Abstract
Purpose of review
Nonhuman primate (NHP) models of AIDS are powerful systems for evaluating HIV vaccine approaches in vivo. Authentic features of HIV-1 transmission, dissemination, target cell tropism, and pathogenesis, and aspects of anti-HIV-1 immune responses, can be recapitulated in NHPs provided the appropriate specific model parameters are considered. Here, we discuss key model parameter options and their implications for HIV-1 vaccine evaluation.
Recent findings
With the availability of several different NHP host species/subspecies, different challenge viruses and challenge stock production methods, and various challenge routes and schema, multiple NHP models of AIDS exist for HIV vaccine evaluation. The recent development of multiple new challenge viruses, including chimeric simian-human immunodeficiency viruses (SHIVs) and simian immunodeficiency virus (SIV) clones, improved characterization of challenge stocks and production methods, and increased insight into specific challenge parameters have resulted in an increase in the number of available models and a better understanding of the implications of specific study design choices.
Summary
Recent progress and technical developments promise new insights into basic disease mechanisms and improved models for better preclinical evaluation of interventions to prevent HIV transmission.
Keywords: macaque, vaccine challenge, SIV, SHIV
Introduction
Since the earliest evaluations of classical vaccination approaches, nonhuman primate (NHP) models of AIDS have played a key role in HIV vaccine design and development, providing safety and proof-of-concept data for proposed HIV vaccine strategies. Although NHP/AIDS models have been utilized to evaluate both prophylactic vaccine modalities and therapeutic vaccination approaches, this review will focus primarily on NHP challenge models for use in studies of viral transmission and the evaluation of acquisition prevention approaches.
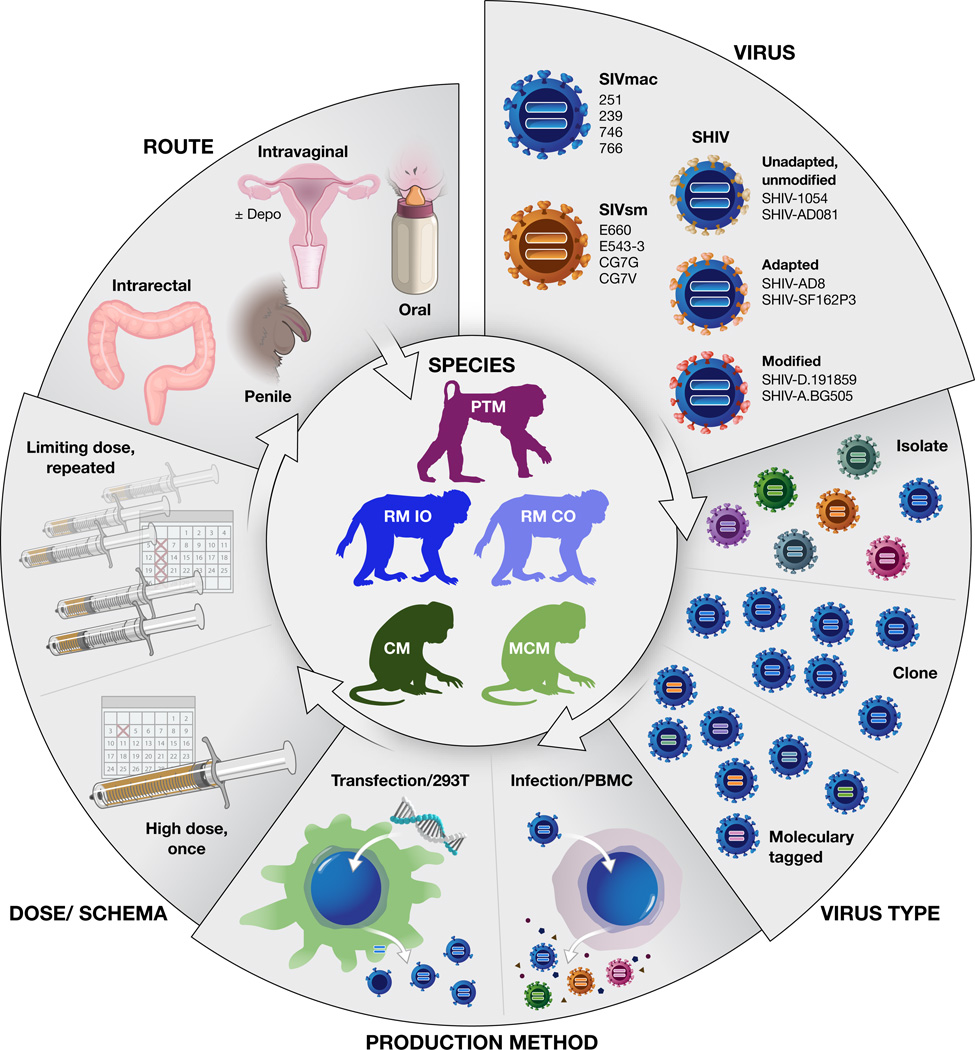
When designing an in vivo NHP experiment, it is critical to select from among the many available NHP/AIDS models that which is best suited to address the specific research questions of interest. The choice of NHP host species/subspecies, challenge virus, challenge route, virus dose, and challenge schema (Fig. 1) can all impact key biological features and outcomes and should be carefully considered. This review will cover our current perspectives on these parameters and considerations.
Figure 1.
Schematic representation of NHP/AIDS virus challenge study design considerations. Example SIV challenge viruses shown include those of the SIVmac and SIVsm lineages, including recently described transmitted/founder clones (SIVmac746, 766, SIVsmCG7G, CG7V) derived from these lineages [5]. Representative SHIV challenge viruses are also shown; additional SHIVs have also been recently described [1,2*–4*]. Depo, Depo-Provera. PTM, pig-tailed macaque. RM IO, rhesus macaque Indian-origin. RM CO, rhesus macaque Chinese-origin. CM, cynomolgus macaque. MCM, Mauritian cynomolgus macaque.
NHP Host Species
Vaccine studies conducted in NHPs typically involve experimental AIDS virus challenge of Asian macaque species, including rhesus macaques (RMs; Macaca mulatta), cynomolgus macaques (CMs; Macaca fascicularis), and pig-tailed macaques (PTMs; Macaca nemestrina) (Fig. 1), that are not naturally infected with simian immunodeficiency virus (SIV). RMs, specifically those of Indian-origin (IO), are the most widely-used macaques for AIDS research and HIV vaccine studies [6], with an associated large body of historical data describing SIV infection and vaccine immunogenicity within these hosts, and with a greater availability of immunological reagents and genomic information [7,8] than is available for other species. When infected with benchmark pathogenic viruses, such as the SIVmac lineage viruses, IO RMs undergo a generally consistent disease course with characteristically high acute viral loads [9] that provide a robust and reproducible readout for prevention studies. Chinese-origin (CO) RMs can also be used for SIV challenge studies [10–12], but acute viral loads may be 1 to 2 logs lower and chronic phase viral loads may be several orders of magnitude lower in this subspecies when infected with the most widely used viruses, which have been optimized for use in their IO relatives [13–18]. It is unclear if the underlying biological cause of these lower viral loads might also impact viral transmission. Although a less stringent model involving superior control of viral replication even in the absence of vaccination may have advantages for some studies, this apparent limitation for using CO RMs may be surmountable through the use of alternative viruses specifically adapted for robust infection of CO rhesus [18].
Cynomolgus macaques, which based on practical and logistical considerations have been used predominantly in European laboratories, have been used less extensively for AIDS research than RMs. Following infection with SIV strains that are pathogenic in IO RMs, CMs have an infection course that is less consistently pathogenic, with viral load profiles that may be several logs lower and more variable [19], though, like CO RMs, it is possible that this limitation may be overcome by using viruses adapted for this species [20–22]. Despite the more limited historical use of CMs for AIDS research, CMs from the island of Mauritius, which are descended from a small number of founder animals subject to insular geographic isolation resulting in limited genetic MHC class I and class II diversity [23,24], have emerged as a valuable research tool for studies seeking to more completely control for animal MHC haplotypes than is readily feasible with other, more outbred macaque hosts and populations [25,26*,27].
In recent years there has been increased interest in the use of PTMs for intravaginal challenge studies because female PTMs have a normal lunar menstrual cycle with associated hormonal changes that are more akin to human menstrual cycles than other available macaque species [28]. The relevance of PTM vaginal physiology has allowed researchers to assess in this species the impact of factors such as coinfections and contraceptive use on susceptibility to vaginal infection [29*,30], and to evaluate modalities to prevent vaginal virus transmission, including microbicides and other pharmacologic interventions [31–34], all while accounting for menstrual cycle phases [30,35,36*–38]. However, within-species comparisons of intravaginal and intrarectal challenges using PTMs may be confounded by the higher levels of baseline immune activation associated with elevated intestinal permeability and microbial translocation in the absence of infection in PTMs when compared with RMs [39]. This characteristic of PTM intestinal biology may impact fundamental aspects of mucosal viral transmission and early viral replication, although environmental factors may contribute to the described phenotype. Intriguingly, PTMs express a variant form of the TRIM5 viral restriction factor that, unlike rhesus TRIM5α, does not restrict HIV replication [40–43]. This finding has led to ongoing efforts to derive minimally chimeric HIV-1 strains capable of high level replication and pathogenesis in PTMs [44,45], which with further development may become highly useful for HIV-1 vaccine evaluation in NHPs.
Challenge viruses
Factors relevant to the characterization and choice of challenge viruses for a given experiment include identity, clonal or swarm composition, extent of genetic diversity, neutralization sensitivity, pathogenicity, restriction factor susceptibility, and mode of production. To date, the majority of NHP/AIDS research, including vaccine studies, has utilized experimental challenge with SIV isolates and clones derived from chronically infected RMs, though very recent efforts have resulted in the generation of several new pathogenic transmitted/founder SIV clones that may represent more relevant vaccine targets [5] (Fig. 1).
Homologous vs. Heterologous Challenge
Initial NHP vaccine studies relied upon homologous challenges in which animals were challenged with either uncloned SIVmac251 swarm virus or the SIVmac239 infectious molecular clone following vaccination with immunogens derived from the same SIVmac251/239 lineage viruses [46,47]. Although such studies generated important immunogenicity and proof-of-concept data, given the extensive diversity of circulating HIV-1, demonstrating protection against a virus challenge heterologous to the vaccine immunogen has long been of both interest and importance. To evaluate heterologous protection in NHPs, heterologous immunization/challenge designs have been implemented, with SIVmac-based immunogens and challenge(s) with the SIVsmE660 isolate or SIVsmE543 clone, pathogenic viruses which genetically diverge from the SIVmac lineage to an extent approximating the degree of divergence between different HIV-1 subtypes [48,49*–51]. Alternatively, immunization with SIVsm-based immunogens and subsequent challenge with SIVmac viruses may be used. While SIVmac239 and SIVmac251 have a degree of resistance to antibody-mediated neutralization comparable to or exceeding typical circulating HIV-1 strains [52–54**], the SIVsmE660 isolate is composed of viral clones most of which are relatively more neutralization sensitive [48], making it a more tractable target for antibody-based prevention studies. However, unlike the SIVmac viruses, SIVsmE660 and E543 are highly susceptible to restriction by specific rhesus macaque TRIM5α alleles [55–58], which can impact virus acquisition after mucosal challenge [57,58], confounding the interpretation of vaccine efficacy [59,60]. Thus, when using SIVsmE660 and related challenge viruses, it is critical to genotype study animals for their TRIM5α alleles and either exclude those with restrictive TRIM5α alleles or account for the distribution of restrictive and permissive alleles among the study arms.
SIV vs. SHIV
The extreme neutralization resistance of many of the best characterized SIVmac viruses [52–54**], the limited availability until quite recently [54**] of well-pedigreed antibody reagents specific for SIV Env epitopes of interest, and the growing collection of broadly reactive anti-HIV-1 Env-specific neutralizing antibodies that do not cross-react with SIV have led to renewed enthusiasm for the development of relevant simian-human immunodeficiency viruses (SHIVs). These chimeric viruses, which conceptually comprise an SIV backbone genome containing an inserted HIV-1 Env gene, allow for the direct evaluation of HIV-1 Env targeted prevention strategies. The first efforts toward generating pathogenic CCR5-using SHIVs focused on animal-to-animal serial virus passage to develop SHIVs capable of high level viral replication in macaques [1,61,62]. Although some of these viruses have been highly valuable research tools for proof-of-concept evaluations of antibody-mediated infection prevention [63**,64**,65*–68*,69–71], they represent limited genotypic breadth, were derived from Envs cloned from chronically-infected people, often after in vitro passage, and thus do not represent the most clinically-relevant Envs for transmission studies and vaccine assessment. More recent efforts have taken advantage of the greater availability of HIV-1 Env clones from transmitted/founder (T/F) viruses, which initiated individual human infections. Through the use of in vivo competition, subtype B and C T/F Envs capable of mediating mucosal SHIV transmission and robust acute viral replication in RMs without any animal-to-animal serial passage or alteration have been identified [2*,72], with one subtype B SHIV even demonstrating the ability to induce progressive disease with AIDS defining clinical endpoints in the absence of any Env adaptation or alteration [72]. Mucosally transmissible SHIVs bearing unpassaged T/F CRF01_AE Envs have also been identified [3], as have SHIVs bearing T/F subtype A, B, C, and D Envs containing specific mutations proposed to improve interaction with the RM CD4 receptor [4*], which may prove to be a critical determinant of SHIV replication capacity [73*].
Although sustained elevated chronic viral loads with authentic pathogenesis and a progressive infection course is not essential for most prevention studies, these would be preferred features for new viruses and additional characterization and development of such viruses will be important. Taken together, though, the current generation of new SHIVs represent substantial progress, providing a promising panel with broad viral diversity and range of neutralization profiles for use in NHP studies assessing Env-targeting vaccine modalities.
Generation and Characterization of Challenge Virus Stocks
Once a challenge virus is selected, choice of an infectious stock of that virus is a critical aspect of NHP vaccine challenge studies (Fig. 1). The specific challenge virus stock and the method used to generate it can dictate feasible downstream assays and impact study results. Virus stocks used for vaccine studies can be clones or swarms, produced by transfection or by short term expansion in an infected culture of mitogen activated primary macaque CD4+ T lymphocytes or PBMC host cells, although human cells have also been used.
Viral swarms
The ability to distinguish transmitted variants by viral genome sequencing allows for verification that the virus challenge dose appropriately recapitulates the limited number of transmitted variants that initiate most human sexual transmissions [74–76]. It may also increase the statistical power of a study without an increase in animal numbers. Enumerating the number of distinct transmitted variants can serve as a minimum estimate of infection/transmission-events per animal [77*,78**–80], with reduction of this parameter interpreted as a measure of partial vaccine efficacy, even if acquisition was not completely prevented. This type of analysis has traditionally required the use of an uncloned viral isolate swarm, such as SIVmac251 or SIVsmE660, containing sufficient viral diversity for individual variants to be genetically distinguished through single genome amplification and sequencing techniques, typically over full length Env sequences [80–82].
Challenge with phenotypically heterogeneous viral isolate swarms may also enable the elucidation of vaccine efficacy mechanisms through “sieve analyses”. Evaluating the genotypes and associated phenotypes of “breakthrough” viral variants in vaccinated animals may provide insights into vaccine mechanism of action and viral evasion [49*,78**]. However, to identify genotypic signatures of breakthrough virus, the combination of genotype frequency within the stock, virus dose, and numbers of animals and challenges must be such that the specific genotype will be significantly selected for in vaccinees relative to control animals. Thus this type of analysis is critically dependent upon the genetic composition of the challenge stock used, and the presence and relative proportion of the pertinent variants can vary dramatically in unpredictable and uncontrolled ways from stock to stock [83].
There are other potential pitfalls to consider when utilizing uncloned virus swarms as challenge stocks, including variability in viral diversity and non-viral stock constituents from stock to stock [83]. This may confound comparisons between studies using different stocks of nominally the same virus. In addition, when animals are challenged with virus at limiting dilutions, resulting in the transmission of only one or a few viral variants from within the swarm, the specific transmitted variants infecting each animal are highly subject to stochastic selection, and thus each animal may be infected with one or several viral variants that are phenotypically distinct from the other study animals. While the complete range of phenotypic breadth within a given stock is difficult to capture, recent studies have shown that there are considerable variant-to-variant phenotypic differences within such swarm stocks [48,83]. In addition, discriminating variants drawn from an uncloned swarm often requires the sequencing of large genomic regions, thereby limiting the depth of sequence sampling or dramatically increasing the costs of sequencing.
Viral clones
The use of clonal virus stocks, produced by transfecting non-permissive cells, typically 293T cells, with a molecularly cloned viral genome can obviate many of these issues while potentially creating others. By using a non-permissive cell type to produce the virus, viral replication in culture is avoided, resulting in a virus population that is genetically homogeneous, allowing greater stock-to-stock reproducibility. As a result, each animal will be challenged with and infected by a genetically identical virus, making comparisons between study groups and individual study animals more straightforward. However, potential drawbacks to consider for the use of clonal, transfection produced virus stocks include increased sensitivity to antibody-mediated neutralization [48,84–87] and lower apparent mucosal infectivity than matched viruses generated in infected PBMCs, despite comparable in vitro infectivity titers, two features which may be related to the relatively lower virion-associated Env glycoprotein content for virus produced in 293T cells [83,84]. Moreover, the clonal genetic composition of unmodified transfection-produced virus stocks precludes the discrimination and enumeration of transmitted variants following challenge.
Sequence tagged synthetic swarms
To allow for discrimination and enumeration of transmitted variants while minimizing the phenotypic differences from variant-to-variant, we recently described the generation of a synthetic viral swarm, generated by incorporating a series of sequence-identifiable synonymous mutations into a small region of the otherwise isogenic SIVmac239 viral genome [88]. This approach, which can be applied to other molecularly-cloned viruses such as SHIVs, allows distinct variants to be easily identified and enumerated after transmission, with increased feasibility for deep sequencing due to the short unique sequence region. If made by transfection, stocks of this synthetic swarm containing equivalent relative proportions of each of the genetic tags can be easily generated, though many of the other limitations of transfection-derived viruses still apply. Short term expansion in infected PBMCs of such molecularly tagged-virus populations would likely obviate many of these issues, and, unlike standard infectious molecular-clones that would not acquire sufficient genetic diversity for T/F virus discrimination following short term expansion in infected cells [83*], genetically-tagged viruses would already possess the needed diversity.
Challenge route, study design and interpretation
NHP/AIDS models have been used to varying degrees to evaluate virus transmission and preventative measures for most of the routes relevant to HIV-1 transmission, including intravenous (IV), intrarectal (IR), intravaginal (Ivag), penile, oral, and intrauterine transmission (Fig. 1). While some of these approaches have become routine for the field, they often rely on historical precedent and are still poorly understood, in part reflecting a limited understanding of the human transmission scenarios that scientists hope to model in NHPs. For example, the vast majority of transmission and vaccine challenge studies performed in NHPs to date have utilized cell-free virus stocks that can be conveniently characterized, titered in vitro, and stored, and that behave reproducibly in animals. However, a role for the transmission of infected cells remains unclear, and may merit further consideration [89], but is currently omitted from most NHP studies.
Challenge route, dose, and schema
IV virus transmission represents the most consistent and straightforward transmission route in NHPs, but it also represents one of the least clinically relevant and most stringent routes for HIV vaccine research. Though there may be some utility for evaluating vaccine-mediated prevention of IV transmission in NHPs to model protection against transmission through needle sharing, standard NHP IV infection and challenge protocols involve the IV delivery of relatively large volumes of fluid containing relatively large numbers of virions, which does not ideally model this transmission route in humans.
Given the central role that sexual transmission plays in the HIV-1 pandemic, much of the HIV vaccine research currently conducted in NHPs focuses on the use of mucosal challenge studies to model sexual transmission. Of the available mucosal challenge routes in NHP study animals, IR challenges represent the most consistent and practically feasible. However, even for IR challenges, there are likely refinements to consider, as demonstrated by a recent study highlighting the impact of simple parameters such as inoculum volume and the presence of rectal/colonic feces on inoculum distribution along the mucosal surface [90]. Ivag and penile challenge models represent somewhat more demanding systems. Consistent with human transmission rates, successful Ivag and penile challenges in NHPs require relatively higher doses of virus than IR transmission and are more prone to animal-to-animal variability [81,82,91]. Efforts to improve the consistency of virus infection rates in unvaccinated control animals via the penile and Ivag routes have included, respectively, the use of two sequential penile challenges per day [82,92] and the administration of the progestin drug Depo Provera [93,94], which has been shown to thin the vaginal epithelium and increase susceptibility to infection [95]. While the relevance of either practice may be limited, avoiding Depo Provera creates logistical challenges as it becomes critical to monitor and control for animal menstrual cycles, which can substantially impact susceptibility to vaginal infection [30,35,36*–38,96]. Although efforts are usually made to perform mucosal challenges atraumatically, to avoid harm to study animals and confounding effects from transmission inconsistencies due to mucosal damage, it is also worth considering whether this practice best models mucosal transmission associated with coital acts in humans.
Following the discovery that the majority of sexual HIV-1 transmission in humans results in only one or a few viral variants establishing systemic infection [75,76], and the demonstration that this phenomena could be recapitulated through the use of titered inocula doses in NHPs [80,81,91], much of the HIV prevention research conducted in NHPs has moved in recent years to the use of repeated limiting dose challenge studies. In a repeated limiting dose challenge paradigm, the titer of the challenge stock must be determined in animals to identify a dose that infects only a fraction of unvaccinated control animals per exposure (typically half or fewer), with animals repeatedly exposed until evidence of infection, or through a predetermined number of challenges. While the size of the inoculum used even in limiting-dose challenge studies may exceed that of typical human mucosal HIV exposures, this model paradigm allows for the approximation of limited numbers of founder variants following transmission, simulating typical human mucosal transmission, while satisfying practical considerations, such as study duration and costs associated with numbers of exposures required to infect control animals. Use of a repeat challenge model impacts statistical analysis and interpretation of results, and while calculation of “per challenge vaccine efficacy” may provide a measure of partial vaccine efficacy, this would seem of limited value in a study where all vaccinees ultimately become infected after a small number of challenges. Between labs, there is considerable variability in the challenge frequency, challenge stock used, and whether or not virus doses escalate throughout the study, and the potential impact of these parameters on subsequent challenges remains unclear, although most data argue against immunological sensitization from challenges not resulting in a take of infection. An alternative approach involves the use of somewhat higher doses of viruses, approximating a minimal dose required to infect the majority of unvaccinated control animals with a single challenge. In this type of study design, researchers can utilize sequencing approaches as described above to assess vaccine efficacy by combining evaluations of infection rates, number of transmitted variants per animal, and sieving analysis [49*,78**,97].
Conclusions
In recent years, substantial advances have been made toward the development of additional NHP/AIDS model options, with the potential for greater model relevance and translatability. However, with this increase in the number of existing model parameters and the increasing availability of assay technologies comes a greater need and opportunity for additional model development and characterization. Given the many NHP/AIDS models available for use in HIV vaccine studies, it is important that the most appropriate model, based on relevance and feasibility, be selected for each specific experimental question and that premature standardization for its own sake is avoided. Through the use of relevant, well-characterized parameters, in NHP/AIDS models HIV vaccine researchers have robust in vivo experimental systems that may provide key insights toward the preclinical development of a successful HIV vaccine.
Key Points.
Multiple challenge models, each consisting of a unique combination of a NHP host species, a challenge virus prepared by a particular method, and a specific challenge route and schema, are available for vaccine and other prevention studies.
Each of these factors can significantly impact study logistics, feasible analyses, outcomes and interpretations.
Investigators should thoughtfully select the model whose features best address the specific experimental question of interest.
Recent developments, including new SHIVs incorporating more clinically relevant HIV-1 Env sequences, generation of transmitted SIV clones, and sequence tagged “synthetic swarms” of challenge viruses offer new possibilities for conducting more sophisticated and potentially clinically relevant studies of vaccines and other prevention approaches.
Acknowledgments
The authors thank Adrienne Swanstrom and Joseph Meyer for assistance with manuscript preparation.
Financial Support and Sponsorship.
This work was supported with federal funds from the National Cancer Institute, National Institutes of Health under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government
Footnotes
Conflicts of Interest.
None.
References
- 1.Song RJ, Chenine AL, Rasmussen RA, Ruprecht CR, Mirshahidi S, Grisson RD, Xu W, Whitney JB, Goins LM, Ong H, et al. Molecularly cloned SHIV-1157ipd3N4: a highly replication- competent, mucosally transmissible R5 simian-human immunodeficiency virus encoding HIV clade C Env. J Virol. 2006;80:8729–8738. doi: 10.1128/JVI.00558-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asmal M, Luedemann C, Lavine CL, Mach LV, Balachandran H, Brinkley C, Denny TN, Lewis MG, Anderson H, Pal R, et al. Infection of monkeys by simian-human immunodeficiency viruses with transmitted/founder clade C HIV-1 envelopes. Virology. 2015;475:37–45. doi: 10.1016/j.virol.2014.10.032.. Through the use of pooled virus infections and in vivo virus competition, these authors identify a subset of SHIVs bearing transmitted/founder subtype C HIV-1 Envs capable of mucosal transmission and sustained viremia, though without documented pathogenicity.
- 3.Tartaglia LJ, Chang HW, Lee BC, Abbink P, Ng'ang'a D, Boyd M, Lavine CL, Lim SY, Sanisetty S, Whitney JB, et al. Production of Mucosally Transmissible SHIV Challenge Stocks from HIV-1 Circulating Recombinant Form 01_AE env Sequences. PLoS Pathog. 2016;12:e1005431. doi: 10.1371/journal.ppat.1005431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li H, Wang S, Kong R, Ding W, Lee FH, Parker Z, Kim E, Learn GH, Hahn P, Policicchio B, et al. Envelope residue 375 substitutions in simian-human immunodeficiency viruses enhance CD4 binding and replication in rhesus macaques. Proc Natl Acad Sci U S A. 2016;113:E3413–E3422. doi: 10.1073/pnas.1606636113.. Through the introduction of assorted modifications at a site in the HIV-1 gp120 proposed to influence interactions with rhesus CD4, these authors derived replication competent, mucosally-transmissible SHIVs bearing modified Envs from multiple HIV-1 subtypes, suggesting a potential avenue for the generation of “designer” SHIVs bearing specific Envs of interest.
- 5.Lopker MJ, Del Prete GQ, Estes JD, Li H, Reid C, Newman L, Lipkey L, Camus C, Easlick JL, Wang S, et al. Derivation and characterization of pathogenic transmitted/founder molecular clones from SIVsmE660 and SIVmac251 following mucosal infection. J Virol. 2016 doi: 10.1128/JVI.00718-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nonhuman Primate HIV/SIV Vaccine Trials Database. www.hiv.lanl.gov/content/vaccine/home.html.
- 7.Rhesus Genome Assembly: Nov. 2015 (BCM Mmul_8.0.1/rheMac8) https://genome.ucsc.edu/cgi-bin/hgGateway?db=rheMac8.
- 8.Speir ML, Zweig AS, Rosenbloom KR, Raney BJ, Paten B, Nejad P, Lee BT, Learned K, Karolchik D, Hinrichs AS, et al. The UCSC Genome Browser database: 2016 update. Nucleic Acids Res. 2016;44:D717–D725. doi: 10.1093/nar/gkv1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch VM, Lifson JD, et al. Simian immunodeficiency virus infection of monkeys as a model system for the study of AIDS pathogenesis, treatment, and prevention. Adv Pharmacol. 2000;49:437–477. doi: 10.1016/s1054-3589(00)49034-4. [DOI] [PubMed] [Google Scholar]
- 10.Guo J, Zuo T, Cheng L, Wu X, Tang J, Sun C, Feng L, Chen L, Zhang L, Chen Z. Simian immunodeficiency virus infection evades vaccine-elicited antibody responses to V2 region. J Acquir Immune Defic Syndr. 2015;68:502–510. doi: 10.1097/QAI.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 11.Hutnick NA, Myles DJ, Hirao L, Scott VL, Ferraro B, Khan AS, Lewis MG, Miller CJ, Bett AJ, Casimiro D, et al. An optimized SIV DNA vaccine can serve as a boost for Ad5 and provide partial protection from a high-dose SIVmac251 challenge. Vaccine. 2012;30:3202–3208. doi: 10.1016/j.vaccine.2012.02.069. [DOI] [PubMed] [Google Scholar]
- 12.Sun C, Chen Z, Tang X, Zhang Y, Feng L, Du Y, Xiao L, Liu L, Zhu W, Chen L, et al. Mucosal priming with a replicating-vaccinia virus-based vaccine elicits protective immunity to simian immunodeficiency virus challenge in rhesus monkeys. J Virol. 2013;87:5669–5677. doi: 10.1128/JVI.03247-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ling B, Veazey RS, Luckay A, Penedo C, Xu K, Lifson JD, Marx PA. SIV(mac) pathogenesis in rhesus macaques of Chinese and Indian origin compared with primary HIV infections in humans. AIDS. 2002;16:1489–1496. doi: 10.1097/00002030-200207260-00005. [DOI] [PubMed] [Google Scholar]
- 14.Sanders-Beer B, Babas T, Mansfield K, Golightly D, Kramer J, Bowlsbey A, Sites D, Nieves-Duran L, Lin S, Rippeon S, et al. Depo-Provera does not alter disease progression in SIVmac-infected female Chinese rhesus macaques. AIDS Res Hum Retroviruses. 2010;26:433–443. doi: 10.1089/aid.2009.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trichel AM, Rajakumar PA, Murphey-Corb M. Species-specific variation in SIV disease progression between Chinese and Indian subspecies of rhesus macaque. J Med Primatol. 2002;31:171–178. doi: 10.1034/j.1600-0684.2002.02003.x. [DOI] [PubMed] [Google Scholar]
- 16.Marthas ML, Lu D, Penedo MC, Hendrickx AG, Miller CJ. Titration of an SIVmac251 stock by vaginal inoculation of Indian and Chinese origin rhesus macaques: transmission efficiency, viral loads, and antibody responses. AIDS Res Hum Retroviruses. 2001;17:1455–1466. doi: 10.1089/088922201753197123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joag SV, Stephens EB, Adams RJ, Foresman L, Narayan O. Pathogenesis of SIVmac infection in Chinese and Indian rhesus macaques: effects of splenectomy on virus burden. Virology. 1994;200:436–446. doi: 10.1006/viro.1994.1207. [DOI] [PubMed] [Google Scholar]
- 18.Burdo TH, Marcondes MC, Lanigan CM, Penedo MC, Fox HS. Susceptibility of Chinese rhesus monkeys to SIV infection. AIDS. 2005;19:1704–1706. doi: 10.1097/01.aids.0000186823.76230.33. [DOI] [PubMed] [Google Scholar]
- 19.Reimann KA, Parker RA, Seaman MS, Beaudry K, Beddall M, Peterson L, Williams KC, Veazey RS, Montefiori DC, Mascola JR, et al. Pathogenicity of simian-human immunodeficiency virus SHIV-89.6P and SIVmac is attenuated in cynomolgus macaques and associated with early T-lymphocyte responses. J Virol. 2005;79:8878–8885. doi: 10.1128/JVI.79.14.8878-8885.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinohara K, Sakai K, Ando S, Ami Y, Yoshino N, Takahashi E, Someya K, Suzaki Y, Nakasone T, Sasaki Y, et al. A highly pathogenic simian/human immunodeficiency virus with genetic changes in cynomolgus monkey. J Gen Virol. 1999;80(Pt 5):1231–1240. doi: 10.1099/0022-1317-80-5-1231. [DOI] [PubMed] [Google Scholar]
- 21.Kaizu M, Ami Y, Nakasone T, Sasaki Y, Izumi Y, Sato H, Takahashi E, Sakai K, Shinohara K, Nakanishi K, et al. Higher levels of IL-18 circulate during primary infection of monkeys with a pathogenic SHIV than with a nonpathogenic SHIV. Virology. 2003;313:8–12. doi: 10.1016/s0042-6822(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 22.Borsetti A, Baroncelli S, Maggiorella MT, Bellino S, Moretti S, Sernicola L, Belli R, Ridolfi B, Farcomeni S, Negri DR, et al. Viral outcome of simian-human immunodeficiency virus SHIV-89.6P adapted to cynomolgus monkeys. Arch Virol. 2008;153:463–472. doi: 10.1007/s00705-007-0009-2. [DOI] [PubMed] [Google Scholar]
- 23.Krebs KC, Jin Z, Rudersdorf R, Hughes AL, O'Connor DH. Unusually high frequency MHC class I alleles in Mauritian origin cynomolgus macaques. J Immunol. 2005;175:5230–5239. doi: 10.4049/jimmunol.175.8.5230. [DOI] [PubMed] [Google Scholar]
- 24.O'Connor SL, Blasky AJ, Pendley CJ, Becker EA, Wiseman RW, Karl JA, Hughes AL, O'Connor DH. Comprehensive characterization of MHC class II haplotypes in Mauritian cynomolgus macaques. Immunogenetics. 2007;59:449–462. doi: 10.1007/s00251-007-0209-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cain BT, Pham NH, Budde ML, Greene JM, Weinfurter JT, Scarlotta M, Harris M, Chin E, O'Connor SL, Friedrich TC, et al. T cell response specificity and magnitude against SIVmac239 are not concordant in major histocompatibility complex-matched animals. Retrovirology. 2013;10:116. doi: 10.1186/1742-4690-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mohns MS, Greene JM, Cain BT, Pham NH, Gostick E, Price DA, O'Connor DH. Expansion of Simian Immunodeficiency Virus (SIV)-Specific CD8 T Cell Lines from SIV-Naive Mauritian Cynomolgus Macaques for Adoptive Transfer. J Virol. 2015;89:9748–9757. doi: 10.1128/JVI.00993-15.. Potential experimental advantages of limited MHC diversity in MCMs for addressing some questions of interest are nicely demonstrated in this study, in which SIV-specific CD8+ T cell clones could be isolated and expanded from unvaccinated, uninfected MCMs.
- 27.Ferguson D, Mattiuzzo G, Ham C, Stebbings R, Li B, Rose NJ, Mee ET, Smith D, Page M, Cranage MP, et al. Early biodistribution and persistence of a protective live attenuated SIV vaccine elicits localised innate responses in multiple lymphoid tissues. PLoS One. 2014;9:e104390. doi: 10.1371/journal.pone.0104390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blakley GB, Beamer TW, Dukelow WR. Characteristics of the menstrual cycle in nonhuman primates. IV. Timed mating in Macaca nemestrina. Lab Anim. 1981;15:351–353. doi: 10.1258/002367781780953059. [DOI] [PubMed] [Google Scholar]
- 29. Radzio J, Henning T, Jenkins L, Ellis S, Farshy C, Phillips C, Holder A, Kuklenyik S, Dinh C, Hanson D, et al. Combination Emtricitabine and Tenofovir Disoproxil Fumarate Prevents Vaginal Simian/Human Immunodeficiency Virus Infection in Macaques Harboring Chlamydia trachomatis and Trichomonas vaginalis. J Infect Dis. 2016;213:1541–1545. doi: 10.1093/infdis/jiw002.. Study conducted in the PTM/intravaginal challenge model evaluating the impact of inflammatory coinfections on the efficacy of pre-exposure prophylaxis against multiple intravaginal challenges over the course of multiple menstrual cycles.
- 30.Vishwanathan SA, Burgener A, Bosinger SE, Tharp GK, Guenthner PC, Patel NB, Birse K, Hanson DL, Westmacott GR, Henning TR, et al. Cataloguing of Potential HIV Susceptibility Factors during the Menstrual Cycle of Pig-Tailed Macaques by Using a Systems Biology Approach. J Virol. 2015;89:9167–9177. doi: 10.1128/JVI.00263-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radzio J, Spreen W, Yueh YL, Mitchell J, Jenkins L, Garcia-Lerma JG, Heneine W. The long-acting integrase inhibitor GSK744 protects macaques from repeated intravaginal SHIV challenge. Sci Transl Med. 2015;7:270–275. doi: 10.1126/scitranslmed.3010297. [DOI] [PubMed] [Google Scholar]
- 32.Dobard CW, Sharma S, Cong ME, West R, Makarova N, Holder A, Pau CP, Hanson DL, Novembre FJ, Garcia-Lerma JG, et al. Efficacy of topical tenofovir against transmission of a tenofovir-resistant SHIV in macaques. Retrovirology. 2015;12:69. doi: 10.1186/s12977-015-0195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radzio J, Aung W, Holder A, Martin A, Sweeney E, Mitchell J, Bachman S, Pau CP, Heneine W, Garcia-Lerma JG. Prevention of vaginal SHIV transmission in macaques by a coitally-dependent Truvada regimen. PLoS One. 2012;7:e50632. doi: 10.1371/journal.pone.0050632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moss JA, Srinivasan P, Smith TJ, Butkyavichene I, Lopez G, Brooks AA, Martin A, Dinh CT, Smith JM, Baum MM. Pharmacokinetics and preliminary safety study of pod-intravaginal rings delivering antiretroviral combinations for HIV prophylaxis in a macaque model. Antimicrob Agents Chemother. 2014;58:5125–5135. doi: 10.1128/AAC.02871-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kersh EN, Henning T, Vishwanathan SA, Morris M, Butler K, Adams DR, Guenthner P, Srinivasan P, Smith J, Radzio J, et al. SHIV susceptibility changes during the menstrual cycle of pigtail macaques. J Med Primatol. 2014;43:310–316. doi: 10.1111/jmp.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kersh EN, Henning TR, Dobard C, Heneine W, McNicholl JM. Short Communication: Practical Experience with Analysis and Design of Repeat Low-Dose SHIVSF162P3 Exposure Studies in Female Pigtail Macaques with Varying Susceptibility During Menstrual Cycling. AIDS Res Hum Retroviruses. 2015;31:1166–1169. doi: 10.1089/aid.2014.0373.. Retrospective analysis demonstrating the impact of menstrual cycle on intravaginal challenge rates and the critical importance of accounting for timing of intravaginal challenge relative to menstrual cycle phase.
- 37.Kersh EN, Ritter J, Butler K, Ostergaard SD, Hanson D, Ellis S, Zaki S, McNicholl JM. Relationship of Estimated SHIV Acquisition Time Points During the Menstrual Cycle and Thinning of Vaginal Epithelial Layers in Pigtail Macaques. Sex Transm Dis. 2015;42:694–701. doi: 10.1097/OLQ.0000000000000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morris MR, Byrareddy SN, Villinger F, Henning TC, Butler K, Ansari AA, McNicholl JM, Kersh EN. Relationship of menstrual cycle and vaginal infection in female rhesus macaques challenged with repeated, low doses of SIVmac251. J Med Primatol. 2015;44:301–305. doi: 10.1111/jmp.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klatt NR, Harris LD, Vinton CL, Sung H, Briant JA, Tabb B, Morcock D, McGinty JW, Lifson JD, Lafont BA, et al. Compromised gastrointestinal integrity in pigtail macaques is associated with increased microbial translocation, immune activation, and IL-17 production in the absence of SIV infection. Mucosal Immunol. 2010;3:387–398. doi: 10.1038/mi.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brennan G, Kozyrev Y, Hu SL. TRIMCyp expression in Old World primates Macaca nemestrina and Macaca fascicularis. Proc Natl Acad Sci U S A. 2008;105:3569–3574. doi: 10.1073/pnas.0709511105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newman RM, Hall L, Kirmaier A, Pozzi LA, Pery E, Farzan M, O'Neil SP, Johnson W. Evolution of a TRIM5-CypA splice isoform in old world monkeys. PLoS Pathog. 2008;4:e1000003. doi: 10.1371/journal.ppat.1000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson SJ, Webb BL, Ylinen LM, Verschoor E, Heeney JL, Towers GJ. Independent evolution of an antiviral TRIMCyp in rhesus macaques. Proc Natl Acad Sci U S A. 2008;105:3557–3562. doi: 10.1073/pnas.0709003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Virgen CA, Kratovac Z, Bieniasz PD, Hatziioannou T. Independent genesis of chimeric TRIM5-cyclophilin proteins in two primate species. Proc Natl Acad Sci U S A. 2008;105:3563–3568. doi: 10.1073/pnas.0709258105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hatziioannou T, Del Prete GQ, Keele BF, Estes JD, McNatt MW, Bitzegeio J, Raymond A, Rodriguez A, Schmidt F, Mac Trubey C, et al. HIV-1-induced AIDS in monkeys. Science. 2014;344:1401–1405. doi: 10.1126/science.1250761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thippeshappa R, Ruan H, Wang W, Zhou P, Kimata JT. A variant macaque-tropic human immunodeficiency virus type 1 is resistant to alpha interferon-induced restriction in pig-tailed macaque CD4+ T cells. J Virol. 2013;87:6678–6692. doi: 10.1128/JVI.00338-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daniel MD, Kirchhoff F, Czajak SC, Sehgal PK, Desrosiers RC. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 47.Marthas ML, Sutjipto S, Higgins J, Lohman B, Torten J, Luciw PA, Marx PA, Pedersen NC. Immunization with a live, attenuated simian immunodeficiency virus (SIV) prevents early disease but not infection in rhesus macaques challenged with pathogenic SIV. J Virol. 1990;64:3694–3700. doi: 10.1128/jvi.64.8.3694-3700.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lopker M, Easlick J, Sterrett S, Decker JM, Barbian H, Learn G, Keele BF, Robinson JE, Li H, Hahn BH, et al. Heterogeneity in neutralization sensitivities of viruses comprising the simian immunodeficiency virus SIVsmE660 isolate and vaccine challenge stock. J Virol. 2013;87:5477–5492. doi: 10.1128/JVI.03419-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee FH, Mason R, Welles H, Learn GH, Keele BF, Roederer M, Bar KJ. Breakthrough Virus Neutralization Resistance as a Correlate of Protection in a Nonhuman Primate Heterologous Simian Immunodeficiency Virus Vaccine Challenge Study. J Virol. 2015;89:12388–12400. doi: 10.1128/JVI.01531-15.. Follow up study to reference 78, phenotypically evaluating the neutralization profile of breakthrough viruses transmitted to vaccinated animals, confirming and extending the proposed sieve effect
- 50.Manrique J, Piatak M, Lauer W, Johnson W, Mansfield K, Lifson J, Desrosiers R. Influence of mismatch of Env sequences on vaccine protection by live attenuated simian immunodeficiency virus. J Virol. 2013;87:7246–7254. doi: 10.1128/JVI.00798-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shedlock DJ, Silvestri G, Weiner DB. Monkeying around with HIV vaccines: using rhesus macaques to define 'gatekeepers' for clinical trials. Nat Rev Immunol. 2009;9:717–728. doi: 10.1038/nri2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burns DP, Collignon C, Desrosiers RC. Simian immunodeficiency virus mutants resistant to serum neutralization arise during persistent infection of rhesus monkeys. J Virol. 1993;67:4104–4113. doi: 10.1128/jvi.67.7.4104-4113.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Means RE, Greenough T, Desrosiers RC. Neutralization sensitivity of cell culture-passaged simian immunodeficiency virus. J Virol. 1997;71:7895–7902. doi: 10.1128/jvi.71.10.7895-7902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mason RD, Welles HC, Adams C, Chakrabarti BK, Gorman J, Zhou T, Nguyen R, O'Dell S, Lusvarghi S, Bewley CA, et al. Targeted Isolation of Antibodies Directed against Major Sites of SIV Env Vulnerability. PLoS Pathog. 2016;12:e1005537. doi: 10.1371/journal.ppat.1005537.. Seminal study that filled a longstanding need within the SIV research field for well-characterized macaque monoclonal antibodies targeting defined SIV Env epitopes analogous to epitopes targeted by many of the best-defined human neutralizing antibodies targeting HIV Env.
- 55.de Groot NG, Heijmans CM, Koopman G, Verschoor EJ, Bogers WM, Bontrop RE. TRIM5 allelic polymorphism in macaque species/populations of different geographic origins: its impact on SIV vaccine studies. Tissue Antigens. 2011;78:256–262. doi: 10.1111/j.1399-0039.2011.01768.x. [DOI] [PubMed] [Google Scholar]
- 56.Kirmaier A, Wu F, Newman RM, Hall LR, Morgan JS, O'Connor S, Marx PA, Meythaler M, Goldstein S, Buckler-White A, et al. TRIM5 suppresses cross-species transmission of a primate immunodeficiency virus and selects for emergence of resistant variants in the new species. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reynolds MR, Sacha JB, Weiler AM, Borchardt GJ, Glidden CE, Sheppard NC, Norante FA, Castrovinci PA, Harris JJ, Robertson HT, et al. The TRIM5{alpha} genotype of rhesus macaques affects acquisition of simian immunodeficiency virus SIVsmE660 infection after repeated limiting-dose intrarectal challenge. J Virol. 2011;85:9637–9640. doi: 10.1128/JVI.05074-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yeh WW, Rao SS, Lim SY, Zhang J, Hraber PT, Brassard LM, Luedemann C, Todd JP, Dodson A, Shen L, et al. The TRIM5 gene modulates penile mucosal acquisition of simian immunodeficiency virus in rhesus monkeys. J Virol. 2011;85:10389–10398. doi: 10.1128/JVI.00854-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Letvin NL, Rao SS, Montefiori DC, Seaman MS, Sun Y, Lim SY, Yeh WW, Asmal M, Gelman RS, Shen L, et al. Immune and Genetic Correlates of Vaccine Protection Against Mucosal Infection by SIV in Monkeys. Sci Transl Med. 2011;3:81–36. doi: 10.1126/scitranslmed.3002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheppard NC, Jones RB, Burwitz BJ, Nimityongskul FA, Newman LP, Buechler MB, Reed JS, Piaskowski SM, Weisgrau KL, Castrovinci PA, et al. Vaccination against endogenous retrotransposable element consensus sequences does not protect rhesus macaques from SIVsmE660 infection and replication. PLoS One. 2014;9:e92012. doi: 10.1371/journal.pone.0092012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tan RC, Harouse JM, Gettie A, Cheng-Mayer C. In vivo adaptation of SHIV(SF162): chimeric virus expressing a NSI, CCR5-specific envelope protein. J Med Primatol. 1999;28:164–168. doi: 10.1111/j.1600-0684.1999.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 62.Nishimura Y, Shingai M, Willey R, Sadjadpour R, Lee WR, Brown CR, Brenchley JM, Buckler-White A, Petros R, Eckhaus M, et al. Generation of the pathogenic R5-tropic simian/human immunodeficiency virus SHIVAD8 by serial passaging in rhesus macaques. J Virol. 2010;84:4769–4781. doi: 10.1128/JVI.02279-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Barouch DH, Alter G, Broge T, Linde C, Ackerman ME, Brown EP, Borducchi EN, Smith KM, Nkolola JP, Liu J, et al. Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science. 2015;349:320–324. doi: 10.1126/science.aab3886.. Study evaluating the efficacy of an adenovirus-26 prime, Env protein boost vaccination strategy in two different repeated, limiting dose intrarectal heterologous challenge models, one involving SIVmac251 challenge and another involving SHIV-SF162P3 challenge.
- 64. Gardner MR, Kattenhorn LM, Kondur HR, von Schaewen M, Dorfman T, Chiang JJ, Haworth KG, Decker JM, Alpert MD, Bailey CC, et al. AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature. 2015;519:87–91. doi: 10.1038/nature14264.. Demonstrates prevention of SHIV-AD8 acquisition by a chimeric CD4-based protein stably expressed by an adeno-associated virus in a repeated, dose-escalation intravenous challenge study design in RMs.
- 65. Gautam R, Nishimura Y, Pegu A, Nason MC, Klein F, Gazumyan A, Golijanin J, Buckler-White A, Sadjadpour R, Wang K, et al. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature. 2016;533:105–109. doi: 10.1038/nature17677.. Utilizing repeated, limiting dose intrarectal SHIV-AD8 challenges, this study demonstrates remarkable durability of acquisition prevention effects afforded by single passive infusions of several different broadly neutralizing anti-HIV antibodies, particularly for those animals with slower plasma antibody decay kinetics.
- 66.Hessell AJ, Jaworski JP, Epson E, Matsuda K, Pandey S, Kahl C, Reed J, Sutton WF, Hammond KB, Cheever TA, et al. Early short-term treatment with neutralizing human monoclonal antibodies halts SHIV infection in infant macaques. Nat Med. 2016;22:362–368. doi: 10.1038/nm.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lakhashe SK, Byrareddy SN, Zhou M, Bachler BC, Hemashettar G, Hu SL, Villinger F, Else JG, Stock S, Lee SJ, et al. Multimodality vaccination against clade C SHIV: partial protection against mucosal challenges with a heterologous tier 2 virus. Vaccine. 2014;32:6527–6536. doi: 10.1016/j.vaccine.2014.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Moldt B, Le KM, Carnathan DG, Whitney JB, Schultz N, Lewis MG, Borducchi EN, Smith KM, Mackel JJ, Sweat SL, et al. Neutralizing antibody affords comparable protection against vaginal and rectal simian/human immunodeficiency virus challenge in macaques. AIDS. 2016;30:1543–1551. doi: 10.1097/QAD.0000000000001102.. Comparative examination of the capacity of passive immunization with a broadly neutralizing anti-HIV-1 antibody to protect against a single high dose SHIV-SF162P3 challenge via the intrarectal and intravaginal routes, with animals Depo-Provera treated, showing similar protective efficacy irrespective of route.
- 69.Sholukh AM, Byrareddy SN, Shanmuganathan V, Hemashettar G, Lakhashe SK, Rasmussen RA, Watkins JD, Vyas HK, Thorat S, Brandstoetter T, et al. Passive immunization of macaques with polyclonal anti-SHIV IgG against a heterologous tier 2 SHIV: outcome depends on IgG dose. Retrovirology. 2014;11:8. doi: 10.1186/1742-4690-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sholukh AM, Watkins JD, Vyas HK, Gupta S, Lakhashe SK, Thorat S, Zhou M, Hemashettar G, Bachler BC, Forthal DN, et al. Defense-in-depth by mucosally administered anti-HIV dimeric IgA2 and systemic IgG1 mAbs: complete protection of rhesus monkeys from mucosal SHIV challenge. Vaccine. 2015;33:2086–2095. doi: 10.1016/j.vaccine.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thippeshappa R, Tian B, Cleveland B, Guo W, Polacino P, Hu SL. Oral Immunization with Recombinant Vaccinia Virus Prime and Intramuscular Protein Boost Provides Protection against Intrarectal Simian-Human Immunodeficiency Virus Challenge in Macaques. Clin Vaccine Immunol. 2015;23:204–212. doi: 10.1128/CVI.00597-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Del Prete GQ, Ailers B, Moldt B, Keele BF, Estes JD, Rodriguez A, Sampias M, Oswald K, Fast R, Trubey CM, et al. Selection of unadapted, pathogenic SHIVs encoding newly transmitted HIV-1 envelope proteins. Cell Host Microbe. 2014;16:412–418. doi: 10.1016/j.chom.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Boyd DF, Peterson D, Haggarty BS, Jordan AP, Hogan MJ, Goo L, Hoxie JA, Overbaugh J. Mutations in HIV-1 envelope that enhance entry with the macaque CD4 receptor alter antibody recognition by disrupting quaternary interactions within the trimer. J Virol. 2015;89:894–907. doi: 10.1128/JVI.02680-14.. Shows that a specific set of adaptive changes that improve rhesus CD4 binding acquired in HIV-1 Envs during in vitro culture passage on macaque cells alter Env structure and antibody binding, highlighting the potential value in identifying SHIVs with Envs that need not be adapted for use in NHPs and the importance of carefully characterizing new viruses.
- 74.Abrahams MR, Anderson JA, Giorgi EE, Seoighe C, Mlisana K, Ping LH, Athreya GS, Treurnicht FK, Keele BF, Wood N, et al. Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-poisson distribution of transmitted variants. J Virol. 2009;83:3556–3567. doi: 10.1128/JVI.02132-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li H, Bar KJ, Wang S, Decker JM, Chen Y, Sun C, Salazar-Gonzalez JF, Salazar MG, Learn GH, Morgan CJ, et al. High Multiplicity Infection by HIV-1 in Men Who Have Sex with Men. PLoS Pathog. 2010;6:e1000890. doi: 10.1371/journal.ppat.1000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Santra S, Tomaras GD, Warrier R, Nicely NI, Liao HX, Pollara J, Liu P, Alam SM, Zhang R, Cocklin SL, et al. Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques. PLoS Pathog. 2015;11:e1005042. doi: 10.1371/journal.ppat.1005042.. Illustrative of the added power and biological insight afforded by quantification of unique transmitted/founder variants by demonstrating that while two non-neutralizing anti-HIV-1 antibodies did not alter acquisition rates, they did induce a significant reduction in the number of transmitted variants in a single high dose intrarectal SHIV-BAL challenge model.
- 78. Roederer M, Keele BF, Schmidt SD, Mason RD, Welles HC, Fischer W, Labranche C, Foulds KE, Louder MK, Yang ZY, et al. Immunological and virological mechanisms of vaccine-mediated protection against SIV and HIV. Nature. 2014;505:502–508. doi: 10.1038/nature12893.. Comprehensive repeated limiting-dose intrarectal heterologous SIV challenge study demonstrating significant DNA-prime/adenovirus boost vaccine effects in both the rates of acquisition and in number of unique transmitted/variants, with key biological insights revealed by sequence-based “sieve” analysis.
- 79.Vaccari M, Keele BF, Bosinger SE, Doster MN, Ma ZM, Pollara J, Hryniewicz A, Ferrari G, Guan Y, Forthal DN, et al. Protection afforded by an HIV vaccine candidate in macaques depends on the dose of SIVmac251 at challenge exposure. J Virol. 2013;87:3538–3548. doi: 10.1128/JVI.02863-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu J, Keele BF, Li H, Keating S, Norris PJ, Carville A, Mansfield KG, Tomaras GD, Haynes BF, Kolodkin-Gal D, et al. Low-dose mucosal simian immunodeficiency virus infection restricts early replication kinetics and transmitted virus variants in rhesus monkeys. J Virol. 2010;84:10406–10412. doi: 10.1128/JVI.01155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Keele BF, Li H, Learn GH, Hraber P, Giorgi EE, Grayson T, Sun C, Chen Y, Yeh WW, Letvin NL, et al. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J Exp Med. 2009;206:1117–1134. doi: 10.1084/jem.20082831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma ZM, Keele BF, Qureshi H, Stone M, Desilva V, Fritts L, Lifson JD, Miller CJ. SIVmac251 is inefficiently transmitted to rhesus macaques by penile inoculation with a single SIVenv variant found in ramp-up phase plasma. AIDS Res Hum Retroviruses. 2011;27:1259–1269. doi: 10.1089/aid.2011.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Del Prete GQ, Scarlotta M, Newman L, Reid C, Parodi LM, Roser JD, Oswald K, Marx PA, Miller CJ, Desrosiers RC, et al. Comparative characterization of transfection- and infection-derived simian immunodeficiency virus challenge stocks for in vivo nonhuman primate studies. J Virol. 2013;87:4584–4595. doi: 10.1128/JVI.03507-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Louder MK, Sambor A, Chertova E, Hunte T, Barrett S, Ojong F, Sanders-Buell E, Zolla-Pazner S, McCutchan FE, Roser JD, et al. HIV-1 envelope pseudotyped viral vectors and infectious molecular clones expressing the same envelope glycoprotein have a similar neutralization phenotype, but culture in peripheral blood mononuclear cells is associated with decreased neutralization sensitivity. Virology. 2005;339:226–238. doi: 10.1016/j.virol.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 85.Provine NM, Cortez V, Chohan V, Overbaugh J. The neutralization sensitivity of viruses representing human immunodeficiency virus type 1 variants of diverse subtypes from early in infection is dependent on producer cell, as well as characteristics of the specific antibody and envelope variant. Virology. 2012;427:25–33. doi: 10.1016/j.virol.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Provine NM, Puryear WB, Wu X, Overbaugh J, Haigwood NL. The infectious molecular clone and pseudotyped virus models of human immunodeficiency virus type 1 exhibit significant differences in virion composition with only moderate differences in infectivity and inhibition sensitivity. J Virol. 2009;83:9002–9007. doi: 10.1128/JVI.00423-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Willey R, Nason MC, Nishimura Y, Follmann DA, Martin MA. Neutralizing antibody titers conferring protection to macaques from a simian/human immunodeficiency virus challenge using the TZM-bl assay. AIDS Res Hum Retroviruses. 2010;26:89–98. doi: 10.1089/aid.2009.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Del Prete GQ, Park H, Fennessey CM, Reid C, Lipkey L, Newman L, Oswald K, Kahl C, Piatak M, Jr, Quinones OA, et al. Molecularly tagged simian immunodeficiency virus SIVmac239 synthetic swarm for tracking independent infection events. J Virol. 2014;88:8077–8090. doi: 10.1128/JVI.01026-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bernard-Stoecklin S, Gommet C, Cavarelli M, Le Grand R. Nonhuman primate models for cell-associated simian immunodeficiency virus transmission: the need to better understand the complexity of HIV mucosal transmission. J Infect Dis. 2014;210(Suppl 3):S660–S666. doi: 10.1093/infdis/jiu536.. Review article presenting a case for the development of NHP models of AIDS virus transmission through the transmission of infected cells, rather than only cell-free virus.
- 90.Smedley J, Turkbey B, Bernardo ML, Del Prete GQ, Estes JD, Griffiths GL, Kobayashi H, Choyke PL, Lifson JD, Keele BF. Tracking the luminal exposure and lymphatic drainage pathways of intravaginal and intrarectal inocula used in nonhuman primate models of HIV transmission. PLoS One. 2014;9:e92830. doi: 10.1371/journal.pone.0092830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stone M, Keele BF, Ma ZM, Bailes E, Dutra J, Hahn BH, Shaw GM, Miller CJ. A limited number of simian immunodeficiency virus (SIV) env variants are transmitted to rhesus macaques vaginally inoculated with SIVmac251. J Virol. 2010;84:7083–7095. doi: 10.1128/JVI.00481-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Qureshi H, Ma ZM, Huang Y, Hodge G, Thomas MA, DiPasquale J, DeSilva V, Fritts L, Bett AJ, Casimiro DR, et al. Low-dose penile SIVmac251 exposure of rhesus macaques infected with adenovirus type 5 (Ad5) and then immunized with a replication-defective Ad5-based SIV gag/pol/nef vaccine recapitulates the results of the phase IIb step trial of a similar HIV-1 vaccine. J Virol. 2012;86:2239–2250. doi: 10.1128/JVI.06175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pegu A, Yang ZY, Boyington JC, Wu L, Ko SY, Schmidt SD, McKee K, Kong WP, Shi W, Chen X, et al. Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci Transl Med. 2014;6:243–288. doi: 10.1126/scitranslmed.3008992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Andrews CD, Yueh YL, Spreen WR, St Bernard L, Boente-Carrera M, Rodriguez K, Gettie A, Russell-Lodrigue K, Blanchard J, Ford S, et al. A long-acting integrase inhibitor protects female macaques from repeated high-dose intravaginal SHIV challenge. Sci Transl Med. 2015;7:270–274. doi: 10.1126/scitranslmed.3010298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marx PA, Spira AI, Gettie A, Dailey PJ, Veazey RS, Lackner AA, Mahoney CJ, Miller CJ, Claypool LE, Ho DD, et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2:1084–1089. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 96.Vishwanathan SA, Guenthner PC, Lin CY, Dobard C, Sharma S, Adams DR, Otten RA, Heneine W, Hendry RM, McNicholl JM, et al. High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J Acquir Immune Defic Syndr. 2011;57:261–264. doi: 10.1097/QAI.0b013e318220ebd3. [DOI] [PubMed] [Google Scholar]
- 97.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]