Abstract
Coriandrum sativum L. (CS) seeds are known to possess therapeutic potentials against a variety of physiological disorders. This study assesses acute and sub-chronic toxicity profile of hydro-methanolic extract of CS seeds using OECD guidelines. In acute toxicity study, mice were once orally administered 1000, 3000 and 5000 mg/kg body weight of CS extract. There were no any behavioral alterations or mortality recorded in CS treated groups. The LD50 value was more than 5000 mg/kg body weight. In the sub-chronic oral toxicity study, the animals were orally administered with CS extract (1000, 2000 and 3000mg/kg body weight) daily for 28 days whereas; vehicle control group received 0.5 % carboxy methyl cellulose. There was significant reduction in food intake, body weight gain and plasma lipid profiles of CS2 and CS3 (2000 and 3000 mg/kg body weight respectively) groups as compared to the control group. However, there were no alterations in haematological profile, relative organ weights, histology and plasma markers of damage of vital organs (heart, liver and kidney). The overall finding of this study indicates that CS extract is non-toxic up to 3000 mg/kg body weight and can be considered as safe for consumption.
Keywords: Coriandrum sativum L., hydro-methanolic seed extract, acute toxicity, sub-chronic toxicity
Introduction
The traditional systems of medicine such as the Ayurveda, Unani and Sidda have been a treasure trove for development of majority of modern medicines. Also the medicinal research relies on ethnobotany and ethnopharmacognocy for discovery of new molecules for that conventionally result in drugs developments (Gurib-Fakim, 2006[25]). World Health Organization (WHO) estimates that approximately 80 % of the developing world's population is using traditional medicine for primary healthcare (Bannerman, 1982[5]). However, there is a prevalent misunderstanding that herbal medicines are devoid of toxic effects (WHO, 2004[55]). Adverse effects of herbs have been reported including allergic reactions, hepatotoxicity (Saad et al., 2006[49]), nephrotoxicity (Colson and De Broe, 2005[12]; Kwan et al., 2006[35]; Zhu, 2002[56]; Vanherweghem, 1998[54]), cardiac toxicity (Horowitz et al., 1996[27]; Moritz et al., 2005[41]; Gaibazzi et al., 2002[21]), neurotoxicity (Ernst, 2003[19]; Benjamin et al., 2001[7]) and even death (Jensen and Allen, 1981[32]) have been reported. Therefore, a pre-clinical toxicity study is indispensible to validate their safe medicinal use.
Coriandrum sativum L. (Apiaceae) (CS) is an annual herb, that is widely distributed. Its fresh leaves and dried seeds are extensively used in Middle Eastern, Mediterranean, Indian, Latin American, African and Southeast Asian cuisines. Decoction and tincture of powdered seeds of CS alone or in combination with other herbal agents are recommended for dyspeptic complaints, loss of appetite, convulsion, insomnia and anxiety (Grieve, 1971[24]). It is also used as medication against diabetes, indigestion, flatulence, renal disorders and a diuretic agent (Grieve, 1971[24]; Emamghoreishi et al., 2005[18]). Its therapeutic potential in the treatment of urethritis, cystitis, urinary tract infection, urticaria, rashes, burns, sore throat, vomiting, indigestion, nosebleed, cough, allergies, hay fever, dizziness and amoebic dysentery has also been reported (Grieve, 1971[24]; PDR for Herbal Medicines, 1998[47]).
Phytochemical constituents of CS seeds have been studied extensively and their analysis had revealed presence of polyphenols (rutin, caffeic acid derivatives, ferulic acid, galic acid, and chlorogenic acid), flavonoids (quercetin and isoquercetin) and β-carotenoids (Melo et al., 2003[39]). The essential oil obtained from CS seeds contains α and β-pinene, camphor, citronellol, coriandrol, p-cymene, geraniol, geranyl acetate, limonene, linalool, myrcene, α and β phellandrene and α and β-terpinene along with many fatty acids. Presence of water soluble compounds such as monoterpenoid glycosides, monoterpenoid glucose sulfate and other glycosides have been reported (Sergeeva, 1975[50]; Ishikawa et al., 2003[28]). The pharmacological activities of various extracts and essential oils of CS seeds have been studied wherein; the essential oils have been found to possess antimicrobial (Baratta et al., 1998[6]) and antifungal properties (Garg and Siddiqui, 1992[22]). Its efficacy as a hypoglycemic (Gray and Flatt, 1999[23]), hypolipidemic (Chithra and Leelamma, 1997[11], 1999[10]; Lal et al., 2004[36]), hypocholesterolemic (Dhanapakiam et al., 2008[17]), antihypertensive (Medhin et al., 1986[38]), antioxidant (Melo et al., 2003[39]; Ramadan et al., 2003[48]; Bajpai et al., 2005[4]), antimutagenic (Cortes-Eslava et al., 2004[14]), anxiolytic (Emamghoreishi et al., 2005[18]), antimicrobial (Kubo et al., 2004[34]; Cantore et al., 2004[9]), larvicidal (Consoli et al., 1988[13]) and post-coital antifertility agent (Al-Said et al., 1987[3]) have also been reported.
We had recently reported anti-insulin resistance (Patel et al., 2011[45]) and cardioprotective (Patel et al., 2012[46]) potentials of CS seed extract. Since toxicological evaluation of CS seed extract is not studied, the present study evaluates possible toxicity of CS seed extract using Economic Co-operation and Development (OECD) guidelines.
Material and Methods
Plant material and preparation of extract
Seeds of CS were collected (in the month of February and March) and identified by Dr. P.S. Nagar, Department of Botany, The M.S. University of Baroda. A herbarium of plant was deposited in the Department of Botany. One hundred grams of powdered dry seeds were soaked in methanol:water (80:20 v/v) at room temperature and allowed to stand for seven days. The resultant extract was filtered through a muslin cloth and then concentrated in a rotary evaporator under reduced pressure to obtain a thick semisolid brown paste (Hashim et al., 2005[26]). The final yield was 8.3 g (w/w).
Experimental animals
Adult female Swiss albino mice (20-25 g) were obtained from Zydus Cadila Research Centre, Ahmedabad, Gujarat, India. They were housed under standard animal house conditions (temperature: 23 ± 2 °C; photoperiod: 12 h light and 12 h dark; humidity: 45-50 %). They were fed with standard laboratory pellets (M/S Pranav agro, Ltd., Baroda, India) and water ad libitum. The animals were maintained as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) India and the experimental protocol approved by the animal ethical committee of the Department of Zoology, The M. S. University of Baroda, Vadodara (Approval No.827/ac/04/ CPCSEA).
Acute oral toxicity
Acute oral toxicity study was conducted according to the guidelines of Organization for Economic Co-operation and Development (OECD, 401[42]). Twenty four animals were randomly allocated into four groups of six animals each. Group I (Control): animals were administered orally with vehicle (0.05 % Carboxy methyl cellulose; CMC). Remaining groups (II, III and IV) were administered with 1000, 3000 and 5000 mg/ kg body weight of CS extract respectively via gastric intubation. Doses were prepared using 0.05 % CMC and dose volume was not more than 1 ml/kg body weight. Cage side observations (tremors, convulsions, salivation, diarrhea, lethargy, sleep and coma) were recorded during first four hours and mortality was recorded after 24 h (Jadeja et al., 2011[30]).
Sub-chronic oral toxicity
The sub-chronic oral toxicity study was conducted according to the guidelines of the Organization for Economic Co-operation and Development (OECD, 407[43]). Twenty four animals were randomly divided into four groups of six animals each. Group I (CS0) served as a control and received 0.5 % CMC (vehicle) for 28 days whereas the remaining groups (Group II- CS1, Group III- CS2 and Group IV- CS3) were orally administered 1000, 2000 and 3000 mg/kg body weight respectively of CS extract daily for 28 consecutive days. Food and water intake were recorded daily, whereas, body weight was recorded once in a week throughout study period (Thounaojam et al., 2010[52]; Thounaojam et al., 2011[53]).
Plasma isolation and haematology
At the end of 28 days, blood samples were collected from overnight fasted animals through retro-orbital sinus puncture in ethylene diamine tetra acetic acid (EDTA) coated vials and plasma was separated by cold centrifugation (Plasto Crafts Superspin-R centrifuge) at 3000 rpm for 10 min. Blood was also collected for the analysis of haematological parameters such as white blood cell (WBC) count, red blood cell (RBC) count, haemoglobin (Hb) levels, mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC) and red cell distribution width (RCDW) using BC 2300 Haematology Analyzer (Shezhen Mindray Biomedical Electronics Co., Ltd., China).
Plasma biochemical parameters
Creatinine kinase-MB (cardiac damage), aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, total protein (liver damage) and urea and creatinine (kidney damage) were analyzed using commercially available kits (Recon diagnostic Ltd., Vadodara, India). Also, plasma glucose and lipid profile [total cholesterol (TC), triglyceride (TG) and high density lipoprotein (HDL-C)] were assessed and low density lipoprotein (LDL-C) and very low density lipoprotein (VLDL-C) were calculated by Friedewald's formula (Friedewald et al., 1972[20]).
Relative organ weights and histopathology
Animals were later sacrificed by cervical dislocation under mild ether anesthesia for autopsy and liver, kidney, heart, lung and spleen were excised, rinsed in 0.9 % saline and weighed. After sacrifice, organ weights (lungs, heart, liver, kidney and spleen) were recorded and relative organ weights (ROW) were calculated as follows.
ROW =
Absolute organ weight (g) X 100
Body weight on the day of sacrifice (g)
Tissue pieces of vital organs (heart, liver and kidneys) were fixed in 10 % paraformaldehyde for paraffin histology and processed in paraffin embedding as per the standard protocol. 7 µm thick sections of each tissue were stained with hematoxylin and eosin, and observed for possible histopathological damages.
Results
Acute oral toxicity
Cage side observations did not record any behavioral changes such as tremor, convulsion, salivation, diarrhea, lethargy or sleep during the first four hours of CS extract (1000, 2000 or 3000 mg/kg body weight) administration. After 24 h there was no mortality recorded in plant extract administered groups. However, urine output was found to be increased in CS treated animals (1000, 2000 or 3000 mg/kg body weight) as compared to the control (data not shown).
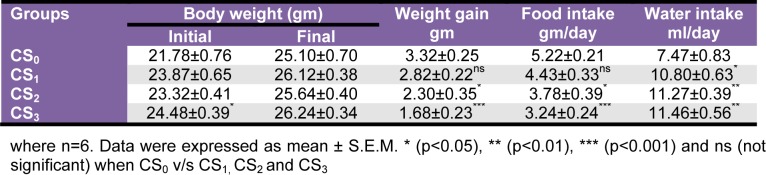
Sub-chronic oral toxicity - Body weight gain, food and water intake
CS1 and CS2 groups did not record any significant alterations in body weight gain. However, CS3 group (3000 mg/kg body weight) recorded significant (p<0.001) decrement in body weight gain. Further, there was significant (p<0.05 and p<0.001 respectively) reduction in food intake of CS2 and CS3 groups as compared to CS0. Water intake was significantly (p<0.05, p<0.01 and p<0.001 respectively) increased in all the CS extract administered groups as compared to CS0 group (Table 1(Tab. 1)).
Table 1. Effect of Coriandrum sativum L. seed extract sub-chronic oral administration on food intake, water intake and body weight.
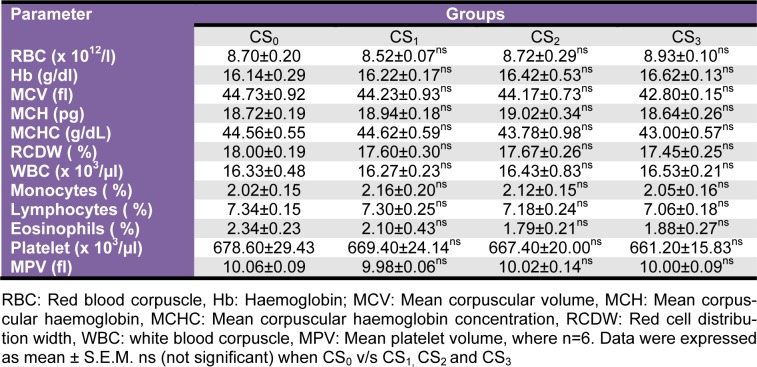
Haematology
The haematological parameters (RBC, WBC, Hb, MCV, MCH, MCHC, RCDW, monocytes, lymphocytes, eosinophil) did not record any significant alterations in any of CS administered groups (Table 2(Tab. 2)).
Table 2. Effect of Coriandrum sativum L. seed extract sub-chronic oral administration on haematological parameters.
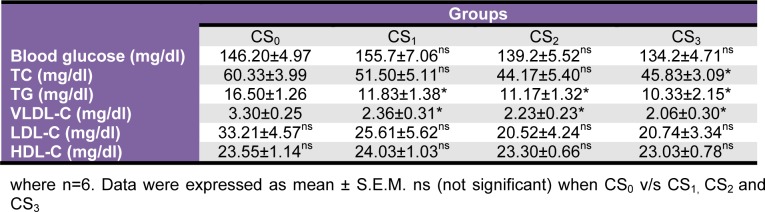
Biochemical parameters
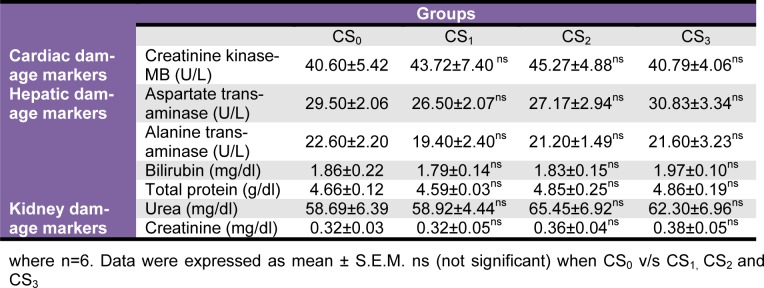
Plasma glucose recorded moderate non-significant decrement in CS2 and CS3 groups. Also, plasma TC, TG, LDL and VLDL levels recorded moderate to significant reductions in all the CS treated groups but, plasma HDL levels were unaltered (Table 3(Tab. 3)). Plasma marker of creatinine kinase-MB, AST, ALT, bilirubin, total protein, urea and creatinine did not record significant alterations in any of the CS treated groups as compared to the CS0 group (Table 4(Tab. 4)).
Table 3. Effect of Coriandrum sativum L. seed extract sub-chronic oral administration on plasma glucose, lipid profile and lipoprotein profile.
Table 4. Effect of Coriandrum sativum L. seed extract sub-chronic oral administration on plasma markers of heart, liver and kidney damage.
Relative organ weights and histopathology
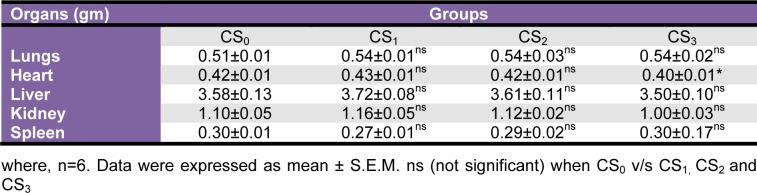
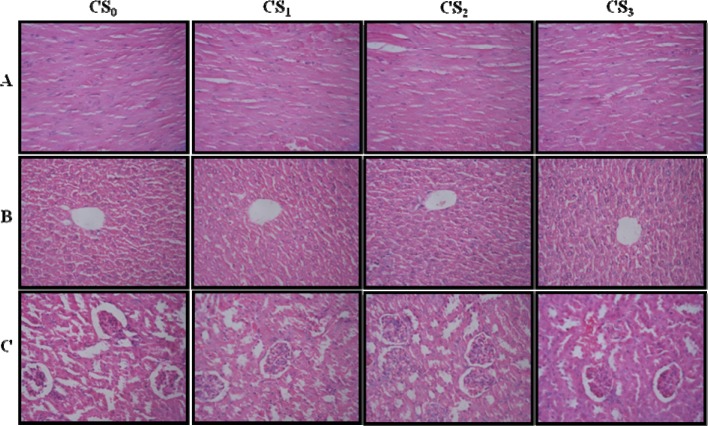
There were no significant changes in ROW of CS treated groups as compared to CS0 group (Table 5(Tab. 5)). A detailed scrutiny of histoarchitecture of the heart, liver and kidney did not reveal any observable cellular damage. The cellular morphology, nuclear characteristics and tissue integrity of organs of CS treated groups were comparable to the CS0 group (Figure 1(Fig. 1)).
Table 5. Effect of Coriandrum sativum L. seed extract sub-chronic oral administration on relative organ weight.
Figure 1. Photomicrographs of the sections of the heart (A), liver (B) and kidney (C) of control (CS0) and CS administered (CS1, CS2 and CS3) mice for 28 days showing no histoarchitecture change in CS treated groups as compared to control.
Discussion
Acute toxicity study recorded zero mortality at the end of 24 h period, following CS extracts administration. No behavioral alterations were recorded during the first four hours after administration of CS extract. Hence, the LD50 of CS extract is thought to be greater than 5000 mg and therefore CS extract can be considered as non-toxic up to the said dose (OECD 401[42]).
Sub-chronic oral toxicity studies have provided information on drugs that can possibly pose health risks (Ministério de Saúde/Brasil, 2004[40]). Twenty eight days of oral administration of CS (CS2 and CS3) extract showed significant decrement in food intake and body weight gain as compared to CS0 mice. Significant reduction in food intake is suggested as responsible for the observed decrement in body weight gain. Loss of appetite is often synonymous with weight loss due to disturbances in carbohydrate, protein or fat metabolisms (Klaassen, 2001[33]) and the same might be a possible reason for the weight loss in our study. CS (CS2 and CS3) treated mice also showed significant decrement in plasma TC, TG, LDL and VLDL whereas glucose and HDL levels were unchanged. These results indicate that higher doses of CS (CS2 and CS3) results in a reduction of food intake and subsequent decrement in lipid profile whereas a lower dose (CS1) does not lead to any such negative impact on metabolism.
CK-MB is an enzyme present in the myocardium that leaks out only under conditions of massive myocardial damage resulting from disintegration of contractile apparatus and increased sarcoplasmic permeability (Mair et al., 1994[37]; Jadeja et al., 2010[31]). Observed normal levels of CK-MB under all doses CS administration is reflective of its normal functional status and negligible damage. The same is further validated through the histology of heart of CS treated groups that reveals presence of intact myocardium. However, marginal increment in ROW of heart in CS3 group is inexplicable and warrants further scrutiny.
High levels of AST and ALT are reported in liver diseases or hepatotoxicity (Brautbar and Williams, 2002[8]; Desai et al., 2012[16]). Plasma AST, ALT and bilirubin of CS0 and CS treated groups were comparable thus indicative of normal functional status of liver. The ROW and histopathological observations of liver showed no significant changes following CS treatment.
Renal dysfunction can be assessed by concurrent measurements of urea and creatinine and their normal levels reflect at reduced likelihood of renal problems (Davis and Bredt, 1994[15]; Thounaojam et al., 2010[51]). In the present study, changes in plasma urea and createnine levels in CS treated groups showed non-significant differences on a dose dependent manner indicating a normal renal function. Healthy status of the kidneys of CS treated groups was further confirmed by their histoarchitecture and ROW. However, higher urine output observed in CS2 and CS3 treated groups can be attributed to its diuretic property as reported earlier by other research groups (Aissaoui et al., 2008[2]; Jabeen et al., 2009[29]).
The haematopoietic system is one of the most sensitive targets for toxic compounds and hence it is mandatory to record any possible alterations resulting from a test substance (Olson et al., 2000[44]). Change in haematological parameters has a higher predictive value, when the data of drug toxicity on animal studies are translated for clinical usage (Adeneye and Adokiye, 2008[1]). A normal haematological profile of CS treated groups also further justified the non-toxic nature of CS extract.
Conclusion
In light of these findings, we may conclude that CS extract is not toxic in all the doses studied herein. This study is the first report that evaluates toxicity of CS extract and defines it as non-toxic up to a dose of 3000 mg/kg body weight.
Acknowledgement
First author acknowledges University Grants Commission, New Delhi for providing Financial Assistance in the form of JRFSMS scholarship.
References
- 1.Adeneye AA, Adokiye SB. Protective effect of the aqueous leaf and seed extract of Phyllanthus amarus on gentamicin and acetaminophen-induced nephrotoxic rats. J Ethnopharmacol. 2008;118:318–32. doi: 10.1016/j.jep.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 2.Aissaoui A, El-Hilaly J, Israili ZH, Lyoussi B. Acute diuretic effect of continuous intravenous infusion of an aqueous extract of Coriandrum sativum L. in anesthetized rats. J Ethnopharmacol. 2008;115:89–95. doi: 10.1016/j.jep.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Al-Said MS, Al-Khamis KI, Islam MW, Parmar NS, Tariq M, Ageel AM. Post-coital antifertility activity of the seeds of Coriandrum sativum in rats. J Ethnopharmacol. 1987;21:165–173. doi: 10.1016/0378-8741(87)90126-7. [DOI] [PubMed] [Google Scholar]
- 4.Bajpai M, Mishra A, Prakash D. Antioxidant and free radical scavenging activities of some leafy vegetables. Int J Food Sci Nutr. 2005;56:473–481. doi: 10.1080/09637480500524299. [DOI] [PubMed] [Google Scholar]
- 5.Bannerman RH. Traditional medicine in modern healthcare. World Health Forum. 1982;3(1):8–13. [Google Scholar]
- 6.Baratta MT, Dorman HJD, Deans SG, Biondi DM, Ruberto G. Chemical composition, antimicrobial and antioxidative activity of laurel, sage, rosemary, oregano and coriander essential oils. J Essent Oil Res. 1998;10:18–27. [Google Scholar]
- 7.Benjamin J, Muir T, Briggs K, Pentland B. A case of cerebral haemorrhage - can Ginkgo biloba be implicated? Postgrad Med J. 2001;77:112–3. doi: 10.1136/pmj.77.904.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brautbar N, Williams J., II Industrial solvents and solvent and liver toxicity: rick assessment, rick factors and mechanisms: review. Int J Hyg Environ Health. 2002;205:479–91. doi: 10.1078/1438-4639-00175. [DOI] [PubMed] [Google Scholar]
- 9.Cantore PL, Iacobellis NS, De Marco A, Capasso F, Senatore F. Antibacterial activity of Coriandrum sativum L. and Foeniculum vulgare Miller var. vulgare (Miller) essential oils. J Agric Food Chem. 2004;52:7862–7866. doi: 10.1021/jf0493122. [DOI] [PubMed] [Google Scholar]
- 10.Chithra V, Leelamma S. Coriandrum sativum changes the levels of lipid peroxides and activity of antioxidant enzymes in experimental animals. Ind J Biochem Biophys. 1999;36:59–61. [PubMed] [Google Scholar]
- 11.Chithra V, Leelamma S. Hypolipidemic effect of coriander seeds (Coriandrum sativum): mechanism of action. Plant Food Hum Nutr. 1997;51:167–172. doi: 10.1023/a:1007975430328. [DOI] [PubMed] [Google Scholar]
- 12.Colson CR, De Broe ME. Kidney injury from alternative medicines. Adv Chronic Kidney Dis. 2005;12:261–275. doi: 10.1016/j.ackd.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Consoli RAGB, Mendes NM, Pereira JP, Santos BDS, Lamounier MA. Larvicidal properties of plant extracts against Aedes fluviatilis (Lutz) (Diptera: Culicidae) in the laboratory. Mem Inst Oswaldo Cruz Rio De Janeiro. 1988;83:87–94. doi: 10.1590/s0074-02761988000100012. [DOI] [PubMed] [Google Scholar]
- 14.Cortes-Eslava J, Gomez-Arroyo S, Villalobos-Pietrini R, Espinosa-Aguirre JJ. Antimutagenicity of coriander (Coriandrum sativum) juice on the mutagenesis produced by plant metabolites of aromatic amines. Toxicol Lett. 2004;153:283–292. doi: 10.1016/j.toxlet.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Davis ME, Bredt ND. Renal methods for toxicity. In: Hayes AWC, editor. Principles and methods of toxicology. 3rd. New York: Raven Press; 1994. p. 871. [Google Scholar]
- 16.Desai SN, Patel DK, Devkar RV, Patel PV, Ramachandran AV. Hepatoprotective potential of polyphenol rich extract of Murraya koenigii L.: An in vivo study. Food Chem Toxicol. 2012;50:310–4. doi: 10.1016/j.fct.2011.10.063. [DOI] [PubMed] [Google Scholar]
- 17.Dhanapakiam P, Mini Joseph J, Ramaswamy VK, Moorthi M, Senthi KA. The cholesterol lowering property of coriander seeds (Coriandrum sativum): Mechanism of action. J Environ Biol. 2008;29:53–56. [PubMed] [Google Scholar]
- 18.Emamghoreishi M, Khasaki M, Aazam MF. Coriandrum sativum: Evaluation of its anxiolytic effect in the elevated plus-maze. J Ethnopharmacol. 2005;96:365–70. doi: 10.1016/j.jep.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 19.Ernst E. Serious psychiatric and neurological adverse effects of herbal medicines - a systemic review. Acta Psychiatr Scand. 2003;108:83–91. doi: 10.1034/j.1600-0447.2003.00158.x. [DOI] [PubMed] [Google Scholar]
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low- density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 21.Gaibazzi N, Gelmini GP, Montresor G, Canel D, Comini T, Fracalossi C, et al. Long QRS tachycardia secondary to Aconitum napellus alkaloid ingestion. Ital Heart J Suppl. 2002;3:874–7. [PubMed] [Google Scholar]
- 22.Garg SC, Siddiqui N. In-vitro antifungal activity of the essential oil of Coriandrum sativum. J Res Educ Ind Med. 1992;11:11–13. [Google Scholar]
- 23.Gray AM, Flatt PR. Insulin-releasing and insulin-like activity of the traditional anti-diabetic plant Coriandrum sativum (Coriander) Br J Nutr. 1999;81:203–209. doi: 10.1017/s0007114599000392. [DOI] [PubMed] [Google Scholar]
- 24.Grieve M. A modern herbal. Mineola, NY: Dover Publ; 1971. [Google Scholar]
- 25.Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Aspects Med. 2006;27:1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Hashim MS, Lincy S, Remya V, Teena M, Anila L. Effect of polyphenolic compounds from Coriandrum sativum on H2O2-induced oxidative stress in human lymphocytes. Food Chem. 2005;92:653–60. [Google Scholar]
- 27.Horowitz RS, Feldhaus K, Dart RC, Stermitz FR, Beck JJ. The clinical spectrum of Jin Bu Huan toxicity. Arch Intern Med. 1996;156:899–903. [PubMed] [Google Scholar]
- 28.Ishikawa T, Kondo K, Kitajima J. Water-soluble constituents of coriander. Chem Pharml Bull. 2003;51:32–9. doi: 10.1248/cpb.51.32. [DOI] [PubMed] [Google Scholar]
- 29.Jabeen Q, Bashira S, Lyoussic B, Gilani AH. Coriander fruit exhibits gut modulatory, blood pressure lowering and diuretic activities. J Ethnopharmacol. 2009;122:123–30. doi: 10.1016/j.jep.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Jadeja RN, Thounaojam MC, Ansarullah, Jadav SV, Patel MD, Patel DK, Salunke SP, et al. Toxicological evaluation and hepatoprotective potential of Clerodendron glandulosum Coleb leaf extract. Hum Exp Toxicol. 2011;30(1):63–70. doi: 10.1177/0960327110368420. [DOI] [PubMed] [Google Scholar]
- 31.Jadeja RN, Thounaojam MC, Patel DK, Devkar RV, Ramachandran AV. Pomegranate (Punica granatum L.) juice supplementation attenuates isoproterenol induced cardiac necrosis in rats. Cardiovasc Toxicol. 2010;10(3):174–180. doi: 10.1007/s12012-010-9076-9. [DOI] [PubMed] [Google Scholar]
- 32.Jensen WI, Allen JP. Naturally occurring and experimentally induced castor bean (Ricinus communis) poisoning in ducks. Avian Dis. 1981;5:184–194. [PubMed] [Google Scholar]
- 33.Klaassen CD. Casarett and Doull’s toxicology. The basic science of poisons. New York: McGraw-Hill; 2001. [Google Scholar]
- 34.Kubo I, Fujita KI, Kubo A, Nihei KI, Ogura T. Antibacterial activity of coriander volatile compounds against Salmonella choleraesuis. J Agric Food Chem. 2004;52:3329–32. doi: 10.1021/jf0354186. [DOI] [PubMed] [Google Scholar]
- 35.Kwan TH, Tong MK, Leung KT, Lai CK, Poon WT, Chan YW, et al. Acute renal failure associated with prolonged intake of slimming pills containing anthraquinones. Hong Kong Med J. 2006;12:394–7. [PubMed] [Google Scholar]
- 36.Lal AAS, Tkumar PBM, Pillai KS. Hypolipidemic effect of Coriandrum sativum L. intriton-induced hyperlipidemic rats. Indian J Exp Biol. 2004;42:909–12. [PubMed] [Google Scholar]
- 37.Mair J, Wagner I, Jakob G, Lechleitner P, Drenstl F, Paschenctort B, et al. Different time courses of cardiac contractile proteins after acute myocardial infarction. Clin Chim Acta. 1994;231:47–60. doi: 10.1016/0009-8981(94)90253-4. [DOI] [PubMed] [Google Scholar]
- 38.Medhin DG, Hadhazy BP, Verzar-Petri G. Hypotensive effects of Lupinus termis and Coriandrum sativum in anaesthetized rats. Acta Pharm Hung. 1986;56:59–63. [PubMed] [Google Scholar]
- 39.Melo EA, Bion FM, Filho JM, Guerra NB. In vivo antioxidant effect of aqueous and etheric coriander (Coriandrum sativum L.) extracts. Eur J Lipid Sci Technol. 2003;105:483–7. [Google Scholar]
- 40.Ministério da Saúde/Brasil. Agência Nacional de Vigilância Sanitária. Resolução RE n_ 90 de 16.3.2004. Guia para a realização de estudos de toxicidade pré-clínica de fitoterápicos. Diário Oficial da União. 2004. [Google Scholar]
- 41.Moritz F, Compagnon P, Kaliszczak IG, Kaliszczak Y, Caliskan V, Girault C. Severe acute poisoning with homemade Aconitum napellus capsules: toxicokinetic and clinical data. Clin Toxicol. 2005;43:873–6. doi: 10.1080/15563650500357594. [DOI] [PubMed] [Google Scholar]
- 42.OECD. Guidelines for the testing of chemicals. OECD 401. Acute oral toxicity. Paris: Organisation for Economic Cooperation and Development; 1981. Available from: http://masetto.sourceoecd.org/vl=80569188/cl=12/nw=1/rpsv/ij/oecdjournals/1607310x/v1n4/s2/p1. [Google Scholar]
- 43.OECD. Repeat dose 28 days oral toxicity study in rodents; In-Guidance document for the development of OECD guideline for testing of chemicals. 1995. (Environmental monogr No. 76). Available from: http://masetto.sourceoecd.org/vl=80569188/cl=12/nw=1/rpsv/ij/oecdjournals/1607310x/v1n4/s8/p1.
- 44.Olson H, Betton G, Robinson D, Thomas K, Monro A, Kolaja G, et al. Concordance of toxicity of pharmaceuticals in humans and in animals. Reg Toxicol Pharmacol. 2000;32:56–67. doi: 10.1006/rtph.2000.1399. [DOI] [PubMed] [Google Scholar]
- 45.Patel DK, Desai SN, Devkar RV, Ramachandran AV. Coriandrum sativum L. aqueous extract mitigates high fat diet induced insulin resistance by controlling visceral adiposity in C57BL/6J Mice. Bol Latinoam Caribe Plant Med Aromat. 2011;10:127–135. [Google Scholar]
- 46.Patel DK, Desai SN, Gandhi HP, Devkar RV, Ramachandran AV. Cardio protective effect of Coriandrum sativum L. on isoproterenol induced myocardial necrosis in rats. Food Chem Toxicol. 2012;50:3120–3125. doi: 10.1016/j.fct.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 47.PDR for Herbal Medicines. Physician's Desk Reference for Herbal Medicines. 3rd. Thomson Healthcare; 1998. [Google Scholar]
- 48.Ramadan MF, Kroh LW, Morsel JT. Radical scavenging activity of black cumin (Nigella sativa L.), coriander (Coriandrum sativum L.), and niger (Guizotia abyssinica Cass.) crude seed oils and oil fractions. J Agric Food Chem. 2003;51:6961–9. doi: 10.1021/jf0346713. [DOI] [PubMed] [Google Scholar]
- 49.Saad B, Azaizeh H, Abu-Hijleh G, Said O. Safety of traditional Arab herbal. Evid Based Complement Alternat Med. 2006;3:433–9. doi: 10.1093/ecam/nel058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sergeeva NV. Rutin and other polyphenols of the herbage of Coriandrum sativum. Chem Nat Compd. 1975;10:98. [Google Scholar]
- 51.Thounaojam MC, Jadeja RN, Devkar RV, Ramachandran AV. Sida rhomboidea. Roxb leaf extract ameliorates gentamicin induced nephrotoxicity and renal dysfunction in rats. J Ethnopharmacol. 2010;132:365–367. doi: 10.1016/j.jep.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 52.Thounaojam MC, Jadeja RN, Patel DK, Devkar RV, Ramchandran AV. Acute and subchronic oral toxicity of Sida rhomboidea Roxb. leaf extract. J Complement Integrat Med. 2010;7(1):1. [Google Scholar]
- 53.Thounaojam MC, Jadeja RN, Sankhari JM, Devkar RV, Ramachandran AV. Safety evaluations on ethanolic extract of red cabbage (Brassica oleracea l.) in mice. J Food Sci. 2011;76:T35–9. doi: 10.1111/j.1750-3841.2010.01962.x. [DOI] [PubMed] [Google Scholar]
- 54.Vanherweghem LJ. Misuse of herbal remedies: the case of an outbreak of terminal renal failure in Belgium (Chinese herbs nephropathy) J Altern Complement Med. 1998;4:9–13. doi: 10.1089/acm.1998.4.1-9. [DOI] [PubMed] [Google Scholar]
- 55.WHO. Guidelines on safety monitoring of herbal medicines in pharmacovigilance systems. Geneva: WHO; 2004. [Google Scholar]
- 56.Zhu YP. Toxicology of the Chinese herb mu tong (Aristolochia manshuriensis). What history tells us. Adverse Drug React Toxicol Rev. 2002;21:171–7. doi: 10.1007/BF03256194. [DOI] [PubMed] [Google Scholar]