Abstract
A recent paper published in Nature demonstrates a multifaceted relation between enteric glial cells (EGC), intestinal epithelia, and ILC3, via the EGC release of neurotrophic factors, a structurally related group of ligands within the TGF-β superfamily of signaling molecules and IL-22 produced by ILC3.
The enteric nervous system (ENS) acts largely autonomously to regulate intestinal motility and secretion and is often referred to as our “second brain”, because of its complexity and structure. The interconnecting neurons of the ENS are supported by a manifold of enteric glial cells (EGC) that encompass the entire intestinal wall. Similar to astrocytes that surround endothelia of the blood-brain barrier to protect the brain extracellular fluid from circulating blood mediators, it has been hypothesized that interactions between EGC and epithelia regulate barrier function. In support of this purported function, EGC depletion showed a fulminant enterocolitis in mice and diminished intestinal barrier function1. Hence EGC certainly plays an eminent role in mucosal immune homeostasis although their immune-supportive function is incompletely understood at present.
Innate lymphoid cells (ILCs) are a recently identified family of immune cell types comprising three groups: group 1 ILCs, including ILC1 and NK cells, group 2 ILCs (ILC2) and group 3 ILCs, including CCR6− ILC3 and CCR6+ Lymphoid Tissue inducer (LTi) cells2. These can be distinguished from each other on the basis of cytokine production and transcription factors needed for their development and functions. Both ILC3 and LTi cells depend on RORγt and produce IL-17 and IL-22, but LTi cells are important for the formation of secondary lymphoid organs such as Peyer's patches, and ILC3 plays protective roles in innate immunity at barrier surfaces and maintaining the intestinal homeostasis. Two subsets of ILC3 can be distinguished based on expression of the natural cytotoxicity receptor (NCR) NKp46. IL-22 is produced by ILC3 and Th22 cells and acts on non-hematopoietic cells expressing the IL-22 receptor including intestinal epithelial cells (IEC). Activation of the IL-22R complex by IL-22 acts on intestinal stem cells to promote epithelial regeneration, and stimulates the secretion of antimicrobial peptides (AMPs)3.
In their exciting study Ibiza et al.4 connect ILC to neurons by identifying a group of IL-22-producing ILC3 that express the receptor tyrosine kinase Ret, which has a non-redundant role in providing protection against the pathogenic bacterium Citrobacter rodentium and intestinal damage induced by the compound Dextran Sulphate Sodium (DSS). Ret is predominantly expressed on LTi cells and NKp46− ILC3. Ablation of Ret in ILC3 strongly reduced IL-22 production by ILC3, resulting in decreased production of AMPs by IECs and increased susceptibility to intestinal inflammation. Somewhat surprising, the production of another ILC3 signature cytokine IL-17 is not affected by Ret ablation in ILC3 (Figure 1).
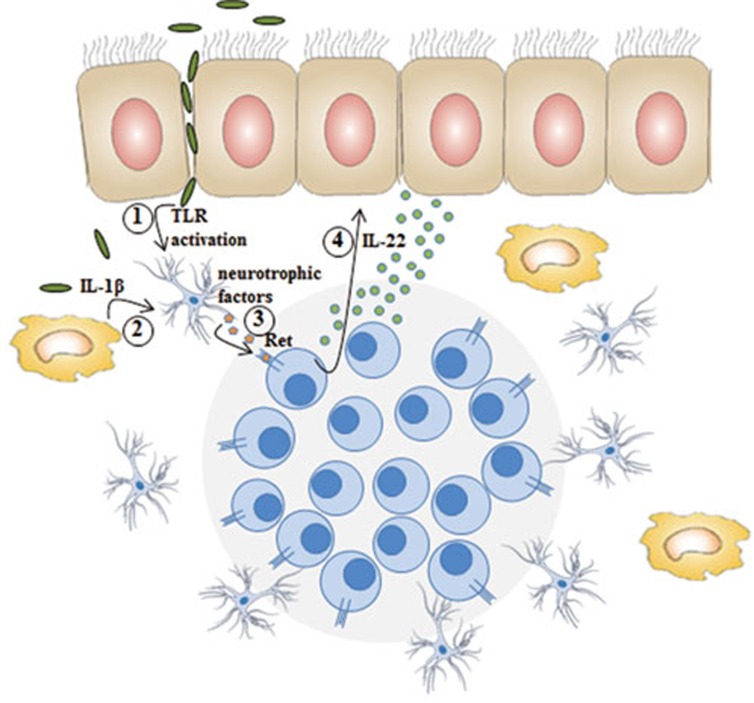
Figure 1.
Glial cells that surround ILC3 aggregates such as in cryptopatches and ILF are directly activated by (1) TLR ligands and (2) IL-1β, which results in the secretion of (3) neurotrophic factors that bind to the RTK on ILC3. Ret-activated ILC3 in turn secretes IL-22 (4) enhancing epithelial cell reactivity.
Using double reporter mice for Ret+ ILC3 (RetGFP) and for glial cells (including EGC; glial fibrillary acidic protein (Gfap)-CreRosa26RFP), they observed that stellate-shaped projections of EGC are in close proximity to RetGFP ILC3 aggregates in cryptopatches (CP) and isolated lymphoid follicles (ILF), enabling paracrine activation of Ret+ILC3 subsets. The ligands for Ret are glial cell-derived neurotrophic factor (GDNF) family ligands (GFL) that also include neurturin and artemin, proteins controlling neuron survival and differentiation5, and are secreted by EGC upon stimulation by Toll like receptors (TLRs) or the cytokines IL-1β and IL-334. Engagement of ILC3-expressed Ret by GFLs results in phosphorylation of the MAP kinase cascade and activation of STAT3, which then binds to the IL-22 promoter. Thus ILC3 can respond to signals from EGCs through Ret, in addition to a variety of previously identified mediators derived from myeloid cells such as IL-1β, IL-23, or dietary products such as retinoic acids and aryl hydrocarbon receptor agonists. It is unclear whether all these signals act on the same cell or on different subsets. For instance it seems that Ret is predominantly expressed on NKp46− ILC3. Thus acquisition of NKp46, which correlates with an enhanced IL-22 production3, seems to parallel loss of Ret. One could speculate that Ret activation is involved in this transition, and thus that the absence of Ret signaling impaired the transition towards IL-22-producing NKp46+ ILC3, resulting in the enhanced susceptibility to tissue damage and infection. Dissecting the composition of the distinct ILC3 subsets in wild-type mice, Ret-deficient mice, and mice that have a gain-of-function mutation in Ret would further contribute to our understanding of how this kinase is involved in the regulation of IL-22 production in the distinct CCR6− ILC3 subsets.
Ibiza et al. focused on the protective properties of Ret-induced IL-22 production, orchestrated by an EGC-ILC3-IEC interplay. However, Ret signaling has also been reported to be essential for the organogenesis of Peyer's patches, survival, expansion and function of hematopoietic cells, and function of certain T cells6, indicating a more general control mechanism of lymphocyte function. Ret has recently been reported to be expressed on intestinal ILC2 as well7, which among other compartments reside in secondary and tertiary lymphoid structures. It would be of interest to explore whether Ret signaling may also regulate innate type 2 cytokine functions, for example upon parasitic worm infections.
There are multiple seminal observations from the study that open new avenues of investigation. As described by Ibiza et al., Myd88-mediated signaling seemed to be required for EGC survival, as specific deletion of MyD88 in GFAP-expressing cells decreased expression of all EGC markers in the gut4. Thus, this study reveals that the role of EGC in immune surveillance may be much more prominent than expected from previous studies. Furthermore, neurotrophin expression has been reported in many tissues5, which opens the possibility that a glial-ILC3 regulatory arm exists for barrier function in other organs as well, such as in skin, lung, and liver. Finally, a better understanding of the regulation of expression of Ret on ILC subsets or other immune cells will be relevant for chronic inflammatory disease pathogenesis and possibly treatment.
References
- Bush TG, Savidge TC, Freeman TC, et al. Cell 1998; 93:189–201. [DOI] [PubMed]
- Spits H, Artis D, Colonna M, et al. Nat Rev Immunol 2013; 13:145–149. [DOI] [PubMed]
- Artis D, Spits H. Nature 2015; 517:293–301. [DOI] [PubMed]
- Ibiza S, Garcia-Cassani B, Ribeiro H, et al. Nature 2016; 535:440–443. [DOI] [PMC free article] [PubMed]
- Mulligan LM. Nat Rev Cancer 2014; 14:173–186. [DOI] [PubMed]
- Veiga-Fernandes H, Mucida D. Cell 2016; 165:801–811. [DOI] [PMC free article] [PubMed]
- Robinette ML, Fuchs A, Cortez VS, et al. Nat Immunol 2015; 16:306–317. [DOI] [PMC free article] [PubMed]