Abstract
The Saccharomyces cerevisiae viruses are noninfectious double-stranded RNA viruses whose segments are separately encapsidated. A large viral double-stranded RNA (L1; 4580 base pairs) encodes all required viral functions. M1, a double-stranded RNA of 1.9 kilobases, encodes an extracellular toxin (killer toxin) and cellular immunity to that toxin. Some strains contain smaller, S, double-stranded RNAs, derived from M1 by internal deletion. Particles containing these defective interfering RNAs can displace M1 particles by faster replication and thus convert the host strain to a nonkiller phenotype. In this work, we report the development of an assay in which the expression of S plus-strand from an inducible plasmid causes the loss of M1 particles. This assay provides a convenient method for identifying in vivo cis-acting sequences important in viral replication and packaging. We have mapped the sequence involved in interference to a region of 132 base pairs that includes two sequences similar to the viral binding site sequence previously identified in L1 by in vitro experiments.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boone C., Bussey H., Greene D., Thomas D. Y., Vernet T. Yeast killer toxin: site-directed mutations implicate the precursor protein as the immunity component. Cell. 1986 Jul 4;46(1):105–113. doi: 10.1016/0092-8674(86)90864-0. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Elliott Q., Bussey H., Burn V., Smith A., Tipper D. J. Sequence of the preprotoxin dsRNA gene of type I killer yeast: multiple processing events produce a two-component toxin. Cell. 1984 Mar;36(3):741–751. doi: 10.1016/0092-8674(84)90354-4. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Hopper J. E., Rogers D. T., Tipper D. J. Translational analysis of the killer-associated virus-like particle dsRNA genome of S. cerevisiae: M dsRNA encodes toxin. Cell. 1980 Feb;19(2):403–414. doi: 10.1016/0092-8674(80)90514-0. [DOI] [PubMed] [Google Scholar]
- Bruenn J. A., Brennan V. E. Yeast viral double-stranded RNAs have heterogeneous 3' termini. Cell. 1980 Apr;19(4):923–933. doi: 10.1016/0092-8674(80)90084-7. [DOI] [PubMed] [Google Scholar]
- Bruenn J. A. Virus-like particles of yeast. Annu Rev Microbiol. 1980;34:49–68. doi: 10.1146/annurev.mi.34.100180.000405. [DOI] [PubMed] [Google Scholar]
- Bruenn J., Kane W. Relatedness of the double-stranded RNAs present in yeast virus-like particles. J Virol. 1978 Jun;26(3):762–772. doi: 10.1128/jvi.26.3.762-772.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M. E., Dowhanick J. J., Nemeroff M. E., Pietras D. F., Tu C. L., Bruenn J. A. Overlapping genes in a yeast double-stranded RNA virus. J Virol. 1989 Sep;63(9):3983–3990. doi: 10.1128/jvi.63.9.3983-3990.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban R., Fujimura T., Wickner R. B. Internal and terminal cis-acting sites are necessary for in vitro replication of the L-A double-stranded RNA virus of yeast. EMBO J. 1989 Mar;8(3):947–954. doi: 10.1002/j.1460-2075.1989.tb03456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban R., Fujimura T., Wickner R. B. Site-specific binding of viral plus single-stranded RNA to replicase-containing open virus-like particles of yeast. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4411–4415. doi: 10.1073/pnas.85.12.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban R., Wickner R. B. A deletion mutant of L-A double-stranded RNA replicates like M1 double-stranded RNA. J Virol. 1988 Apr;62(4):1278–1285. doi: 10.1128/jvi.62.4.1278-1285.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban R., Wickner R. B. Three different M1 RNA-containing viruslike particle types in Saccharomyces cerevisiae: in vitro M1 double-stranded RNA synthesis. Mol Cell Biol. 1986 May;6(5):1552–1561. doi: 10.1128/mcb.6.5.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field L. J., Bobek L. A., Brennan V. E., Reilly J. D., Bruenn J. A. There are at least two yeast viral double-stranded RNAs of the same size: an explanation for viral exclusion. Cell. 1982 Nov;31(1):193–200. doi: 10.1016/0092-8674(82)90419-6. [DOI] [PubMed] [Google Scholar]
- Fried H. M., Fink G. R. Electron microscopic heteroduplex analysis of "killer" double-stranded RNA species from yeast. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4224–4228. doi: 10.1073/pnas.75.9.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T., Esteban R., Wickner R. B. In vitro L-A double-stranded RNA synthesis in virus-like particles from Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4433–4437. doi: 10.1073/pnas.83.12.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T., Wickner R. B. Gene overlap results in a viral protein having an RNA binding domain and a major coat protein domain. Cell. 1988 Nov 18;55(4):663–671. doi: 10.1016/0092-8674(88)90225-5. [DOI] [PubMed] [Google Scholar]
- Goff C. G., Moir D. T., Kohno T., Gravius T. C., Smith R. A., Yamasaki E., Taunton-Rigby A. Expression of calf prochymosin in Saccharomyces cerevisiae. Gene. 1984 Jan;27(1):35–46. doi: 10.1016/0378-1119(84)90236-1. [DOI] [PubMed] [Google Scholar]
- Hanes S. D., Burn V. E., Sturley S. L., Tipper D. J., Bostian K. A. Expression of a cDNA derived from the yeast killer preprotoxin gene: implications for processing and immunity. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1675–1679. doi: 10.1073/pnas.83.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper J. E., Bostian K. A., Rowe L. B., Tipper D. J. Translation of the L-species dsRNA genome of the killer-associated virus-like particles of Saccharomyces cerevisiae. J Biol Chem. 1977 Dec 25;252(24):9010–9017. [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane W. P., Pietras D. F., Bruenn J. A. Evolution of defective-interfering double-stranded RNAs of the yeast killer virus. J Virol. 1979 Nov;32(2):692–696. doi: 10.1128/jvi.32.2.692-696.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M., Pietras D. F., Nemeroff M. E., Corstanje B. J., Field L. J., Bruenn J. A. Conserved regions in defective interfering viral double-stranded RNAs from a yeast virus. J Virol. 1986 May;58(2):402–407. doi: 10.1128/jvi.58.2.402-407.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolle S., Skipper N., Bussey H., Thomas D. Y. The expression of cDNA clones of yeast M1 double-stranded RNA in yeast confers both killer and immunity phenotypes. EMBO J. 1984 Jun;3(6):1383–1387. doi: 10.1002/j.1460-2075.1984.tb01981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff M. E., Bruenn J. A. Conservative replication and transcription of Saccharomyces cerevisiae viral double-stranded RNA in vitro. J Virol. 1986 Mar;57(3):754–758. doi: 10.1128/jvi.57.3.754-758.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff M. E., Pietras D. F., Bruenn J. A. Construction of full-length cDNA copies of viral double-stranded RNA. Virus Genes. 1988 Jun;1(3):243–253. doi: 10.1007/BF00572703. [DOI] [PubMed] [Google Scholar]
- Oliver S. G., McCREADY S. J., Holm C., Sutherland P. A., McLaughlin C. S., Cox B. S. Biochemical and physiological studies of the yeast virus-like particle. J Bacteriol. 1977 Jun;130(3):1303–1309. doi: 10.1128/jb.130.3.1303-1309.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
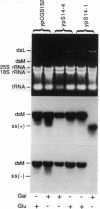
- Ridley S. P., Wickner R. B. Defective Interference in the Killer System of Saccharomyces cerevisiae. J Virol. 1983 Feb;45(2):800–812. doi: 10.1128/jvi.45.2.800-812.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz P., Stucka R., Feldmann H., Combriato G., Klobeck H. G., Fittler F. Sequence of a cDNA clone encompassing the complete mature human prostate specific antigen (PSA) and an unspliced leader sequence. Nucleic Acids Res. 1988 Jul 11;16(13):6226–6226. doi: 10.1093/nar/16.13.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper N., Thomas D. Y., Lau P. C. Cloning and sequencing of the preprotoxin-coding region of the yeast M1 double-stranded RNA. EMBO J. 1984 Jan;3(1):107–111. doi: 10.1002/j.1460-2075.1984.tb01769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers J. M. Isolation of Suppressive Sensitive Mutants from Killer and Neutral Strains of SACCHAROMYCES CEREVISIAE. Genetics. 1973 Aug;74(4):571–579. doi: 10.1093/genetics/74.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney T. K., Tate A., Fink G. R. A study of the transmission and structure of double stranded RNAs associated with the killer phenomenon in Saccharomyces cerevisiae. Genetics. 1976 Sep;84(1):27–42. doi: 10.1093/genetics/84.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele D. J., Hannig E. M., Leibowitz M. J. Genome structure and expression of a defective interfering mutant of the killer virus of yeast. Virology. 1984 Aug;137(1):20–31. doi: 10.1016/0042-6822(84)90004-7. [DOI] [PubMed] [Google Scholar]
- Vodkin M. Induction of yeast killer factor mutations. J Bacteriol. 1977 Oct;132(1):346–348. doi: 10.1128/jb.132.1.346-348.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Double-stranded RNA replication in yeast: the killer system. Annu Rev Biochem. 1986;55:373–395. doi: 10.1146/annurev.bi.55.070186.002105. [DOI] [PubMed] [Google Scholar]
- Williams T. L., Leibowitz M. J. Conservative mechanism of the in vitro transcription of killer virus of yeast. Virology. 1987 May;158(1):231–234. doi: 10.1016/0042-6822(87)90258-3. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]