doi: 10.1103/PhysRev.54.899. [DOI] [Google Scholar]
- Kübler J. Oxford: Clarendon; 2000. Theory of Itinerant Electron Magnetism. [Google Scholar]
- Fecher G H, Kandpal H C, Wurmehl S, Felser C. and Schönhense G. J. Appl. Phys. 2006;99:08J106. doi: 10.1063/1.2167629. [DOI] [Google Scholar]
- Kübler J. 1984. PhysicaB 127 257 10.1016/S0921-4526(84)93082-5 [DOI] [Google Scholar]
- Kübler J. 2000. Oxford: Oxford University Press [Google Scholar]
- Balke B, Fecher G H, Kandpal H C and Felser C. 2006. Phys. Rev.B 74 104405 10.1103/PhysRevB.74.104405 [DOI] [Google Scholar]
- Fecher G H. and Felser C. J. Phys. D: Appl. Phys. 2007;40:1582. doi: 10.1088/0022-3727/40/6/S12. [DOI] [Google Scholar]
- Webster P J and Ziebeck K R A. 1988. Landolt-Börnstein—Group III Condensed Matter vol 19CHeidelberg: Springer; p 104 [Google Scholar]
- Ziebeck K R A and Neumann K-U. 2001. Landolt-Börnstein—Group III Condensed Matter vol 32CHeidelberg: Springer; p 64 [Google Scholar]
- Kübler J. J. Phys.: Condens. Matter. 2006;18:9795. doi: 10.1088/0953-8984/18/43/003. [DOI] [Google Scholar]
- Kübler J, Fecher G H and Felser C. 2007. Phys. Rev.B 76 024414 10.1103/PhysRevB.76.024414 [DOI] [Google Scholar]
- Wurmehl S, Fecher G H, Kandpal H C, Ksenofontov V, Felser C, Lin H-J and Morais J. 2005. Phys. Rev.B 72 184434 10.1103/PhysRevB.72.184434 [DOI] [Google Scholar]
- Villars P. and Calvert L D. vol 2nd edn. Materials Park, OH: ASM International; 1991. Pearson's Handbook of Crystallographic Data for Intermetallic Phases. [Google Scholar]
- Bacon G E. and Plant J S. J. Phys. F: Met. Phys. 1971;1:524. doi: 10.1088/0305-4608/1/4/325. [DOI] [Google Scholar]
- Wurmehl S. et al J. Phys. D: Appl. Phys. 2006;39:803. doi: 10.1088/0022-3727/39/5/S06. [DOI] [Google Scholar]
- Wurmehl S, Fecher G H, Kandpal H C, Ksenofontov V, Felser C. and Lin H-J. Appl. Phys. Lett. 2006;88:032503. doi: 10.1063/1.2166205. [DOI] [Google Scholar]
- Jepsen O and Andersen O K. 2000TB-LMTO-ASA Program version 47Stuttgart Germany: MPI für Festkörperforschung [Google Scholar]
- Akai H. and Dederichs P P. J. Phys. C: Solid State Phys. 1985;18:2455. doi: 10.1088/0022-3719/18/12/009. [DOI] [Google Scholar]
- Moruzzi V L, Janak J F and Williams A R. 1978Calculated Properties of MetalsNew York: Pergamon [Google Scholar]
- Perdew J P, Chevary J A, Vosko S H, Jackson K A, Pederson M R, Singh D J and Fiolhais C. 1992. Phys. Rev.B 46 6671 10.1103/PhysRevB.46.6671 [DOI] [PubMed] [Google Scholar]
- Mlynarski P and Salahub D R. 1991. Phys. Rev.B 43 1399 10.1103/PhysRevB.43.1399 [DOI] [PubMed] [Google Scholar]
- Engel E and Vosko S H. 1993. Phys. Rev.B 47 13164 10.1103/PhysRevB.47.13164 [DOI] [PubMed] [Google Scholar]
- Blaha P, Schwarz K, Madsen G K H, Kvasnicka D and Luitz J. 2001. WIEN2k, An Augmented Plane Wave + Local Orbitals Program for Calculating Crystal Properties Karlheinz Schwarz, Techn. Universitaet Wien, Wien Austria [Google Scholar]
- Anisimov V I, Aryasetiawan F. and Lichtenstein A I. J. Phys.: Condens. Matter. 1997;9:767. doi: 10.1088/0953-8984/9/4/002. [DOI] [Google Scholar]
- Solovyev I V and Imada M. 2005. Phys. Rev.B 71 045103 10.1103/PhysRevB.71.045103 [DOI] [PubMed] [Google Scholar]
- Thole B T, Carra P, Sette F. and van der Laan G. Phys. Rev. Lett. 1992;68:1943. doi: 10.1103/PhysRevLett.68.1943. [DOI] [PubMed] [Google Scholar]
- Carra P, Thole B T, Altarelli M. and Wang X. Phys. Rev. Lett. 1993;70:694. doi: 10.1103/PhysRevLett.70.694. [DOI] [PubMed] [Google Scholar]
- Elmers H-J et al. 2003. Phys. Rev.B 67 104412 10.1103/PhysRevB.67.104412 [DOI] [Google Scholar]
- Elmers H J, Wurmehl S, Fecher G H, Jakob G, Felser C and Schönhense G. 2004. Appl. Phys.A 79 557 10.1007/s00339-003-2366-3 [DOI] [Google Scholar]
- Lid D R. 2001CRC Handbook of Chemistry and Pysics 82th ednBoca Raton, FL: CRC Press [Google Scholar]
- Fuji S, Sugimura S, Ishida S. and Asano S. J. Phys.: Condens. Matter. 1990;2:8583. doi: 10.1088/0953-8984/2/43/004. [DOI] [Google Scholar]
- Brown P J, Neumann K-U, Webster P J. and Ziebeck K R A. J. Phys.: Condens. Matter. 2000;12:1827. doi: 10.1088/0953-8984/12/8/325. [DOI] [Google Scholar]
- Raphael M P, Ravel B, Huang Q, Willard M A, Cheng S F, Das B N, Stroud R M, Bussmann K M, Claassen J H and Harris V G. 2002. Phys. Rev.B 66 104429 10.1103/PhysRevB.66.104429 [DOI] [Google Scholar]
- Geiersbach U, Bergmann A. and Westerholt K. J. Magn. Magn. Mater. 2002;240:546. doi: 10.1016/S0304-8853(01)00866-6. [DOI] [Google Scholar]
- Kämmerer S, Heitmann S, Meyners D, Sudfeld D, Thomas A, Hütten A. and Reiss G. J. Appl. Phys. 2003;93:7945. doi: 10.1063/1.1556249. [DOI] [Google Scholar]
- Singh L J, Barber Z H, Miyoshi Y, Bugoslavsky Y, Branford W R. and Cohen L F. Appl. Phys. Lett. 2004;84:2367. doi: 10.1063/1.1690868. [DOI] [Google Scholar]
- Wang W H, Przybylski M, Kuch W, Chelaru L I, Wang J, Lu F, Barthel J, Meyerheim H L and Kirschner J. 2005. Phys. Rev.B 71 144416 10.1103/PhysRevB.71.144416 [DOI] [Google Scholar]
- Wang W H, Przybylskia M, Kuch W, Chelaru L I, Wang J, Lu Y F, Barthel J. and Kirschner J. J. Magn. Magn. Matter. 2005;286:336. doi: 10.1016/j.jmmm.2004.09.089. [DOI] [Google Scholar]
- Sakuraba Y, Hattori M, Oogane M, Ando Y, Kato H, Sakuma A, Miyazaki T. and Kubota H. Appl. Phys. Lett. 2006;88:192508. doi: 10.1063/1.2202724. [DOI] [Google Scholar]
- Schmalhorst J, Kämmerer S, Sacher M, Reiss G, Hütten A. and Scholl A. Phys. Rev. B. 2004;70:024426. doi: 10.1103/PhysRevB.70.024426. [DOI] [Google Scholar]
- Schmalhorst J, Kammerer S, Reiss G. and Hütten A. Appl. Phys. Lett. 2005;86:052501. doi: 10.1063/1.1853526. [DOI] [Google Scholar]
- LeClair P, Swagten H J M, Kohlhepp J T. and de Jonge W J M. Appl. Phys. Lett. 2000;76:3783. doi: 10.1063/1.126780. [DOI] [Google Scholar]
- Kandpal H C, Fecher G H, Felser C and Schönhense G. 2006. Phys. Rev.B 73 094422 10.1103/PhysRevB.73.094422 [DOI] [Google Scholar]
- Dowben P A. and Skomski R. J. Appl. Phys. 2004;95:7453. doi: 10.1063/1.1682911. [DOI] [Google Scholar]
- Kandpal H C, Fecher G H. and Felser C. J. Phys. D: Appl. Phys. 2007;40:1507. doi: 10.1088/0022-3727/40/6/S01. [DOI] [Google Scholar]
- Tanuma S, Powell C J. and Penn D R. Surf. Interface Anal. 1993;21:165. doi: 10.1002/sia.740210302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel W. Hyperfine Interact. 1989;45:73. doi: 10.1007/BF02405873. [DOI] [Google Scholar]
- Kobayashi K. et al Appl. Phys. Lett. 2003;83:1005. doi: 10.1063/1.1595714. [DOI] [Google Scholar]
- Sekiyama A. and Suga S. J. Electron Spectrosc. Relat. Phenom. 2004;137–140:681. doi: 10.1016/j.elspec.2004.02.004. [DOI] [Google Scholar]
- Thiess S, Kunz C, Cowie B C C, Lee T-L, Reniera M. and Zegenhagen J. Solid State Commun. 2004;132:589. doi: 10.1016/j.ssc.2004.09.021. [DOI] [Google Scholar]
- Kobayashi K. 2005. Nucl. Instrum. Methods Phys. Res. Sect. A 547 98 10.1016/j.nima.2005.05.016 [DOI] [Google Scholar]
- Panaccione G. et al J. Phys.: Condens. Matter. 2005;17:2671. doi: 10.1088/0953-8984/17/17/015. [DOI] [Google Scholar]
- Torelli P. et al Rev. Sci. Instrum. 2005;76:023909. doi: 10.1063/1.1852323. [DOI] [Google Scholar]
- Inomata K, Okamura S, Miyazaki A, Kikuchi M, Tezuka N, Wojcik M. and Jedryka E. J. Phys. D: Appl. Phys. 2006;39:816. doi: 10.1088/0022-3727/39/5/S07. [DOI] [Google Scholar]
- Oogane M, Sakuraba Y, Nakata J, Kubota H, Ando Y, Sakuma A. and Miyazaki T. J. Phys. D: Appl. Phys. 2006;39:834. doi: 10.1088/0022-3727/39/5/S09. [DOI] [Google Scholar]
- Ebke D, Schmalhorst J, Liu N-N, Thomas A, Reiss G. and Hütten A. Appl. Phys. Lett. 2006;89:162506. doi: 10.1063/1.2363939. [DOI] [Google Scholar]
- Ishikawa T, Marukame T, Kijima H, Matsuda K-i, Uemura T. and Yamamoto M. Appl. Phys. Lett. 2006;89:192505. doi: 10.1063/1.2378397. [DOI] [Google Scholar]
- Tezuka N, Ikeda N, Miyazaki A, Sugimoto S, Kikuchi M. and Inomata K. Appl. Phys. Lett. 2006;89:112514. doi: 10.1063/1.2354026. [DOI] [Google Scholar]
- Tezuka N, Ikeda N, Sugimoto S. and Inomata K. Appl. Phys. Lett. 2006;89:252508. doi: 10.1063/1.2420793. [DOI] [Google Scholar]
- Balke B, Fecher G H. and Felser C. Appl. Phys. Lett. 2007;90:242503. doi: 10.1063/1.2748341. [DOI] [Google Scholar]
- Niculescu V, Budnick J I, Hines W A, Rajt K, Pickart S and Skalski S. 1979. Phys. Rev.B 19 452 10.1103/PhysRevB.19.452 [DOI] [Google Scholar]
- Bushow K H J, van Engen P G. and Jongebreur R. J. Magn. Magn. Mater. 1983;38:1–22. doi: 10.1016/0304-8853(83)90097-5. [DOI] [Google Scholar]
- Wurmehl S, Alves M C M, Morais J, Ksenofontov V, Teixeira S R, Machado G, Fecher G H. and Felser C. J. Phys. D: Appl. Phys. 2007;40:1524. doi: 10.1088/0022-3727/40/6/S02. [DOI] [Google Scholar]
- Ögüt S and Rabe K M. 1995. Phys. Rev.B 51 10443 10.1103/PhysRevB.51.10443 [DOI] [PubMed] [Google Scholar]
- Balke B, Wurmehl S, Fecher G H, Felser C, Alves M C M, Bernardi F. and Morais J. Appl. Phys. Lett. 2007;90:172501. doi: 10.1063/1.2731314. [DOI] [Google Scholar]


