Abstract
The present investigation reports the protective effects of curcumin (CMN) and vitamin E against CCl4 induced oxidative stress and nephrotoxicity in rats. The toxicant CCl4 was used to induce nephrotoxicity at a dose of 1 ml/kg as 1:1(v/v) mixture with liquid paraffin twice weekly for 8 weeks. Plasma total protein, albumin, urea and creatinine concentrations were measured to assess the renal function. Antioxidant status in the kidney was estimated by determining the activities of glutathione peroxidase (GPx), glutathione reductase (GR), glutathione-S-transferase (GST), catalase (CAT) and superoxide dismutase (SOD) as well as by histopathological examination. CCl4 caused elevated levels of urea and creatinine while it decreased levels of total protein and albumin in plasma. Furthermore, CCl4 treated rats showed marked depletion of renal endogenous antioxidant enzymes. Oral administration of curcumin (100 mg/kg, daily for 8 weeks) and in combination with vitamin E (three times weekly for 8 weeks) showed significantly attenuated renal dysfunction by increased activity of antioxidant enzymes in kidney. It also increased the concentrations of plasma total protein and albumin, while reducing the concentration of urea and creatinine. Kidney sections of CCl4 induced rats showed deleterious alterations in micro anatomy. However, curcumin and vitamin E treatment prevented kidney damage induced by CCl4. This study reveals that curcumin in combination with vitamin E possesses strong antioxidant and kidney protective activity compared to curcumin alone.
Keywords: carbon tetrachloride, curcumin, vitamin E, silymarin
Introduction
Various studies have demonstrated that carbon tetrachloride (CCl4) intoxication causes free radical generation in many tissues such as liver, kidney, heart, lung, testis, brain and blood (Rechnagel et al., 1989[29]; Kumar et al., 2005[19]; Khan and Ahmed, 2009[15]; Khan et al., 2009[16]). CCl4 intoxication in animals is an experimental model that mimics oxidative stress in many physiological situations (Ivor and Schneider, 2005[12]). Cumulative data suggest a role for reactive oxygen metabolites as one of the postulated mechanisms in the pathogenesis of CCl4 nephrotoxicity (Rechnagel et al., 1989[29]). CCl4 is metabolically converted by cytochromes P450 to CCl3 radicals and results in enhanced generation of trichloro methyl peroxyl radicals (Cl3COO-) and hydrogen peroxide in cultured hepatocytes as well as in mesangial cells (Knight et al., 1989[18]). In vitro and in vivo studies indicated that CCl4 enhances lipid peroxidation and reduced the renal reduced/oxidized glutathione ratio in kidney cortex as well as renal microsomes and mitochondria (Adewole et al., 2007[1]; Khan et al., 2009[16]). Antioxidants are vital substances which possess the ability to protect the body from damage caused by free radical induced oxidative stress (Shahani, 1998[35]; Ozsoy et al., 2008[26]). There is an increasing interest in natural antioxidants, e.g. polyphenols, present in medicinal and dietary plants, which might help prevent oxidative damage. In treatment of these diseases, antioxidant therapy has gained an immense importance. Current research is now directed towards finding naturally occurring antioxidants of plant origin (Sundaram and Mitra, 2007[40]). Plant and plant products are being used as a source of medicine since long. Plants used as food and in traditional medicine are more likely to yield pharmacologically active compounds. The medicinal properties of plants have been investigated in the recent scientific developments throughout the world, due to their potent antioxidant activities, without side effects and of their economic viability.
Curcumin is a major yellow pigment in turmeric ground rhizome of Curcuma longa Linn., which is used widely as a spice and colouring agent in several foods such as curry, mustard and potato chips as well as in cosmetics and drugs (Okada et al., 2001[25]; Joe et al., 2004[14]). Curcumin represents a class of anti-inflammatory and antioxidants reported to be a scavanger of formed ROS (Biswas et al., 2005[3]). Curcumin exhibited antioxidant activity in a renal cell line (Cohly et al., 1998[4]) and ameliorated ferric nitrilotriacetic (Fe-NTA) renal oxidative stress in mice (Okada et al., 2001[25]). However, investigations reporting the protective effects of curcumin along with vitamin E on CCl4 induced nephropathy are scanty. Therefore, in continuation of the search for potential modulators of CCl4-mediated renal damage, we have examined the effects of curcumin, a naturally occurring polyphenol and vitamin E on experimentally induced CCl4 renal oxidative stress in rats.
Materials and Methods
Chemicals and drugs
Carbon tetrachloride was purchased from MERCK India Ltd., Curcumin and α-Tocopherol (Vitamin E) purchased from Sigma Chemicals Co. St Louis, MO/USA. Silymarin suspension purchased from Micro labs, Bangalore. Analyzing kits were obtained from Excel Diagnostics Pvt. Ltd, Hyderabad, Span Diagnostics Ltd, Surath. All other chemicals used were of technical grade.
Animals
Adult male albino rats Wistar strain (Rattus norvegicus) weighing 190 ± 30 procured from Sri Raghavendra Animal Supplier, Bangalore, Karnataka, were used for the study after the clearance from Institutional Animal Ethical Committee (CPCSEA) REGD. No. 470/01/a/ CPCSEA, DT. 24th Aug 2001. They were kept in cages under standard laboratory conditions (23 ± 2 ᴼC, 12 h dark/light) and were fed with commercial rat feed supplied by Sai Durga Feeds and Foods, Bangalore and water ad libitum. Before use prior to the experimentation they were allowed to laboratory conditions for ten days.
Experimental protocol
Animals were randomly assigned into seven groups of six each. Group 1 served as normal control and received Phosphate Buffer Saline (p.o. daily for 8 weeks), Group 2 Curcumin control (100 mg/kg wt. p.o.), Group 3 Curcumin (100 mg/kg p.o. daily for 8 weeks) + Vitamin E (40 mg/kg three times weekly for 8 weeks). Group 4 received CCl4 (1 ml/kg 1:1 in liquid parafin p.o. twice weekly for 8 weeks), Group 5 CCl4 + Curcumin (100 mg/kg p.o. for 8 weeks), Group 6 CCl4 + Curcumin (100 mg/ kg p.o., daily, Vitamin E 40 mg/kg weekly three times p.o. for 8 weeks), Group 7 CCl4 + Silymarin (50 mg/kg wt. p.o. for 8 weeks).
Selection of dose
The dose of CCl4 (1 ml/kg wt.) (Mc Lean et al., 1969[22]; Roy et al., 2006[33]), the dose of curcumin 100 mg/kg wt. (Sreepriya and Bali, 2006[39]) and the dose of vitamin E (Moawad, 2007[23]) were selected with minor modifications.
Sacrifice of animals and collection of blood and kidney
At the end of experimentation and 24 h after the last dose of CCl4, rats were sacrificed by cervical dislocation. Just before sacrifice, the animals were anesthetized with pentobarbital (0.6 ml/kg) and the blood collected by heart puncture and transferred into Eppendorf tubes with anticoagulant containing EDTA (1 mg/ml). Plasma was obtained by centrifugation and was used for estimation of total protein, albumin, urea and creatinine.
After sacrifice, immediately kidneys were removed and washed thoroughly with ice-cold 0.9 sodium chloride solution (saline) and cut into micro pieces fixed in 10 % formalin for histopathological examinations.
Biochemical Analysis
Plasma total protein and albumin were estimated by using kits obtained from Span Diagnostics Ltd, Surath, India. Urea and creatinine were estimated by using kits obtained from Excel Diagnostics Pvt. Ltd, Hyderabad, India.
Antioxidant enzyme assays
A ten percent homogenate of kidney was prepared by using a potter-elvehjem homogenizer at 4 °C in 0.15 M KCl. The homogenate was centrifuged (12,000 rpm for 45 min at 0-4 °C) in a Remi (C24-BL) cooling centrifuge to remove the debris and the supernatant was used for enzyme assays.
Glutathione peroxidase (GPx) assay
Glutathione peroxidase activity was estimated as described by Rotruck et al. (1973[32]) and Ellman's (1959[6]). Briefly, to 0.5 ml 0.4 M buffer (pH 7.0), 0.2 ml enzyme source (kidney homogenate), 0.2 ml 2 mM GSH, 0.1 ml 0.2 mM H2O2 were added and incubated at room temperature for 10 min along with a control tube containing all reagents except enzyme source. The reaction was arrested by adding 0.5 ml of 10 % TCA, centrifuged at 4000 rpm for 5 min and the GSH content in 0.5 ml of supernatant was estimated. The activity was expressed as µg of GSH consumed/min/mg protein.
Glutathione reductase (GR) assay
Glutathione reductase activity was estimated by the method of Pinto and Bartley (1969[27]). To 0.5 ml of 0.25 M potassium phosphate buffer (pH 7.4), 0.1 ml of 25 mM EDTA, 0.1 ml of 1 mM NADPH, 0.96 ml of distilled water and 0.1 ml of enzyme source (kidney homogenate). The reaction was initiated by the addition of 0.024 ml GSSG (50 mM). The change in absorbance was recorded at 1 min intervals at 340 nm for 5 min. The specific activity is expressed as µmol of NADPH oxidized/min/mg protein using an extinction coefficient for NADPH of 6.22 cm-1 mmol-1.
Glutathione-S-transferase (GST) assay
Glutathione-S-transferase activity was estimated as described by Habig et al. (1974[8]). To 1.7 ml of 0.14 M buffer (pH 6.5), 0.2 ml 30 mM GSH and 0.04 ml enzyme source (kidney homogenate). The reaction was initiated by 0.06 ml 0.01 M 1-chloro-2,4-dinitrobenzene (CDNB). The activity was calculated using an extinction coefficient of CDNB-GSH conjugate as 9.6 mM-1 and expressed as µmoles of CDNB-GSH conjugate formed/min/mg protein.
Catalase (CAT) assay
Catalase activity was estimated by Beers and Sizer (1952[2]) method. The assay system contained 1.9 ml 0.05 M buffer (pH 7.0) and 1.0 ml 0.059 M H2O2. The reaction was initiated by addition of 0.1 ml enzyme source (kidney homogenate). The decrease in absorbance was monitored at 1 min interval for 5 min at 240 nm and activity was expressed as nmoles of H2O2 decomposed/ min/mg protein.
Superoxide dismutase (SOD) assay
Superoxide dismutase activity was estimated by the method of Marklund and Marklund (1974[21]) adopted as follows by Soon and Tan (2002[36]): To 2.1 ml of 50 mM buffer, 0.02 ml of enzyme source (kidney homogenate) and 0.86 ml of distilled water. The reaction was initiated with 0.02 ml of 10 mM pyrogallol and change in absorbance was monitored at 420 nm. One unit of SOD was defined as that amount of enzyme required to inhibit the auto-oxidation of pyrogallol by 50 % in standard assay system of 3 ml. The specific activity was expressed as units/min/mg protein.
Protein concentration of the supernatant was estimated by Lowry et al. (1951[20]) using crystalline BSA standard.
Histopathology of kidney
The histological sections of the kidney of rats were taken by adopting the procedure as described by Humason (1972[11]). The tissues were isolated and gently rinsed with physiological saline solution (0.9 % NaCl). They were fixed in Bouin's fluid (75 ml saturated aqueous picric acid, 25 ml 40 % formaldehyde and glacial acetic acid) for 24 hours. The fixative was removed by washing through running tap water for overnight. Then the tissues were processed for dehydration. Ethyl alcohol was used as the dehydrating agent. The tissues were passed through successive series containing 30 %, 50 %, 70 %, 80 %, 90 %, 95 % and absolute alcohols. Then the tissues were cleaned in methyl benzoate and embedded in paraffin wax. Sections of 5 µ thickness were cut using “sipcon” rotatory microtome. The sections were stained with Harris hematoxylin (Harris, 1900[9]) and counter stained with eosin, dissolved in 95 % alcohol. After dehydration and cleaning, the sections were mounted in Canada balsam. Photomicrographs of the section preparations were taken using Magnus (MLX) photomicrographing equipment.
Statistical analysis
The results were expressed as mean ± S.D. The results were analyzed using DMR test (Duncan's Multiple Range Test). P<0.05 was considered as statistically significant (Duncan, 1955[5]).
Results
Effect of curcumin and vitamin E on CCl4 induced changes in the relative kidney weight (KW)/100 g body weight (BW) and plasma profile
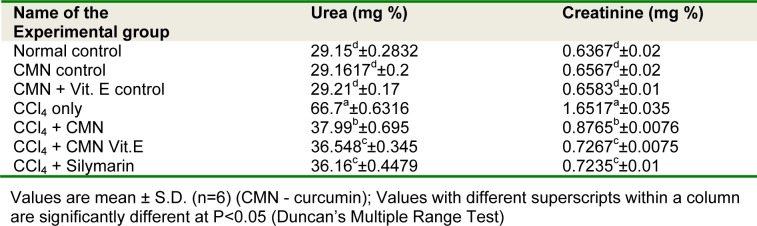
Tables 1(Tab. 1) and 2(Tab. 2) show the relative kidney weights and plasma profile of rats in each group. It was observed that CCl4 induced rats showed significant increase (P<0.05) in relative kidney weights, urea and creatinine, whereas significant (P<0.05) decrease in the concentration of total protein and albumin over normal control. Oral administration of curcumin (group V) and in combination with vitamin E (group VI) in rats treated with CCl4 caused significantly (P<0.05) reduced the relative kidney weights, urea and creatinine concentrations, while significant recovery in the concentrations of total protein and albumin.
Table 1. Effect of curcumin and vitamin E on relative kidney weight and plasma protein and albumin of different treated groups.
Table 2. Effect of curcumin and vitamin E on plasma urea and creatinine of different treated groups.
Effect of curcumin and vitamin E on CCl4 induced changes in antioxidant enzyme activities in the kidney tissue (GPx, GR, GST, CAT and SOD activities)
Table 3(Tab. 3) indicates the activities of antioxidant enzymes in the kidney of seven experimental groups. Nephrotoxicity induced by CCl4 causes a significant decrease in antioxidant enzyme levels (catalase, superoxide dismutase, glutathione peroxidase, glutathione-S-transferase and glutathione reductase) as compared to the normal control group. Whereas oral administration of curcumin (group V) and its combination with vitamin E (group VI) in rats treated with CCl4 showed recovery of the activities of antioxidant enzymes.
Table 3. Effect of curcumin and vitamin E on antioxidant enzymes activities (µg or µmol/min/mg protein) in the kidney of different treated groups.
Effect of curcumin and vitamin E on CCl4 induced changes in kidneys histoarchitecture
The histology of the kidney (Figure 1, A-F(Fig. 1)) from normal, curcumin and in combination with vitamin E control animals showed normal architecture with well developed Bowman's capsule with glomerulus and convoluted tubules enlarged, whereas rats treated with CCl4 (Figure 2, G, H(Fig. 2)) showed degenerative changes in Bowman's capsule and glomerulus, cells with vacuolization and necrotic condition. In contrary oral administration of curcumin and in combination with vitamin E or silymarin ameliorated the renal injuries near to the normal histology of the kidneys (Figure 2, I-N(Fig. 2)).
Figure 1. Histopathology of kidney, A (10 X) & B (40 X) Normal control (Group I), Fig C (10 X) & D (40 X) curcumin control (Group II) and Fig E (10 X) & F (40 X) curcumin and vitamin E control (Group III) showing normal architecture of kidney with prominent Bowman's capsule (BC), epithelial cells (EC), enlarged tubules (ET).

Figure 2. G (10 X) & H (40 X) CCl4 treated (Group IV) showing degenerative changes in renal tubules kidney tissue (DRT/DCG). Structural changes in glomeruli with vacuolization (V). Fig I (10 X) & J (40 X) CCl4 + curcumin (Group V) showing regenerative Bowman's capsule (RBC) and vacuolization. Fig K (10 X) & L (40 X) CCl4 + curcumin along with vitamin E (Group VI) showing regenerative Bowman's capsule (RBC) and reduced vacuolization. Fig M (10 X) & N (40 X) CCl4 + standard drug (silymarin) (Group VII) showing regenerative Bowman's capsule (RBC) and reduced vacuolization.

Discussion
The present study revealed ameliorative effect of curcumin and vitamin E on CCl4 induced renal toxicity in rats. The pathogenesis of kidneys is a crucial public health problem. It is well known that the kidneys play a pivotal role in the regulation of various chemicals. Administration of CCl4 causes nephrotoxicity as indicated by elevation in urine and serum level of urea, creatinine and urobilinogen while it decreased the creatinine clearance. These pathological changes signify the potential damage to liver and kidney cells induced with CCl4 treatment (Ogeturk et al., 2005[24]). From the present study it is evident that elevation in plasma urea and creatinine levels can be attributed to the damage of nephron structural integrity (Khan and Siddique, 2012[17]). In addition, decrease in the plasma total proteins and albumin concentrations in CCl4-treated rats might have resulted from remarkable leakage due to hyper cellularity of both glomeruli and tubules (Adewole et al., 2007[1]). Histopathological examination also revealed the degenerative changes in glomerulus, renal tubules and vacuolization of cells.
Curcumin with or without vitamin E treatment significantly improved the concentrations of total proteins and albumin in plasma, while significant recovery was noticed in the levels of urea and creatinine. Earlier studies have also shown that curcumin treatment decreased serum creatinine and urea concentrations in cyclosporine induced renal injury in rats in dose dependent (Tirkey et al., 2005[41]). This effect may be related to the antioxidant properties of curcumin since it has been found that ROS may be involved in the impairment of glomerular filtration rate (Hughes et al., 1996[10]; Farombi and Ekor, 2006[7]).
Of the antioxidant enzymes, superoxide dismutase and catalase are extremely effective antioxidant enzymes responsible for catalytic dismutation of highly reactive toxic superoxide radicals to H2O2 and for the catalytic decomposition of H2O2 to oxygen and water, respectively (Reiter et al., 2000[31]). CCl4 induced oxidative stress in renal tissues led to accumulation of superoxides and hydrogen peroxides. In this study it is evidenced decline in the activities of GPx, GR, GST, CAT and SOD in the kidney tissue. These results were in agreement with earlier findings (Khan and Siddique, 2012[17]). Curcumin and vitamin E treatment significantly improved the antioxidant enzyme activity in the kidney tissue. This suggests that antioxidant potential of curcumin. More and more studies now established the ability of curcumin to mainly eliminate the hydroxyl radical (Reddy and Lokesh, 1994[30]), superoxide radical (Sreejayan and Rao, 1996[37]), singlet oxygen (Rao et al., 1995[28]), nitrogen dioxide (Unnikrishnan and Rao, 1995[42]) and NO (Sreejayan and Rao, 1997[38]). It has also been demonstrated that curcumin inhibits the generation of the superoxide radical (Ruby et al., 1995[34]).
In our studies simultaneous supplementation of vitamin E and curcumin to CCl4-treated rats was found to ameliorate the renal toxicity. Therefore, it is assumed that administration of both vitamin E and curcumin co-operatively act on ROS induced by CCl4, curcumin alone also shown positive effect, however, it is less effective compared with curcumin combination with vitamin E. In support of our results earlier findings also have suggested that curcumin and vitamin E effectively ameliorates the L-Thyrixine (T4) induced oxidative stress in renal cortex of male rats, whereas curcumin alone unable to do so (Jena and Chainy, 2011[13]). Histopathological examinations are in agreement with biochemical analysis. Regenerative changes in glomerulus and convoluted tubules observed in curcumin and vitamin E supplemented rats. Comparatively curcumin in combination with vitamin E showed almost normal architecture of kidney than curcumin treatment alone.
Conclusion
Our study suggests that curcumin combination with vitamin E may be considered as potentially combating oxidative stress and nephrotoxicity induced by CCl4.
Acknowledgement
The author, Venkata Narayana G, acknowledges the financial assistance rendered as Senior Research Fellow by Department of Zoology (UGC RFSMS), Government of India. And also acknowledges research supervisor Prof. P. Indira for fabulous support throughout author's research work.
References
- 1.Adewole SO, Salako AA, Doherty OW, Naicker T. Effect of melatonin on carbon tetrachloride-induced kidney injury in wistar rats. BMC Public Health. 2007;10:153–164. [Google Scholar]
- 2.Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of ydrogen peroxide by catalase. J Biol Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- 3.Biswas SK, McClure D, Jimenez LA, Megson IL, Rahman I. Curcumin induces glutathione biosynthesis and inhibits NFkappaB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxidant Redox Signaling. 2005;7:32–41. doi: 10.1089/ars.2005.7.32. [DOI] [PubMed] [Google Scholar]
- 4.Cohly HHP, Taylor A, Angel MF, Salahudeen AK. Effect of turmeric, tumerin and curcumin on H2O2-induced renal epithelial (LLC-PK1) cell injury. Free Radic Biol Med. 1998;24:49–54. doi: 10.1016/s0891-5849(97)00140-8. [DOI] [PubMed] [Google Scholar]
- 5.Duncan DB. Multiple range and multiple F tests. Biometrics. 1955;11:1–42. [Google Scholar]
- 6.Ellman G. Tissue sulphhydril. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 7.Farombi EO, Ekor M. Curcumin attenuates gentamicin induced renal oxidative damage in rats. Food Chem Toxicol. 2006;44:1443–1448. doi: 10.1016/j.fct.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;246:7130–7139. [PubMed] [Google Scholar]
- 9.Harris HR. On the rapid conversion of haematoxylin into haematein in staining reactions. J Appl Microsc. 1900;3:777–780. [Google Scholar]
- 10.Hughes AK, Stricklett K, Padilla E, Kohan DE. Effect of reactive oxygen species on endothelin-1 production by human mesangial cells. Kidney Int. 1996;49:181–189. doi: 10.1038/ki.1996.25. [DOI] [PubMed] [Google Scholar]
- 11.Humason GL. Animal tissue techniques. 3rd. San Francisco, CA: Freeman; 1972. [Google Scholar]
- 12.Ivor JB, Schneider MD. Learning from failure: congestive heart failure in the post genomic age. Review series introduction. J Clin Invest. 2005;115:495–499. doi: 10.1172/JCI200524477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jena S, Chainy GB. Regulation of expression of antioxidant enzymes by vitamin E and curcumin in L-thyroxine-induced oxidative stress in rat renal cortex. Mol Biol Rep. 2011;38:1047–1054. doi: 10.1007/s11033-010-0201-4. [DOI] [PubMed] [Google Scholar]
- 14.Joe B, Vijaykumar M, Lokesh BR. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit Rev Food Sci Nutr. 2004;44:97–111. doi: 10.1080/10408690490424702. [DOI] [PubMed] [Google Scholar]
- 15.Khan MR, Ahmed D. Protective effects of Digera muricata (L.) Mart. on testis against oxidative stress of carbon tetrachloride in rat. Food Chem Toxicol. 2009;47:1393–1399. doi: 10.1016/j.fct.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Khan MR, Rizvi W, Khan GN, Khan RA, Shaheen S. Carbon tetrachloride induced nephrotoxicity in rat: protective role of Digera muricata. J Ethnopharmacol. 2009;122:91–99. doi: 10.1016/j.jep.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Khan MR, Siddique F. Antioxidant effects of Citharexylum spinosum in CCl4 induced nephrotoxicity in rat. Exp Toxicol Pathol. 2012;64:349–355. doi: 10.1016/j.etp.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Knight JA, Cheung AK, Pieper RK, Servilla K. Increased urinary lipoperoxide levels in renal transplant patients. Ann Clin Lab Sci. 1989;19:238–241. [PubMed] [Google Scholar]
- 19.Kumar G, Banu GS, Pandian MR. Evaluation of the antioxidant activity of Trianthema portulacastrum L. Ind J Pharmacol. 2005;37:331–333. [Google Scholar]
- 20.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin Phenol Reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 21.Marklund SL, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 22.McLean EK, McLean AE, Sutton PM. Instant cirrhosis. Improved method for producing liver cirrhosis of rats by stimulus administration of carbon tetrachloride and pontobarbitone. Br J Exp Pathol. 1969;50:502–506. [PMC free article] [PubMed] [Google Scholar]
- 23.Moawad KM. Possible prophylactic effects of vitamin E or lycopene treatment on renal toxicity induced by CCL4 administration in albino rats. World J Zool. 2007;2(2):19–28. [Google Scholar]
- 24.Ogeturk M, Kus I, Colakoglu N, Zararsiz I, Ilhan N, Sarsilmaz M. Caffeic acid henethyl ester protects kidneys against carbon tetrachloride toxicity in rats. J Ethnopharmacol. 2005;28:97:273–280. doi: 10.1016/j.jep.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Okada K, Wangpoentrakul C, Tanaka T, Toyokuni S, Uchida K, Osawa T. Curcumin and especially tetrahydrocurcumin ameliorate oxidative stress-induced renal injury in mice. J Nutr. 2001;131:2090–5. doi: 10.1093/jn/131.8.2090. [DOI] [PubMed] [Google Scholar]
- 26.Ozsoy N, Can A, Yanardag R, Akev N. Antioxidant activity of Smilax excelsa L. leaf extracts. Food Chem. 2008;110:571–583. [Google Scholar]
- 27.Pinto RE, Bartley W. The effect of age and sex on glutathione reductase and glutathione peroxidase activities and on aerobic GSH oxidation in rat liver homogenates. Biochem J. 1969;112:109–115. doi: 10.1042/bj1120109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao CV, Rivenson A, Simi B, Reddy BS. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995;55:259–266. [PubMed] [Google Scholar]
- 29.Rechnagel RO, Glende EA, Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. J Pharmacol Exp Ther. 1989;43:139–154. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 30.Reddy AC, Lokesh BR. Studies on the inhibitory effects of curcumin and eugenol on the formation of reactive oxygen species and the oxidation of ferrous iron. Mol Cell Biochem. 1994;137:1–8. doi: 10.1007/BF00926033. [DOI] [PubMed] [Google Scholar]
- 31.Reiter RJ, Tan D, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. J Biomed Sci. 2000;7:444–458. doi: 10.1007/BF02253360. [DOI] [PubMed] [Google Scholar]
- 32.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DC, Hoekstra WG. Selenium: biochemical roles as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 33.Roy CK, Kamath JV, Asad M. Hepatoprotective activity of Psidium guajava Linn. leaf extract. Indian J Exp Biol. 2006;44:305–311. [PubMed] [Google Scholar]
- 34.Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R. Antitumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995;94:79–83. doi: 10.1016/0304-3835(95)03827-j. [DOI] [PubMed] [Google Scholar]
- 35.Shahani S. Evaluation of hepatoprotective efficacy of APCL-a polyherbal formulation in vivo in rats. Indian Drugs. 1998;36:628–631. [Google Scholar]
- 36.Soon YY, Tan BKH. Evaluation of the hypoglycemic and anti-oxidant activities of Morinda officinalis in streptozotocin-induced diabetic rats. Singapore Med J. 2002;43:77–85. [PubMed] [Google Scholar]
- 37.Sreejayan N, Rao MN. Free radical scavenging activity of curcuminoids. Arzneimittelforschung. 1996;46:169–171. [PubMed] [Google Scholar]
- 38.Sreejayan N, Rao MN. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol. 1997;49:105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- 39.Sreepriya M, Bali G. Effects of administration of embelin and curcumin on lipid peroxidation, hepatic glutathione antioxidant defense and hematopoietic system during N-nitrosodiethylamine/phenobarbital-induced hepato carcinogenesis in Wistar rats. Mol Cell Biochem. 2006;284:49–55. doi: 10.1007/s11010-005-9012-7. [DOI] [PubMed] [Google Scholar]
- 40.Sundaram R, Mitra SK. Antioxidant activity of ethyl acetate soluble fraction of Acacia arabica bark in rats. Indian J Pharmacol. 2007;39:33–38. [Google Scholar]
- 41.Tirkey N, Kaur G, Vij G, Chopra K. Curcumin, a diferuloylmethane, attenuates cyclosporine-induced renal dysfunction and oxidative stress in rat kidneys. BMC Pharmacol. 2005;5:15. doi: 10.1186/1471-2210-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Unnikrishnan MK, Rao MN. Curcumin inhibits nitrogen dioxide induced oxidation of hemoglobin. Mol Cell Biochem. 1995;146:35–37. doi: 10.1007/BF00926878. [DOI] [PubMed] [Google Scholar]