Abstract
Primary amebic meningoencephalitis (PAM) is a devastating infection of the brain caused by the thermophilic free-living ameba, Naegleria fowleri. Infection can occur when water containing the ameba enters the body through the nose, usually during recreational water activities such as swimming or diving. Historically, in the United States, cases were mostly reported from the warmer southern-tier states. In the last five years, several notable changes have been documented in PAM epidemiology including a northward expansion of infections and new types of water exposures. The recent reports of two PAM survivors provide hope for improved outcomes with early diagnosis and aggressive treatment. Advanced molecular laboratory tools such as genome sequencing might provide more insight into the pathogenicity of Naegleria fowleri. Clinicians treating patients with meningitis and warm freshwater exposure are encouraged to consider PAM in their differential diagnoses.
Keywords: Naegleria fowleri, primary amebic meningoencephalitis
Introduction
Primary amebic meningoencephalitis (PAM) is a devastating infection of the brain caused by the free -living ameba, Naegleria fowleri. This ameba is commonly found in warm freshwater such as lakes, rivers, and hot springs and rarely infects humans. Infection can occur when water containing the ameba enters the body through the nose, usually during recreational water activities such as swimming or diving. The highly fatal and fulminant nature of this infection is devastating to patient families, puts a burden on already strapped local and state public health departments, and can undermine the public's confidence in the safety of their recreational and drinking water.
Epidemiology of PAM
The first case of PAM was described by Fowler and Carter in Australia in 1965 followed by the first case described in the United States in 1966 [1, 2]. While PAM cases have been reported from several countries and Naegleria fowleri is thought to be distributed worldwide, the most complete data on PAM comes from the United States and therefore, this review will focus predominantly on PAM in the United States. From 1962 through 2015, 138 cases of PAM have been reported in the United States with a range of 0–8 cases annually. A review of cases reported in the United States through 2008 showed that PAM primarily occurs in previously healthy young males exposed to warm, recreational waters, predominantly in lakes, ponds, and reservoirs, in southern-tier states during the summer months [3]. As a thermophilic organism, it has been postulated that changes in the occurrence of Naegleria fowleri and the epidemiology of PAM might be an indicator of climate change.
Changing geography
In the last five years, several notable changes have been documented in PAM epidemiology. In 2010, a 7-year-old Minnesota resident died of PAM after swimming in local lakes and a river [4]. The patient's parents reported the child had not traveled outside of Minnesota in the two weeks prior to becoming ill. An environmental investigation of the lakes and river where the patient swam documented warm water temperatures, algal blooms, and poor water clarity in one lake. Water samples taken from this lake were positive for Naegleria fowleri. Two years later, in August 2012, a 9-year-old Minnesota resident died of PAM. This patient also had no travel outside of Minnesota in the two weeks prior to becoming ill and swam in the same lake as the patient in 2010 which had tested positive for Naegleria fowleri. These cases represent the northernmost documented occurrence of PAM in North America, to date, nearly 600 miles north of the previous northernmost reported case in Missouri. Since 2010, PAM cases have also been reported from other more northern U.S. states including Kansas and Indiana. With climate data showing consistently warming temperatures, the reports of PAM cases outside of the southern-tier states is cause for concern and argues that, regardless of geography, PAM should be considered in the differential for meningitis.
Tap water-associated cases
In addition to changes in the geography of PAM cases, there have also been changes documented in the route of transmission. The 2010 Yoder et al. review of PAM cases reported in the United States since 1962 through 2008 found that over 80% of cases were associated with exposure to natural waters. Tap water was an uncommon exposure (<2%) but had been reported to be associated with two infections from Arizona in 2002 where patients were exposed to untreated drinking water from a geothermal well [5]. In 2011, two cases of PAM were reported from Louisiana in which neither patient had recent recreational water exposure. However, both patients were regular users of neti pots for nasal irrigation [6]. Water samples taken from both of the patients’ homes were positive for Naegleria fowleri including water from a kitchen bathroom sink, bathtub faucets, shower nozzle, and water heater; however, water samples taken from the municipal water treatment plant and distribution system serving the homes were negative at that time. In order to remediate the household plumbing and kill any remaining Naegleria fowleri, the homeowners were instructed to set the water heater to 160° F (71° C) and to run all taps (one faucet at a time) for at least 5 minutes before using the water, repeating this procedure every few weeks to prevent recolonization. No further cases were reported until July 2013, when a 4-year-old child was confirmed to have died from PAM. During the epidemiologic investigation, it was noted that the child had no recreational water exposure in the two weeks prior to becoming ill outside of playing for several hours on a backyard slip-n-slide supplied with tap water from the home. The home where the child played on the slip-n-slide was located in the same Louisiana parish as one of the 2011 PAM cases associated with neti pot use [7]. This finding prompted an environmental investigation of both the home plumbing system as well as the municipal water distribution system that served the home. Water samples were collected and tested for both the presence of Naegleria fowleri as well as total chlorine residual. In this case, Naegleria fowleri was detected in water samples from both the home plumbing system and the municipal water distribution system. Additionally, in the water samples where N. fowleri was detected, the water temperature was noted to be ≥30°C and the chlorine residual was below the level of detection. N. fowleri cysts and trophozoites are inactivated by free chlorine concentrations ≥0.5 mg/L [8, 9]; therefore, maintaining adequate levels of chlorine disinfectant in water distribution systems, particularly in those with elevated water temperatures, is thought to be an important strategy in preventing PAM cases related to tap water use. A similar strategy was adopted by parts of Australia in response to several PAM deaths in the 1960s and 1970s associated with tap water used to fill backyard pools or submerged heads during bathing. Since implementing increased water quality monitoring, ameba testing, and chlorine booster stations to maintain disinfectant residual of ≥0.5 mg/L, Australia has not reported any PAM deaths associated with treated tap water [10].
The use of tap water for the practice of ritual ablution that includes nasal rinsing has also been associated with PAM cases. Ritual nasal rinsing is included in Yogic, Ayurvedic, and Islamic traditions. Within the Islamic faith, ritual nasal rinsing is included in a cleansing process called “wudu” or “ablution” and is usually performed several times a day in preparation for prayer. The association of PAM with ritual ablution was first described by Shakoor et al. in their publication describing 13 cases of PAM in Karachi, Pakistan in 2008 and 2009 who had no history of recreational water use but did perform ablution with tap water [11]. Since that time, PAM cases continue to be reported in the news from Pakistan and subsequent studies have demonstrated that Naegleria fowleri can be found in Karachi water [12]. In 2012, a PAM case was reported from St. Thomas, U.S. Virgin Islands in a 47-year-old male whose only reported nasal freshwater exposure was tap water used for practicing ritual ablution [13]. He practiced ablution both in his home, where the water source was both untreated groundwater from a well and untreated rainwater from a cistern, and at a mosque, where the water source was desalinated and chlorinated municipal water. N. fowleri was detected in household water samples but not in the mosque water samples.
Additional water exposures
Two PAM cases reported in the United States in the last two years highlight additional issues when considering exposures related to the diagnosis of PAM. In 2014, PAM was confirmed as the cause of death in an 11-year-old Florida resident who had traveled to Costa Rica and participated in swimming, zip lining, and water slide use at a resort hot springs in the two weeks preceding his illness onset [14]. Naegleria fowleri was subsequently identified in water samples taken from the resort hot spring and river pond [15]. While the patient's parents were aware of the risk of PAM in Florida waters and took appropriate precautions, they did not know of the risk outside of the United States. This case highlights the importance of considering travel history when PAM is suspected and making the public aware of the risk in warm recreational waters around the world.
In June 2015, a 21-year-old California resident died of PAM after swimming in a privately-owned pool located in a desert environment [16]. The source water for the pool was piped overland for approximately 1.5 miles from a mountain spring via an aging metal pipe that was rusted out and compromised by root systems in many places. The water temperature at the spring source was 50°F but had risen to 98°F where it entered the pool. Moments before swimming began, the participants had added an unknown amount of commercial liquid chlorine to the pool water in an attempt to disinfect the pool. While properly maintained swimming pools should not pose a risk for PAM, this case highlights how a poorly maintained swimming pool can harbor Naegleria fowleri. Additionally, this case demonstrates how water distribution systems can become ideal environments for the growth of Naegleria fowleri.
Clinical presentation and treatment
The diagnosis of PAM carries a high mortality rate of greater than 97%. The clinical presentation of PAM is often indistinguishable from bacterial meningitis with headache, fever, nausea, and vomiting being the most common presenting signs and symptoms [17]. By the time other more common causes of meningitis are ruled out and the diagnosis of PAM is considered, it is often too late to save the patient from the cerebral edema that quickly develops and causes death. U.S. data show a median incubation period of 5 days and a median time from onset of symptoms to death of five days [17]. Most patients presented to medical care with signs or symptoms indicative of central nervous system involvement. Analysis of cerebrospinal fluid (CSF) usually reveals a high opening pressure, a predominantly neutrophilic pleocytosis, elevated protein concentration, and low glucose levels. Only 27% of U.S. cases were diagnosed before death [17]. These findings indicate the diagnosis is often considered too late or not at all in patients with meningitis.
Prior to 2013, there had been only three well-documented survivors worldwide, including one from the United States in 1978. These three early survivors were all treated with conventional (non-deoxycholate formulation) amphotericin B (intravenous and intrathecal) alone or in combination with rifampin, an azole drug (miconazole or fluconazole), and adjunctive therapy with steroids to control cerebral edema [18-20]. While this combination of drugs continued to be used as first-line therapy in patients diagnosed with PAM prior to death, no additional survivors were reported until July 2013, when a 12-year-old female from Arkansas was diagnosed with PAM within 48 hours of symptom onset. Her treatment included intravenous and intrathecal conventional amphotericin B, azithromycin, fluconazole, rifampin, and miltefosine, which, at that time, was an investigational drug available through the Centers for Disease Control and Prevention (CDC). She was also treated aggressively for cerebral edema and the resulting elevated intracranial pressure with dexamethasone, CSF drainage via an external ventricular drain, hyperosmolar therapy with mannitol and 3% saline, moderate hyperventiliation, and induced hypothermia (32–34°C)[21]. After 55 days in the hospital, this patient made a full recovery, returning to school with no residual deficits. During the same summer, another PAM patient, an 8-year-old male in Texas, also survived, although with significant neurologic deficits [22]. He received the same treatment (except for induced hypothermia) as the 12-year-old Arkansas survivor, but was not diagnosed and treated until approximately five days after his symptoms began.
Given changes in the geography of PAM, new modes of transmission via tap water, and the possibility of survival if treatment is initiated early and aggressively, clinicians in all regions of the United States treating patients with meningitis should consider the diagnosis of PAM and inquire about water exposure in the two weeks prior to symptom onset. A presumptive diagnosis can be attempted by examining a wet mount of CSF to look for motile amebae. Regardless of CSF findings, clinicians are encouraged to consult with experts at CDC 24/7 if the diagnosis of PAM is being considered by calling the CDC Emergency Operations Center at 770-488-7100. CDC can assist with treatment recommendations including facilitating the use of miltefosine and arranging for shipment of specimens for diagnostic testing. If motile amebae are seen in the CSF, laboratory staff are encouraged to obtain high-resolution images for examination by CDC experts. Sending images to CDC can facilitate a preliminary diagnosis while specimens are sent to CDC for confirmatory testing.
Diagnostic testing
Clinical specimens
For PAM, CSF and brain biopsy tissue remain the most useful specimen types for diagnosis [23, 24]. These specimens should be collected aseptically, and kept at room temperature (~25°C) prior to any laboratory examinations. These conditions favor survival of the thermophilic amebae in the specimens, facilitating direct observation of live amebae upon microscopic examination, and enabling growth in appropriate culture media. Personnel handling these specimens should wear appropriate protective equipment such as gloves and surgical masks, and should open the containers containing the specimens only inside a biological safety cabinet to minimize risk of unintentional infection. For molecular diagnosis, both CSF and brain biopsy tissue are useful. However, fresh or frozen brain tissue is preferred over formalin-fixed tissue as the formalin treatment adversely affects the DNA quality. Formalin-fixed tissue is useful for the detection of amebae by immunohistochemical assays.
Microscopic identification of amebae in CSF
In suspected PAM patients, a wet mount of CSF should be examined under a microscope immediately after collection for the presence of motile trophozoites. If an immediate microscopic examination is not possible, then the CSF may be stored at room temperature (~25°C) until it is viewed under the microscope. A gentle agitation of the container holding the CSF may be helpful to dislodge any amebae that are adhered to the container before observation under the microscope. Also, brief centrifugation of the CSF specimen at 5,000 × g for 5 minutes may be helpful to concentrate amebae at the bottom of the container prior to placing a small volume on a microscopic slide. Following centrifugation, the supernatant should be carefully removed, leaving about 200—300 μL of residual liquid without dislodging any visible pellet. The microscopic slide containing the CSF may be placed inside a 35-37°C incubator to warm it, which facilitates the movement of any N. fowleri amebae. Smears of CSF should be stained with Giemsa or Wright stains to identify the trophozoite of N. fowleri. The ameba can be clearly differentiated from host cells by the nucleus with its centrally placed large nucleolus. Gram staining of CSF is generally not useful. The three main advantages of microscopic identification of N. fowleri in CSF or brain tissue are its simplicity, rapidity, and low cost. However, the major disadvantage is that an expert microscopist or pathologist is needed to properly identify an ameba from other things such as host cells. This method is also dependent on the morphological integrity of the ameba on the test specimens.
Molecular detection
Recently, molecular techniques based on polymerase chain reaction (PCR) and isothermal DNA amplification have been developed for the specific identification of N. fowleri in clinical and environmental samples [25-33]. Both multicopy mitochondrial 5.8S and 18S rRNA genes and internal transcribed spacers (ITS), as well as single copy genomic DNA have been used to develop these PCR assays. Streby et al. compared four real-time PCR assays in their ability to detect N. fowleri and other Naegleria spp. in environmental samples. They concluded that methods developed by Qvarnstrom et al. (utilizing 18S rRNA gene) and Mull et al. (utilizing 5.8S rRNA gene and ITS region) performed equally well and showed similar sensitivities and specificities. The TaqMan real-time PCR assay by Qvarnstrom et al. has also been widely used to test clinical specimens such as CSF or brain tissue collected from PAM patients, especially in the United States. For CSF and fresh or frozen brain tissue samples, it takes about 2–3 hours to identify N. fowleri from the time the specimen is received in the laboratory. This TaqMan real-time PCR can detect a single ameba in a patient sample because there are hundreds of copies of target DNA per ameba. This method is also highly specific for N. fowleri and it does not amplify DNA from two other pathogenic free-living amebae, Acanthamoeba castellanii or Balamuthia mandrillaris that have been implicated in human infections. Mahittikorn et al. developed a novel loop-mediated isothermal amplification (LAMP) assay for the detection of N. fowleri. LAMP assays do not need sophisticated PCR instrument, and the amplification products can be visualized by the naked eye. The N. fowleri LAMP assay was applied to both environmental water samples and ameba-spiked CSFs, appeared to be specific for N. fowleri, and could detect a single ameba per reaction [27].
PCR is now considered the gold standard for diagnosis and is the first diagnostic test that is performed at CDC on specimens from a suspected PAM case. With proper notification of specimen shipment, the CDC Free-Living and Intestinal Amebas (FLIA) Laboratory can provide same-day PCR results for clinical decision-making.
Antigen detection
Mouse monoclonal antibodies (mAbs) have been developed against N. fowleri. In 1987, Visvesvara and colleagues [34] first developed the mAbs against N. fowleri and used these to detect N. fowleri amebae in brain sections of PAM patients. In the indirect immunofluorescence (IIF) assay, these mAbs reacted intensely with all strains of N. fowleri originating from different geographic areas, but showed no reactivity with four other species of Naegleria (N. gruberi, N. jadini, N. lovaniensis, and N. australiensis) that were never documented to cause disease in humans. These mAbs also did not show any reactivity towards a strain of Acanthamoeba castellanii. N. fowleri amebae concentrated directly from contaminated water can be identified quickly by this IIF assay, suggesting good sensitivity of the test. The advantage of the IIF assay is its ability to mark the amebae with fluorescence so that the microscopist can more easily distinguish amebae from other forms. The CDC FLIA Laboratory usually reserves antigen detection IIF assay for use on formalin-preserved autopsy specimens for the diagnosis of PAM.
Several enzyme-linked immunosorbent assays (ELISAs) have been developed based on monoclonal and polyclonal antibodies for the detection of Naegleria fowleri. However, they have not been tested in clinical specimens for the diagnosis of PAM. Advantages of using antigen detection ELISA assay over microscopic detection include the ability to detect amebic antigens in the absence of intact amebae, rapid turnaround, and ability to be used by laboratory staff otherwise not trained in ameba morphology. One caveat of ELISA is its poor sensitivity compared to PCR assays.
Serologic tests
Seidel [19] reported a specific antibody response to N. fowleri in the 1978 California PAM survivor with a titer of 1:4096 demonstrated by immunofluorescence-based antibody (IFA) assay in the serum samples collected as early as 7 days after admission to hospital. The high antibody titers persisted >4 years in this survivor. However, serologic tests are generally not suitable for PAM diagnosis for several reasons: (1) disease progression in PAM cases is so rapid that they may not mount an antibody response, (2) even if there is an antibody response (assuming specificity against N. fowleri), serology will not be able to distinguish between an acute infection and a past exposure, and (3) the test is time consuming and not useful for making a rapid diagnosis.
Advanced molecular techniques
Genome sequencing
Genome sequencing of free-living amebae is still in its infancy. Accurately annotated genome sequences may provide crucial information about the pathogenicity and virulence of an organism. Sometimes, a genomic comparison between a pathogenic species and a closely related non-pathogenic species can help identify pathogenicity or virulence factors. The genome of a non-pathogenic Naegleria species, N. gruberi, was sequenced in 2010 and the genome sequence of N. fowleri was reported in 2014 [35, 36]. However, comparison of the two suggest that the N. gruberi genome is too diverse to compare to N. fowleri to identify pathogenicity or virulence factors in the latter ameba. It has been suggested that N. lovaniensis, another non-pathogenic species of Naegleria, is possibly the closest to N. fowleri based on the small subunit (SSU) rDNA and 5.8S rDNA sequences (the difference are only 0.8% and 0.6%, respectively) [37]. However, the genome sequence of N. lovaniensis has not yet been reported. Once optimized, genome comparison of N. fowleri strains isolated from PAM patients and those from the environmental sources should provide important information in identifying pathogenicity and virulence factors in this species. Current genotyping tools classify all N. fowleri strains from the United States into three categories [38] and are not useful for linking cases with sources of exposure. Next generation sequencing of environmental and clinical strains may help identify regions that would allow the development of more discriminatory genotyping tools for infection source-tracking in clinical PAM cases and to conduct molecular epidemiology surveys.
Transcriptomic (RNA sequencing) and proteomic analyses
Comparative studies using transcriptomic and proteomic data from highly and weakly virulent N. fowleri trophozoites have identified about two dozen potential targets that may be directly involved in amebic pathogenicity and virulence [36]. Recently, a matrix-assisted laser-desorption-ionization-time-of-flight (MALDITOF) mass spectrometry approach has been used to identify protein biomarkers for pathogenic N. fowleri using 18 N. fowleri strains (including 14 from PAM cases and 4 from environmental sources) and 6 non-pathogenic Naegleria species: N. gruberi, N. lovaniensis, N. australiensis, N. dunnebackei, N. jadinii, and N. italica [39]. This study identified several biomarkers that can differentiate N. fowleri from other species within the genus Naegleria, as well as some common biomarkers for all N. fowleri isolates. Some of the N. fowleri-specific biomarkers (proteins) deserve further characterization in relation to their roles in amebic pathogenicity and virulence.
Conclusions
The last five years have shown that PAM remains a devastating central nervous system infection associated with warm freshwater exposure. Though most clinicians may never encounter a case, the almost always fatal outcome and possibility for survival if treated early and aggressively argue that this infection should be considered in the differential diagnosis of meningitis, particularly in the warm summer months in patients with recent freshwater exposure. Because of the changing geography and types of water exposures, clinicians in all regions should also consider PAM as the cause for meningitis. Diagnostic assistance and treatment recommendations are available 24/7 through consultation with CDC. New insights into this pathogen will be gained through the use of advanced molecular methods.
Figure 1.
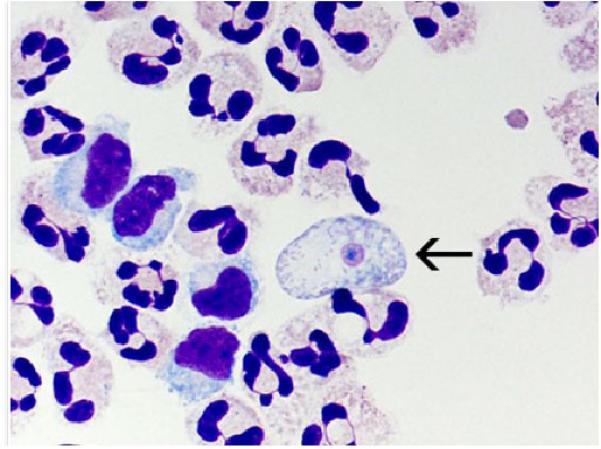
A cytospin of fixed CSF showing a N. fowleri trophozoite (arrow) stained with Giemsa-Wright amidst polymorphonuclear leukocytes and a few lymphocytes. Within the trophozoite, the nucleus and nucleolus can be seen. Magnification ×1000 [image is from CDC and can found at http://www.cdc.gov/parasites/naegleria/naegleria-fowleri-images.html]
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Contributor Information
Jennifer R. Cope, Waterborne Disease Prevention Branch, Division of Foodborne, Waterborne, and Environmental Diseases, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, MS C-09, Atlanta, GA 30329.
Ibne K. Ali, Waterborne Disease Prevention Branch, Division of Foodborne, Waterborne, and Environmental Diseases, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, MS D-66, Atlanta, GA 30329, 404-718-4157, iali@cdc.gov.
References
* = Of particular interest.
** = Of significant interest.
- 1.Fowler M, Carter RF. Acute pyogenic meningitis probably due to Acanthamoeba sp.: a preliminary report. Br Med J. 1965;2(5464):740–2. doi: 10.1136/bmj.2.5464.734-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butt CG. Primary amebic meningoencephalitis. N Engl J Med. 1966;274(26):1473–6. doi: 10.1056/NEJM196606302742605. [DOI] [PubMed] [Google Scholar]
- 3.Yoder JS, Eddy BA, Visvesvara GS, et al. The epidemiology of primary amoebic meningoencephalitis in the USA, 1962-2008. Epidemiol Infect. 2010;138(7):968–75. doi: 10.1017/S0950268809991014. [DOI] [PubMed] [Google Scholar]
- 4.Kemble SK, Lynfield R, DeVries AS, et al. Fatal Naegleria fowleri infection acquired in Minnesota: possible expanded range of a deadly thermophilic organism. Clin Infect Dis. 2012;54(6):805–9. doi: 10.1093/cid/cir961. [DOI] [PubMed] [Google Scholar]
- 5.Marciano-Cabral F, MacLean R, Mensah A, LaPat-Polasko L. Identification of Naegleria fowleri in domestic water sources by nested PCR. Appl Environ Microbiol. 2003;69(10):5864–9. doi: 10.1128/AEM.69.10.5864-5869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoder JS, Straif-Bourgeois S, Roy SL, et al. Primary amebic meningoencephalitis deaths associated with sinus irrigation using contaminated tap water. Clin Infect Dis. 2012;55(9):e79–85. doi: 10.1093/cid/cis626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7*.Cope JR, Ratard RC, Hill VR, et al. The first association of a primary amebic meningoencephalitis death with culturable Naegleria fowleri in tap water from a US treated public drinking water system. Clin Infect Dis. 2015;60(8):e36–42. doi: 10.1093/cid/civ017. [Description of PAM case associated with first documentation of Naegleria fowleri in a U.S. treated public drinking water system.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang SL. Resistance of pathogenic Naegleria to some common physical and chemical agents. Appl Environ Microbiol. 1978;35(2):368–75. doi: 10.1128/aem.35.2.368-375.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarkar P, Gerba CP. Inactivation of Naegleria fowleri by chlorine and ultraviolet light. Journal of the American Water Works Association. 2012;104(3):51–2. [Google Scholar]
- 10.Robinson BS, Christy PE. Disinfection of water for control of amoebae. Water. 1984:21–4. [Google Scholar]
- 11.Shakoor S, Beg MA, Mahmood SF, et al. Primary amebic meningoencephalitis caused by Naegleria fowleri, Karachi, Pakistan. Emerg Infect Dis. 2011;17(2):258–61. doi: 10.3201/eid1702.100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yousuf FA, Siddiqui R, Subhani F, Khan NA. Status of free-living amoebae (Acanthamoeba spp., Naegleria fowleri, Balamuthia mandrillaris) in drinking water supplies in Karachi, Pakistan. J Water Health. 2013;11(2):371–5. doi: 10.2166/wh.2013.112. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention Notes from the field: primary amebic meningoencephalitis associated with ritual nasal rinsing--St. Thomas, U.S. Virgin islands, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(45):903. [PMC free article] [PubMed] [Google Scholar]
- 14.Booth PJ, Bodager D, Slade TA, Jett S. Primary amebic meningoencephalitis associated with hot spring exposure during international travel - Seminole County, Florida, July 2014. MMWR Morb Mortal Wkly Rep. 2015;64(43):1226. doi: 10.15585/mmwr.mm6443a5. [DOI] [PubMed] [Google Scholar]
- 15.Abrahams-Sandi E, Retana-Moreira L, Castro-Castillo A, et al. Fatal meningoencephalitis in child and isolation of Naegleria fowleri from hot springs in Costa Rica. Emerg Infect Dis. 2015;21(2):382–4. doi: 10.3201/eid2102.141576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson RO, Cope JR, Moskowitz M, et al. Notes from the field: Primary amebic meningoencephalitis associated with exposure to swimming pool water supplied by an overland pipe - Inyo County, California, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(16):424. doi: 10.15585/mmwr.mm6516a4. [DOI] [PubMed] [Google Scholar]
- 17.Capewell LG, Harris AM, Yoder JS, et al. Diagnosis, clinical course, and treatment of primary amoebic meningoencephalitis in the United States, 1937-2013. J Pediatric Infect Dis Soc. 2015;4(4):e68–75. doi: 10.1093/jpids/piu103. [DOI] [PubMed] [Google Scholar]
- 18.Anderson K, Jamieson A. Primary amoebic meningoencephalitis. Lancet. 1972;2(7773):379. doi: 10.1016/s0140-6736(72)91763-1. [DOI] [PubMed] [Google Scholar]
- 19.Seidel JS, Harmatz P, Visvesvara GS, et al. Successful treatment of primary amebic meningoencephalitis. N Engl J Med. 1982;306(6):346–8. doi: 10.1056/NEJM198202113060607. [DOI] [PubMed] [Google Scholar]
- 20.Vargas-Zepeda J, Gomez-Alcala AV, Vasquez-Morales JA, et al. Successful treatment of Naegleria fowleri meningoencephalitis by using intravenous amphotericin B, fluconazole and rifampicin. Arch Med Res. 2005;36(1):83–6. doi: 10.1016/j.arcmed.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 21**.Linam WM, Ahmed M, Cope JR, et al. Successful treatment of an adolescent with Naegleria fowleri primary amebic meningoencephalitis. Pediatrics. 2015;135(3):e744–8. doi: 10.1542/peds.2014-2292. [Case report of the 2013 PAM survivor who made a complete neurologic recovery.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cope JRCD, Cohen N, Cotilla M, DaSilva A, Jackson J, Visvesvara GS. Use of the novel therapeutic agent miltefosine for the treatment of primary amebic meningoencephalitis: report of 1 fatal and 1 surviving case. Clinical Infectious Diseases. 2016;62(6):774–6. doi: 10.1093/cid/civ1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Visvesvara GS. Pathogenic and opportunistic free-living amebae. ASM Press; Washington, DC: 2007. [Google Scholar]
- 24.Visvesvara GS. Infections with free-living amebae. Handb Clin Neurol. 2013;114:153–68. doi: 10.1016/B978-0-444-53490-3.00010-8. [DOI] [PubMed] [Google Scholar]
- 25.Streby A, Mull BJ, Levy K, Hill VR. Comparison of real-time PCR methods for the detection of Naegleria fowleri in surface water and sediment. Parasitol Res. 2015;114(5):1739–46. doi: 10.1007/s00436-015-4359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang H, Seong GS, Sohn HJ, et al. Effective PCR-based detection of Naegleria fowleri from cultured sample and PAM-developed mouse. Eur J Protistol. 2015;51(5):401–8. doi: 10.1016/j.ejop.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Mahittikorn A, Mori H, Popruk S, et al. Development of a rapid, simple method for detecting Naegleria fowleri visually in water samples by loop-mediated isothermal amplification (LAMP). PLoS One. 2015;10(3):e0120997. doi: 10.1371/journal.pone.0120997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kao PM, Hsu BM, Hsu TK, et al. Application of TaqMan qPCR for the detection and monitoring of Naegleria species in reservoirs used as a source for drinking water. Parasitol Res. 2014;113(10):3765–71. doi: 10.1007/s00436-014-4042-2. [DOI] [PubMed] [Google Scholar]
- 29.Kao PM, Tung MC, Hsu BM, et al. Quantitative detection and identification of Naegleria spp. in various environmental water samples using real-time quantitative PCR assay. Parasitol Res. 2013;112(4):1467–74. doi: 10.1007/s00436-013-3290-x. [DOI] [PubMed] [Google Scholar]
- 30.Wang W, Wei F, Li J, et al. Isolation and identification of Naegleria species from environmental water in Changchun, northeastern China. Iran J Parasitol. 2014;9(2):254–9. [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmad AF, Lonnen J, Andrew PW, Kilvington S. Development of a rapid DNA extraction method and one-step nested PCR for the detection of Naegleria fowleri from the environment. Water Res. 2011;45(16):5211–7. doi: 10.1016/j.watres.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 32.Garcia A, Goni P, Cieloszyk J, et al. Identification of free-living amoebae and amoeba-associated bacteria from reservoirs and water treatment plants by molecular techniques. Environ Sci Technol. 2013;47(7):3132–40. doi: 10.1021/es400160k. [DOI] [PubMed] [Google Scholar]
- 33.Mull BJ, Narayanan J, Hill VR. Improved method for the detection and quantification of Naegleria fowleri in water and sediment using immunomagnetic separation and real-time PCR. J Parasitol Res. 2013;2013:608367. doi: 10.1155/2013/608367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Visvesvara GS, Peralta MJ, Brandt FH, et al. Production of monoclonal antibodies to Naegleria fowleri, agent of primary amebic meningoencephalitis. J Clin Microbiol. 1987;25(9):1629–34. doi: 10.1128/jcm.25.9.1629-1634.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fritz-Laylin LK, Prochnik SE, Ginger ML, et al. The genome of Naegleria gruberi illuminates early eukaryotic versatility. Cell. 2010;140(5):631–42. doi: 10.1016/j.cell.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 36**.Zysset-Burri DC, Muller N, Beuret C, et al. Genome-wide identification of pathogenicity factors of the free-living amoeba Naegleria fowleri. BMC Genomics. 2014;15:496. doi: 10.1186/1471-2164-15-496. [This is the first article to report on genome sequencing of Naegleria fowleri. The authors also identified proteins potentially involved in N. fowleri pathogenicity.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Jonckheere JF. What do we know by now about the genus Naegleria? Exp Parasitol. 2014;145(Suppl):S2–9. doi: 10.1016/j.exppara.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Zhou L, Sriram R, Visvesvara GS, Xiao L. Genetic variations in the internal transcribed spacer and mitochondrial small subunit rRNA gene of Naegleria spp. J Eukaryot Microbiol. 2003;(50 Suppl):522–6. doi: 10.1111/j.1550-7408.2003.tb00617.x. [DOI] [PubMed] [Google Scholar]
- 39.Moura H, Izquierdo F, Woolfitt AR, et al. Detection of biomarkers of pathogenic Naegleria fowleri through mass spectrometry and proteomics. J Eukaryot Microbiol. 2015;62(1):12–20. doi: 10.1111/jeu.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]


