Abstract
Background
Preemptive analgesia may be considered as a method not only to alleviate postoperative pain but also to decrease analgesic consumption. Different regimens are suggested, but there is currently no standard.
Objectives
The aim was to measure the efficacy of preemptive analgesia with pregabalin, acetaminophen, naproxen, and dextromethorphan in radical neck dissection surgery for reducing the intensity of pain and morphine consumption.
Patients and Methods
This study was conducted as a randomized double-blind clinical trial. Eighty adult patients (18 to 60 years of age) under the American society of anesthesiologists (ASA) physical status I and II undergoing elective radical neck dissection were enrolled. Patients were randomized into two groups of 40 with a simple randomization method. The case group received a combination of 15 mg/kg acetaminophen, 2.5 mg/kg pregabalin, 7 mg/kg naproxen, and 0.3 mg/kg dextromethorphan administered orally one hour prior to surgery. Postoperative pain was assessed with the universal pain assessment tool (UPAT) at 0, 2, 4, 6, 12, and 24 hours after surgery. Subjects received morphine based on postoperative pain control protocol. Total administered morphine doses were noted.
Results
Postoperative pain rates at 0, 2, 4, 6, 12, and 24 hours after surgery were significantly lower for the case group than the control group (P values = 0.014, 0.003, 0.00, 0.00, and 0.00, respectively). Total morphine doses for the preemptive analgesia group were 45% lower than those of the other group. Side effects were similar for both groups.
Conclusions
A single preoperative oral dose of pregabalin, acetaminophen, dextromethorphan, and naproxen one hour before surgery is an effective method for reducing postoperative pain and morphine consumption in patients undergoing radical neck dissection.
Keywords: Pain Management, Preoperative Care, Analgesia, Neck Dissection, Cancer
1. Background
The experience of acute pain in daily life is like an alarm system. It can inform one of the presence of life-threatening situations and cause alterations in individual behaviors in order to prevent future injuries. However, pain after surgery is a maladaptive response. This pain does not afford any significant benefit to the patient, but can actually do harm to various systems within the body (1). Postoperative pain can lengthen the duration of hospital stays, increase the risk of infection, and postpone the return of the patient to normal daily life. Acute postoperative pain is an important predictive factor for chronic post-surgical pain, which is observed in 10% - 65% of patients after surgery (2, 3). Therefore, even though pain is generally a positive body response, controlling pain after surgery is very important. Reducing postoperative pain will result in more patient satisfaction, decreased hospitalization periods, and less complications due to immobility (4).
In current medical practice, opioid analgesics are generally used for controlling postoperative pain. Opioid analgesics can cause many complications, including nausea, vomiting, and pruritus. Furthermore, this category of drugs has actually shown inadequate efficacy in pain management after surgery (5, 6). This inefficacy necessitates the consideration of other methods to control pain after surgery, such as preemptive analgesia.
Preemptive analgesia is the use of analgesics prior to the painful stimulus. Therefore, it decreases the changes in the central sensory processes that are responsible for hyperalgesia and allodynia (7). Different medications and methods have been used for preemptive analgesia, and the molecular pain mechanism is the underlying basis for choosing the agents of preemptive analgesia.
Surgery causes tissue injuries which result in histamine and inflammatory mediators being released at the site of the incision. Afterwards, local chemical mediators such as prostaglandin and bradykinin are released, and the neural receptors are stimulated. The pain signal then enters the spinal cord via the dorsal horn. N-Methyl-D-aspartate (NMDA) receptors in the spinal cord are exclusively dedicated to the management of painful stimuli. The persistent release of inflammatory mediators sensitizes the functional nociceptors. This sensitization decreases the activation threshold and increases the rate of basal spontaneous discharge (8, 9). Multi-modal analgesia works by influencing all of the responsible components in the pain pathway (10).
Naproxen is a well-known drug of the nonsteroidal anti-inflammatory drug (NSAID) group and inhibits cyclooxygenase (COX) 1 and 2. Acetaminophen is widely used for managing pain and fever. It inhibits many of the enzymes in the inflammatory pathways, including COX1, COX2, and myeloperoxidases (11, 12). The exact mechanism of acetaminophen is not understood. In some studies, a different variant of the COX enzyme is suggested to be the primary site of action (13). Gabapentinoid medications were primarily used as anti-epileptic drugs. Pregabalin decreases the concentration of the neurotransmitters related to pain, such as substance P. This can explain the analgesic effect of pregabalin (14).
Dextromethorphan is a NMDA receptor antagonist. This drug acts similarly to ketamine but has less affinity to the NMDA receptors. It has been safely used for preemptive analgesia (15), and can also play a role in reducing neuropathic pain and improving the analgesic effect of morphine on acute pain after surgery (16).
All of these agents have previously been used either separately or in different combinations in various surgeries. In neck dissection surgery, massive tissue incisions can cause severe pain postoperatively, which necessitates the use of some form of analgesia.
2. Objectives
In this study, we investigated the efficacy of preemptive analgesia comprised of pregabalin, acetaminophen, naproxen, and dextromethorphan (PAND) in radical neck dissection surgery for reducing the intensity of pain and morphine consumption.
3. Patients and Methods
This study was a randomized double-blind clinical trial on patients under the American society of anesthesiologists (ASA) physical status I and II who underwent radical neck dissection in a cancer institute. The study design was approved by the ethics committee of the hospital. Informed consent was obtained from all patients at the beginning of the study.
The exclusion criteria included opioid addiction, history of using antidepressants and anti-epileptic medications, chronic head and neck pain, and sensitivity to any of preemptive analgesic agents.
The sample size was calculated with α = 0.05 and β = 0.2 using the following formula:
| Equation 1: |
Eighty patients were enrolled in the study. Each patient was given a code according to a computer-generated list, and then the codes were randomized into two groups based on a simple randomization method. The first group received 2.5 mg/kg pregabalin, 15 mg/kg acetaminophen, 7 mg/kg naproxen, and 0.3 mg/kg dextromethorphan, administered orally one hour prior to surgery (PAND group). In the operating room, blood pressure, pulse rate, ECG, oxygen saturation, and end tidal CO2 were monitored. Patients were premedicated with 2.5 mg midazolam and 2 μg/kg fentanyl. The induction of anesthesia consisted of 3-5 mg/kg thiopental Na and 5 mg/kg atracurium besylate. Anesthesia was maintained with 0.8% - 1.2% isoflurane in 50% N2O-O2 mixture. A dose of 1 μg/kg fentanyl was administered before skin incision. Atracurium and fentanyl were injected if needed during the operation. After skin closure, neostigmine (35 μg/kg) was used to antagonize the residual neuromuscular blockade. The duration of each operation was noted, and patients were moved to the recovery hall. After full consciousness was regained by the patient, pain intensity was measured with the universal pain assessment tool (UPAT) (time = 0). This measurement was repeated at 2, 4, 6, 12, and 24 hours thereafter. For pain control, all patients received morphine based on postoperative pain control protocol. The total doses of morphine needed for pain control were noted. Patients and staff responsible for delivering medications were blinded to the study details.
SPSS version 16 was used for analyzing the data. Descriptive statistics including means and standard deviations were calculated for all numerical data. An unpaired t-test was used for comparing the pain scores at different times and the morphine doses between the two groups. A P value of less than 0.05 was considered statistically significant.
4. Results
The mean ages of the patients in the PAND and control groups was 49.58 ± 13.96 and 49.81 ± 14.59 years, respectively. There were 18 females and 22 males in the control group, and 21 females and 19 males in the preemptive analgesia group. The mean time of surgery duration was 315.83 ± 68.09 and 335 ± 96.72 minutes in the PAND and control groups, respectively. Differences in age, sex, BMI, and surgery duration were not statistically significant between the two groups (Table 1).
Table 1. Age, Sex, and BMI in the PAND and Control Groups.
| Variable | PAND Group | Control Group | P Value |
|---|---|---|---|
| Age (years) | 49.58 ± 13.96 | 49.86 ± 14.59 | 0.93 |
| Sex (m/f) | 22/18 | 19/21 | 0.81 |
| BMI (kg/m 2 ) | 22.41 ± 1.53 | 21.75 ± 1.62 | 0.08 |
| Surgery duration (min) | 315.83 ± 68.09 | 335 ± 96.72 | 0.32 |
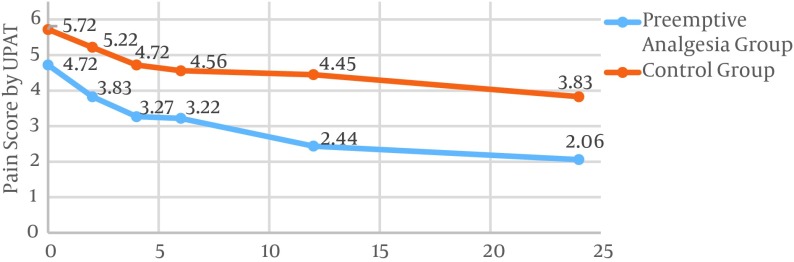
The mean pain scores for all times were 3.26 ± 1.98 and 4.75 ± 1.70 for the PAND and the control groups, respectively. This difference was statistically significant (P value = 0.001). The pain intensity for the preemptive analgesia group at 0, 2, 4, 6, 12, and 24 hours after surgery was lower as compared to the corresponding rates for the control group. This difference in pain scores was significant for all times except time = 0 (Table 2). The pain score was reduced from 4.72 to 2.06 in the case group. This value decreased from 5.72 to 3.83 in the control group (Figure 1).
Table 2. Pain Scores Assessed by the Universal Pain Assessment Tool in the PAND and Control Groups at 0, 2, 4, 6, 12, and 24 Hours After Radical Neck Dissection Surgery.
| Time (Hour) | PAND Group | Control Group | P Value | Statistical Significance |
|---|---|---|---|---|
| 0 | 4.72 ± 2.25 | 5.72 ± 2.09 | 0.055 | Not significant |
| 2 | 3.83 ± 2.59 | 5.22 ± 2.04 | 0.014 | Significant |
| 4 | 3.27 ± 2.15 | 4.72 ± 1.80 | 0.003 | Significant |
| 6 | 3.22 ± 2.31 | 4.56 ± 1.89 | 0.009 | Significant |
| 12 | 2.44 ± 1.98 | 4.45 ± 1.91 | 0.000 | Significant |
| 24 | 2.06 ± 1.69 | 3.83 ± 2.16 | 0.000 | Significant |
Figure 1. Pain Scores Assessed by the Universal Pain Assessment Tool at 0, 2, 4, 6, 12, and 24 Hours After Radical Neck Dissection Surgery for the PAND and Control Groups.
Pain reduction in the time intervals between assessments was significantly different only in the period 6 - 12 hours after surgery (Table 3). We also compared the pain intensity at 2, 4, 6, 12, 24, after 48 hours after surgery to the intensity at time = 0 for both groups. Pain reduction was more substantial for the case group at all time periods, but it was only significant for the period 0 - 12 hours after surgery and insignificant thereafter (Table 4).
Table 3. Pain Score Reduction for the Time Periods of 0-2, 2-4, 4-6, 6-12, and 12-24 Hours After Radical Neck Dissection Surgery for the PAND and Control Groups.
| Time Period (Hours) | PAND Group | Control Group | P Value | Statistical Significance |
|---|---|---|---|---|
| 0 - 2 | 0.89 ± 1.47 | 0.50 ± 1.61 | 0.29 | Not significant |
| 2 - 4 | 0.56 ± 1.23 | 0.50 ± 1.38 | 0.86 | Not significant |
| 4 - 6 | 0.06 ± 1.12 | 0.17 ± 1.30 | 0.70 | Not significant |
| 6 - 12 | 0.78 ± 1.20 | 0.11 ± 1.35 | 0.03 | Significant |
| 12 - 24 | 0.39 ± 0.93 | 0.61 ± 1.41 | 0.44 | Not Significant |
Table 4. Pain Score Reduction for the Time Periods of 0-2, 0-4, 0-6, 0-12, and 0-24 Hours After Radical Neck Dissection Surgery for the PAND and Control Groups.
| Time Period (Hours) | PAND Group | Control Group | P Value | Statistical Significance |
|---|---|---|---|---|
| 0 - 2 | 0.89 ± 1.47 | 0.50 ± 1.61 | 0.28 | Not significant |
| 0 - 4 | 1.44 ± 1.48 | 1.00 ± 1.76 | 0.25 | Not significant |
| 0 - 6 | 1.50 ± 1.54 | 1.16 ± 1.61 | 0.37 | Not significant |
| 0 - 12 | 2.28 ± 1.52 | 1.28 ± 1.80 | 0.01 | Significant |
| 0 - 24 | 2.66 ± 1.51 | 1.88 ± 2.08 | 0.07 | Not Significant |
The mean dose of administered morphine was 7.40 and 13.48 mg for the PAND and control groups, respectively. The difference in the doses of morphine between the two groups was statistically significant (P value = 0.00). No considerable complications relating to the analgesic agents were observed.
5. Discussion
In this study, postoperative pain was reduced significantly for the PAND group. The total dose of administered opioid analgesics was also 54% lower for the case group. This is consistent with similar studies on the effects of preemptive analgesia.
Different agents for preemptive analgesia have been used in previous studies. The preemptive use of dextromethorphan has been associated with reduced visual analogue scale (VAS) scores and decreased meperidine use in elective upper abdominal surgery (17, 18). Preemptive administration of dextromethorphan has also been shown to reduce postoperative pain in elective tonsillectomies (19). The preemptive use of gabapentin has been associated with decreased pain in some studies (20-22). In two other studies, gabapentin was not associated with a significant pain difference between the case and control groups, but it did appear to decrease opioid consumption (23, 24). Another study reported that despite pain reduction in the gabapentin group, opioid consumption was not significantly different (25). It seems that preemptive gabapentinoids can have a positive effect on reducing postoperative pain and opioid consumption (26).
In a study on patients undergoing lower extremity surgery, acetaminophen administered half an hour before surgery or prior to skin closure was associated with enhanced analgesia and decreased postoperative analgesic consumption (27). When comparing the use of acetaminophen with celecoxib for preemptive analgesia, celecoxib was superior to acetaminophen for reducing postoperative pain in patients undergoing lower extremity orthopedic surgery (28).
Multi-modal analgesics have been shown to be superior to single-agent preemptive analgesia in some studies. Regarding spontaneous and movement-evoked pain and opioid sparing, gabapentin with NSAIDs was superior to either of these two agents administered alone (29). In another systematic review of 21 studies, combining acetaminophen with NSAIDs was shown to be more effective than acetaminophen or NSAIDs alone in terms of alleviating postoperative pain (30). This effect has also been tested for perioperative use in another review (31). In another pathophysiologic study, pregabalin with naproxen or gabapentin with naproxen had additive or synergic effects on reversing hyperalgesia in cases of peripheral inflammation (32).
The significant 0 - 12 hours pain relief can be attributed to the delayed effect of the drug combination used in this study. Therefore, by changing the time of administration or changing the drug combination, better pain management could possibly be achieved.
The UPAT is a comprehensive combination of various scoring systems for pain intensity and severity assessment. Different scoring systems for pain have been used in various studies, including numerical rating scales, visual analogue scales, or verbal analogue scales. In these scales, the maximum amount of pain which can be imagined by a patient is compared with the patient’s current pain level. For children and patients who are less cooperative, scales such as facial grimace assessment or a quest scale are used (33).
As there is no established protocol in terms of preemptive analgesic consumption, drug selection seems to be partially based on personal experience and choice. This may be considered as another limitation of this study.
5.1. Conclusion
Using a combination of acetaminophen, dextromethorphan, naproxen, and pregabalin as preemptive analgesia can decrease the need for opioid analgesics and improve pain control for radical neck dissection surgery patients. Further studies are required to establish more definitive guidelines on recommended dosages and choices of agents for preemptive analgesia.
References
- 1.Brunicardi FC. Schwartz's principles of surgery. McGraw-Hill; 2005. [Google Scholar]
- 2.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 3.Macrae WA. Chronic pain after surgery. Br J Anaesth. 2001;87(1):88–98. doi: 10.1093/bja/87.1.88. [DOI] [PubMed] [Google Scholar]
- 4.Dashputra AV, Badwaik RT. Utilization of analgesics in perioperative cases of teaching hospital. Inter J Med Pharma Sci. 2013;3(6):14–9. [Google Scholar]
- 5.Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97(2):534–40. doi: 10.1213/01.ANE.0000068822.10113.9E. [DOI] [PubMed] [Google Scholar]
- 6.Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30(1):149–60. doi: 10.1185/03007995.2013.860019. [DOI] [PubMed] [Google Scholar]
- 7.Hariharan S, Moseley H, Kumar A, Raju S. The effect of preemptive analgesia in postoperative pain relief--a prospective double-blind randomized study. Pain Med. 2009;10(1):49–53. doi: 10.1111/j.1526-4637.2008.00547.x. [DOI] [PubMed] [Google Scholar]
- 8.Carr DB, Goudas LC. Acute pain. Lancet. 1999;353(9169):2051–8. doi: 10.1016/S0140-6736(99)03313-9. [DOI] [PubMed] [Google Scholar]
- 9.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413(6852):203–10. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 10.Gandhi K, Viscusi E. Multimodal pain management techniques in hip and knee arthroplasty. J New York School Regional Anesthesia. 2009;13:1–10. [Google Scholar]
- 11.Anderson BJ. Paracetamol (Acetaminophen): mechanisms of action. Paediatr Anaesth. 2008;18(10):915–21. doi: 10.1111/j.1460-9592.2008.02764.x. [DOI] [PubMed] [Google Scholar]
- 12.Graham GG, Davies MJ, Day RO, Mohamudally A, Scott KF. The modern pharmacology of paracetamol: therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology. 2013;21(3):201–32. doi: 10.1007/s10787-013-0172-x. [DOI] [PubMed] [Google Scholar]
- 13.Kis B, Snipes JA, Busija DW. Acetaminophen and the cyclooxygenase-3 puzzle: sorting out facts, fictions, and uncertainties. J Pharmacol Exp Ther. 2005;315(1):1–7. doi: 10.1124/jpet.105.085431. [DOI] [PubMed] [Google Scholar]
- 14.Maneuf YP, Gonzalez MI, Sutton KS, Chung FZ, Pinnock RD, Lee K. Cellular and molecular action of the putative GABA-mimetic, gabapentin. Cell Mol Life Sci. 2003;60(4):742–50. doi: 10.1007/s00018-003-2108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinbroum AA, Gorodetzky A, Nirkin A, Kollender Y, Bickels J, Marouani N, et al. Dextromethorphan for the reduction of immediate and late postoperative pain and morphine consumption in orthopedic oncology patients: a randomized, placebo-controlled, double-blind study. Cancer. 2002;95(5):1164–70. doi: 10.1002/cncr.10784. [DOI] [PubMed] [Google Scholar]
- 16.Suski M, Bujak-Gizycka B, Madej J, Kacka K, Dobrogowski J, Woron J, et al. Co-administration of dextromethorphan and morphine: reduction of post-operative pain and lack of influence on morphine metabolism. Basic Clin Pharmacol Toxicol. 2010;107(2):680–4. doi: 10.1111/j.1742-7843.2010.00559.x. [DOI] [PubMed] [Google Scholar]
- 17.Helmy SA, Bali A. The effect of the preemptive use of the NMDA receptor antagonist dextromethorphan on postoperative analgesic requirements. Anesth Analg. 2001;92(3):739–44. doi: 10.1097/00000539-200103000-00035. [DOI] [PubMed] [Google Scholar]
- 18.Imani F, Faiz HR, Sedaghat M, Hajiashrafi M. Effects of adding ketamine to fentanyl plus acetaminophen on postoperative pain by patient controlled analgesia in abdominal surgery. Anesth Pain Med. 2014;4(1):12162. doi: 10.5812/aapm.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rafiei MR, Aghadavoudi O, Rezvani M, Poorqasemian M. Evaluation of preemptive analgesia with dextromethorphan gurgling in patients undergoing tonsillectomy. J Rese Med Sci. 2012;17 [Google Scholar]
- 20.Alimian M, Imani F, Faiz SH, Pournajafian A, Navadegi SF, Safari S. Effect of oral pregabalin premedication on post-operative pain in laparoscopic gastric bypass surgery. Anesth Pain Med. 2012;2(1):12–6. doi: 10.5812/aapm.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandey CK, Navkar DV, Giri PJ, Raza M, Behari S, Singh RB, et al. Evaluation of the optimal preemptive dose of gabapentin for postoperative pain relief after lumbar diskectomy: a randomized, double-blind, placebo-controlled study. J Neurosurg Anesthesiol. 2005;17(2):65–8. doi: 10.1097/01.ana.0000151407.62650.51. [DOI] [PubMed] [Google Scholar]
- 22.Alimian M, Imani F, Hassani V, Rahimzadeh P, Sharifian M, Safari S. Effects of single-dose pregabalin on postoperative pain in dacryocystorhinostomy surgery. Anesth Pain Med. 2012;2(2):72–6. doi: 10.5812/aapm.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dierking G, Duedahl TH, Rasmussen ML, Fomsgaard JS, Moiniche S, Romsing J, et al. Effects of gabapentin on postoperative morphine consumption and pain after abdominal hysterectomy: a randomized, double-blind trial. Acta Anaesthesiol Scand. 2004;48(3):322–7. doi: 10.1111/j.0001-5172.2004.0329.x. [DOI] [PubMed] [Google Scholar]
- 24.Mikkelsen S, Hilsted KL, Andersen PJ, Hjortso NC, Enggaard TP, Jorgensen DG, et al. The effect of gabapentin on post-operative pain following tonsillectomy in adults. Acta Anaesthesiol Scand. 2006;50(7):809–15. doi: 10.1111/j.1399-6576.2006.01057.x. [DOI] [PubMed] [Google Scholar]
- 25.Fassoulaki A, Stamatakis E, Petropoulos G, Siafaka I, Hassiakos D, Sarantopoulos C. Gabapentin attenuates late but not acute pain after abdominal hysterectomy. Eur J Anaesthesiol. 2006;23(2):136–41. doi: 10.1017/S0265021505002048. [DOI] [PubMed] [Google Scholar]
- 26.Imani F, Rahimzadeh P. Gabapentinoids: gabapentin and pregabalin for postoperative pain management. Anesth Pain Med. 2012;2(2):52–3. doi: 10.5812/aapm.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khalili G, Janghorbani M, Saryazdi H, Emaminejad A. Effect of preemptive and preventive acetaminophen on postoperative pain score: a randomized, double-blind trial of patients undergoing lower extremity surgery. J Clin Anesth. 2013;25(3):188–92. doi: 10.1016/j.jclinane.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Kashefi P, Honarmand A, Safavi M. Effects of preemptive analgesia with celecoxib or acetaminophen on postoperative pain relief following lower extremity orthopedic surgery. Adv Biomed Res. 2012;1:66. doi: 10.4103/2277-9175.100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilron I, Orr E, Tu D, O'Neill JP, Zamora JE, Bell AC. A placebo-controlled randomized clinical trial of perioperative administration of gabapentin, rofecoxib and their combination for spontaneous and movement-evoked pain after abdominal hysterectomy. Pain. 2005;113(1-2):191–200. doi: 10.1016/j.pain.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110(4):1170–9. doi: 10.1213/ANE.0b013e3181cf9281. [DOI] [PubMed] [Google Scholar]
- 31.Wong I, St John-Green C, Walker SM. Opioid-sparing effects of perioperative paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs) in children. Paediatr Anaesth. 2013;23(6):475–95. doi: 10.1111/pan.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hurley RW, Chatterjea D, Rose Feng M, Taylor CP, Hammond DL. Gabapentin and pregabalin can interact synergistically with naproxen to produce antihyperalgesia. Anesthesiology. 2002;97(5):1263–73. doi: 10.1097/00000542-200211000-00033. [DOI] [PubMed] [Google Scholar]
- 33.Kemp J, Despres O, Dufour A. Unreliability of the visual analog scale in experimental pain assessment: a sensitivity and evoked potentials study. Pain Physician. 2012;15(5):693–9. [PubMed] [Google Scholar]