Abstract
The aim of the present study was to investigate whether the in vitro pro-oxidant effect of ascorbic acid towards thiol groups could be mediated by free radicals formed during its auto-oxidation and/or by a direct oxidation of -SH groups by its oxidized form (dehydroascorbic acid). This hypothesis was examined by measuring the rate of AA (ascorbic acid) oxidation in MOPS (3-morpholinepropanesulfonic acid buffer) and phosphate buffer (PB). Here we have used dithiothreitol (DTT) as model of vicinal thiol-containing enzymes, namely δ-aminolevulinate dehydratase. The rate of AA and DTT oxidation was more pronounced in the presence of PB than in the MOPS. AA oxidation induced by iron/EDTA complex was significantly reduced by addition of superoxide dismutase, catalase and DTT to the reaction medium. H2O2 alone did not stimulate the oxidation of AA; however, AA oxidation was enhanced significantly with the addition of crescent concentrations of iron. Conversely, in DTT oxidation assay (without AA) the addition of iron, EDTA and H2O2, did not promote the oxidation of -SH groups. Our findings suggest that in the presence of physiological concentrations of AA and thiols, the oxidation of -SH groups is mediated by AA conversion to dehydroascorbic acid with the participation of iron. Furthermore, free radical species formed during the auto-oxidation of AA apparently did not oxidize thiol groups to a significant extent.
Keywords: delta-ALA-D, ascorbic acid, thiol groups, oxidation-reduction, free radicals
Introduction
Ascorbic acid (AA) functions as an electron donor and scavenges free radicals such as superoxide radicals and hydroxyl radicals in vitro (Duarte and Lunec, 2005[7]; Frei et al., 1990[10]; Sato et al., 2010[28]; Halliwell, 1996[12]). In normal cell physiology, it can donate one electron to vitamin E radicals (Packer et al., 1979[21]). At physiological pH, the ene-diol structure of ascorbic acid AH2 is entirely in the form of its conjugate base AH-, which explains why it reacts as a one-electron donor in free radical scavenging. After donating an electron, ascorbate is oxidized to a semidehydroascorbate radical (SDA), a less reactive compound. The SDA radical can either be recycled back to AA or can be transformed in dehydroascorbate (DHA). In the presence of GSH, the enzyme DHA reductase can catalyze the regeneration of ascorbate (Linster and Van Schaftingen, 2007[15]).
In vivo AA has been shown to have a diversity of antioxidant properties, protecting the organism and its biomolecules from oxidative damage (Handelman, 2007[13]; Li and Schellhorn, 2007[14]; Sartori-Valinotti et al., 2007[27]; Rodrigo et al., 2007[26]). However, in vitro AA can exhibit both antioxidant (Halliwell, 1996[12]; Pitarque et al., 2006[22]) or pro-oxidant activities. The pro-oxidant activity is related to the maintenance of iron as Fe2+ and, consequently, in a redox state that can generate reactive species (Halliwell, 1996[12]; Buettner and Jurkiewicz, 1996[4]; Valko et al., 2005[31]; Giulivi and Cadenas, 1993[11]). In addition, in this reaction ascorbyl radicals are produced (Mauricio et al., 2003[16]). Literature data have indicated that the auto-oxidation of AA is accompanied by hydroxyl radical generation and this reaction can be increased by Fe2+-EDTA (Mauricio et al., 2003[16]). In contrast, the acute intake of a high dose of vitamin C with a high dose of iron has not been associated with oxidative stress in human blood (Colpo et al., 2008[5]).
Previous in vitro studies from our research group have demonstrated that supraphysiological concentrations of AA inactivate δ-aminolevulinate dehydratase (δ-ALA-D) (Rocha et al., 2005[23], 2012[24]; Beber et al., 1998[2]). The inhibition of this sulfhydryl-containing enzyme can impair heme biosynthesis and result in accumulation of aminolevulinic acid (ALA). Of toxicological significance, literature data have indicated that ALA has some pro-oxidant activity under in vitro and in vivo relevant physiological situations (Bechara et al., 1996[3]; Douki et al, 1998[6]; Emanuelli et al., 2001[9], Rocha et al, 2003[25], 2012[24]).
δ-ALA-D inhibition caused by AA was considerably decreased when 3-morpholinepropanesulfonic acid buffer (MOPS) was used in the assay instead of potassium phosphate buffer (PB) (Rocha et al., 2005[26]; Beber et al., 1998[2]). However, in these studies, the exact mechanisms via which AA inhibited the enzyme activity were not examined and supraphysiological concentrations of AA and thiols were used. Thus, it was not possible to conclude whether the effects of AA were mediated by an excessive formation of dehydroascorbic acid or by HO• and/or O2- radicals formed during ascorbic and thiol groups oxidation.
Therefore, the aim of this study was to investigate whether dithiothreitol (DTT) oxidation (which was used here as a model of vicinal thiol-containing enzymes) by physiological concentrations of AA occurs via free radical formed during its auto-oxidation and/or by oxidation of -SH groups directly by dehydroascorbic acid formed during the oxidation of AA both in MOPS and phosphate buffers. Furthermore, the possible participation of Fenton reaction on DTT oxidation was also investigated.
Materials and Methods
Chemicals
5-5'-dithio-bis(2-nitrobenzoic) acid (DTNB), dithiotreitol (DTT), ascorbic acid (AA), iron sulfate, ethylenediamintetracetic acid (EDTA), 3-morpholinepropanesulfonic acid buffer (MOPS), catalase (CAT), superoxide dismutase (SOD) and potassium phosphate buffer (PB) were obtained from Sigma (St. Louis, MO., USA). All other chemicals were of analytical grade and obtained from standard commercial suppliers.
AA oxidation rate
AA (200 μmol/L) oxidation rate was determined at 265 nm (Mauricio et al., 2003[16]).
DTT oxidation rate
The oxidation rate of thiol groups was determined according to Ellman's method (Ellman, 1959[8]) . The incubation was carried at room temperature in 100 mmol/L PB (pH 6.8) or 100 mmol/L MOPS (pH 6.8) containing 400 μmol/L DTT. At different times (0; 3.3; 6.6; 10; 13.3; 16.6 and 20 min), aliquots of the reaction mixture were sampled for -SH groups quantification.
Statistical analysis
AA and DTT oxidation and the effect of superoxide dismutase, catalase, iron and EDTA were analysed by two-way analysis of variance (treatments x time) followed by Duncan's multiple range test when appropriate. Differences were considered to be significant when p< 0.05.
Results
AA oxidation rate determined in the absence of DTT
Fe(II) (50 μmol/L) plus EDTA (100 μmol/L) caused a rapid oxidation of AA (Figure 1(Fig. 1)). This result suggests that dehydroascorbic acid is produced when iron/ EDTA are present in the medium. The AA oxidation rate was more pronounced in the presence of PB buffer when compared to MOPS buffer (compare Figure 1A(Fig. 1) and 1B(Fig. 1)).
Figure 1. Oxidation of AA (200 μmol/l) in the presence of EDTA (100 μmol/l); Fe2+ (50 μmol/l); SOD (300 U/ml) and catalase (200 U/ml); in PB (100 mmol/l) (1A) or MOPS (100 mmol/l) (1B) buffer. AA oxidation rate was determined by measuring the AA absorbance at 265 nm. Data show means ± SEM values from 3 to 4 independent experiments performed in duplicate. SEM cannot be seen when it is smaller than symbols.

In PB buffer (Figure 1A(Fig. 1)), SOD (300 U/ml) diminished partially the oxidation of AA in presence of iron/EDTA complex (about 30 % compared to AA/Fe/ EDTA after 20 min). This effect was less accentuated than caused by CAT (200 U/mL) (about 75 % compared to AA/Fe/EDTA after 20 min). The simultaneous addition of CAT and SOD abolished the auto-oxidation of AA induced by iron/ EDTA (about 95 %, compared to AA/Fe/ EDTA; p<0.05), in all tested times (Figure 1A(Fig. 1)). In MOPS buffer (Figure 1B(Fig. 1)), the AA oxidation rate induced by iron/EDTA was marginally reduced by addition of CAT (200 U/mL). However, SOD alone or in association with CAT did not modify significantly AA oxidation (Figure 1B(Fig. 1)).
AA oxidation rate in the presence of DTT
The results of Figure 2 (A and B)(Fig. 2) were obtained under the same assay conditions used in Figure 1(Fig. 1); except that DTT (400 μmol/L) was added to the medium. In the presence of DTT, the apparent oxidation of AA was reduced considerably in PB (Figure 2A(Fig. 2)) and almost abolished in MOPS buffer (Figure 2B(Fig. 2)). Similar to that observed in PB in the absence of DTT, there was practically no AA oxidation in the presence of SOD+CAT (Figure 2A(Fig. 2)).
Figure 2. Oxidation of AA (200 μmol/l) in the presence of EDTA (100 μmol/l); Fe2+ (50 μmol/l); SOD (300 U/ml); catalase (200 U/ml) and DTT (200 μmol/l), in PB (100 mmol/l) (2A) or MOPS (100 mmol/l) (2B) buffer. The oxidation rate of AA was determined by measuring the AA absorbance at 265 nm. Data show means ± SEM values from 3 to 4 independent experiments performed in duplicate. SEM cannot be seen when it is smaller than symbols.
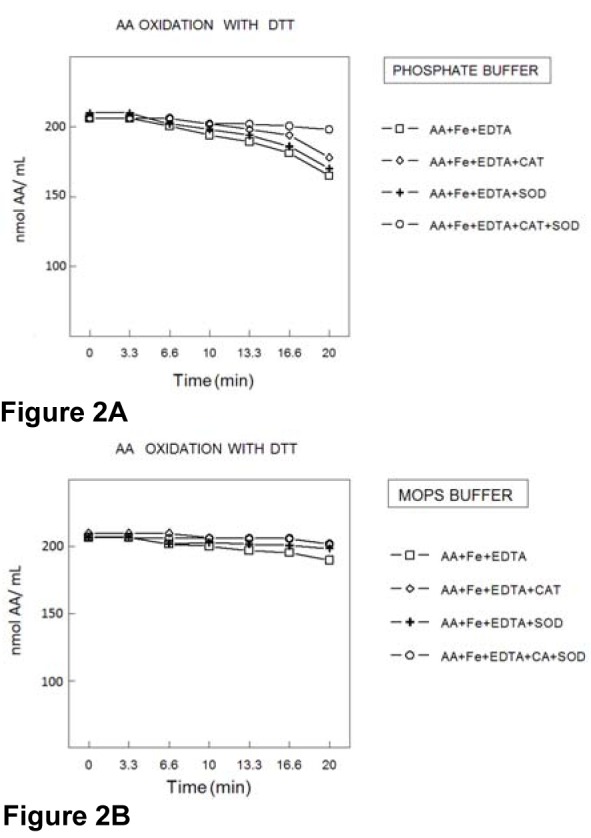
DTT oxidation rate
In the presence of AA and in PB, thiol groups of DTT were oxidized by Fe(II)/ EDTA (Figure 3A(Fig. 3)) and SOD did not modify DTT oxidation. Catalase causes a small but significant reduction in DTT oxidation (p<0.05) and the association of SOD+CAT produced a more marked antioxidant effect than CAT alone (p<0.01). DTT oxidation was less accentuated in the presence of MOPS buffer than in PB buffer and the antioxidant enzymes alone or in combination did not reduce the rate of DTT oxidation in the presence of MOPS buffer (Figure 3B(Fig. 3)).
Figure 3. DTT oxidation in the presence of AA. DTT (200 μmol/l) oxidation was determined in the presence of EDTA (100 μmol/l); Fe2+ (50 μmol/l); SOD (300 U/ml) and catalase (200 U/mL), in PB (100 mmol/L) (3A) or MOPS (100 mmol/l) (3B) buffer. The rate of thiol oxidation was determined by measuring the amount sulfhydryl groups at different times. Data show means ± SEM values from 3 to 4 independent experiments performed in duplicate. SEM cannot be seen when it is smaller than symbols.
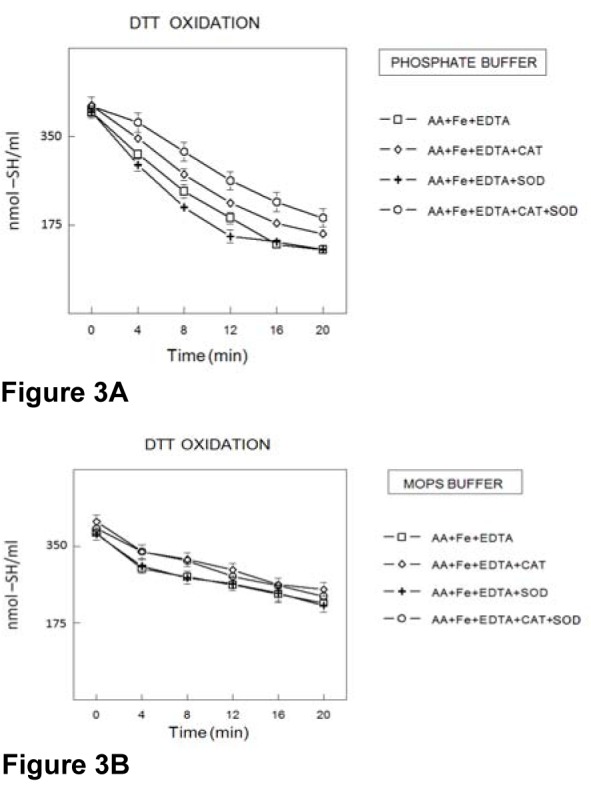
DTT oxidation in the presence of H2O2 and Fe2+ (in the absence of AA and EDTA)
The potential oxidation of DTT by Fenton reaction (in the absence of AA and EDTA in the reaction mixture) in PB (Figure 4A(Fig. 4)) or MOPS buffer (Figure 4B(Fig. 4)) was investigated. However, hydrogen peroxide alone or in the presence of up to 0.2 mmol/l Fe(II) did not cause significant acceleration of DTT oxidation (Figure 4A(Fig. 4) and B(Fig. 4)).
Figure 4. Oxidation of DTT (400 μmol/l) in the presence of H2O2 (50 μmol/l) plus Fe2+ (concentrations expressed in μmol/l) in PB (100 mmol/l) (4A) and/or MOPS (100 mmol/l) (4B) buffers. The rate of thiol oxidations was determined by measuring the amount sulfhydryl groups at different times. Data show means ± SEM values from 3 to 4 independent experiments performed in duplicate. SEM cannot be seen when it is smaller than symbol.
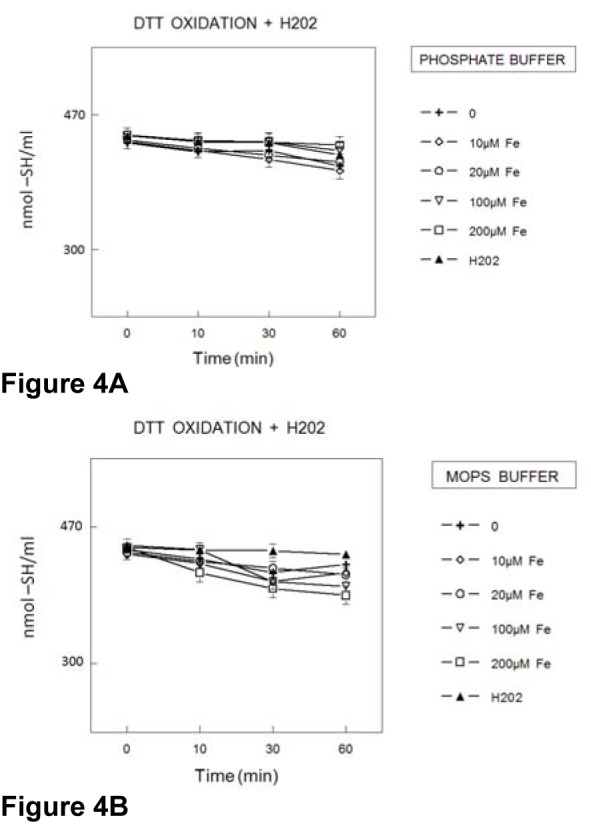
DTT oxidation in the presence of H2O2 and Fe2+ (in the presence of EDTA and absence of AA)
In the presence of PB and EDTA, the rate of DTT oxidation was increased by hydrogen peroxide and the simultaneous addition of Fe(II) and hydrogen peroxide did not cause further increase in the rate of DTT oxidation (Figure 5A(Fig. 5)). However, in the presence of MOPS buffer, the rate of DTT oxidation was not modified by EDTA and hydrogen peroxide (regardless of the addition of Fe(II); Figure 5B(Fig. 5)).
Figure 5. Oxidation of DTT (400 μmol/l) in the presence of EDTA (100 μmol/l) and H2O2 (50 μmol/l) plus Fe2+ (concentrations expressed in μmol/l) in PB (100 mmol/l) (5A) or MOPS (100 mmol/l) (5B) buffer. The rate of thiol oxidation was determined by measuring the amount sulfhydryl groups at different times. Data show means ± SEM values from 3 to 4 independent experiments performed in duplicate. SEM cannot be seen when it is smaller than symbols.
Oxidation of AA in the presence of H2O2 and Fe2+ (in the absence of EDTA)
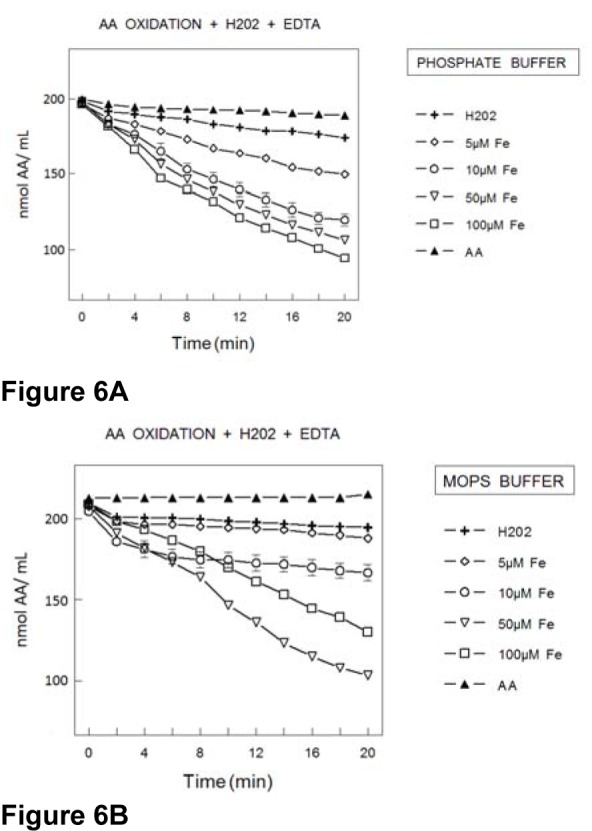
In the presence of PB (Figure 6A(Fig. 6)), the addition of H2O2 did not increase significantly the rate of AA oxidation. Conversely, the simultaneous inclusion of iron in the reaction medium caused a concentration dependent increase in AA oxidation (Figure 6A(Fig. 6)). In the presence of MOPS buffer, hydrogen peroxide did not modify AA and the simultaneous addition of Fe(II) and hydrogen peroxide was associated with an increase in the rate of AA oxidation (Figure 6B(Fig. 6)). However, in MOPS buffer, AA oxidation rate was less pronounced than in PB buffer (Figure 6A(Fig. 6) and B(Fig. 6)). In fact, the rate of AA oxidation was increased only in the presence of concentrations of Fe(II) higher than 10 μmol/l, whereas in PB 5 μmol/l Fe(II) caused a significant increase in the rate of AA oxidation (Figure 6A(Fig. 6)).
Figure 6. Oxidation of AA (200 μmol/l) in the presence of EDTA (100 μmol/l) and H2O2 (50 μmol/l) plus Fe2+ (concentrations expressed in μmol/l) in PB (100 mmol/l) (6A) or MOPS (100 mmol/l) (6B) buffer. The rate of AA oxidation was determined by measuring the AA absorbance at 265 nm. Data show means ± SEM values from 3 to 4 independent experiments performed in duplicate. SEM cannot be seen when it is smaller than symbols.
Discussion
Previously, literature data have clearly indicated that transition metals can react with reducing agents, namely with AA in aerobic solutions, to generate oxygen free radicals via reactions (1)-(4), where M is usually Fe or Cu.
3 AAred + 3 M(n + 1)+ → 3 AAox + 3 Mn+ + 3 H+ (1)
2 Mn+ + 2 O2 → 2 M(n + 1)+ + 2O2• (2)
2O2• + 2 H+ → H2O2 + O2 (3)
Mn+ + H2O2 → M(n + 1)+ + HO• + HO- (4)
According to these reactions, AA oxidation is stimulated by a transition metal and can drive the formation of reactive oxygen species, including O• and HO• (Van Dyke et al., 1996[32]). Here, when phosphate buffer (PB) was used, we have clearly demonstrated that reactive oxygen species were involved in the oxidation reactions of AA induced by transition metals (i.e. Fe2+), because SOD and CAT abolished AA oxidation. However, in the presence of MOPS, the oxidation of AA acid was less accentuated than in the presence of PB. Furthermore, a protective effect of SOD and CAT on AA oxidation was not observed. This can be related to the fact that MOPS buffer blocks HO• formation (Van Dyke et al., 1996[32]) and, consequently, by slowing down reaction 4 (see scheme above) can diminish the role of SOD and CAT as inhibitors of AA oxidation.
Previously, we reported that supraphysiological concentrations of AA inhibited the thiol-containing enzyme δ-aminolevulinate (ALA-D) and this effect was more evident when phosphate buffer was used as a buffer than in the presence of MOPS buffer (Rocha et al., 2005[23]). In fact, in the presence of MOPS, the rate of AA oxidation was lower than that in PB and correspondingly, the enzyme inhibition was diminished in this buffer. MOPS seems to be able to scavenge HO• and, consequently, the decrease in AA oxidation and ALA-D inhibition could be related to a blockage or reduction in reaction (4), which is essential in the regeneration of oxidized metal that will oxidize AA in reaction (1). However, in our previous study, we have used supraphysiological concentrations of AA and the protective effect of antioxidant enzymes was negligible.
Here we have used physiological concentrations of AA and we have observed that, under physiological conditions, reactive oxygen species are involved in AA oxidation. However, since reactive oxygen species could oxidize -SH group, we could not support our early assumption that AA inhibition of ALA-D was mediated by its oxidation to dehydroascorbic acid. Consequently, in order to enlarge our knowledge about the interaction of AA and -SH groups using physiological concentrations of AA, we designed a set of simple experiments to determine whether or not reactive oxygen species could be involved in thiol oxidation. In fact, we have used a classical dithiol and we were able to observe that hydrogen peroxide, in the presence or not of Fe(II), cannot oxidize significantly DTT. Furthermore, hydrogen peroxide by itself cannot oxidize AA, but in the presence of iron the rate of AA oxidation is increased considerably, which is in accordance with reaction 1-4. Thus, it seems clear from experiments here described that using physiological concentrations of AA and thiols, the oxidation of thiol groups by AA is not mediated by reactive oxygen species, but by dehydroascorbic acid that is formed via reactions 1-4. In fact, literature data have indicated the importance of chemical and physiological interactions between endogenous thiols-disulfide and ascorbic acid-dehydroascorbic acid (May et al., 1996[17]; Winkler, 1992[34], 1994[35]; Baker and Smiley, 1984[1]). Here we have indicated that DTT can protect AA oxidation possibly by its regeneration from dehydroascorbic acid or by reducing its oxidation via inhibition of some of the steps of reactions 1 to 4 (see the scheme at the beginning of Discussion). In fact, thiols can either act as antioxidant or pro-oxidant agents (Misra, 1974[19]; Milne et al., 1993[18]; Spear and Aust, 1995[29]); however, under the conditions used in the present investigation DTT has predominantly exhibited antioxidant activity possibly by inhibiting AA-mediated free-radical formation.
The antioxidant effect of MOPS buffer, in addition to its previously described ability to scavenge reactive oxygen species (Van Dyke et al., 1996[32]) can also be related to an interference with the availability of iron for participation in reactions 1 to 4. In line with this, literature data have indicated that buffers can interfere considerably with iron (II) reactivity (Mlakar et al., 1996[20]; Welch et al.,2002[33]; Spear and Aust, 1998[30]). Here in the experiments described in Figure 6(Fig. 6), we have demonstrated that MOPS can decrease the capacity of Fe(II) to induce AA oxidation (Figure 6B(Fig. 6)). Consequently, our previous conclusion that MOPS buffer had antioxidant activity by acting as a free radical scavenger, should also encompass the possible “chelation” of free iron ions (II or III).
In summary, the results presented here indicated that AA can oxidize thiols by its auto-oxidation to dehydroascorbic acid. This auto-oxidation requires a transition metal that, under most common experimenttal situations may be attributed to trace amounts of contaminant iron, via a set of reactions (described at the begin of Discussion section) generates free radicals. These free radicals could potentially also contribute to thiol oxidation; however, experiments described in Figure 4(Fig. 4) and 5(Fig. 5) suggest that HO• is not an important intermediate involved in DTT oxidation. Consequently, we can suppose that in the presence of AA and iron, thiol oxidation occur via regeneration AA.
Acknowledgements
This study was supported by FAPERGS-PRONEX-CNPQ, the FINEP research grant “Rede Instituto Brasileiro de Neurociência (IBN-Net)”# 01.06.0842-00, INCT FOR EXCITOTOXICITY AND NEUROPROTECTION, CNPQ, CAPES/ SAUX/ PROAP/ PROCAD, VITAE Fundation and FAPERGS.
References
- 1.Baker WL, Smiley KL. Possible explanation and implications of the reaction of ascorbic acid with some disulphide reagents. Biochem J. 1984;218:995–996. doi: 10.1042/bj2180995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beber FA, Wollmeister J, Brigo MJ, Silva MC, Pereira CN, Rocha JB. delta-Aminolevulinatedehydratase inhibition by ascorbic acid is mediated by an oxidation system existing in the hepatic supernatant. Int J Vitam Nutr Res. 1998;68:181–188. [PubMed] [Google Scholar]
- 3.Bechara EJ. Oxidative stress in acute intermittent porphyria and lead poisoning may be triggered by 5-aminolevulinic acid. Braz J Med Biol Res. 1996;29:841–851. [PubMed] [Google Scholar]
- 4.Buettner GR, Jurkiewicz BA. Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiat Res. 1996;145:532–541. [PubMed] [Google Scholar]
- 5.Colpo E, de Bem AF, Pieniz S, Schettert SD, dos Santos RM, Farias IL, et al. A single high dose of ascorbic acid and iron is not correlated with oxidative stress in healthy volunteers. Ann Nutr Metab. 2008;53:79–85. doi: 10.1159/000162257. [DOI] [PubMed] [Google Scholar]
- 6.Douki T, Onuki J, Medeiros MH, Bechara EJ, Cadet J, Di Mascio P. Hydroxyl radicals are involved in the oxidation of isolated and cellular DNA bases by 5-aminolevulinic acid. FEBS Lett. 1998;428:93–96. doi: 10.1016/s0014-5793(98)00504-3. [DOI] [PubMed] [Google Scholar]
- 7.Duarte TL, Lunec J. Review: When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic Res. 2005;39:671–686. doi: 10.1080/10715760500104025. [DOI] [PubMed] [Google Scholar]
- 8.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 9.Emanuelli T, Pagel FW, Alves LB, Regner A, Souza DO. Inhibition of adenylate cyclase activity by 5-aminolevulinic acid in rat and human brain. Neurochem Int. 2001;38:213–218. doi: 10.1016/s0197-0186(00)00092-9. [DOI] [PubMed] [Google Scholar]
- 10.Frei B, Stocker R, England L, Ames BN. Ascorbate: the most effective antioxidant in human blood plasma. Adv Exp Med Biol. 1990;264:155–163. doi: 10.1007/978-1-4684-5730-8_24. [DOI] [PubMed] [Google Scholar]
- 11.Giulivi C, Cadenas E. The reaction of ascorbic acid with different heme iron redox states of myoglobin. Antioxidant and prooxidant aspects. FEBS Lett. 1993;332:287–290. doi: 10.1016/0014-5793(93)80651-a. [DOI] [PubMed] [Google Scholar]
- 12.Halliwell B. Vitamin C: antioxidant or pro-oxidant in vivo? Free Radic Res. 1996;25:439–454. doi: 10.3109/10715769609149066. [DOI] [PubMed] [Google Scholar]
- 13.Handelman GJ. Vitamin C deficiency in dialysis patients—are we perceiving the tip of an iceberg? Nephrol Dial Transplant. 2007;22:328–331. doi: 10.1093/ndt/gfl534. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for vitamin C. J Nutr. 2007;137:2171–2184. doi: 10.1093/jn/137.10.2171. [DOI] [PubMed] [Google Scholar]
- 15.Linster CL, Van Schaftingen E. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J. 2007;274:1–22. doi: 10.1111/j.1742-4658.2006.05607.x. [DOI] [PubMed] [Google Scholar]
- 16.Maurício AQ, Lopes GK, Gomes CS, Oliveira RG, Alonso A, Hermes-Lima M. Pyridoxal isonicotinoyl hydrazone inhibits iron-induced ascorbate oxidation and ascorbyl radical formation. Biochim Biophys Acta. 2003;1620:15–24. doi: 10.1016/s0304-4165(02)00502-0. [DOI] [PubMed] [Google Scholar]
- 17.May JM, Qu ZC, Whitesell RR, Cobb CE. Ascorbate recycling in human erythrocytes: role of GSH in reducing dehydroascorbate. Free Radic Biol Med. 1996;20:543–551. doi: 10.1016/0891-5849(95)02130-2. [DOI] [PubMed] [Google Scholar]
- 18.Milne L, Nicotera P, Orrenius S, Burkitt MJ. Effects of glutathione and chelating agents on copper-mediated DNA oxidation: pro-oxidant and antioxidant properties of glutathione. Arch Biochem Biophys. 1993;304:102–109. doi: 10.1006/abbi.1993.1327. [DOI] [PubMed] [Google Scholar]
- 19.Misra HP. Generation of superoxide free radical during the autoxidation of thiols. J Biol Chem. 1974;249:2151–2155. [PubMed] [Google Scholar]
- 20.Mlakar A, Batna A, Dudda A, Spiteller G. Iron (II) ions induced oxidation of ascorbic acid and glucose. Free Radic Res. 1996;25:525–539. doi: 10.3109/10715769609149074. [DOI] [PubMed] [Google Scholar]
- 21.Packer JE, Slater TF, Willson RL. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature. 1979;278:737–738. doi: 10.1038/278737a0. [DOI] [PubMed] [Google Scholar]
- 22.Pitarque M, Creus A, Marcos R. Analysis of glutathione and vitamin C effects on the benzenetriol-induced DNA damage in isolated human lymphocytes. Sci World J. 2006;6:1191–1201. doi: 10.1100/tsw.2006.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rocha JB, Lissner LA, Puntel RL, Fachinetto R, Emanuelli T, Nogueira CW, et al. Oxidation of delta-ALA-D and DTT mediated by ascorbic acid: modulation by buffers depends on free iron. Biol Pharm Bull. 2005;28:1485–1489. doi: 10.1248/bpb.28.1485. [DOI] [PubMed] [Google Scholar]
- 24.Rocha JBT, Saraiva RA, Garcia SC, Gravina FS, Nogueira CW. Aminolevulinate dehydratase (δ-ALA-D) as marker protein of intoxication with metals and other pro-oxidant situations. Toxicol Res. 2012;1:85–102. [Google Scholar]
- 25.Rocha ME, Dutra F, Bandy B, Baldini RL, Gomes SL, Faljoni-Alário A, et al. Oxidative damage to ferritin by 5-aminolevulinic acid. Arch Biochem Biophys. 2003;409:349–356. doi: 10.1016/s0003-9861(02)00633-1. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigo R, Guichard C, Charles R. Clinical pharmacology and therapeutic use of antioxidant vitamins. Fundam Clin Pharmacol. 2007;21:111–127. doi: 10.1111/j.1472-8206.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 27.Sartori-Valinotti JC, Iliescu R, Fortepiani LA, Yanes LL, Reckelhoff JF. Sex differences in oxidative stress and the impact on blood pressure control and cardiovascular disease. Clin Exp Pharmacol Physiol. 2007;34:938–945. doi: 10.1111/j.1440-1681.2007.04643.x. [DOI] [PubMed] [Google Scholar]
- 28.Sato Y, Uchiki T, Iwama M, Kishimoto Y, Takahashi R, Ishigami A. Determination of dehydroascorbic acid in mouse tissues and plasma by using tris(2-carboxyethyl)phosphine hydrochloride as reductant in metaphosphoric acid/ethylenediaminetetraacetic acid solution. Biol Pharm Bull. 2010;33:364–369. doi: 10.1248/bpb.33.364. [DOI] [PubMed] [Google Scholar]
- 29.Spear N, Aust SD. Effects of glutathione on Fenton reagent-dependent radical production and DNA oxidation. Arch Biochem Biophys. 1995;324:111–116. doi: 10.1006/abbi.1995.9921. [DOI] [PubMed] [Google Scholar]
- 30.Spear N, Aust SD. The effects of different buffers on the oxidation of DNA by thiols and ferric iron. J Biochem Mol Toxicol. 1998;12:125–132. doi: 10.1002/(sici)1099-0461(1998)12:2<125::aid-jbt7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 31.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 32.Van Dyke BR, Clopton DA, Saltman P. Buffer-induced anomalies in the Fenton chemistry of iron and copper. Inorg Chim Acta. 1996;242:57–61. [Google Scholar]
- 33.Welch KD, Davis TZ, Aust SD. Iron autoxidation and free radical generation: effects of buffers, ligands, and chelators. Arch Biochem Biophys. 2002;397:360–369. doi: 10.1006/abbi.2001.2694. [DOI] [PubMed] [Google Scholar]
- 34.Winkler BS. Unequivocal evidence in support of the nonenzymatic redox coupling between glutathione/glutathione disulfide and ascorbic acid/dehydroascorbic acid. Biochim Biophys Acta. 1992;1117:287–290. doi: 10.1016/0304-4165(92)90026-q. [DOI] [PubMed] [Google Scholar]
- 35.Winkler BS, Orselli SM, Rex TS. The redox couple between glutathione and ascorbic acid: a chemical and physiological perspective. Free Radic Biol Med. 1994;17:333–349. doi: 10.1016/0891-5849(94)90019-1. [DOI] [PubMed] [Google Scholar]


