Abstract
To investigate the subchronic effect of cadmium intoxication on lipid metabolism and the inflammatory responses accompanying it, rats were administered 50 and 100 ppm cadmium through their drinking water for 7 weeks. At both concentrations, cadmium exposure resulted in significant elevation (p < 0.05) of total cholesterol and gave rise to hypertriglyceridemia in the plasma of the animals. The proinflammatory cytokines, IL-2, IL-6 and TNF-α, were highly expressed in the animals. At the 50 ppm dose level, plasma IL-2, IL-6 and TNF-α levels were increased by 20 %, 87 % and 336 % respectively, while the 100 ppm dose yielded 32 %, 57 % and 470 % increases, respectively. A drastic build-up of MDA in the liver elicited by the metal led to an 85 % increase in lipid peroxidation at high dose. A 3-fold increase of lipid hydroperoxidation (LOOH) products was obtained on exposure to cadmium at 100 ppm. Cadmium caused more than a 2-fold increase in oxLDL levels at both doses tested. Paraoxonase activity was also significantly repressed, culminating in a 43 % reduction in activity at 100 ppm dose. Disruption of lipid metabolism, increased lipid peroxidation as well as imbalance in proinflammatory cytokine levels may thus, be means by which cadmium induces its toxicity.
Keywords: cadmium, cytokines, cholesterol, hypertriglyceridemia, oxLDL, paraoxonase
Introduction
Cadmium is a toxic heavy metal that is widely distributed in the environment. It is naturally found in the environment as a constituent of ocean water and to a lesser level in surface water and groundwater (ATSDR, 2008[5]). It can be naturally emitted into the environment through volcanic activities, forest fires, and generation of sea salt aerosols (ATSDR, 2008[5]). Anthropogenically, cadmium is introduced into the environment as a result of non-ferrous mining and refining processes, manufacture and application of phosphate fertilizers, fossil fuel combustion and manufacture and use of nickel-cadmium batteries (Järup, 2003[25]; Zhang et al., 2008[55]). Because of its wide application in industrial processes, cadmium has become one of the most important environmental pollutants in the world. One major route of exposure is the gastrointestinal ingestion of the metal through food and drinking water, especially in non-smoking and non-occupationally exposed populations (ATSDR, 2008[5]). Cadmium exposure results in accumulation of the metal in sensitive target organs and the metal has been shown to affect cell physiology and growth (Ramirez and Giménez, 2002[40]; Lafuente et al., 2003[28]).
Studies have linked environmental and occupational cadmium exposure with cardiovascular disease mortality (Nakagawa et al., 2006[35]; Nawroth et al., 2008[36]). Heavy metals are generally immunotoxic, among the many effects displayed (Villanueva et al., 2000[46]) and cadmium especially, has been demonstrated to induce disorders in the humoral and cellular immune responses (Kataranovski et al., 1998[26]; Dan et al., 2000[11]; Marth et al., 2000[33]). Cytokines, which are mediators of inflammatory and immune reactions, are known to alter endothelium functions (Ait-Oufella et al., 2011[3]). They also induce the expression of chemokines and adhesion molecules on the vascular endothelium (Weber et al., 2008[49]). They are normally produced as part of the immune system but excessive secretion in response to several agents, such as microorganisms and their products as well as xenobiotics can lead to immune cell dysfunctions (Shook and Laskin, 1994[43]; Weglarz et al., 2006[50]). However, there is a paucity of data regarding cytokine response during cadmium exposure.
Information on the effects of cadmium on cardiovascular system and its attendant inflammatory response is sparse; this study was, therefore, undertaken to evaluate the effect of sub-chronic cadmium exposure in drinking water on the plasma lipids, lipoprotein contents and cytokine levels in rats.
Materials and Methods
Animals and treatment
Twenty four male Wistar rats (bred in the Animal House of the Faculty of Basic Medical Sciences, Ladoke Akintola University of Technology, Ogbomoso, Nigeria) with body weight between 120 and 140 g were used for the experiment. The animals were housed in clean plastic cages and maintained on a standard pellet diet and distilled water ad libitum. They were allowed to acclimatize for 2 weeks, after which, the body weight was recorded and the rats were randomly picked into three groups, comprised of eight animals each. Group one served as the control, while the rats in groups two and three were exposed to cadmium in the form of cadmium chloride in their drinking water at 50 and 100 ppm concentration respectively for 7 weeks. Although the levels far exceeded cadmium levels to which humans are exposed to in food and water (EFSA, 2009[14]; WHO, 2011[51]), the concentrations represented levels of cadmium that have been reported in literature to have elicited toxic effects in rats (Waalkes and Rehm, 1992[48]; Lafuente et al., 2004[29]). At the end of cadmium exposure, the rats were fasted overnight and the body weights were recorded. Blood was collected from the animals into heparinized tubes by cardiac puncture under light diethyl ether anesthesia. The blood samples were centrifuged at 2000 x g for 10 minutes to obtain the plasma. All samples were stored at -20 °C until biochemical analyses were performed.
Biochemical analyses
Plasma concentrations of total cholesterol, triglyceride and phospholipids were determined with commercial kits (Chemelex®, Barcelona, Spain). HDL cholesterol and triglycerides were analyzed with the same commercial kits for total cholesterol and triglycerides after very low density lipoproteins (VLDL) and LDL were precipitated with heparin-MnCl2 solution as described by Gidez et al. (1982[17]). LDL and VLDL cholesterol was estimated by subtracting HDL cholesterol from total cholesterol. Plasma oxidized LDL (oxLDL) concentrations were measured using the method described by Ahotupa and Vasankari (1999[2]). Briefly, buffered heparin (0.064 M trisodium citrate adjusted to pH 5.05 with 5 M HCl, and containing 50000 IU/L heparin) was used to precipitate LDL. Insoluble lipoproteins were sedimented by centrifugation at 1000×g for 10 min and the pellet was resuspended in 1.0 mL of 0.1 M sodium phosphate buffer (pH 8.0, containing 0.9 % NaCl). Lipids were extracted from 100 μL of LDL by chloroform/methanol, dried under nitrogen, redissolved in cyclohexane and analyzed spectrophotometrically at 234 nm. A molar absorption coefficient of 2.95×104 M−1cm−1 was used for calculations. Level of lipid peroxidation was measured by the method of Ohkawa et al. (1979[38]). Briefly, 0.2 ml of tissue homogenate was added to the reaction mixture consisting of 0.2 ml 8 % SDS, 1.5 ml 20 % acetic acid and 0.6 ml distilled water. Reaction was initiated by adding 1.5 ml of 1 % TBA and terminated by 10 % TCA. The mixture was then centrifuged and absorbance of the supernatant was read at 532 nm. LPO was expressed in terms of nmoles MDA formed/mg tissue using an extinction coefficient of 1.56×105 M−1 cm−1. Lipid hydroperoxides in plasma were assayed by the method of Nourooz-Zadeh et al. (1994[37]). Samples (90 μl) of plasma were mixed with either 10 μl of 10 mM triphenylphosphine (TPP) in methanol or with 10 μl of methanol and incubated for 30 min at a room temperature. Then, 900 μl of FOX2 reagent (250 μM ammonium ferrous sulfate, 100 μM. xylenol orange, 25 mM H2SO4 and 4 mM butylated hydroxytoluene in 90 % methanol) was added and the sample was incubated for another 30 min. The mixture was centrifuged at 12 000 × g for 10 min to remove flocculated material and the absorbance was read at 560 nm. The absorbance of the sample with TPP was subtracted from the sample without TPP and hydroperoxides concentration was calculated from the standard curve prepared using different concentrations (1-20 μM) of H2O2. The paraoxonase activity of PON1 was determined using paraoxon (O, O-diethyl-o-p-nitrophenylphosphate) as the substrate. The increase in absorbance at 405 nm due to the formation of 4-nitrophenol following the hydrolysis of paraoxon was measured as described by Furlong et al. (1989[16]). The molar extinction co-efficient of 18050 M-1cm-1 was used to calculate enzyme activity. Plasma concentrations of interleukin 2 (IL-2), 6 (IL-6) and Tumor Necrosis Factor-alpha (TNF-α) were analyzed by enzyme-linked immunosorbent assay (ELISA) methods with commercially available assay kits (RayBiotech, Inc. USA).
Statistical evaluation
Results are expressed as mean ± S.E. One-way analysis of variance (ANOVA) followed by Tukey's test was used to analyze the results with p < 0.05 considered significant.
Results
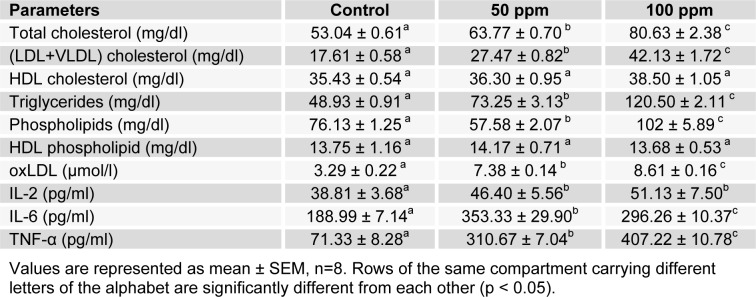
The administration of Cd through drinking water caused distortion in the plasma lipid contents of the treated animals compared to control. Cadmium exposure at the two levels of concentration tested resulted in significantly increased plasma total cholesterol, triglyceride, and (LDL+VLDL)-cholesterol fraction concentrations when compared with control (Table 1(Tab. 1)). There was a steady increase in plasma cholesterol on exposure to 50 ppm cadmium which was sustained in the 100 ppm cadmium-exposed animals (p < 0.05). Cadmium-exposure induced hypertriglyceridemia in the animals with 50 % and 146 % increases observed at 50 and 100 ppm level respectively (p < 0.05). The phospholipid content in the plasma was disturbed by cadmium intoxication. Exposure to cadmium at 50 ppm level resulted in a 25 % depletion of the lipid while 100 ppm caused a significant 34 % increase (p < 0.05) in concentration. In the HDL particles, there were no significant differences in the cholesterol and phospholipid contents between control and cadmium-exposed animals.
Table 1. Effect of cadmium on plasma lipids and lipoproteins, oxLDL and plasma cytokine levels.
Cytokine levels were increased significantly (p < 0.05) by cadmium at all concentrations employed in the study (Table 1(Tab. 1)). There were significant increases in IL-2 concentrations on exposure to cadmium at both 50 and 100 ppm levels. With IL-6, the increase in concentration was higher in the 50 ppm group, giving an 87 % elevation above the control. However, the increase elicited by exposure to 100 ppm cadmium was not as much, with a yield of 57 % above the control. Cadmium administration elicited a dose dependent increase in TNF-α concentrations. Exposure to 50 and 100 ppm cadmium resulted in 4- and 6-fold increases respectively.
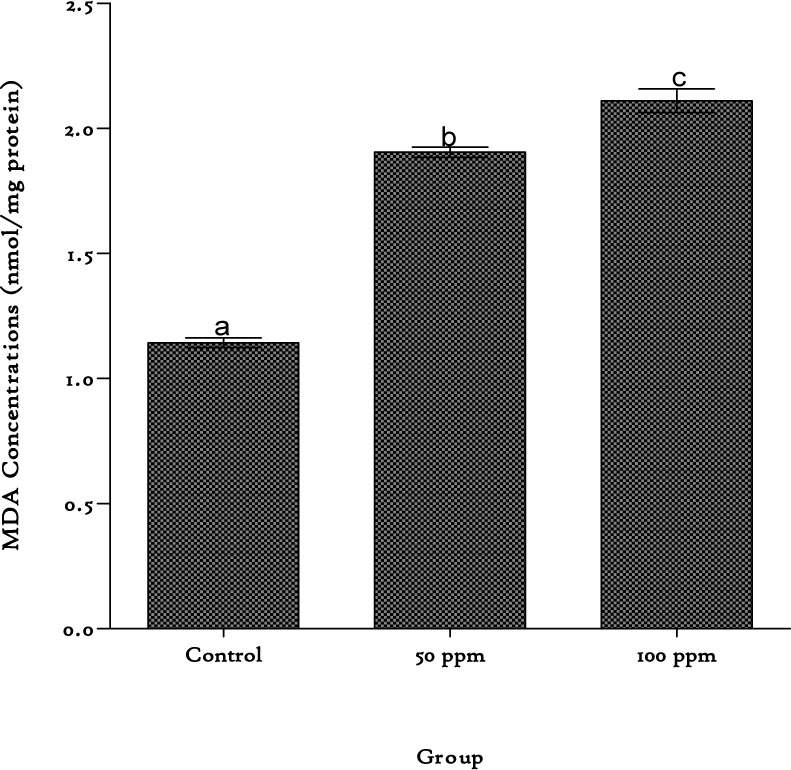
Cadmium produced significant increases in MDA levels in the liver, producing 67 % and 85 % increases at the 50 and 100 ppm exposure level respectively (Figure 1(Fig. 1)).
Figure 1. Lipid peroxidation levels in the liver of rats exposed to cadmium in their drinking water. Values are represented as mean ± SEM, n=8. Bars of the same compartment carrying different letters of the alphabet are significantly different from each other (p < 0.05).
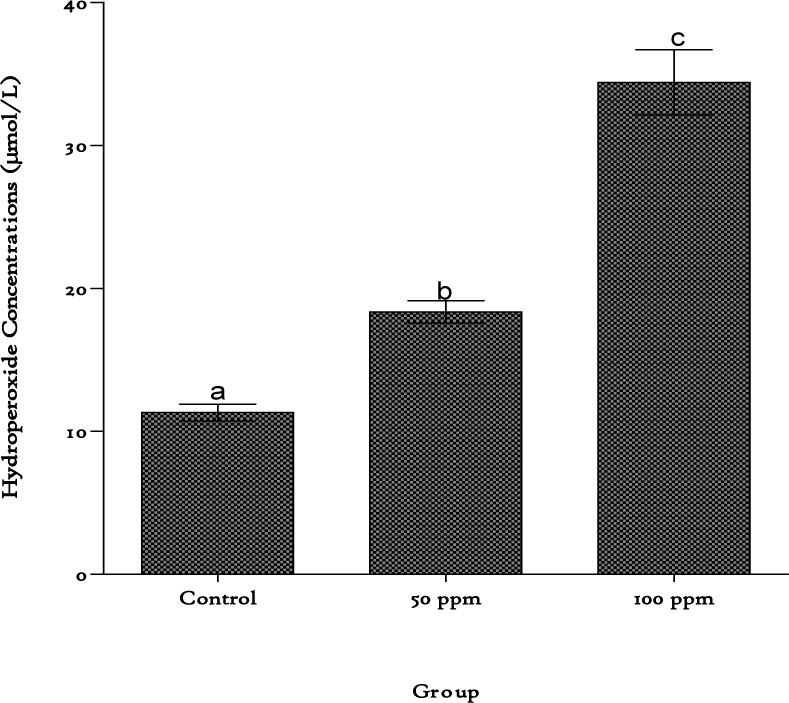
These increases were accompanied by elevation in plasma lipid hydroperoxide contents with a 1.6-fold increase obtained at 50 ppm cadmium exposure level and a 3-fold increase with the 100 ppm level (Figure 2(Fig. 2)).
Figure 2. Lipid hydroperoxide concentrations in plasma of rats exposed to cadmium in their drinking water. Values are represented as mean ± SEM, n=8. Bars of the same compartment carrying different letters of the alphabet are significantly different from each other (p < 0.05).
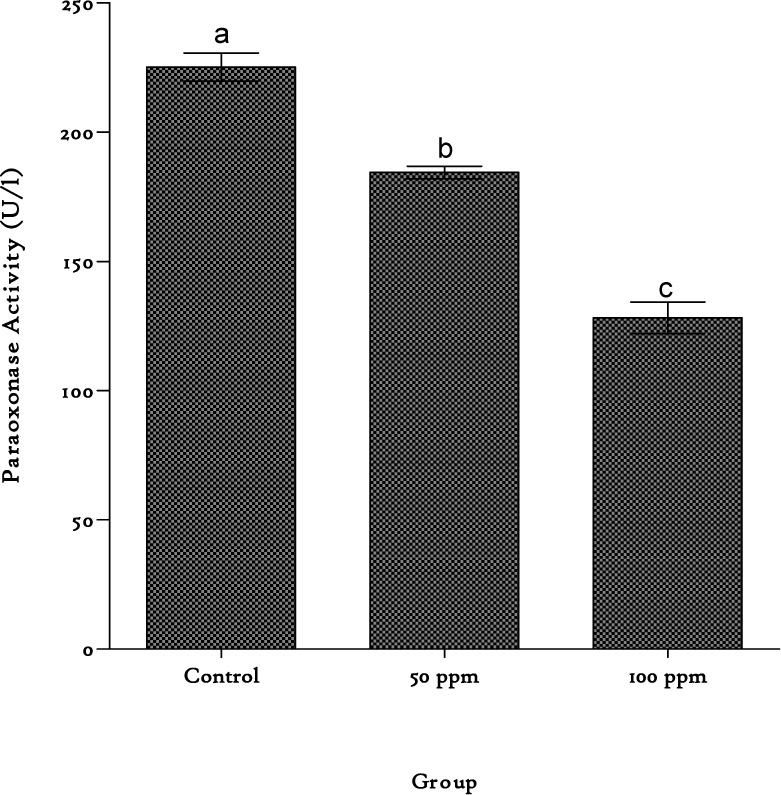
OxLDL levels were significantly (p < 0.05) elevated in the plasma by 124 % and 162 % on cadmium exposure at 50 and 100 ppm, respectively (Table 1(Tab. 1)). The activity of the enzyme paraoxonase was drastically reduced by cadmium exposure. An 18 % loss of enzyme activity was observed in the 50 ppm cadmium exposed group while in the 100 ppm group exposure yielded a 43 % reduction in activity (Figure 3(Fig. 3)).
Figure 3. Paraoxonase activities in the plasma of rats exposed to cadmium in their drinking water. Values are represented as mean ± SEM, n=8. Bars of the same compartment carrying different letters of the alphabet are significantly different from each other (p < 0.05).
Discussion
Evidence abound that lipid and lipoprotein abnormalities as well as cytokines play significant roles in the pathogenesis and progression of atherosclerosis and cardiovascular diseases (Ginsberg, 1994[18]; Glew et al., 2002[20]). There is also increasing evidence that environmental factors contribute to these conditions (Aguilar-Salinas et al., 2001[1]). In this study, we evaluated the effect of cadmium exposure on lipids and some pro-inflammatory cytokines in rats. Cadmium exposure caused a dose-dependent increase in plasma triglyceride levels and this is associated with increased concentration of the TG-rich lipoproteins i.e. LDL+VLDL fraction. The observed increase may, therefore, be a result of an increase in the hepatic synthesis of TG and/or a lower clearance rate of TG-rich lipoproteins. Increases in both plasma triglyceride and LDL and VLDL concentrations after cadmium exposure in rats have been reported earlier by Larregle et al. (2008[30]). They attributed the increases to a decreased lipoprotein lipase (LPL) activity in the animals which resulted in an increase in the circulating triglyceride-rich VLDL. Studies have shown a strong independent relation between plasma triglyceride concentrations and the likelihood of cardiovascular disease (Assmann et al., 1996[4]). The increase in TG-rich lipoproteins observed in this study has been cited as one of the biochemical abnormalities often associated with elevated levels of triglyceride (Hokanson and Austin, 1996[23]). These triglyceride-rich lipoproteins and their remnants, which are known to be atherogenic, may directly contribute to the formation of arterial wall foam cells (Zilversmit, 1979[56]).
Plasma total cholesterol contents were significantly elevated in this study. The observed increase in plasma cholesterol was associated with an increase in LDL and VLDL cholesterol fraction in the cadmium-treated rats. The observed increased total plasma cholesterol and LDL and VLDL cholesterol levels, and normal HDL cholesterol levels in the rats in this study suggest that reverse cholesterol transport was not affected by cadmium exposure, but rather cholesterol synthesis. HMG CoA reductase is the rate limiting enzyme in cholesterol biosynthesis. The activity of this enzyme might have been stimulated by cadmium, leading to the increased cholesterol levels observed. The increase could partly be attributed to a reduced efflux of circulatory VLDL cholesterol particles as a consequence of a decreased LPL activity or a loss of LDL receptor function, leading to decreased LDL catabolism and elevated LDL levels (Twisk et al., 2000[44]).
Low density lipoprotein cholesterol has been shown to have an essential role in the development of atherosclerosis (Brown et al., 1990[8]). This atherogenic property of LDL is partly a result of its oxidative modification as the uptake of oxidized low lipoproteins (oxLDL) has been identified as the major cause of foam cell formation (Witztum and Steinberg, 1991[52]). In this study, cadmium exposure resulted in a significant increase in the concentrations of oxLDL. The presence of high levels of oxLDL which are derived from lipid abnormalities and oxidative stress was accompanied with increases in lipid peroxidation levels in the rats.
To examine the lipid peroxidation levels, hepatic MDA concentration in all the groups was estimated. Cadmium at both doses significantly elevated the MDA concentrations in the liver with the highest degree of lipid peroxidation obtained at the 100 ppm dose. Another indicator of lipid peroxidation is the formation of hydroperoxides (LOOH). The present study shows that cadmium-induced oxidative stress also affects plasma lipids, as indicated by the high levels of LOOH. Lipoproteins account for the majority of plasma lipids, the results suggest that oxidized lipoproteins could contribute to the atherosclerotic process induced by cadmium. This is strongly supported by the oxLDL contents obtained in the study. OxLDL, an atherogenic indicator, was measured by analyzing baseline level of diene conjugation in lipid fraction of LDL which reportedly gives a direct and accurate index of oxidative stress (Cerne and Lukac-Bajalo, 2006[10]). Low density lipoprotein cholesterol has been shown to have an essential role in the development of atherosclerosis (Brown et al., 1990[8]). This atherogenic property of LDL is partly a result of its oxidative modification (Witztum and Steinberg, 1991[52]). In this study, cadmium exposure resulted in a significant increase in concentrations of oxLDL. This data is consistent with earlier studies which report high lipid peroxidation levels in many tissues after cadmium exposure (Sacan et al., 2007[42]).
Paraoxonase has been shown to be important in the metabolism of phospholipid and cholesteryl ester hydroperoxides, thereby preventing the oxidation of low density lipoprotein (LDL) and retarding atherogenesis (Mackness et al., 1998[32]). It has also been suggested that there is an inverse relationship between PON activity and the risk of cardiovascular diseases (Mackness et al., 2003[32]). In our study there are marked and significant decreases in plasma paraoxonase activities in the rats, following exposure to cadmium at two high dose levels. As paraoxonase is entirely complexed to HDL in the plasma and HDL level was not affected by cadmium exposure in this study, the reduction in the enzyme activity could thus not be a consequence of a reduction in HDL levels, but instead, be due to reduced expression/synthesis of the enzyme in the liver.
Another explanation is that increased oxidative stress may be responsible for the low paraoxonase activity observed during cadmium exposure. Aviram et al. (1999[6]) have suggested that PON ability to protect LDL against oxidation is accompanied by inactivation of the enzyme, due to an interaction between the enzyme free sulfhydryl group and oxidized lipids formed during LDL oxidation. Accompanying this is the significant increase in oxLDL of the cadmium-exposed rats. There was also an appreciable increase in the LOOH levels in the plasma of the animals, suggestive of an increase in lipid peroxidation with resultant oxidative stress. It has been shown that PON1 is sensitive to oxidation and is inactivated by oxidized lipids (Aviram et al., 1999[6]). The decrease in plasma PON activity in cadmium-exposed rats might have been from increased inactivation of PON by an increased generation of reactive oxygen species during cadmium intoxication. From these data, increased LOOH levels and decreased paraoxonase activities may be implicated in cadmium toxicity. Reduction in paraoxonase activities renders HDL susceptible to oxidation, which may compromise its function (Deakin et al., 2007[12]). Several studies suggest that a low-level plasma PON1 activity is associated with increased prevalence of atherosclerosis and could be an independent risk factor for coronary events (Mackness et al., 1998[32]). Proinflammatory cytokines such as TNF-α have been shown to down-regulate mRNA expression of PON1 in HepG2 cells (Kumon et al., 2002[27]). The increase in the proinflammatory cytokine levels may thus partly be responsible for the reduction in plasma PON1 activity seen in our study.
Cadmium intoxication caused increased expressions of IL-2, IL-6 and TNF-α in the animals. A similar result was reported by Villanueva et al. (2000[46]) where cadmium exposure led to increased production of TNF-α and IL-6. The elevations of these proinflammatory cytokines could be due in part, to an increase in ox-LDL caused by cadmium. oxLDL has chemoattractant activity on monocytes and it promotes their differentiation into macrophages (Quinn et al., 1987[39]). Its binding to CD36 has been reported to trigger the release of proinflammatory cytokines in macrophages (Janabi et al., 2000[24]). Atherosclerosis is widely recognized as an inflammatory disease involving large arteries (Glass and Witztum, 2001[19]; Hansson, 2005[22]). The increase in the levels of these proinflammatory cytokines could tilt the balance between them and the anti-inflammatory cytokines, increasing the atherogenic potential in the system. The activation of NK-κB in macrophages is also known to control the expression of proinflammatory cytokines, such as IL-6 and TNF-α (Monaco et al., 2004[34]). NK-κB is a redox-sensitive transcription factor; the increase in oxidative stress induced by cadmium could have, therefore, favored its activation in the animals. This could have resulted in the elevated levels of IL-6 and TNF-α seen in our study. IL-6 and TNF-α production have significant correlation with development of atherosclerosis and have been implicated as an atherosclerotic indicator (Haddy et al., 2003[21]).
IL-6 can act as both pro-inflammatory and anti-inflammatory cytokine, depending on its level of expression (Febbraio and Pedersen, 2005[15]). IL-6 is required for resistance against infections, however, at high concentrations, it has been implicated in pathological conditions such as increased risk of atherosclerosis and heart attack (Dubinski and Zdrojewicz, 2007[13]). IL-6 is one of the most consistent biomarker of peripheral arterial disease (PAD) because it enhances cell adhesion molecule (CAM) expression by endothelial and smooth muscle cells, and also, the production of acute phase reactants such as C-reactive protein and TNF-α by the hepatocytes. It correlates with metabolic and inflammatory factors (CRP and TNF-α) which are considered relevant to atherosclerotic progression (Haddy et al., 2003[21]). It has also been implicated in the progression of stimulated smooth muscle cells into foam cells (von der Thusen et al., 2003[47]). Zahid et al. (2007[54]) reported a reciprocal regulation of IL-6 by TNF-α at high concentrations. The increase observed in serum IL-6 levels in rats administered cadmium at the doses of 50 and 100 ppm could thus, be due to this reciprocal regulation of this cytokine by TNF-α.
On the other hand, TNF-α has been associated with an elevated risk of recurrent myocardial infarction and cardiovascular death (Ridker et al., 2000[41]). Its levels also correlate with the burden of atherosclerosis (Bruunsgaard et al., 1999[9]). TNF-α has been shown to alter the distribution of adhesion receptors involved in cell-cell adhesion, preventing the formation of F-actin stress fibers. The resulting restructuring of the intercellular junction eventual leads to loss of endothelial permeability, favoring leukocyte transmigration (Wojciak-Stothard et al., 1998[53]). The dose dependent increase in TNF-α concentration strengthens the fact that cadmium intoxication induced inflammatory responses in the rats, which could have been a result of oxidative stress.
IL-2 plays a role integral in vascular inflammatory processes. It is found to be expressed in atherosclerotic plaques and a study had shown that intraperitoneal injections of IL-2 increased lesion size in mice fed atherogenic diet (Upadhya et al., 2004[45]). Increase in IL-2 concentration has been shown to sensitize the expression of IL-2 receptors (IL-2R) on cells and IL-2/IL-2R interaction are reported to induce the proliferation, differentiation, maturation and survival of antigen-selected cytotoxic T cells via the activation of the expression of specific genes (Beadling and Smith, 2002[7]). Although the increase in dose of administered cadmium did not aggravate the severity of the expression of IL-2, the administration of cadmium to rat must have emanated into systemic and/or localized inflammation that triggered immunological response.
The study demonstrated that the toxicity of cadmium may partly be due to its disruption of lipid metabolism in addition to a perturbation of proinflammatory cytokine levels. The exposure resulted in modulation of cholesterol homeostasis as well as interference with lipid transport. Some of these dysfunctional states elicited by cadmium may be linked to its ability to induce oxidative stress in cellular systems, giving rise to both systemic and localized inflammatory responses.
References
- 1.Aguilar-Salinas CA, Olaiz G, Valles V, Torres JMR, Pérez FJG, Rull JA, et al. High prevalence of low HDL cholesterol concentrations and mixed hyperlipidemia in a Mexican nationwide survey. J Lipid Res. 2001;42:1298–1307. [PubMed] [Google Scholar]
- 2.Ahotupa M, Vasankari TJ. Baseline diene conjugation in LDL lipids: an indicator of circulating oxidized LDL. Free Radic Biol Med. 1999;27:1141–50. doi: 10.1016/s0891-5849(99)00201-4. [DOI] [PubMed] [Google Scholar]
- 3.Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:969–979. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- 4.Assmann G, Schulte H, von Eckardstein A. Hypertriglyceridemia and elevated lipoprotein (A) are risk factors for major coronary events in middle-aged men. Am J Cardiol. 1996;77:1179–84. doi: 10.1016/s0002-9149(96)00159-2. [DOI] [PubMed] [Google Scholar]
- 5.ATSDR, Agency for Toxic Substances and Disease Registry. Draft toxicological profile for cadmium. Atlanta, GA: ATSDR; 2008. [PubMed] [Google Scholar]
- 6.Aviram M, Rosenblat M, Billecke S, Erogul J, Sorenson R, Bisgaier CL, et al. Human serum paraoxonase (PON 1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free Radic Biol Med. 1999;26:892–904. doi: 10.1016/s0891-5849(98)00272-x. [DOI] [PubMed] [Google Scholar]
- 7.Beadling CB, Smith KA. DNA array analysis of interleukin-2-regulated immediate/ early genes. Med Immunol. 2002;1(1):2. doi: 10.1186/1476-9433-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown G, Albers JJ, Fisher LD, Schaefer SM, Lin JT, Kaplan C, et al. Regression of the coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990;323:1289–1298. doi: 10.1056/NEJM199011083231901. [DOI] [PubMed] [Google Scholar]
- 9.Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen AN, Skinhoj P, Pedersen BK. A high plasma concentration of TNF-alpha is associated with dementia in centenarians. J Gerontol A Biol Sci Med Sci. 1999;54:357–64. doi: 10.1093/gerona/54.7.m357. [DOI] [PubMed] [Google Scholar]
- 10.Cerne D, Lukac-Bajalo J. Oxidative stress assays for disease risk stratification. Acta Pharm. 2006;56:1–17. [PubMed] [Google Scholar]
- 11.Dan G, Lall SB, Rao DN. Humoral and cell mediated immune response to cadmium in mice. Drug Chem Toxicol. 2000;23:349–360. doi: 10.1081/dct-100100120. [DOI] [PubMed] [Google Scholar]
- 12.Deakin S, Moren X, James RW. HDL oxidation compromises its influence on paraoxonase-1 secretion and its capacity to modulate enzyme activity. Arterioscler Thromb Vasc Biol. 2007;27:1146–1152. doi: 10.1161/ATVBAHA.107.141747. [DOI] [PubMed] [Google Scholar]
- 13.Dubinski A, Zdrojewicz Z. The role of interleukin-6 in development and progression of atherosclerosis. Pol Merkur Lekarski. 2007;22:291–294. [PubMed] [Google Scholar]
- 14.EFSA. Cadmium in food. EFSA Journal. 2009;980:1–139. [Google Scholar]
- 15.Febbraio MA, Pedersen BK. Contraction-induced myokine production and release: is skeletal muscle an endocrine organ? Exerc Sport Sci Rev. 2005;33:114–149. doi: 10.1097/00003677-200507000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Furlong CE, Richter RJ, Seidel, Motulsky AG. Spectrophotometric assays for the enzymatic hydrolysis of the active metabolites of chlorpyrifos and parathion by plasma paraoxonase/arylesterase. Anal Biochem. 1989;180:242–7. doi: 10.1016/0003-2697(89)90424-7. [DOI] [PubMed] [Google Scholar]
- 17.Gidez LT, Miller GH, Burnstein M, Slagle S, Eder HA. Separation and quantitation of subclasses of human plasma high density lipoproteins by a simple precipitation procedure. J Lipid Res. 1982;23:1206–23. [PubMed] [Google Scholar]
- 18.Ginsberg HN. Lipoprorein metabolism and its relationship to atherosclerosis. Med Clin North Am. 1994;78:1–20. doi: 10.1016/s0025-7125(16)30174-2. [DOI] [PubMed] [Google Scholar]
- 19.Glass CK, Witztum JL. Atherosclerosis the road ahead. Cell. 2001;104:503–16. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 20.Glew RH, Kassam HA, Bhanji RA, Okorodudu A, VanderJagt DJ. Serum lipid profiles and risk of cardiovascular disease in three different male populations in northern Nigeria. J Health Popul Nutr. 2002;20:166–174. [PubMed] [Google Scholar]
- 21.Haddy N, Sass C, Droesch S, Zaiou M, Siest G, Ponthieux A, et al. IL-6, TNF-alpha and atherosclerosis risk indicators in a healthy family population: the STANISLAS cohort. Atherosclerosis. 2003;170:277–83. doi: 10.1016/s0021-9150(03)00287-9. [DOI] [PubMed] [Google Scholar]
- 22.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 23.Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3:213–219. [PubMed] [Google Scholar]
- 24.Janabi M, Yamashita S, Hirano K, Sakai N, Hiraoka H, Matsumoto K, et al. Oxidized LDL-induced NF-kappa B activation and subsequent expression of proinflammatory genes are defective in monocyte-derived macrophages from CD36-deficient patients. Arterioscler Thromb Vasc Biol. 2000;20:1953–60. doi: 10.1161/01.atv.20.8.1953. [DOI] [PubMed] [Google Scholar]
- 25.Järup L. Hazards of heavy metal contamination. Br Med Bull. 2003;68:167–82. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 26.Kataranovski M, Kataranovski D, Savic D, Jovcic G, Bogdanovic Z, Jovanovic T. Granulocyte and plasma cytokine activity in acute cadmium intoxication in rats. Physiol Res. 1998;47:453–461. [PubMed] [Google Scholar]
- 27.Kumon Y, Nakauchi Y, Suehiro T, Suehiro T, Shiinoki T, Tanimoto N, et al. Proinflammatory cytokines but not acute phase serum amyloid A or reactive protein, down-regulates paraoxonase 1 (PON1) expression by HepG2. Amyloid. 2002;9:160–164. doi: 10.3109/13506120209114817. [DOI] [PubMed] [Google Scholar]
- 28.Lafuente A, Cano P, Esquifino A. Are cadmium effects on plasma gonadotropins, prolactin, ACTH, GH and TSH levels, dose dependent? Biometals. 2003;16:243–50. doi: 10.1023/a:1020658128413. [DOI] [PubMed] [Google Scholar]
- 29.Lafuente A, González-Carracedol A, Esquifino AI. Differential effects of cadmium on blood lymphocyte subsets. Biometals. 2004;17:451–456. doi: 10.1023/b:biom.0000029441.20037.72. [DOI] [PubMed] [Google Scholar]
- 30.Larregle EV, Varas SM, Oliveros LB, Martinez LD, Antón R, Marchevsky E, et al. Lipid in liver of rat exposed to cadmium. Food Chem Toxicol. 2008;46:1786–92. doi: 10.1016/j.fct.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 31.Mackness B, Durrington P, McElduff P, Yarnell J, Azam N, Watt M, et al. Low paraoxonase activity coronary events in the Caerphilly Prospect Study. Circulation. 2003;107:2775–2779. doi: 10.1161/01.CIR.0000070954.00271.13. [DOI] [PubMed] [Google Scholar]
- 32.Mackness MI, Mackness B, Durrington PN, Fogelman AM, Berliner J, Lusis AJ, et al. Paraoxonase and coronary heart disease. Curr Opin Lipidol. 1998;9:319–24. doi: 10.1097/00041433-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Marth E, Barth S, Jelovcan S. Influence of cadmium on the immune system. Description of stimulating reactions. Centr Eur J Public Health. 2000;8:40–44. [PubMed] [Google Scholar]
- 34.Monaco C, Andreakos E, Kiriakidis S, Mauri C, Bicknell C, Foxwell B, et al. Canonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc Natl Acad Sci USA. 2004;101:5634–9. doi: 10.1073/pnas.0401060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakagawa H, Nishijo M, Morikawa Y, Miura K, Tawara K, Kuriwaki J, et al. Urinary cadmium and mortality among inhabitants of a cadmium-polluted area in Japan. Environ Res. 2006;100:323–329. doi: 10.1016/j.envres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Nawrot TS, Van Hecke E, Thijs L, Richart T, Kuznetsova T, Jin Y, et al. Cadmium-related mortality and long-term secular trends in the cadmium body burden of an environmentally exposed population. Environ Health Perspect. 2008;116:1620–1628. doi: 10.1289/ehp.11667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nourooz-Zadeh J, Tajaddini-Sarmadi J, Wolff SP. Measurement of plasma hydroperoxide concentrations by the ferrous oxidation-xylenol orange assay in conjunction with triphenylphosphine. Anal Biochem. 1994;220:403–409. doi: 10.1006/abio.1994.1357. [DOI] [PubMed] [Google Scholar]
- 38.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Ann Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 39.Quinn MT, Parthasarathy S, Fong LG, Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/ macrophages during atherogenesis. Proc Natl Acad Sci USA. 1987;84:2995–8. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramirez DC, Giménez MS. Lipid modification in mouse peritoneal macrophages alter chronic cadmium exposure. Toxicology. 2002;172:1–12. doi: 10.1016/s0300-483x(01)00560-1. [DOI] [PubMed] [Google Scholar]
- 41.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–72. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 42.Sacan O, Yanardag R, Bolkent S, Bilgin-Sokmen B, Tunali S. Protective effects of selenium, vitamin C and E combination on cadmium-induced toxicity in various tissues of rats. Fresenius Environ Bull. 2007;16:839–845. [Google Scholar]
- 43.Shook LB, Laskin DL. Xenobiotics and inflammation. New York: Academic Press; 1994. [Google Scholar]
- 44.Twisk J, Gillian-Daniel DL, Tebon A, Wang L, Barrett PHR, Attie AD. The role of the LDL receptor in apolipoprotein B secretion. J Clin Invest. 2000;105:521–32. doi: 10.1172/JCI8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Upadhya S, Mooteri S, Peckham N, Pai RG. Atherogenic effect of interleukin-2 and antiatherogenic effect of interleukin-2 antibody in apo-E-deficient mice. Angiology. 2004;55:289–94. doi: 10.1177/000331970405500308. [DOI] [PubMed] [Google Scholar]
- 46.Villanueva MBG, Koizumi S, Jonai H. Cytokine production by human peripheral blood mononuclear cells after exposure to heavy metals. J Health Sci. 2000;46:358–62. [Google Scholar]
- 47.von der Thusen JH, Kuiper J, van Berkel TJ, Biessen EA. Interleukins in atherosclerosis: molecular pathways and therapeutic potential. Pharmacol Rev. 2003;55:133–66. doi: 10.1124/pr.55.1.5. [DOI] [PubMed] [Google Scholar]
- 48.Waalkes MP, Rehm S. Carcinogenicity of oral cadmium in the male Wistar (WF/NCr) rat: Effect of chronic dietary zinc deficiency. Fund Appl Toxicol. 1992;19:512–520. doi: 10.1016/0272-0590(92)90089-z. [DOI] [PubMed] [Google Scholar]
- 49.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–15. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 50.Weglarz L, Parfiniewicz B, Mertas A, Kondera-Anasz Z, Jaworska-Kik M, Dzierzewicz Z, et al. Effect of endotoxins isolated from desulfovibrio desulfuricans soil and intestinal strain on the secretion of TNF-α by human mononuclear cells. Pol J Environ Stud. 2006;15:615–622. [Google Scholar]
- 51.WHO, World Health Organization. Cadmium in drinking-water, background document for development of WHO Guidelines for Drinking-water Quality. 2011; 2011. pp. 1–16. [Google Scholar]
- 52.Witztum JL, Steinberg D. Role of oxidised LDL in atherogenesis. J Clin Invest. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wojciak-Stothard B, Entwistle A, Garg R, Ridley AJ. Regulation of TNF-alpha-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. J Cell Physiol. 1998;176:150–65. doi: 10.1002/(SICI)1097-4652(199807)176:1<150::AID-JCP17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 54.Zahid IR, Song-hua HU, Chen-wen X, Abdullah GA. Adjuvant effects of saponins on animals immune responses. J Zhejiang Univ Sci B. 2007;8:153–161. doi: 10.1631/jzus.2007.B0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang W, Pang F, Huang Y, Ping Y, Lin W. Cadmium exerts toxic effects on ovarian steroid hormone release in rats. Toxicol Lett. 2008;182:18–23. doi: 10.1016/j.toxlet.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 56.Zilversmit, DB Atherogenesis: a postprandial phenomenon. Circulation. 1979;60:473–485. doi: 10.1161/01.cir.60.3.473. [DOI] [PubMed] [Google Scholar]