Abstract
Abnormalities in endothelial cell structure and function may lead to diseases such as thrombosis and atherosclerosis. Oxidative stress plays an important role in the pathogenesis of various cardiovascular diseases including atherosclerosis. Previous studies have shown a relationship between a diet rich in flavonoid and a reduced incidence of cardiovascular diseases. Piper sarmentosum (PS) is a plant with high flavonoid content and it possesses antioxidant and anti-atherosclerotic activities. Therefore this study aimed to investigate the flavonoids present in aqueous extract of PS (AEPS) and its cytoprotective effects in oxidative stress-induced human umbilical vein endothelial cells (HUVEC). AEPS contained high total phenolic content (91.02 ± 0.02 mg QE/g DM) and total flavonoid content (48.57 ± 0.03 mg GAE/g DM). Screening using high performance liquid chromatography (HPLC) technique showed the presence of rutin and vitexin as the main flavonoids in AEPS. HUVEC were exposed to 180 µM H2O2 and treated with various concentrations of rutin or vitexin (10 to 400 µM) for 24 hours. Both rutin and vitexin at the concentration of 150-400 µM significantly increased the viability of H2O2-induced HUVEC as denoted by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Therefore rutin and vitexin as the main flavonoids present in PS may be involved in the protective effects of PS against oxidative stress.
Keywords: Piper sarmentosum, flavonoid, rutin, vitexin, oxidative stress, human umbilical vein endothelial cells
Introduction
The endothelial cells serve as a crucial barrier between the vascular wall and the circulation. The cells also secrete a variety of substances in response to physiological and pathological stimuli (Cines et al., 1998[7]). Abnormalities in endothelial cell structure and function may lead to diseases such as thrombosis, atherosclerosis, and vasculitis (Jaffe et al., 1973[19]). Human umbilical vein endothelial cells (HUVEC) is the most commonly used cell culture model for in vitro experiments related to cardiovascular diseases (Park et al., 2008[28]; Potdar and Kavdia, 2009[29]).
Oxidative stress plays an important role in the pathogenesis of various cardiovascular diseases including atherosclerosis (Ross, 1999[32]). Oxidative stress results from imbalance between the prooxidant and the antioxidative defense mechanisms of the body. At high concentrations, reactive oxygen species (ROS) can cause severe damage to cellular structures and components including nucleic acids, proteins and lipids, thereby leading to apoptosis (Estany et al., 2007[10]). The major source of endogenous ROS is generated from H2O2 (Nohl et al., 2003[27]), which has been extensively used to induce oxidative stress in in vitro experiments (Xiao et al., 2000[37]; Yang et al., 2006[38]).
Flavonoids are a group of phenolic compounds which can be found naturally in plants. Epidemiological studies indicate that increased intake of dietary flavonoids is associated with a decrease in the risk of cardiovascular diseases (Arts and Hollman, 2005[3]). The cardiovascular protective effects of flavonoids may be mediated by multiple mechanisms such as antioxidant, anti-platelet, anti-hypertensive and hypoglycaemic effects (Ushida et al., 2008[35]).
Piper sarmentosum (PS) is a herbaceous plant which is commonly found in the tropical and subtropical regions. Phytochemically, the plant contains constituents like alkaloids, phenols, flavonoids, vitamin C, vitamin E, xanthophylls and tannins (Chanwitheesuk et al., 2005[6]; Hussain et al., 2009[16]). The plant extracts have been reported to possess multiple cardiovascular protective effects. Recent research has demonstrated that aqueous extract of Piper sarmentosum (AEPS) improved endothelial function by promoting nitric oxide production in HUVEC (Ugusman et al., 2010[34]). Moreover, PS reduced neointimal foam cell infiltration and atherosclerotic lesion in hypercholesterolemic rabbits (Amran et al., 2010[2], 2012[1]).
Previous studies have shown that PS has high antioxidant activity (Vimala et al., 2003[36]; Hussain et al., 2009[16]; Hafizah et al., 2010[14]). AEPS reduced endothelial oxidative stress by decreasing the expression of intercellular adhesion molecule-1 (ICAM-1) and NADPH oxidase-4 (Nox4) while enhancing the expression of the antioxidant enzymes (Ugusman et al., 2011[34]). AEPS also exhibited potent effects as antioxidant in preventing apoptosis in H2O2-induced HUVEC (Hafizah et al., 2010[14]). There is a positive correlation between antioxidant activity of PS and its phenolic and flavonoid contents (Hussain et al., 2009[16]). Therefore the aim of this study is to identify the flavonoids present in AEPS and its cytoprotective effects in oxidative stress-induced HUVEC.
Materials and Methods
Collection and extraction of plant material
Leaves of Piper sarmentosum were collected in Sungai Buloh, Malaysia and identified by a plant taxonomist from Forest Research Insitute of Malaysia (voucher specimen: FRI 45870). The leaves were washed with tap water, cut into small pieces, sun-dried and grounded to powder form. Ten percent of AEPS was prepared by soaking 100 g of the powdered leaves in 900 ml of purified water and incubated in a high speed mixer at 80 °C for 3 hours. After being left to cool, the extract was filtered using a mesh and further concentrated. The aqueous extract was then freeze dried to the powdered form of the extract and kept at 4 °C until used.
Determination of total phenolic content
Total phenolic content (TPC) was determined by the Folin-Ciocalteau method as described previously (Nabavi et al., 2008[26]). The extract sample (0.5 ml of 1 mg/ml AEPS) was mixed with Folin-Ciocalteau reagent (5 ml, 1:10 diluted with distilled water) for 5 min and aqueous Na2CO3 (4 ml, 1 M) were then added. The mixture was allowed to stand for 1 hour and the phenol content was determined by colorimetry at 765 nm. The standard curve was prepared by using 0 to 250 mg/ml solutions of gallic acid in methanol: water (50:50, v/v). Total phenolic content was expressed in terms of milligram of gallic acid equivalent per gram dry matter (mg GAE/g DM).
Determination of total flavonoid content
Colorimetric aluminum chloride method was used for total flavonoid content (TFC) determination as described previously (Nabavi et al., 2008[26]). Briefly, 0.5 ml solution of 1 mg/ml AEPS was mixed with 1.5 ml of methanol, 0.1 ml of 10 % aluminum chloride, 0.1 ml of 1 M potassium acetate and 2.8 ml of distilled water. The mixture was left at room temperature for 30 minutes. The absorbance of the reaction mixture was measured at 415 nm with a spectrophotometer (Versamax, USA). The standard curve was prepared by using quercetin solutions at concentrations of 12.5 to 100 mg/ml. Total flavonoid content was expressed in terms of milligram of quercetin equivalent per gram dry matter (mg QE/g DM).
High performance liquid chromatography (HPLC) analysis of AEPS
Firstly, 10 mg AEPS was dissolved in 1 ml water. The solution was filtered through a membrane filter (pore size 0.45 µm) prior to analysis. The sample was analysed by means of a HPLC system (Waters Delta 600 with 600 Controller) with photodiode array detector (Waters 996). A Phenomenex-Luna (5 µm) column was used (4.6 mm i.d. x 250 mm) and for elution of the constituents, a gradient of two solvents denoted as A and B was employed. A was 0.1 % aqueous formic acid, whereas B was 0.1 % formic acid in acetonitrile. Initial conditions were 85 % A and 15 % B, with a linear gradient reaching 25 % B at t=12 min. This was followed by an isocratic elution until t=22 min, after which the programme returned to the initial solvent composition at t=25 min and continued until t=35 min. The flow rate used was 1.0 ml/min and the injection volume was 10 µl. Detection was carried out at 366 nm. The retention times of the major peaks in the chromatograms of AEPS were compared with those of standards (naringin, genistein, fisetin, myricetin, vitexin, isovitexin, apigenin, quercetin and naringenin) (Sigma, USA).
Primary HUVEC culture
Human umbilical cords were obtained from healthy mothers who underwent spontaneous vaginal deliveries. Informed consent was obtained from each subject and the present study was approved by the Ethical Research Committee of Universiti Kebangsaan Malaysia Medical Centre (FF-092-2010). HUVEC were obtained from umbilical cord veins by 0.1 % Collagenase Type I (Gibco-Invitrogen Corp., Grand Island, N.Y.) digestion as described previously (Ugusman et al., 2010[33]). Cells were grown in medium 200 (Cascade Biologics, USA) supplemented with low serum growth supplement (Cascade Biologics, USA) at 37 °C in a humidified atmosphere of 5 % CO2 and 95 % air. HUVEC were confirmed by the typical endothelial cell cobblestone morphology and the positive expressions of von Willebrand factor and CD31 in immunocytochemistry. The culture medium was changed every other day until the cells reached confluence. HUVEC from passage 3 at 80 % confluency were used for experiment.
Determination of HUVEC viability
The viability of HUVEC was determined using 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay kit (Sigma, USA). Rutin (purity: 95 %) and vitexin (purity: 95 %) were purchased from Sigma, USA. HUVEC were seeded at concentration of 2 × 104 cells/well in a 96-well plate. The MTT assay was done in two stages. During the first stage, HUVEC were treated with various concentrations of rutin or vitexin (0 to 1000 µM) for 24 hours to determine the IC50 of rutin and vitexin. The second stage was performed to determine the cytoprotective effects of rutin and vitexin in H2O2-induced oxidative cell damage. Therefore, HUVEC were exposed to 180 µM H2O2 and treated with various concentrations of rutin or vitexin (10 to 400 µM) for 24 hours. The dose of H2O2 used was based on the IC50 of H2O2 adopted from a previous study (Hafizah et al., 2010[14]). After 24 hours of treatment, 200 µl of 5 mg/ml MTT solution was added to each well and the mixture was incubated for 4 hours. Subsequently, the supernatants were aspirated and the purple formazan crystal formed in each well was dissolved in 200 µl of MTT solvent. The absorbance of each well was read at 570 nm with a colorimetric microplate reader (Versamax, USA).
Statistical analysis
Data was tested for normality using Kolmogorov-Smirnov test and all variables were normally distributed. Data was expressed as mean ± SEM. Statistical analysis between two groups was performed using paired t-test using SPSS version 16.0 software. Values of p < 0.05 were considered statistically significant.
Results
Total phenolic and flavonoid contents of AEPS
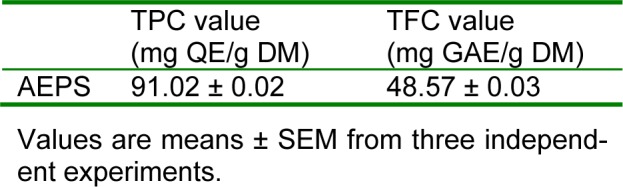
The TPC and TFC values of AEPS are presented in Table 1(Tab. 1). AEPS contained high TPC (91.02 ± 0.02 mg QE/g DM) and TFC (48.57 ± 0.03 mg GAE/g DM).
Table 1. Total phenolic and flavonoid contents of aqueous extract of Piper sarmentosum.
HPLC analysis of AEPS
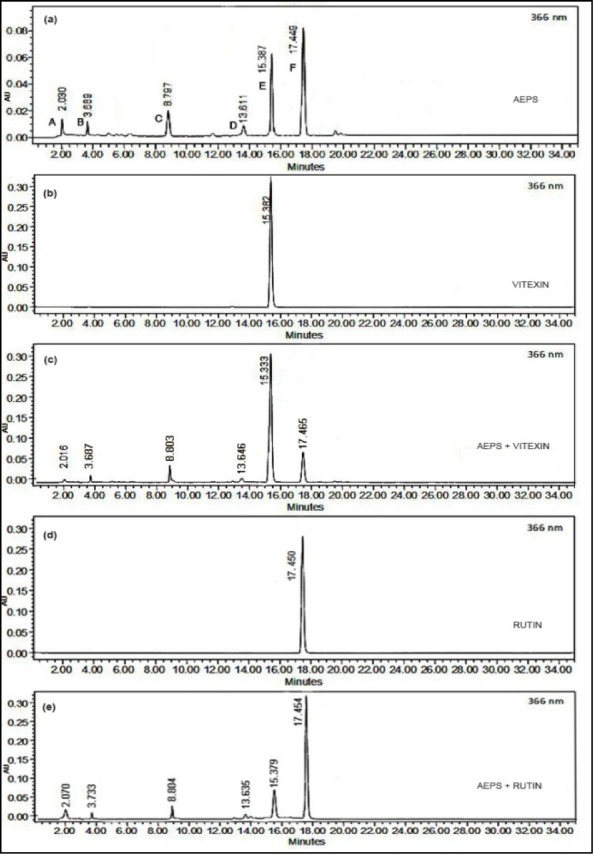
Figure 1a(Fig. 1) shows the chromatogram of AEPS. The retention times (Rt) of the major peaks in the chromatogram of AEPS were compared with those of standards (naringin, genistein, fisetin, myricetin, rutin, vitexin, isovitexin, apigenin, quercetin and naringenin). Peak E in the chromatogram (Rt = 15.387 min) corresponded to vitexin (Rt = 15.382 min) (Figure 1b(Fig. 1)) while peak F (Rt = 17.449 min) corresponded to rutin (Rt = 17.450 min) (Figure 1d(Fig. 1)). The sample was spiked with vitexin and rutin and the results confirmed the presence of vitexin (Figure 1c(Fig. 1)) and rutin (Figure 1e(Fig. 1)). The Rt of other peaks in the chromatogram of AEPS did not correspond to the Rt of other standards used (naringin, genistein, fisetin, myricetin, isovitexin, apigenin, quercetin and naringenin). Quantitative analysis of rutin and vitexin was carried out with reference to the standard curves of rutin (y = 13487x - 181561, r2 = 0.995) and vitexin (y = 12313x - 18598, r2 = 0.998). The concentration of vitexin in AEPS was 51.93 ± 0.55 ppm (0.5193 %) while the concentration of rutin was 75.70 ± 0.50 ppm (0.7570 %).
Figure 1. HPLC chromatograms of (a) AEPS (b) vitexin (c) AEPS + vitexin (d) rutin (e) AEPS + rutin.
Effects of rutin on HUVEC viability
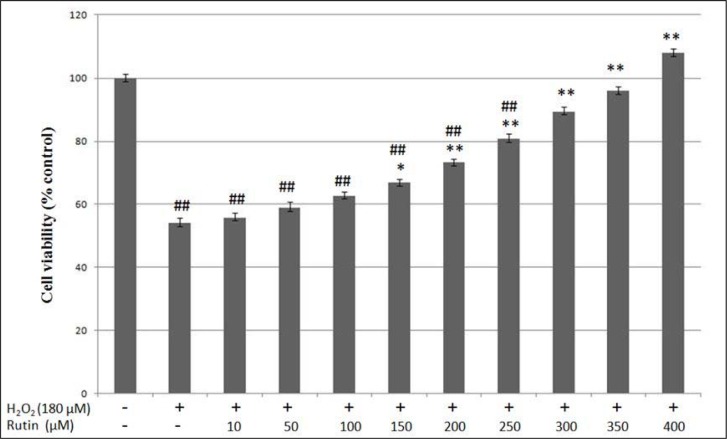
Rutin up to the concentration of 1000 µM was not toxic to HUVEC after 24 hours of incubation (Figure 2(Fig. 2)). In the next stage of MTT assay, HUVEC induced with 180 µM H2O2 was treated with rutin at the concentration of 10-400 µM to determine the cytoprotective effects of rutin on H2O2-induced oxidative cell damage. Exposure to 180 µM H2O2 for 24 hours significantly reduced the viability of HUVEC to 54.0 ± 1.6 % compared to the control group (HUVEC without exposure to H2O2 and rutin) (p < 0.01) (Figure 3(Fig. 3)). However, treatment of H2O2-induced HUVEC with different concentrations of rutin (150-400 µM) significantly increased HUVEC viability in a dose-dependent manner compared to the cells induced with only H2O2. Treatment with 150, 200, 250, 300, 350 and 400 µM rutin increased the viability of HUVEC to 66.9 ± 1.1 %, 73.2 ± 1.3 %, 80.7 ± 1.6 %, 90.4 ± 1.5 %, 95.9 ± 1.4 % and 107.9 ± 1.4 %, respectively. In addition, HUVEC treated with 300, 350 and 400 µM rutin showed no significant difference in cell viability compared to the control group.
Figure 2. Effects of rutin on the viability of HUVEC after 24 hours of incubation. Values are means ± SEM of six independent experiments.
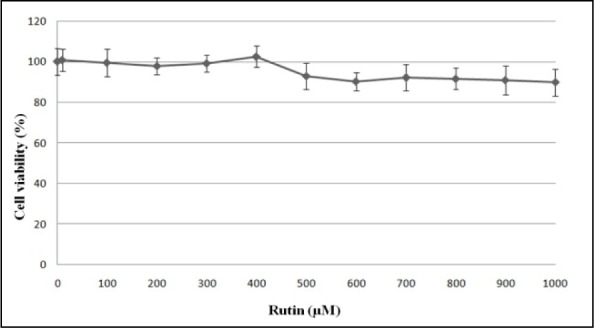
Figure 3. Protective effects of rutin on HUVEC viability against oxidative injury induced by 180 µM H2O2. HUVEC were co-treated with 10-400 µM rutin for 24 hours. Values are means ± SEM of six independent experiments. (##) p < 0.01 vs. control group; (*) p < 0.05 vs. H2O2 only group; (**) p < 0.01 vs. H2O2 only group.
Effects of vitexin on HUVEC viability
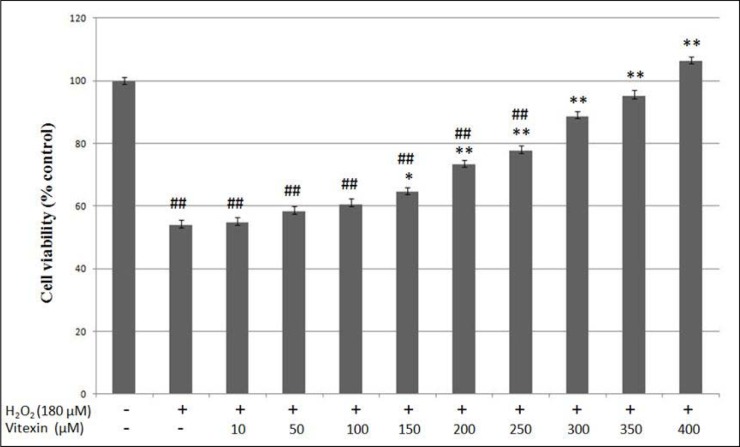
Vitexin up to the concentration of 1000 µM was not toxic to HUVEC after 24 hours of incubation (Figure 4(Fig. 4)). In the next stage of MTT assay, HUVEC induced with 180 µM H2O2 was treated with vitexin at the concentration of 10-400 µM to determine the cytoprotective effects of vitexin on H2O2-induced oxidative cell damage. Exposure to 180 µM H2O2 for 24 hours significantly reduced the viability of HUVEC to 54.0 ± 1.6 % compared to the control group (HUVEC without exposure to H2O2 and vitexin) (p < 0.01) (Figure 5(Fig. 5)). However, treatment of H2O2-induced HUVEC with different concentrations of vitexin (150-400 µM) significantly increased HUVEC viability in a dose-dependent manner compared to the cells induced with only H2O2. Treatment with 150, 200, 250, 300, 350 and 400 µM vitexin increased the viability of HUVEC to 64.7 ± 1.1 %, 73.4 ± 1.4 %, 77.8 ± 1.6 %, 89.3 ± 1.2 %, 95.3 ± 1.7 % and 106.4 ± 1.1 %, respectively. In addition, HUVEC treated with 300, 350 and 400 µM vitexin showed no significant difference in cell viability compared to the control group.
Figure 4. Effects of vitexin on the viability of HUVEC after 24 hours of incubation. Values are means ± SEM of six independent experiments.
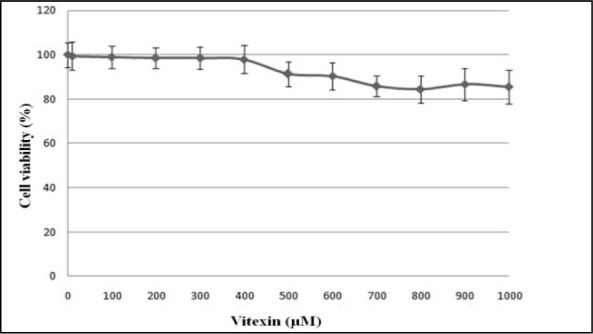
Figure 5. Protective effects of vitexin on HUVEC viability against oxidative injury induced by 180 µM H2O2. HUVEC were co-treated with 10-400 µM vitexin for 24 hours. Values are means ± SEM of six independent experiments. (##) p < 0.01 vs. control group; (*) p < 0.05 vs. H2O2 only group; (**) p < 0.01 vs. H2O2 only group.
Discussion
Screening of AEPS using HPLC showed the presence of rutin and vitexin as the main flavonoids present in the extract (Figure 1(Fig. 1)). Another study also reported the presence of rutin in AEPS (Hussain et al., 2009[16]). To the best of our knowledge, this is the first study to report the presence of vitexin in AEPS. There are a few unidentified peaks in the chromatogram of AEPS (Figure 1a(Fig. 1)). The flavonoids represented by the peaks can be identified by doing isolation work or by comparing the Rt with more standards.
In other studies, aqueous-methanol extract of PS contains the flavonoids myricetin, quercetin and apigenin (Miean and Mohamed, 2001[25]) while methanol extract of PS contains naringenin (Vimala et al., 2003[36]). However, aqueous extract of PS used in this study does not contain myricetin, quercetin, apigenin and naringenin. There are internal and external factors that can contribute to the variations in the type of flavonoids found in PS sample. Intrinsically, metabolic content of a plant is influenced by ontogenetic, ecotypic, genotypic and chemotypic factors. Extrinsically, it can be influenced by variations in the growth conditions, age of the plant, collection time, drying and storage conditions (Hussain et al., 2009[17]). The type of solvent used for extraction of PS can also influence its flavonoid content. Solvent with different polarity can cause different types of flavonoids being extracted (Bimakr at al., 2009[4]). The sample used in this study is aqueous extract of PS while the samples that contain myricetin, quercetin and apigenin is aqueous-methanol extract of PS (Miean and Mohamed, 2001[25]) and naringenin is found in methanol extract of PS (Vimala et al., 2003[36]).
Rutin (3,3′,4′,5,7-pentahydroxyflavone-3-rhamnoglucoside) is a flavonoid of the flavonol type. Rutin is abundantly present in onions, apples, tea and red wine (Hertog et al., 1992[15]). It was reported that rutin has several pharmacological properties including antioxidant, anti-platelet, anti-thrombus, vasoprotective and cardioprotective activities (Janbaz et al., 2002[20]).
In this study, rutin at the concentration of 150-400 µM significantly increased the viability of H2O2-induced HUVEC (Figure 3(Fig. 3)). In a previous study, rutin significantly attenuated H2O2-induced apoptosis in HUVEC in a concentration-dependant manner (Gong et al., 2010[12]). This indicated that rutin had a potential protective effect on endothelial cells against injury induced by H2O2.
Reactive oxygen species (superoxide, H2O2 and hydroxyl radicals) are potent intracellular oxidants which have been proposed as critical regulators of apoptosis (Danial and Korsmeyer, 2004[8]). Reduced glutathione (GSH) is a major antioxidant that protects cells from oxidative stress by scavenging peroxides in the mitochondria (Kaur et al., 2006[21]). The mitochondrion is an important organelle for the production of cellular energy and has been reported to be involved in programmed cell death (Ishikawa et al., 2008[18]). H2O2 can cause endothelial cell injury by inducing mitochondrial dysfunction which includes loss of mitochondrial membrane potential (Cai 2005[5]; Liu et al., 2008[24]). Rutin protected HUVEC against apoptosis induced by H2O2 through decreasing the intracellular ROS level, increasing the intracellular GSH and restoring the mitochondrial membrane potential (Gong et al., 2010[12]).
Vitexin (apigenin-8-C-β-D-glucopyranoside) is a flavonoid of the flavon type. It is present in hawthorn, passion flower, bamboo leaves, millet and barley (Kim et al., 2005[22]). Vitexin has anti-hypertensive and anti-inflammatory effects (Prabhakar et al., 1981[30]). Additionally, vitexin has anti-thyroid, anti-arteriosclerotic and anti-hepatotoxic effects (Gaitan et al., 1995[11]). It also has anti-cancer (Zhou et al., 2009[39]), anti-nociceptive (Gorzalczany et al., 2011[13]) and anti-bacteria (Quílez et al., 2010[31]) activities.
Previous studies have shown that vitexin is a potent antioxidant. Vitexin at the dose of 100 mg/ml could eliminate 70 % of superoxide anion radical and 60 % of DPPH radical (Kim et al., 2005[22]). At the concentrations of 0.1 dan 1.0 mM, vitexin has higher superoxide scavenging activity than α-tocopherol (Kim et al., 2005[23]).
At high concentrations, ROS can cause severe damage to cellular structures and components including nucleic acids, proteins and lipids, thereby leading to apoptosis (Estany et al., 2007[10]). Malondialdehyde (MDA) is the most abundant product of polyunsaturated lipid peroxidation. The reaction of MDA with thiobarbituric acid produces thiobarbituric acid-reactive substances (TBARS) (Del Rio et al., 2005[9]). Vitexin is able to reduce the levels of MDA and TBARS and it also inhibits LDL oxidation (Kim et al., 2005[23]).
Vitexin's ability to enhance the viability of HUVEC induced by H2O2 is likely to be attributed by its high ROS scavenging activity in addition to its inhibition on lipid peroxidation. However, the underlying molecular mechanisms of vitexin's protective effect against apoptosis in HUVEC induced by H2O2 have not been studied in detail as yet.
Conclusion
AEPS contains the flavonoids rutin and vitexin. Both rutin and vitexin have cytoprotective effects against H2O2-induced oxidative cell damage. Therefore rutin and vitexin may be involved in the protective effects of AEPS against oxidative stress.
Acknowledgement
The authors are grateful to Universiti Kebangsaan Malaysia Medical Centre (FF-092-2010) and Ministry of Higher Education (UKM-FF-03-FRGS0037-2010), Malaysia, for funding this project. The authors also would like to thank Prof. Dr. Srijit Das for his contribution in editing this paper.
References
- 1.Amran AA, Zakaria Z, Othman F, Das S, Al-Mekhlafi HM, Raj S, et al. Effect of methanolic extract of Piper sarmentosum leaves on neointimal foam cell infiltration in rabbits fed with high cholesterol diet. EXCLI J. 2012;11:274–283. [PMC free article] [PubMed] [Google Scholar]
- 2.Amran AA, Zakaria Z, Othman F, Das S, Raj S, Nordin NAMM. Aqueous extract of Piper sarmentosum decreases atherosclerotic lesions in high cholesterolemic experimental rabbits. Lipids Health Dis. 2010;9:44. doi: 10.1186/1476-511X-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arts ICW, Hollman PCH. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81:317S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 4.Bimakr M, Rahman RA, Taip FS, Chuan L, Ganjloo A, Selamat J, et al. Supercritical carbon dioxide (SC-CO2) extraction of bioactive flavonoid compounds from spearmint (Mentha Spicata L.) leaves. Eur J Sci Res. 2009;33:679–690. [Google Scholar]
- 5.Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc Res. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 6.Chanwitheesuk A, Teerawutgulrag A, Rakariyatham N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem. 2005;92:491–497. [Google Scholar]
- 7.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527. [PubMed] [Google Scholar]
- 8.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 9.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Met Cardiovasc Dis. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Estany S, Palacio J, Barnadas R, Sabes M, Iborra A, Martinez P. Antioxidant activity of N-acetylcysteine, flavonoids and α-tocopherol on endometrial cells in culture. J Reprod Immunol. 2007;75:1–10. doi: 10.1016/j.jri.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Gaitan E, Cooksey RC, Legan J, Lindsay RH. Antithyroid effects in vivo and in vitro of vitexin: a C-glucosylflavone in millet. J Clin Endocrinol Met. 1995;80:1144–1147. doi: 10.1210/jcem.80.4.7714083. [DOI] [PubMed] [Google Scholar]
- 12.Gong G, Qin Y, Huang W, Zhou S, Yang X, Li D. Rutin inhibits hydrogen peroxide-induced apoptosis through regulating reactive oxygen species mediated mitochondrial dysfunction pathway in human umbilical vein endothelial cells. Eur J Pharmacol. 2010;628:27–35. doi: 10.1016/j.ejphar.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 13.Gorzalczany S, Marrassini C, Miño J, Acevedo C, Ferraro G. Antinociceptive activity of ethanolic extract and isolated compounds of Urtica circularis. J Ethnopharmacol. 2011;134:733–738. doi: 10.1016/j.jep.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 14.Hafizah A, Zaiton Z, Zulkhairi A, Mohd Ilham A, Nor Anita M, Zaleha A. Piper sarmentosum as an antioxidant on oxidative stress in human umbilical vein endothelial cells induced by hydrogen peroxide. J Zhejiang Univ-Sc B. 2010;11:357–365. doi: 10.1631/jzus.B0900397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hertog MGL, Hollman PCH, Katan MB. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J Agr Food Chem. 1992;40:2379–2383. [Google Scholar]
- 16.Hussain K, Ismail Z, Sadikun A, Ibrahim P. Antioxidant, anti-TB activities, phenolic and amide contents of standardised extracts of Piper sarmentosum Roxb. Nat Prod Res. 2009;23:238–249. doi: 10.1080/14786410801987597. [DOI] [PubMed] [Google Scholar]
- 17.Hussain K, Ismail Z, Sadikun A, Ibrahim P. Evaluation of metabolic changes in fruit of Piper sarmentosum in various seasons by metabolomics using Fourier Transform Infrared (FTIR) spectroscopy. Int J Pharm Clin Res. 2009;1:68–71. [Google Scholar]
- 18.Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320(5876):661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 19.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janbaz KH, Saeed SA, Gilani AH. Protective effect of rutin on paracetamol- and CCl4-induced hepatotoxicity in rodents. Fitoterapia. 2002;73:557–563. doi: 10.1016/s0367-326x(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 21.Kaur P, Aschner M, Syversen T. Glutathione modulation influences methyl mercury induced neurotoxicity in primary cell cultures of neurons and astrocytes. Neurotoxicology. 2006;27:492–500. doi: 10.1016/j.neuro.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Kim JH, Lee BC, Sim GS, Lee DH, Lee KE, Yun YP, et al. The isolation and antioxidative effects of vitexin from Acer palmatum. Arch Pharm Res. 2005;28:195–202. doi: 10.1007/BF02977715. [DOI] [PubMed] [Google Scholar]
- 23.Kim YC, Jun M, Jeong WS, Chung SK. Antioxidant properties of flavone C glycosides from atractylodes japonica leaves in human low density lipoprotein oxidation. J Food Sci. 2005;70:S575–SS80. [Google Scholar]
- 24.Liu YM, Jiang B, Bao YM, An LJ. Protocatechuic acid inhibits apoptosis by mitochondrial dysfunction in rotenone-induced PC12 cells. Toxicol in Vitro. 2008;22:430–437. doi: 10.1016/j.tiv.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem. 2001;49:3106–3112. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- 26.Nabavi SM, Ebrahimzadeh MA, Nabavi SF, Hamidinia A, Bekhradnia AR. Determination of antioxidant activity, phenol and flavonoids content of Parrotia persica Mey. PharmacologyOnline. 2008;2:560–567. [Google Scholar]
- 27.Nohl H, Kozlov A, Gille L, Staniek K. Cell respiration and formation of reactive oxygen species: facts and artefacts. Biochem Soc Trans. 2003;31:1308–1311. doi: 10.1042/bst0311308. [DOI] [PubMed] [Google Scholar]
- 28.Park YJ, Lee T, Ha J, Jung IM, Chung JK, Kim SJ. Effect of nicotine on human umbilical vein endothelial cells (HUVECs) migration and angiogenesis. Vasc Pharmacol. 2008;49:32–36. doi: 10.1016/j.vph.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Potdar S, Kavdia M. NO/peroxynitrite dynamics of high glucose-exposed HUVECs: chemiluminescent measurement and computational model. Microvasc Res. 2009;78:191–198. doi: 10.1016/j.mvr.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prabhakar M, Bano H, Kumar I, Shamsi M, Khan S. Pharmacological investigations on vitexin. Planta Med. 1981;43:396–403. doi: 10.1055/s-2007-971532. [DOI] [PubMed] [Google Scholar]
- 31.Quílez A, Berenguer B, Gilardoni G, Souccar C, de Mendonça S, Oliveira L, et al. Anti-secretory, anti-inflammatory and anti-Helicobacter pylori activities of several fractions isolated from Piper carpunya Ruiz & Pav. J Ethnopharmacol. 2010;128:583–589. doi: 10.1016/j.jep.2010.01.060. [DOI] [PubMed] [Google Scholar]
- 32.Ross R. Atherosclerosis – an inflammatory disease. New Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 33.Ugusman A, Zakaria Z, Hui CK, Nordin NA. Piper sarmentosum increases nitric oxide production in oxidative stress: a study on human umbilical vein endothelial cells. Clinics (Sao Paulo) 2010;65:709–714. doi: 10.1590/S1807-59322010000700010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ugusman A, Zakaria Z, Hui CK, Nordin NAMM. Piper sarmentosum inhibits ICAM-1 and Nox4 gene expression in oxidative stress-induced human umbilical vein endothelial cells. BMC Complement Altern Med. 2011;11:31. doi: 10.1186/1472-6882-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ushida Y, Matsui T, Tanaka M, Matsumoto K, Hosoyama H, Mitomi A, et al. Endothelium-dependent vasorelaxation effect of rutin-free tartary buckwheat extract in isolated rat thoracic aorta. J Nutr Biochem. 2008;19:700–707. doi: 10.1016/j.jnutbio.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Vimala S, Mohd I, Abdull R, Rohana S. Natural antioxidants: Piper sarmentosum (Kadok) and Morinda elliptica. Mal J Nutr. 2003;9(Suppl 1):41–51. [PubMed] [Google Scholar]
- 37.Xiao XQ, Lee NTK, Carlier PR, Pang YP, Han YF. Bis (7)-tacrine, a promising anti-Alzheimer's agent, reduces hydrogen peroxide-induced injury in rat pheochromocytoma cells: comparison with tacrine. Neurosci Lett. 2000;290:197–200. doi: 10.1016/s0304-3940(00)01357-4. [DOI] [PubMed] [Google Scholar]
- 38.Yang B, Oo TN, Rizzo V. Lipid rafts mediate H2O2 prosurvival effects in cultured endothelial cells. FASEB. 2006;20:1501–1503. doi: 10.1096/fj.05-5359fje. [DOI] [PubMed] [Google Scholar]
- 39.Zhou YJ, Liu YE, Cao JG, Zeng GY, Shen C, Li YL, et al. Vitexins, nature-derived lignan compounds, induce apoptosis and suppress tumor growth. Clin Cancer Res. 2009;15:5161–5169. doi: 10.1158/1078-0432.CCR-09-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]