Abstract
Background
Brain radiation necrosis (BRN) can be a complication of radiotherapy for primary and secondary brain tumors, as well as head and neck tumors. Since vascular endothelial growth factor (VEGF) is also a vascular permeability factor in the brain, bevacizumab, a humanized antibody that inhibits VEGF, would be expected to reduce perilesional edema that often accompanies BRN.
Methods
Patients with surgically untreatable, symptomatic BRN refractory to conventional medical treatments (eg, corticosteroid, anticoagulants, or hyperbaric oxygen therapy) were enrolled. We judged that a major cause of perilesional edema with a lesion-to-normal brain ratio ≤1.8 on 11C-methionine or ≤2.5 on 18F-boronophenylalanine PET was BRN, not tumor recurrence, and 6 cycles of biweekly bevacizumab (5 mg/kg) were administered. The primary endpoint was a ≥30% reduction from the patients' registration for perilesional edema continuing for ≥1 month.
Results
Of the 41 patients enrolled, 38 were fully eligible for the response assessment. The primary endpoint was achieved in 30 of the 38 (78.9%) patients at 3.0 months (median) after enrollment. Sixteen patients (42.1%) experienced improvement of their Karnofsy Performance Score. Corticosteroid use could be reduced in 29 patients (76.3%). Adverse events at grade ≥3 occurred in 10 patients (24.4%).
Conclusions
Bevacizumab treatment offers certain clinical benefits for patients with surgically untreatable, symptomatic BRN. The determination of BRN using amino-acid PET, not biopsy, is adequate and less invasive for determining eligibility to receive bevacizumab.
Keywords: Bevacizumab, brain radiation necrosis, positron emission tomography, vascular endothelial growth factor
Radiotherapy contributes to the local control of brain tumors, but high-dose radiotherapy or multiple radiotherapy sessions often increase the incidence of severe radiation injury. Acute radiation injuries can usually be controlled by corticosteroids, but the efficacy of these drugs is limited for late-phase radiation injuries such as brain radiation necrosis (BRN). BRN sometimes impairs patients' clinical status, resulting in worsened quality of life even if the brain tumor is stable. Since vascular endothelial growth factor (VEGF) is also a vascular permeability factor in the brain,1 bevacizumab, a humanized antibody that inhibits VEGF, would be expected to reduce perilesional edema that often accompanies BRN.2–6 However, bevacizumab's optimal dose, dosing interval, and duration of efficacy have not been fully defined.
Symptomatic BRN is accompanied by progressive, perilesional edema volume on T2-weighted MR images, and impairs patients' quality of life. With the use of ordinary imaging modalities such as MRI, it is not easy to determine whether worsening perilesional edema is caused mainly by BRN or by tumor progression; the histological analysis of the lesion is the gold standard for diagnosis. Pure BRN is rare in malignant brain tumors, and some tumor cells usually remain somewhere in or around the BRN (Supplemental material, Fig. S1).
There are a number of noninvasive neuroimaging techniques to evaluate patients for BRN; these include CT, MRI, and PET. The authors have experience with PET using amino acid tracers for CNS lesions7–12 and believe that it is useful for distinguishing BRN from tumor growth. The contrast between tumor and normal tissue shown by PET using some types of amino acids is much higher than that shown by 18F-fluorodeoxyglucose PET. We previously investigated the cut-off value for amino-acid PET that could discriminate tumor progression from pure BRN or BRN with remaining tumor cells.13
Here we conducted a prospective, multicenter, single-arm clinical trial to evaluate bevacizumab's safety and efficacy in patients with surgically untreatable symptomatic BRN refractory to conventional medical treatments. We used amino-acid PET to examine patients suspected of having BRN. For patient eligibility, we classified BRN on the basis of the biological lesion activity as an alternative to histopathological diagnosis requiring invasive surgical procedures.
Patients and Methods
Patients
Patients were eligible if: (i) they had been treated for a brain tumor or head/neck cancer with radiotherapy at least 3 months prior to registration in this trial; (ii) they had progressive perilesional edema that clinically seemed to be caused by BRN; (iii) they had symptoms that were refractory to conventional medical treatment such as corticosteroids and anticoagulants lasting 1 month or longer; (iv) the lesion was enhanced by contrast media, was accompanied by perilesional edema, and was not resectable for any reason; (v) they had a KPS score of 60 or higher; and (vi) they had adequate hematologic, hematic, and renal function. In the present BRN cases after radiotherapy for metastatic brain tumors, systemically or locally active lesions were not detected by radiographic examinations. Tumor markers were within normal ranges otherwise. Further details of the inclusion and exclusion criteria are provided in the Supplementary Materials. The radiation modality and doses in individual cases are listed in Supplementary material, Table S1.
The protocol was approved by the Institutional Review Board at each participating institution. Written informed consent was obtained from all patients.
Assessment of BRN by amino-acid PET
We classified BRN using the uptake of tracer on amino-acid PET, and the lesions were examined by 11C-methionine (MET) or 18F-boronophenylalanine (BPA) PET. Circular regions of interest (ROIs) were placed over the lesion and the contralateral white matter. The lesion-to-normal tissue (L/N) ratio of tracer uptake was calculated by dividing the lesion ROI by the contralateral white matter ROI. A lesion with an L/N ratio less than or equal to 1.8 on 11C-MET PET or greater than or equal to 2.5 on 18F-BPA-PET was classified as BRN, indicating that BRN was the dominant cause of the perilesional edema. These cut-off values were determined by the trial's steering committee, based on previous findings.8,13,14
Treatment
All patients received intravenous bevacizumab at 5 mg/kg biweekly as a cycle, until 6 cycles were completed. After 3 cycles, MRI was conducted. If no negative change was detected by MRI, the patient continued the bevacizumab. Treatment was discontinued if the patient experienced certain adverse events as described in the Supplementary Materials.
Measurement on MRI
The radiological reduction of the perilesional edema was measured by MRI. A volumetric analysis was performed using U.S. FDA-cleared imaging software (Osirix MD v.2.5.1, Pixmeo, Bernex, Switzerland). In each slice, the area of hyperintensity was measured on T2-weighted or fluid-attenuated inversion recovery (FLAIR) images. Hyperintense lesions were automatically traced as ROIs by means of the lower threshold of perilesional edema, and edema was defined referring to the upper limit of the 95% confidence interval (CI) for the normal brain tissue value. Volume was calculated as the sum of all areas of ROIs multiplied by the slice interval.
MR images were obtained at baseline, after 3 cycles, 6 cycles and then at 1 month and every 3 months after the completion of treatment (see Fig. 3). Reductions in contrast-enhanced lesions were also evaluated in the same way as described above. The diagnostic MRI central review was performed by two neurosurgeons, as mentioned in the Acknowledgements.
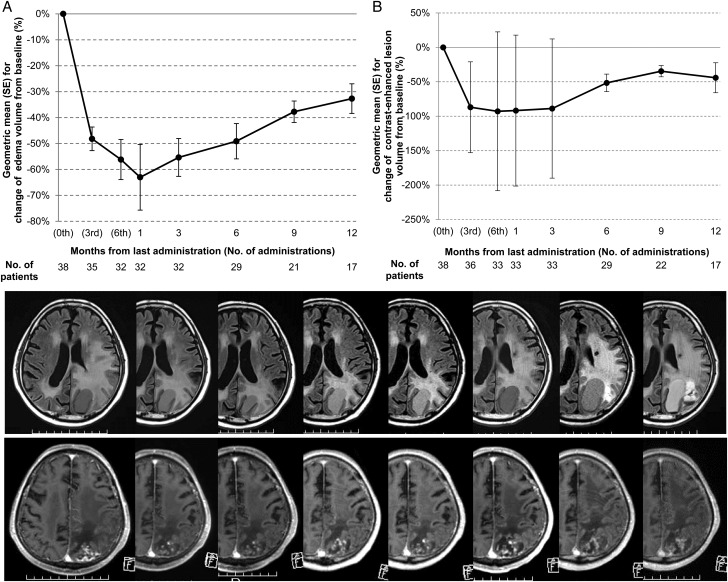
Fig. 3.
Chronological changes in the volumes of (A) the perilesional edema and (B) the contrast-enhanced lesions, compared to the baseline values. In panels A and B, the values are plotted as the geometric mean and SE. MR images show representative chronological changes in perilesional edema on FLAIR images (upper column) and contrast-enhanced lesions on gadolinium-enhanced, T1-weighted images (lower column). Images at baseline, after 3 cycles, 6 cycles, at 1 month, and every 3 months until 12 months after completion of the treatment are shown from left to right.
Evaluation of Efficacy and Safety
The primary endpoint was remission of perilesional edema on MRI. Response was defined as a ≥30% reduction in perilesional edema from the baseline. Remission was defined as a response lasting at least 4 weeks. Progression was defined as a 25% or greater increase in perilesional edema from the minimal value. Secondary endpoints were the reduction of corticosteroid use, improvement of KPS, recurrent radiation necrosis (progression), and reduction in the contrast-enhanced lesion's volume. Steroid usage and KPS were recorded at the time of each scheduled assessment. The incidence of adverse events was assessed until 1 month after treatment completion, according to the Common Terminology Criteria for Adverse Events (CTCAE), v.4.0.
Statistical Analysis
Our primary objective was to elucidate the reduction of perilesional edema by bevacizumab in terms of area measured using MRI. In the 27 symptomatic BRN patients treated at Osaka Medical College between June 2004 and July 2009, the response rate to treatments other than bevacizumab was 14.8% (4/27).15 Thus, the response rate under the null hypothesis was conservatively assumed to be 20%. The response rate to bevacizumab treatment was presumed to be approximately 45% (under the alternative hypothesis). With a type 1 error of ≤5% in the two-sided binomial test and a power of ≥90%, the estimated required number of patients was 37. Using a 7.5% drop-out rate, we set a target sample size of 40.
The efficacy evaluation, which included the primary endpoint, was based on the intent-to-treat principle using the full analysis set (FAS) data. We used the Clopper-Pearson method to calculate the CI of the response rate. The evaluation of adverse events was based on a safety analysis set. The incidence of adverse events is expressed as frequency and percentage. We also calculated the geometric mean and standard error (SE) for the percent changes from the baseline values of perilesional edema volume and contrast-enhanced volume, the frequencies for each KPS category (better, unchanged, worse compared with the baseline), and the mean and SE of corticosteroid dose/day at each time point.
For the time-to-remission and progression evaluations, we estimated the patients' rate curves and median times using the Kaplan-Meier method. As a sensitivity analysis comparing pairs of groups, Fisher's exact test for categorical variables and the Wilcoxon rank sum test for continuous variables were performed. Data were analyzed using SAS v.9.3 software (SAS Institute, Cary, NC) and R v.3.1.2 software (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients and Treatment
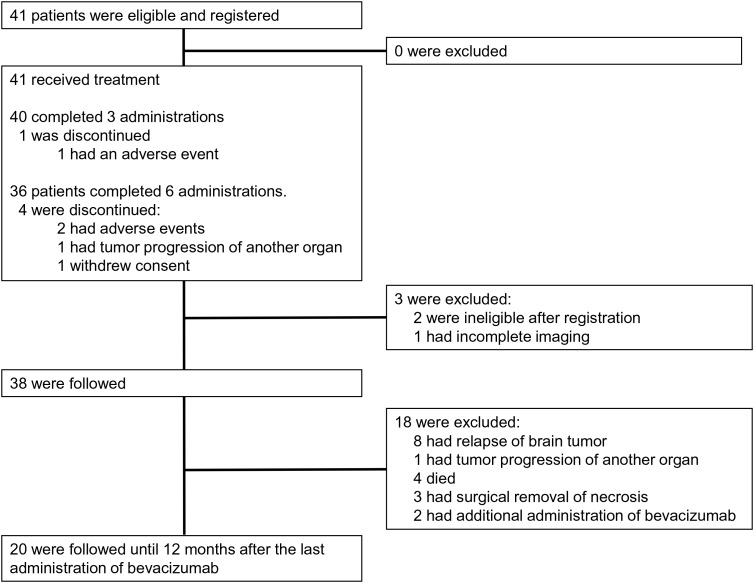
Between April 2011 and February 2013, 41 patients from 16 institutions were enrolled in this trial (Fig. 1). All patients received bevacizumab and none were excluded before treatment. Three cycles of bevacizumab administration were completed in 40 of the patients. Thirty-six of these patients underwent 6 bevacizumab cycles and completed the protocol treatment. Bevacizumab was discontinued due to three adverse events (7.3%): 2 cases of grade 2 intracranial hemorrhages and 1 case of deterioration of neurological status. One patient developed a primary-disease tumor recurrence and 1 patient withdrew consent. Three of the 41 patients were eventually excluded because it was found after registration that they did not meet all of the inclusion criteria. The remaining 38 cases were followed as the FAS.
Fig. 1.
CONSORT diagram. Of the 41 patients enrolled in this trial, 40 underwent 3 cycles of bevacizumab. One patient had an adverse event (AE) leading to the discontinuation of bevacizumab. Thirty-eight patients were followed up as the full analysis set (FAS). Thirty-six patients underwent 6 administrations of bevacizumab. Of the 4 patients who discontinued bevacizumab, 2 had AEs. Primary organ tumor recurred in 1 patient and consent was withdrawn by 1 patient. Thirty-eight patients were followed, and 20 patients were completely followed until 12 months after the last administration of bevacizumab (the per protocol set [PPS]).
Twenty patients completed the 12-month follow-up as the per protocol set (PPS). Eighteen patients were followed but not assessable for a 12-month follow-up due to relapse of brain tumor (8 patients); tumor progression of another organ (1 patient); death (4 patients); and additional interventions (5 patients). The median follow-up period was 13.1 months for the FAS and 12.9 months for the safety analysis set. Table 1 shows the clinical characteristics of the patients.
Table 1.
Patient characteristics
| Variable | n = 38 |
|---|---|
| Age (years), median (range) | 54.5 (17.0–73.0) |
| Sex | |
| Male | 22 (57.9%) |
| Female | 16 (42.1%) |
| Karnofsky Performance Status, median (range) | 70 (60–100) |
| Tumors | |
| Primary brain tumors | 27 (71.1%) |
| Secondary brain tumors | 10 (26.3%) |
| Adjacent organ | 1 (2.6%) |
| Primary brain tumor | |
| Glioblastoma (WHO grade IV) | 11 |
| Anaplastic glioma (WHO grade III) | 7 |
| Glioma (WHO grade II) | 4 |
| Others | 5 |
| Origin of metastatic brain tumor | |
| Lung | 5 (50%) |
| Non-lung | 5 (50%) |
| Time from radiotherapy to diagnosis (months), median (range) | 24.1 (3.0–140.9) |
| Radiotherapy | |
| SRS | 26 |
| EBRT | 17 |
| Hypofractionated IMRT | 4 |
| Proton beam | 3 |
| BNCT | 1 |
| Chemotherapy for tumor | 30 (78.9%) |
| Temozolomide | 20 |
| Other chemotherapeutic agents | 10 |
| Treatment for BRN | |
| Corticosteroids | 38 (100.0%) |
| Vitamin E | 20 (52.6%) |
| Anticoagulants/antiplatelets | 18 (47.4%) |
| Surgical removal of necrosis | 4 (10.5%) |
| Hyperbaric oxygen therapy | 3 (7.9%) |
| Bevacizumab | 1 (2.6%) |
| Osmotic diuretics | 1 (2.6%) |
| Amino-acid PET | |
| 11C-methionine | 30 (78.9%) |
| 18F-boronophenylalanine | 8 (21.1%) |
| Lesion-to-normal tissue ratio, median (range) | |
| 11C-methionine | 1.5 (0.6–1.8) |
| 18F-boronophenylalanine | 1.9 (1.5–2.3) |
| Volume of BRN (mL), median (range) | |
| Perilesional edema | 134.7 (1.9–359.1) |
| Contrast-enhanced lesion | 7.4 (1.6–40.3) |
Abbreviations: BNCT, boron neutron capture therapy; BRN, brain radiation necrosis; EBRT, external beam radiotherapy; IMRT, intensity-modulated radiotherapy; LGG, low-grade glioma; SRS, stereotactic radiosurgery.
Efficacy as Evaluated by MRI
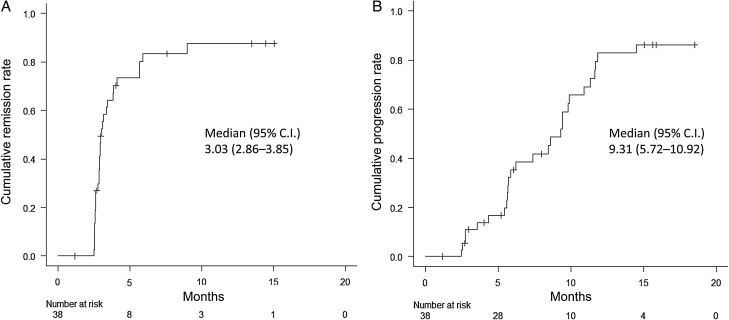
Of the 38 patients in the FAS, 30 achieved remission of their perilesional edema (78.9%; 95% CI, 62.7%–90.4%, P < .0001, binomial test). The median time to remission (responses lasting ≥1 month) from enrollment was 3.03 months (95% CI, 2.86–3.85 months) in the FAS (Fig. 2A). Twenty-seven patients had progression of perilesional edema (71.1%; 95% CI, 54.1%–84.6%). The median time to progression of perilesional edema was 9.31 months (95% CI, 5.72–10.92 months) (Fig. 2B). The average volume of perilesional edema reached the minimum (63.0% decrease from the baseline) at 1 month after the last treatment (Fig. 3A). Subsequently, the volume of perilesional edema increased linearly, but the volume did not reach the baseline value.
Fig. 2.
Kaplan-Meier analysis of (A) the cumulative remission rate and (B) the cumulative progression rate of perilesional edema for the 38 patients in the full analysis set (FAS). The median times to remission and progression from enrollment were 3.03 and 9.31 months, respectively.
Regarding the contrast-enhanced lesions, the average volume reached the minimum (92.8% decrease from baseline) at the last treatment (Fig. 3B). Subgroup analyses revealed no significant clinical factors associated with remission (Supplementary material, Table S2). Fisher's exact test revealed no significant differences in the remission rate related to the original tumor pathology (primary vs metastasis, P = .655) or applied radiotherapeutic modality groups (stereotactic radiosurgery vs others, P = .689; high-dose radiotherapy vs others, P = .660). Likewise, the volume of contrast-enhanced lesions subsequently increased and the final volume of the contrast-enhanced lesions did not reach the baseline volume at 12 months after the completion of treatment.
KPS and Corticosteroids
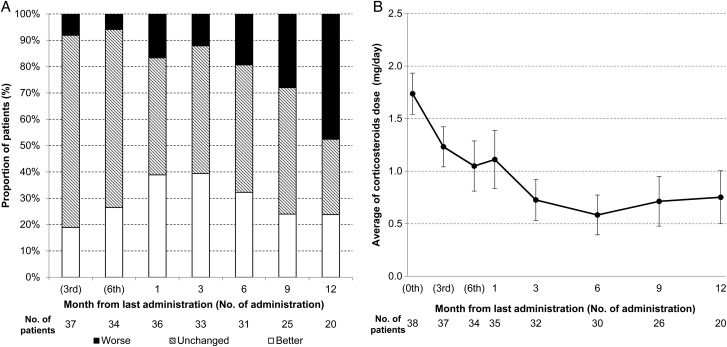
Among the 38 FAS patients, 16 achieved an improved KPS score (42.1%; 95% CI, 21.8%–54%). Ten patients showed 10% improvement and 6 patients showed 20% improvement in KPS, compared with the baseline. The percentage of patients with improved KPS scores increased gradually up to 1 month after the treatment completion (Fig. 4A), but decreased from 6 months after treatment completion; at 12 months posttreatment, one-half of the patients had worsened KPS scores compared with their baseline scores.
Fig. 4.
Chronological changes in (A) Karnofsky performance status (KPS) and (B) corticosteroid dosage. The patients were categorized into 3 KPS groups (better, unchanged, and worse compared with the baseline).
Five patients (23.8%; 95%CI, 8.2%–47.2%) retained better KPS scores at the last follow-up. A corticosteroid was being used by 35 patients (92.1%) at their trial enrollment. The average dexamethasone dose was 1.7 mg/day, and it was eventually reduced in 29 patients (76.3%; 95% CI, 59.8%–88.6%). At 6 months posttreatment, the dose reached the minimum of 0.6 mg/day (Fig. 4B). The final dexamethasone dose (0.8 mg/day) was still lower than the baseline dose.
Safety
Safety data until 1 month posttreatment were available for 41 patients. Adverse events occurred in 36 patients (87.8%, Table 2). Ten patients (24.4%) experienced a grade 3 or greater adverse event. Hypertension was the most common adverse event, occurring in 14 patients (34.1%). Regarding adverse events that are frequently reported following bevacizumab treatment, there were 3 intracranial hemorrhages (7.3%) and 7 mucocutaneous hemorrhages (17.1%). Although 2 patients experienced a venous thromboembolic event, no patient had an arterial thromboembolic event. There were no wound-healing complications. The other grade ≥3 and frequent adverse events are shown in Supplementary material, Table S3.
Table 2.
Summary of adverse events
| Event | Any grade |
Grade ≥3 |
||
|---|---|---|---|---|
| No. of Patients | Percent | No. of Patients | Percent | |
| Any adverse event | 36 | 87.8 | 10 | 24.4 |
| Hypertension | 14 | 34.1 | 2 | 4.9 |
| Elevated ALT | 13 | 31.7 | 2 | 4.9 |
| Convulsion | 5 | 12.2 | 2 | 4.9 |
| Anemia | 12 | 29.3 | 1 | 2.4 |
| Mucocutaneous hemorrhage | 7 | 17.1 | 0 | 0.0 |
| Proteinuria | 5 | 12.2 | 0 | 0.0 |
| Intracranial hemorrhage | 3 | 7.3 | 0 | 0.0 |
| Venous thromboembolic event | 2 | 4.9 | 0 | 0.0 |
Abbreviation: ALT, alanine aminotransferase.
During the 41 patients' follow-up period (median 14.9 months), 6 patients died. The cause of death was related to the CNS in 2 patients: 1 patient's brain tumor progressed and it could not be determined whether the other patient's cerebral lesion was a tumor recurrence or BRN. The other causes of death were sepsis, malnutrition resulting in pneumonia, and pulmonary embolism. These 3 events occurred more than 4 months after the termination of bevacizumab treatments. The cause of death was unknown in 1 patient.
The brain tumors of 8 patients relapsed at the median time of 7.1 months (range, 2.7–18.5 months) (Supplementary material, Fig. S2), and the metastatic brain tumor of 1 patient progressed to an original lesion in another organ.
Discussion
Although BRN is a well-known, radiation-induced disease, and many reports have been published describing the effect of bevacizumab on BRN,3–5,16–19 no formal clinical guidelines regarding BRN have been established. Our previous study's findings15 are thus quoted as the null hypothesis for the treatment response rate. We undertook a systematic review of conventional medical treatments for BRN, as described in the Supplementary Materials. Our search did not turn up any randomized studies for corticosteroids, anticoagulants, or other medications for BRN, and thus the evidence regarding conservative treatments for BRN is limited.20–25 We feel that our previous data for the treatment of 27 patients with BRN15 are sufficient and appropriate as a reference for the treatment response rate. The present study revealed a 79% response to bevacizumab that is similar to other reports of bevacizumab in BRN3–5,16–19 and is also much better compared with our prior study's 15% response rate.15
Most of the reports showing the efficacy of bevacizumab for BRN were case studies or case reports. Only one randomized trial of symptomatic BRN patients was reported (in 2011 by Levin and colleagues).5 They established the first class I evidence that bevacizumab effectively treats BRN, and their carefully conducted study's small patient series (n = 14) was sufficient to provide a P value of .0013, which justified the small series.5 Here we enrolled the largest number of patients to date and had the longest follow-up period among the studies of BRN treated with bevacizumab.
The optimal dose and number of cycles of bevacizumab treatment for BRN are unknown, but many of the reported bevacizumab doses for BRN were the same as those used for the tumors (10 mg/kg, biweekly). As we reported earlier, we suspect that the current dose of bevacizumab (5 mg/kg) is enough to shrink perilesional edema in BRN.2,6 From the viewpoint regarding the AUC, the present bevacizumab administration regimen (5 mg/kg every 2 weeks, 6 times) is equivalent to Levin's study (7.5 mg/kg, every 3 weeks, 4 times).26 The duration bevacizumab's treatment effect and the BRN relapse rate are not clear in previous reports. The main purpose of the present trial was to elucidate the remission rate and safety after 6 cycles of a 5 mg/kg dose of bevacizumab as the minimal treatment. The secondary purpose was to clarify the posttreatment course of perilesional edema after bevacizumab treatment.
Our present data revealed that bevacizumab rapidly decreased perilesional edema and achieved a high cumulative remission rate (Fig. 2A and 3A). Bevacizumab could maintain the decrease in perilesional edema for a certain period; however, progression of the edema was observed in many cases at 9 months (median) after enrollment (Fig. 2B). Sixteen patients of the 38 patients in the FAS showed KPS improvement in the follow-up period, and the dose of corticosteroids decreased in most of the patients (76.3%). However, one-half of the patients did not achieve a 10% improvement in KPS score. Unfortunately, the symptomatic amelioration eventually disappeared as the perilesional edema recurred after the treatment completion. In brief, bevacizumab's therapeutic effectiveness was transient in our dosing regimen (6 cycles of 5 mg/kg). The number of doses might thus need to be increased to maintain bevacizumab's therapeutic efficacy for longer periods. Another possible option is the re-induction of bevacizumab for recurrent BRN after precedent bevacizumab treatment.
In fact, as we and others reported, BRN can recur and require an additional dose of bevacizumab; this re-challenge of bevacizumab to recurred BRN usually works well.2,5 The efficacy of repeated bevacizumab against recurred BRN differed markedly from that against bevacizumab-failed tumor progression.27,28
With regard to safety, in the present trial, the total incidence of serious adverse events (grade ≥3) was 24.4%. This value is similar to the other report of bevacizumab for the treatment of BRN.26 In previous phase II and III trials of glioblastomas treated with bevacizumab,29–31 the incidences of serious adverse events were higher than those of the present trial. One of the possible reasons may be that adverse events were monitored in the present study until 1 month after treatment completion, and it is thus difficult to evaluate whether our dosing schedule as the minimum treatment reduced adverse events because there are no comparable data.
Another important problem related to the management of BRN is its diagnosis. Not only the clinical course and MRI examinations, but also objective diagnostic criteria are necessary to elucidate the effects of bevacizumab on BRN. In clinical oncology, the pathological diagnosis is definitive. Unless the case involves pure BRN, however, residual tumor cells are usually mixed with postirradiated changes in BRN. In such cases, a histological diagnosis based on a small specimen acquired by needle biopsy sometimes gives inadequate information about the lesion compared with that obtained using a whole necrotomy specimen.
In unresectable BRN, it is expected that amino-acid PET can be used to classify BRN by evaluating biological lesion activity. In this situation, the classification of BRN indicates that the perilesional edema is predominantly caused by BRN and does not refer to the absence of tumor cells. 11C-MET is the most widely used amino-acid tracer in the world and in patients with CNS tumors, including gliomas and metastatic brain tumors, 11C-MET PET demonstrated high sensitivity (75%–93%) and high specificity (72.7%–100%) for the differentiation of BRN from tumor recurrence.7–12,32 We adopted 18F-BPA-PET in addition to 11C-MET-PET in the present study. 18F-BPA-PET had shown an excellent diagnostic power for malignant gliomas activity,33 and the tracer uptake in the normal brain is less than that by 11C-MET-PET, which provides high contrast in the L/N ratio between BRN and tumor progression.13,34–36 This helped us predict the effectiveness of bevacizumab for BRN in our previous studies.2,6 In addition, the L/N ratios of both of these types of PET exhibited a close linear relationship.37
While MRI is more widely used for the diagnosis of BRN than PET, our experience with PET-classified BRN led to its use in the present trial and the conclusion that the PET-based determination of BRN was an adequate predictor of bevacizumab treatment benefit. The classification of BRN by the cut-off index of tracer uptake is objective and unlikely to be influenced by inter-rater differences. Another clinical advantage of determining eligibility by amino-acid PET is its less-invasive nature. An invasive diagnostic method such as a biopsy may delay the start of bevacizumab administration due to wound healing troubles. The delayed induction of bevacizumab could make it more difficult to improve the quality of life of patients with symptomatic BRN.
In conclusion, the results obtained in this prospective study with a long observation period indicate that bevacizumab reduced perilesional edema at a high response rate, resulting in the improvement of performance status in patients with surgically untreatable BRN classified by amino-acid PET, although the efficacy of bevacizumab was transient in most of the patients.
Supplementary Material
Funding
This work was supported by a Health and Labour Sciences Research Grant administered by Japan's Ministry of Health, Labour and Welfare (Clinical Trial on Development of New Drugs and Medical Devices, H24-006).
Supplementary Material
Acknowledgments
We thank the study participants and their families, the members of the independent safety monitoring committee (Dr. Yuta Shibamoto, Dept. of Radiology, Nagoya Municipal University, Dr. Teruki Teshima, Dept. of Radiation Oncology, Osaka Medical Center and Cardiovascular Diseases, and Dr. Takashi Daimon, Dept. of Biostatistics, Center for Clinical Support and Education, Hyogo College of Medicine), the members of the diagnostic MRI central review committee (Dr. Toshihiko Wakabayashi, Dept. of Neurosurgery, Nagoya University and Dr. Amami Kato, Dept. of Neurosurgery, Kinki University), Dr. Haruko Yamamoto (Dept. of Advanced Medical Technology Development, Research and Development Initiative Center, National Cerebral and Cardiovascular Center) for the advice regarding the draft of the protocol, and Dr. Masanori Fukushima, the director of the Translational Research Informatics Center, Foundation for Biomedical Research and Innovation (FBRI). We also thank Dr. Tohru Shiga, Dept. of Nuclear Medicine, Hokkaido University Graduate School of Medicine, for the fruitful discussion about the cut-off value in the PET study. We also thank Dr. Jonathan P.S. Knisely, Dept. of Radiation Medicine, Hofstra University, for his critical reading of the manuscript and valuable comments on it. This trial was approved as an Investigational Medical Care System and Advanced Therapy of Type B by the Ministry of Health, Labour and Welfare, Japan and was registered with the University Medical Information Network System with the assigned code UMIN000005391. The data management and statistical analyses were independently performed by the Translational Research Informatics Center, FBRI (Kobe, Japan).
Conflict of interest statement. This study was conducted as a collaboration between Osaka Medical College and the Foundation for Biomedical Research & Innovation (FBRI). As an investigator-initiated clinical study, the principal investigator (S.-I. Miyatake) was responsible for the performance of the study, with operational and technical support provided by the FBRI. The FBRI is a public-interest incorporated foundation committed to the promotion of translational and clinical research in Japan, and it receives financial support from the Japanese government and pharmaceutical/medical device companies. However, none of these companies played a role in the study design, data collection, data analysis or data interpretation of the study, or in the writing of this manuscript.
References
- 1. Schoch HJ, Fischer S, Marti HH. Hypoxia-induced vascular endothelial growth factor expression causes vascular leakage in the brain. Brain. 2002;125 (Pt 11):2549–2557. [DOI] [PubMed] [Google Scholar]
- 2. Furuse M, Kawabata S, Kuroiwa T, Miyatake S. Repeated treatments with bevacizumab for recurrent radiation necrosis in patients with malignant brain tumors: a report of 2 cases. J Neurooncol. 2011;102 (3):471–475. [DOI] [PubMed] [Google Scholar]
- 3. Gonzalez J, Kumar AJ, Conrad CA, Levin VA. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67 (2):323–326. [DOI] [PubMed] [Google Scholar]
- 4. Torcuator R, Zuniga R, Mohan YS et al. Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neurooncol. 2009;94 (1):63–68. [DOI] [PubMed] [Google Scholar]
- 5. Levin VA, Bidaut L, Hou P et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79 (5):1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Furuse M, Nonoguchi N, Kawabata S et al. Bevacizumab treatment for symptomatic radiation necrosis diagnosed by amino acid PET. Jpn J Clin Oncol. 2013;43 (3):337–341. [DOI] [PubMed] [Google Scholar]
- 7. Tsuyuguchi N, Sunada I, Iwai Y et al. Methionine positron emission tomography of recurrent metastatic brain tumor and radiation necrosis after stereotactic radiosurgery: is a differential diagnosis possible? J Neurosurg. 2003;98 (5):1056–1064. [DOI] [PubMed] [Google Scholar]
- 8. Terakawa Y, Tsuyuguchi N, Iwai Y et al. Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med. 2008;49 (5):694–699. [DOI] [PubMed] [Google Scholar]
- 9. Nakajima T, Kumabe T, Kanamori M et al. Differential diagnosis between radiation necrosis and glioma progression using sequential proton magnetic resonance spectroscopy and methionine positron emission tomography. Neurol Med Chir (Tokyo). 2009;49 (9):394–401. [DOI] [PubMed] [Google Scholar]
- 10. Kim YH, Oh SW, Lim YJ et al. Differentiating radiation necrosis from tumor recurrence in high-grade gliomas: assessing the efficacy of 18F-FDG PET, 11C-methionine PET and perfusion MRI. Clin Neurol Neurosurg. 2010;112 (9):758–765. [DOI] [PubMed] [Google Scholar]
- 11. Yamane T, Sakamoto S, Senda M. Clinical impact of (11)C-methionine PET on expected management of patients with brain neoplasm. Eur J Nucl Med Mol Imaging. 2010;37 (4):685–690. [DOI] [PubMed] [Google Scholar]
- 12. Okamoto S, Shiga T, Hattori N et al. Semiquantitative analysis of C-11 methionine PET may distinguish brain tumor recurrence from radiation necrosis even in small lesions. Ann Nucl Med. 2011;25 (3):213–220. [DOI] [PubMed] [Google Scholar]
- 13. Miyashita M, Miyatake S, Imahori Y et al. Evaluation of fluoride-labeled boronophenylalanine-PET imaging for the study of radiation effects in patients with glioblastomas. J Neurooncol. 2008;89 (2):239–246. [DOI] [PubMed] [Google Scholar]
- 14. Tsuyuguchi N, Takami T, Sunada I et al. Methionine positron emission tomography for differentiation of recurrent brain tumor and radiation necrosis after stereotactic radiosurgery–in malignant glioma. Ann Nucl Med. 2004;18 (4):291–296. [DOI] [PubMed] [Google Scholar]
- 15. Nonoguchi N, Miyatake S, Fukumoto M et al. The distribution of vascular endothelial growth factor-producing cells in clinical radiation necrosis of the brain: pathological consideration of their potential roles. J Neurooncol. 2011;105 (2):423–431. [DOI] [PubMed] [Google Scholar]
- 16. Boothe D, Young R, Yamada Y, Prager A, Chan T, Beal K. Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro Oncol. 2013;15 (9):1257–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deibert CP, Ahluwalia MS, Sheehan JP et al. Bevacizumab for refractory adverse radiation effects after stereotactic radiosurgery. J Neurooncol. 2013;115 (2):217–223. [DOI] [PubMed] [Google Scholar]
- 18. Wang Y, Pan L, Sheng X et al. Reversal of cerebral radiation necrosis with bevacizumab treatment in 17 Chinese patients. Eur J Med Res. 2012;17:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yonezawa S, Miwa K, Shinoda J et al. Bevacizumab treatment leads to observable morphological and metabolic changes in brain radiation necrosis. J Neurooncol. 2014;119 (1):101–109. [DOI] [PubMed] [Google Scholar]
- 20. Lorenzo ND, Nolletti A, Palma L. Late cerebral radionecrosis. Surg Neurol. 1978;10 (5):281–290. [PubMed] [Google Scholar]
- 21. Glass JP, Hwang TL, Leavens ME, Libshitz HI. Cerebral radiation necrosis following treatment of extracranial malignancies. Cancer. 1984;54 (9):1966–1972. [DOI] [PubMed] [Google Scholar]
- 22. Woo E, Lam K, Yu YL, Lee PW, Huang CY. Cerebral radionecrosis: is surgery necessary? J Neurol Neurosurg Psychiatry. 1987;50 (11):1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rizzoli HV, Pagnanelli DM. Treatment of delayed radiation necrosis of the brain. A clinical observation. J Neurosurg. 1984;60 (3):589–594. [DOI] [PubMed] [Google Scholar]
- 24. Glantz MJ, Burger PC, Friedman AH, Radtke RA, Massey EW, Schold SC Jr. Treatment of radiation-induced nervous system injury with heparin and warfarin. Neurology. 1994;44 (11):2020–2027. [DOI] [PubMed] [Google Scholar]
- 25. Happold C, Ernemann U, Roth P, Wick W, Weller M, Schmidt F. Anticoagulation for radiation-induced neurotoxicity revisited. J Neurooncol. 2008;90 (3):357–362. [DOI] [PubMed] [Google Scholar]
- 26. Levin VA, Mendelssohn ND, Chan J et al. Impact of bevacizumab administered dose on overall survival of patients with progressive glioblastoma. J Neurooncol. 2015;122 (1):145–150. [DOI] [PubMed] [Google Scholar]
- 27. Han SJ, Rolston JD, Molinaro AM et al. Phase II trial of 7 days on/7 days off temozolmide for recurrent high-grade glioma. Neuro Oncol. 2014;16 (9):1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Omuro A, Chan TA, Abrey LE et al. Phase II trial of continuous low-dose temozolomide for patients with recurrent malignant glioma. Neuro Oncol. 2013;15 (2):242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Friedman HS, Prados MD, Wen PY et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27 (28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 30. Lai A, Filka E, McGibbon B et al. Phase II pilot study of bevacizumab in combination with temozolomide and regional radiation therapy for up-front treatment of patients with newly diagnosed glioblastoma multiforme: interim analysis of safety and tolerability. Int J Radiat Oncol Biol Phys. 2008;71 (5):1372–1380. [DOI] [PubMed] [Google Scholar]
- 31. Chinot OL, Wick W, Mason W et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370 (8):709–722. [DOI] [PubMed] [Google Scholar]
- 32. Van Laere K, Ceyssens S, Van Calenbergh F et al. Direct comparison of 18F-FDG and 11C-methionine PET in suspected recurrence of glioma: sensitivity, inter-observer variability and prognostic value. Eur J Nucl Med Mol Imaging. 2005;32 (1):39–51. [DOI] [PubMed] [Google Scholar]
- 33. Takahashi Y, Imahori Y, Mineura K. Prognostic and therapeutic indicator of fluoroboronophenylalanine positron emission tomography in patients with gliomas. Clin Cancer Res. 2003;9 (16 Pt 1):5888–5895. [PubMed] [Google Scholar]
- 34. Miyatake S, Kawabata S, Nonoguchi N et al. Pseudoprogression in boron neutron capture therapy for malignant gliomas and meningiomas. Neuro Oncol. 2009;11 (4):430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miyatake S, Furuse M, Kawabata S et al. Bevacizumab treatment of symptomatic pseudoprogression after boron neutron capture therapy for recurrent malignant gliomas. Report of 2 cases. Neuro Oncol. 2013;15 (6):650–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miyatake S, Kawabata S, Hiramatsu R, Furuse M, Kuroiwa T, Suzuki M. Boron neutron capture therapy with bevacizumab may prolong the survival of recurrent malignant glioma patients: four cases. Radiat Oncol. 2014;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nariai T, Ishiwata K, Kimura Y et al. PET pharmacokinetic analysis to estimate boron concentration in tumor and brain as a guide to plan BNCT for malignant cerebral glioma. Appl Radiat Isot. 2009;67 (7–8 Suppl):S348–S350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.