Abstract
HIV disease progression appears to be driven by high levels of immune activation. Given observations that fetal exposure to infectious pathogens in utero can result in reduced immune responses, or tolerance, to those pathogens postnatally, we hypothesized that fetal exposure to HIV may tolerize the fetus to the virus, thus reducing damage caused by immune activation if infected later in life. To test this hypothesis, fetal rhesus macaques (Macaca mulatta) were injected with the attenuated virus SIVmac1A11 in utero and then challenged with pathogenic SIVmac239 one year after birth. SIVmac1A11-injected animals had significantly reduced plasma RNA viral loads (p<0.02) up to 35 weeks post-infection. Generalized estimating equations (GEE) analysis was performed to identify immunologic and clinical measurements significantly associated with plasma RNA viral load. A positive association was observed with the proportion of CD8+ T cells expressing the transcription factor, FoxP3, and the proportion of CD4+ T cells producing the lymphoproliferative cytokine, IL-2, while an inverse relationship was found with the levels of circulating CD4+ and CD8+ T cells expressing intermediate levels of the proliferation marker, Ki-67. Further analyses demonstrated that animals exposed to SIV in utero appeared to have enhanced SIV-specific cellular immune responses, expressed lower levels of the exhaustion marker, PD-1, on CD8+ T cells, and had more circulating Th17 cells when compared to controls. While the development of tolerance was not demonstrated, these data suggest that rhesus monkeys exposed to SIVmac1A11 in utero had distinct immune responses associated with the control of viral replication after postnatal challenge.
One Sentence Summary
Animals exposed to attenuated SIV in utero had reduced levels of viral replication and altered immune responses after pathogenic challenge postnatally.
Introduction
Vaccines are traditionally designed to induce neutralizing antibodies and/or cytotoxic T cells that can specifically recognize and destroy a given target, e.g., an infectious agent. While highly successful against many acute infectious agents, this approach has not only failed to protect against HIV but has in some instances been associated with more infections, not fewer (1). Unlike other acute infectious agents, HIV persists and results in progressive disease in the setting of an activated immune system, one that is associated with altered homeostasis of CD4+ T cells (2,3), and with the elicitation of pro-inflammatory cytokines (4,5). The fact that viral replication is necessary but not sufficient for disease progression has been revealed by examination of nonhuman primate responses to infection by simian immunodeficiency virus (SIV). Thus, sooty mangabeys (SM) and African green monkeys (AGM), species that are naturally infected with SIV, exhibit high viral loads but manifest only low levels of chronic inflammation and incur few if any clinical complications post-infection, whereas rhesus and pigtailed macaques, species not naturally infected with SIV in the wild, have equally high viral loads and display high levels of persistent pathological inflammation that are accompanied by disease progression (6–10). Since traditional vaccines generally induce activation of the immune system (11), they may paradoxically favor viral replication and spread (12). If this is the case, then protection against HIV may best be achieved by an immune response that is wholly different from those normally induced by traditional vaccines.
An alternative approach to prevent the replication and spread of HIV in vivo would be to create a vaccine that instead suppresses an immunoreactive response against the virus, e.g., one that generates tolerance in an antigen-specific manner. If such a response were to dampen the rate of viral spread, then the virus might instead be cleared by the normal processes of cell turnover (13). Since lentiviral infection appears to target a number of CD4+ T cell subpopulations, including long-lived memory CD4+ T cells (14,15), prevention of inflammation during the initial stages of infection may reduce the spread of virus to such cells and create a pool of infected cells that can be cleared instead by natural homeostatic mechanisms. Such a dynamic might, indeed, account for the comparatively rapid loss of viral reservoirs found in babies and in some adults treated shortly after infection (16,17).
Several routes of immunization have been historically associated with the induction of tolerance, including the administration of low or high doses of antigen in the absence of co-stimulation, the oral administration of antigen, and exposure to antigen in utero (18–21). Of note, the latter route is one that occurs as a matter of course during gestation of the human fetus in an untreated, HIV-infected mother. Since more that 50% of those around the world who are infected by HIV are women of childbearing age (who, unfortunately, are often not on suppressive antiretroviral therapy during the course of pregnancy), such exposures are also quite common. Yet, remarkably, only about 5–10% of babies born to such mothers are found to have been infected in utero (22,23).
We wondered whether the apparent protection of the human fetus from HIV infection might be related to the fact that the human fetal immune system is more likely to generate a tolerogenic, as opposed to an immunoreactive, response to exogenous antigen. In previous studies, we have shown that the T and myeloid lineages in utero are derived from a hematopoietic progenitor cell that is distinct from that found in the adult (24). When stimulated, fetal naïve CD4+ T cells are polarized towards a FoxP3+ T cell lineage with immunosuppressive properties (25) while fetal CD4+CD14+CD16− monocytes are relatively deficient in their ability to up-regulate surface molecules necessary for antigen presentation and delivery of co-stimulatory signals (26). We have also shown that in utero development of the nonhuman primate (rhesus macaque) immune system has many parallels to that of the human (27). A previous report in which fetal macaques were injected with nonpathogenic SIVmac1A11 in utero also found that exposed animals were better able to control viral replication when challenged with pathogenic virus at one year of age, although the mechanism of protection was unclear (28). We have now extended these studies to evaluate a larger array of immune parameters and to specifically address the possibility that inoculation of nonpathogenic SIV into the developing rhesus macaque fetus might generate a tolerogenic immune response, one that protects against aberrant immune activation and disease progression after postnatal challenge. Our results suggest that in utero exposure to SIV has important effects on the development of the fetal immune system that are associated with, and possibly causal of, partial viral control upon subsequent challenge.
Results
Experimental design
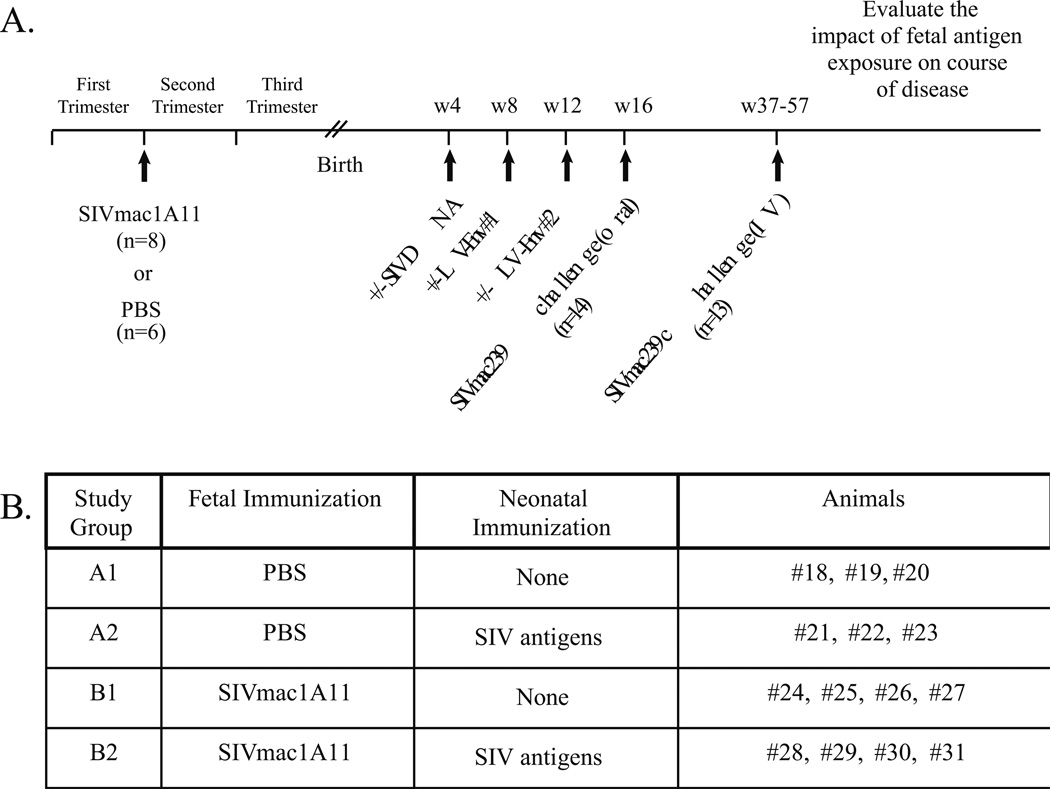
To determine whether exposure to SIV antigens in utero might have an impact on the immune response to pathogenic SIV challenge after birth, six fetal monkeys (Group A) served as sham controls and eight fetal monkeys (Group B) were injected with a nonpathogenic molecular SIV clone, SIVmac1A11, under ultrasound guidance using established methods (Fig. 1A). To specifically explore the hypothesis that prenatal exposure to SIV might induce tolerance to SIV antigens, either because of clonal deletion, the induction of antigen-specific regulatory cells, and/or the generation of antigen-specific anergy, half of the animals in each group were exposed intramuscularly (IM) to a DNA plasmid encoding SIV Env at four weeks after birth, followed by IM injections of a lentivirus (LV) expressing SIV Env at 8 and 12 weeks after birth (LV-Env) (Fig. 1B). All of the monkeys were then orally challenged (at postnatal week 16) with pathogenic SIVmac239 (105 TCID50). When only one of the 14 monkeys was found to be productively infected (even after repeated oral challenges), the remaining 13 monkeys (including six in the control Group A and seven in the experimental Group B) were challenged intravenously (IV) with SIVmac239 (100 TCID50) at an age range of 37–57 weeks. In addition to monitoring viral loads and CD4+ T cell counts, measures of immune phenotype and function were collected over time.
Fig. 1. Study Design.
(A) Timeline of immunizations and virus challenges. Fetal macaques were injected with SIVmac1A11 or PBS in the late first trimester (55±5 days gestation; term 165±10 days). Animals were then delivered by cesarean section at full term and immunized with either PBS or SIV antigens as neonates. At 16 weeks postnatal age, each animal was orally challenged with SIVmac239. Animals that did not demonstrate productive infection were then challenged intravenously between 37 and 57 weeks of age with SIVmac239 and followed for disease progression. (B) Summary of study groups.
Exposure to SIV in utero is associated with lower viral load after challenge
To assess the relative impact of in utero administration of SIVmac1A11 or postnatal immunization on peak and post-peak viral loads, the treatment effects were modeled separately and together using generalized estimating equations (GEE). In utero treatment was significantly associated with lower viral load in all models while postnatal immunization was associated with a trend to higher viral load. No significant interaction between pre- and postnatal treatments was detected, but the study was underpowered to detect such an effect. Given the stronger effect of the in utero treatment on viral load, the remainder of the analyses focused on differences observed between in utero treatment groups.
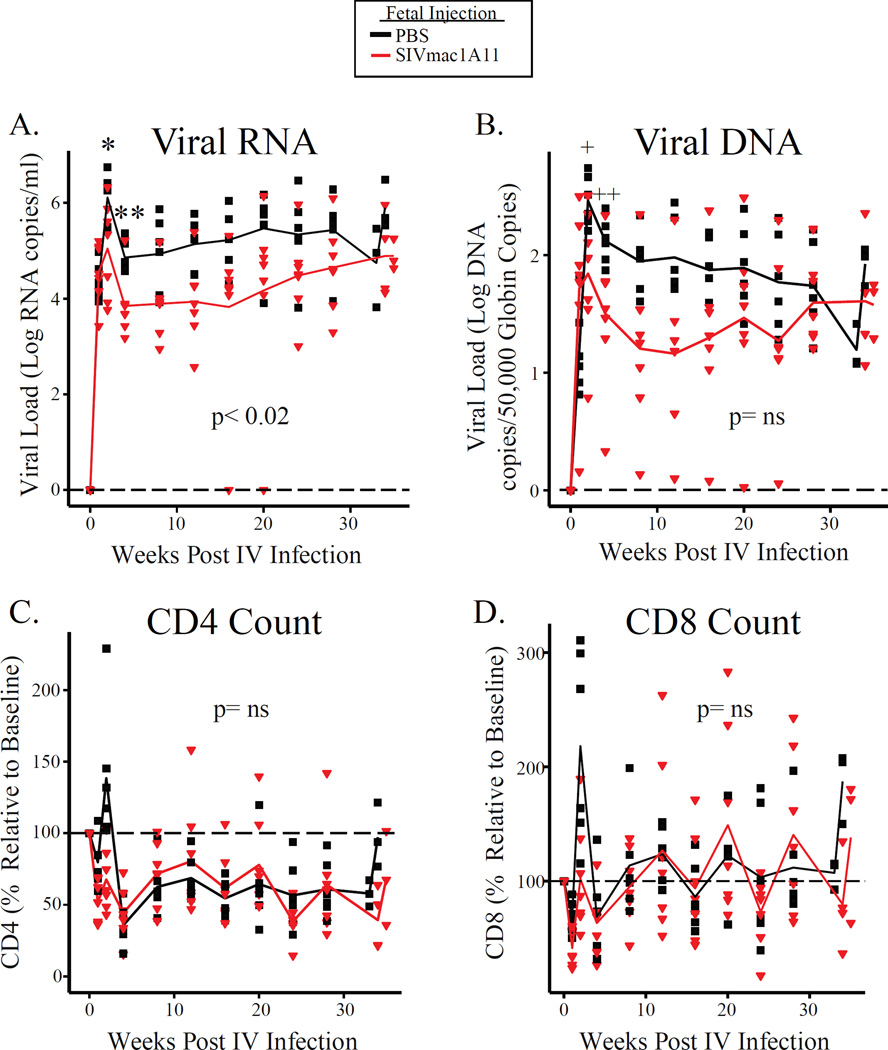
At 37–57 weeks of age, six monkeys in the control Group A and seven monkeys in the experimental Group B were successfully challenged by the IV route. Thereafter, viral loads in the two groups were found to diverge (Fig. 2A) with those exposed to SIVmac1A11 in utero having lower peak plasma RNA viral loads (p<0.04) at two weeks post-infection, lower plasma RNA viral load at four weeks post-infection (p<0.006), and significantly lower plasma RNA viral load over the remainder of the study (p<0.02) (Fig. 2A). Similar reductions in viral loads were also noted for peripheral blood mononuclear cell (PBMC)-associated viral DNA (p=0.03 at peak and p<0.04 at week 4); this difference, however, was not significant over all time points assessed (Fig. 2B). Notably, one monkey that had been exposed to SIVmac1A11 in utero was able to control viral replication to undetectable levels between weeks 16–20 post-challenge (Fig. 2A). Finally, changes in the circulating CD4+ T and CD8+ T cell counts were not different between groups during the course of infection (Fig. 2C–D).
Fig. 2. Animals exposed to SIVmac1A11 in utero have reduced peak and set point viral loads post-infection.
(A) Plasma RNA and (B) cell-associated DNA viral loads over the course of infection. *p<0.04, **p<0.006, +p=0.03, ++p<0.04. P-values for a given time point were calculated using t-tests. (C and D) The number of CD4+ T cells (C) and CD8+ T cells (D) over the course of infection. Y-axes represent the CD4 or CD8 count over the course of infection as a percentage of the CD4 or CD8 count on the day of infection. Longitudinal analyses were calculated with regression using generalized estimating equations (GEE) techniques.
No evidence of immune tolerance
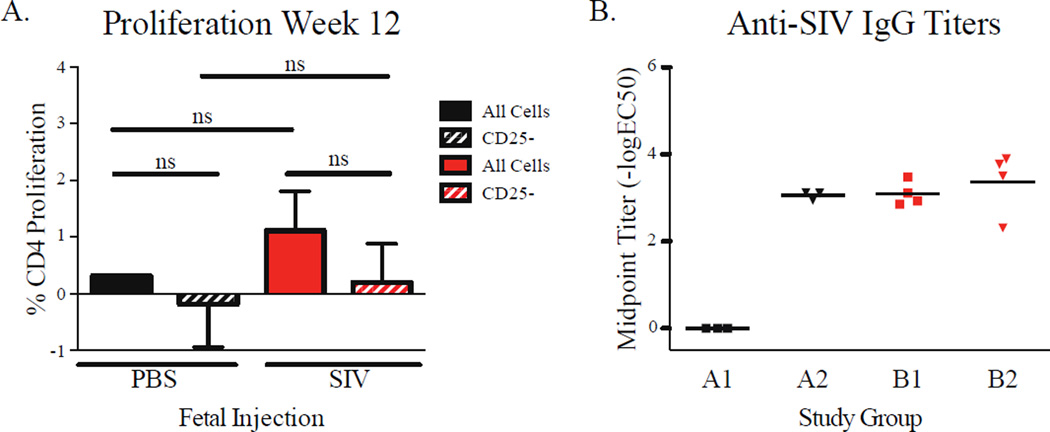
To determine whether exposure to SIVmac1A11 in utero generated a state of tolerance to SIV, animals in Groups A and B were immunized IM with either PBS (Groups A1 and B1) or with SIV DNA at week 4 and LV-Env (Groups A2 and B2) at weeks 8 and 12. At week 12, PBMCs from each animal were stimulated in vitro with aldrithiol-2 inactivated SIV (AT-2 SIV). Proliferative responses of PBMCs, as measured by dilution of CFSE, were found to be small, variable, and indistinguishable between the groups, irrespective of exposure to SIV antigens in utero or postnatally, and whether in the presence or absence of CD25+ Tregs (Fig. 3A). Similar results were obtained when CD4+ and CD8+ T cell cytokine responses (including production of IFNγ, TNFα, IL-2, IL-4, and IL-17) were examined. Upon measuring anti-SIV IgG antibodies in plasma (at week 9), monkeys in the control Group (A1) that had not been immunized with LV-Env after birth showed no detectable circulating IgG specific for AT-2 SIV whereas monkeys in each of the other three groups (A2, B1, and B2) showed levels of circulating SIV-specific IgG that were both detectable and equivalent (Fig. 3B). These observations suggest that exposure to SIVmac1A11 in utero induced an IgG response that was detectable in the 9-week-old infant, and that was not associated with intrinsic or extrinsic (e.g., CD25+ Treg-mediated) T cell tolerance, at least as measured using these assays under the conditions described.
Fig. 3. Fetal exposure to SIVmac1A11 followed by neonatal vaccination elicited humoral but not cellular responses prior to oral challenge.
(A) Proliferation of CD4+ T cells in total PBMCs from 12-week-old rhesus monkeys, labeled with CFSE and incubated with AT-2 SIV for 6 days. (B) IgG titers against AT-2 SIV in plasma from 9-week-old animals. Differences were assessed using t-tests with significance defined as p< 0.05.
Immunologic measures associated with viral load
Measures of immune phenotype and function (including those of cell activation and proliferation as well as of cytokine production by different T cell subsets) were studied as a function of time after challenge with SIVmac239. To explore which of these parameters might be associated with the observed changes in viral load between the two groups after SIVmac239 challenge, GEE were used to identify significant associations with viral load. As most of the divergence in viral load between the groups occurred after the set point (4 weeks post-infection), we restricted our analyses to between 4 and 35 weeks post-infection. Given the small number of animals in this study and the fact that these analyses were exploratory, multivariate models of measured parameters and multiple comparison corrections were not included. Of 192 measures that were analyzed, 15 were found to be associated with viral load. These measurements fell into four main categories (Table 1):
Table 1. Plasma RNA viral loads are associated with various immunologic parameters.
A panel of 192 immunologic and clinical measurements, measured at multiple time points post-infection, was analyzed for associations with log plasma RNA viral load by GEE. Fifteen parameters were identified as being significantly associated with viral load (p<0.05).
| Log RNA | Viral Load | |
|---|---|---|
| Parameter | p-value | Estimate |
| CD8_CM_CD127low_FoxP3+ | 0.0419 | 0.1003 |
| CD8_CM_Ki-67hi_FoxP3+ | 0.0029 | 0.2764 |
| CD8_Ki-67hi_FoxP3+ | 0.0030 | 1.7308 |
| CD8_Naive_Ki-67hi_FoxP3+ | 0.0096 | 7.8656 |
| CD4:CD8 | 0.0475 | −0.4095 |
| Monocytes | 0.0230 | 0.0002 |
| ALT | 0.0097 | 0.0041 |
| Cholesterol | 0.0301 | 0.0091 |
| CD4_IL2+ | 0.0481 | 0.0137 |
| CD4_EM_Ki-67+ | 0.0166 | −0.0052 |
| CD4_EM_Ki-67int | 0.0005 | −0.0155 |
| CD4_Ki-67int | 0.0001 | −0.0588 |
| CD8_CM_Ki-67int | 0.0135 | −0.0272 |
| CD8_EM_Ki-67+ | 2.03E-05 | −0.0082 |
| CD8_EM_Ki-67int | 0.0372 | −0.0308 |
CD8+ T cell subsets expressing FoxP3
While the GEE analysis did not find an association between the frequency of total CD4+ Tregs and viral load (see also Fig. S1), it did identify positive associations between plasma viral load and CD8+ T cells with a central memory phenotype (both CD95+CD28+ and CD45RA−CD27+) expressing the transcription factor FoxP3 and low levels of the IL-7 receptor, CD127 (29). A similar pattern was found for total, naïve (both CD95−CD28+ and CD45RA+CD27+), and central memory CD8+ T cells that expressed FoxP3 and high levels of the proliferation marker, Ki-67. None of these measurements were significantly different between groups (Fig. S2).
Clinical measurements associated with viral load but not different between groups
The only clinical measurements that were identified by our GEE analysis to be associated with viral load were the ratio of CD4+ to CD8+ T cells, the number of circulating monocytes, the levels of alanine aminotransferase (ALT, a measure of liver health), and serum cholesterol. All measurements were made on peripheral blood samples. The CD4:CD8 ratio was the only measurement found to be inversely proportional to viral load while higher levels of all other measures were associated with higher viral loads. However, when analyzed over the course of infection, no significant difference was found between animals exposed to SIVmac1A11 in utero and controls in these measurements (Fig. S3).
CD4+ T cells capable of producing IL-2
As a measure of immune function, PBMCs were stimulated in vitro to assess their ability to produce cytokines (IFNγ, TNFα, IL-2, IL-4, and/or IL-17) after polyclonal stimulation (PMA and ionomycin). Only CD4+ T cells capable of producing the lymphoproliferative cytokine, IL-2, were found to be associated with viral load. This association was positive, such that a stronger IL-2 response was associated with higher viral loads. Interestingly, IL-2 production was lower in PBMCs from animals exposed to SIVmac1A11 in utero when compared to controls (Fig. S4). No differences in IL-2 production were observed immediately prior to infection.
CD4 and CD8 T cell subsets expressing intermediate levels of Ki-67
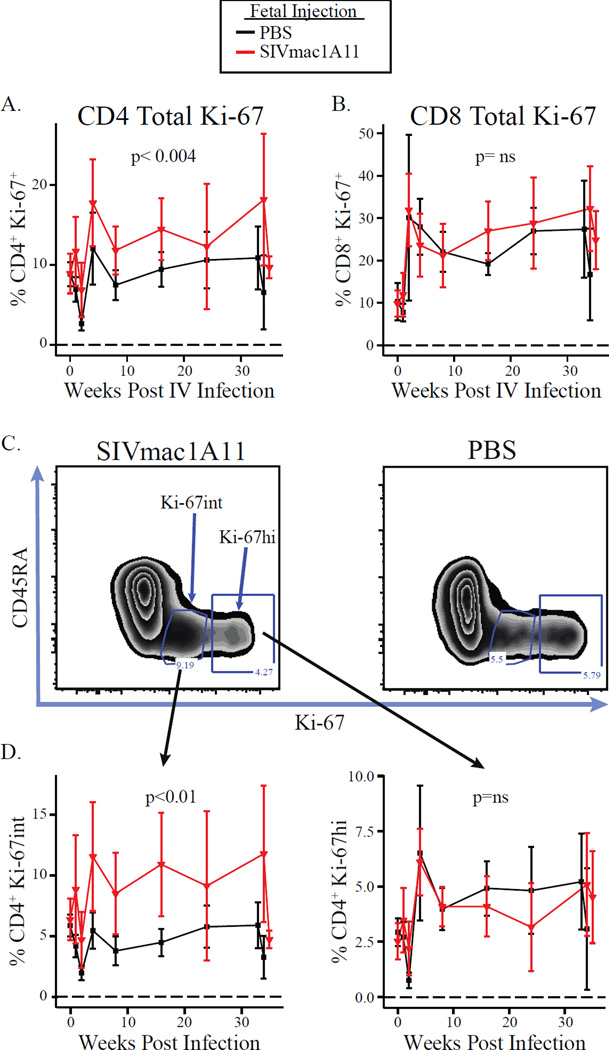
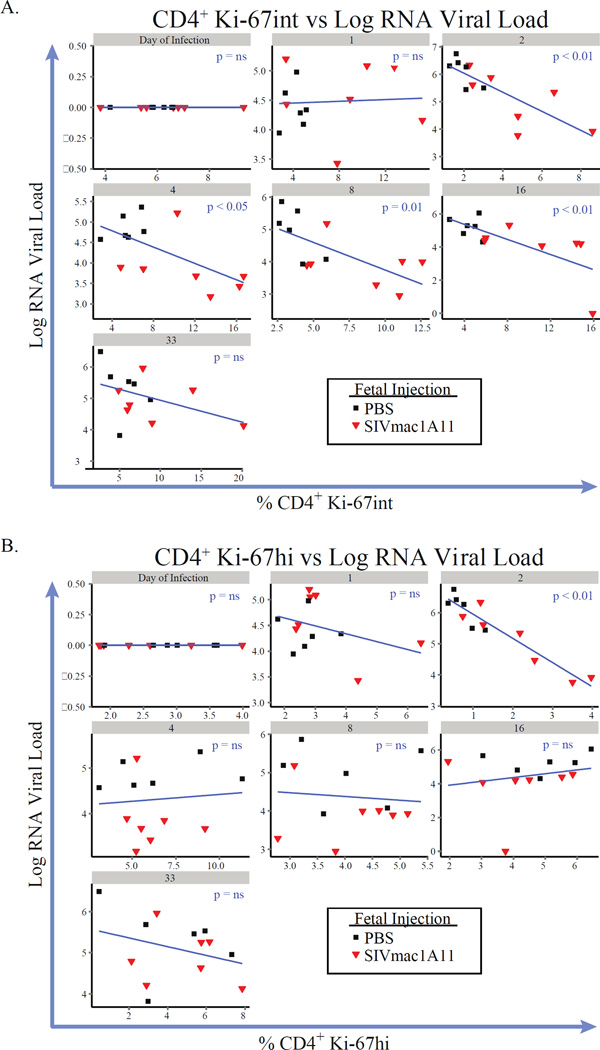
The frequency of both CD4+ and CD8+ T cells expressing Ki-67 was inversely correlated with viral loads over the course of infection, except in the case where Ki-67+ cells co-expressed FoxP3 (as discussed above). When plotted longitudinally, it became apparent that Ki-67-expressing CD4+ T cells were more abundant in rhesus monkeys exposed to SIVmac1A11 in utero (p<0.004) (Fig. 4A). No difference between experimental groups was found in CD8+ T cells (Fig. 4B). Interestingly, Ki-67 was found to be expressed in CD4+ T cells from SIVmac1A11-exposed monkeys in a unique manner, with many more cells expressing intermediate levels of Ki-67 protein relative to those observed in CD4+ T cells from sham control rhesus monkeys (Fig. 4C). Such Ki-67 intermediate (Ki-67int) cells were not differentially abundant at the time of SIVmac239 challenge but did rise in abundance in the SIVmac1A11-exposed animals as a function of time thereafter when compared to the sham control animals (p<0.01) (Fig. 4D, left panel). By contrast, CD4+ T cells expressing high levels of Ki-67 were not different between the groups (Fig. 4D, right panel).
Fig. 4. Ki-67 expression patterns are altered in animals exposed to SIVmac1A11 in utero.
Frequencies of total Ki-67+ cells in (A) CD4+ or (B) CD8+ T cells post-infection. (C) A representative cytogram of the distribution of Ki-67 in memory (CD45RA−) CD4+ T cells found in animals exposed prenatally to SIVmac1A11 (left panel) or PBS (right panel). (D) Frequencies of Ki-67int (left panel) or Ki-67hi (right panel) cells over the course of infection.
Including Ki-67hi and Ki-67int CD4+ and CD8+ T cells into the GEE analysis revealed multiple (and potentially overlapping) subsets that were significantly inversely associated with viral load over the course of infection. Linear regression models were then employed to analyze these associations for every time point measured post-infection. Plots for each measurement of viral load with each measurement of CD4+ Ki-67int cells are shown in Fig. 5A, with significant associations found at weeks 2, 4, 8, 16, and 24. These associations remained significant after removing the animal that controlled viral replication to undetectable levels (animal #27). By contrast, it was only at week 2 that the frequency of CD4+ Ki-67hi cells had an association with viral load (Fig. 5B).
Fig. 5. Ki-67int cells are inversely correlated with viral load at multiple time points post-infection.
Linear regression analyses comparing the frequencies of CD4+ (A) Ki-67int or (B) Ki-67hi cells (x-axes) with log plasma RNA viral loads (y-axes) at each time point over the course of disease. Numbers in grey bars represent weeks post-infection.
Immunologic measures not associated with viral load by GEE but different between groups
Multiple previous studies with SIV-infected rhesus macaques and HIV-infected humans have raised the possibility that viral replication and spread can be suppressed by adaptive immunity (e.g., virus-specific neutralizing antibodies and effector T cells) and augmented by the immunologic effects of chronic activation (e.g., resulting in the release of pro-inflammatory cytokines and the generation of functionally “exhausted” T cells) (30–32). Although the GEE models used in Table 1 did not show that any such measures predicted lower viral loads in the SIVmac1A11-exposed neonates at any time point after challenge with SIVmac239, several merit further discussion:
SIV-specific B cell responses
While monkeys from control Group A1 (that had no exposure to SIVmac1A11 in utero and that did not receive any postnatal vaccinations) did not develop detectable antibody responses against AT-2 SIV until after the peak of viremia (two weeks post-infection), monkeys in each of the other groups had detectable titers by two weeks post-infection (and a single animal had detectable antibody titers on the day of infection) (Fig. S5). In a GEE model, these titers showed no significant relationship to viral RNA levels or to the observed differences in viral load between Group A and Group B monkeys. However, it is interesting to note that the only animal with anti-SIV antibody titers detected on the day of infection (#27) was able to completely control viremia at weeks 16 and 20 post-infection.
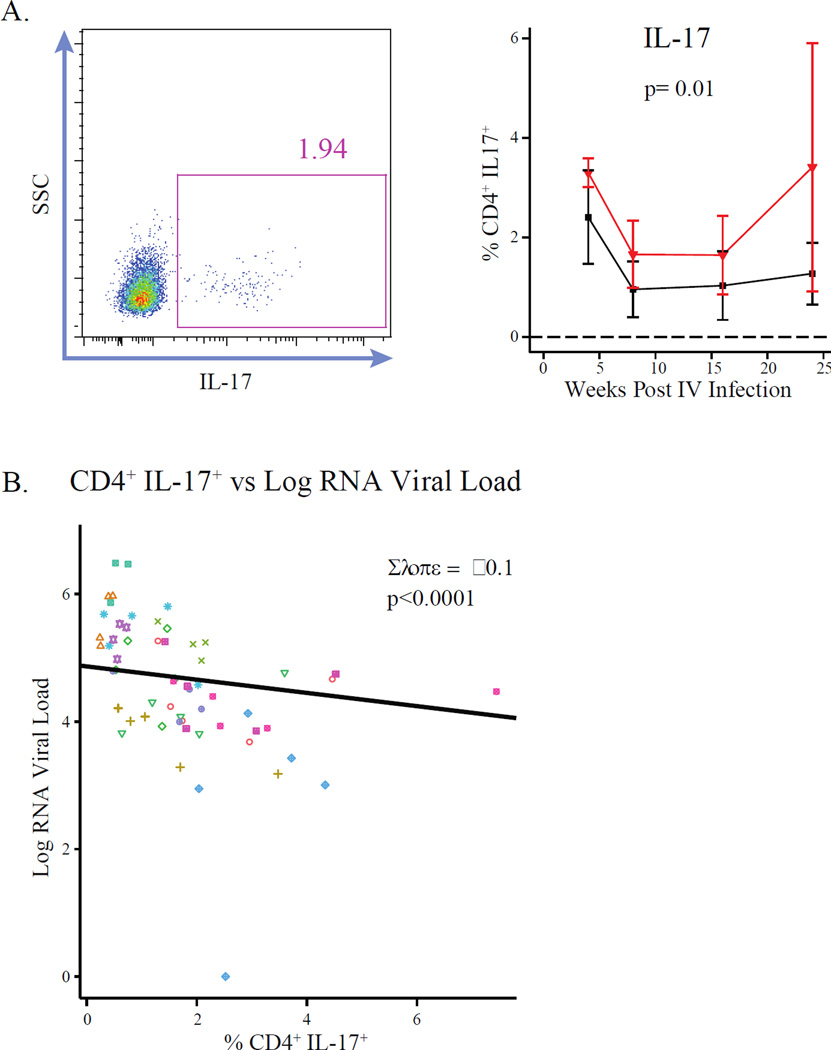
CD4+ T cells capable of secreting the cytokine, IL-17
The frequency of circulating CD4+ T cells producing IL-17 was measured after polyclonal stimulation of PBMCs and staining for intracellular IL-17. The frequency of such cells was not different between groups on the day of challenge but when the frequency of IL-17 producing CD4+ T cells was plotted longitudinally, animals injected with SIVmac1A11 in utero were found to have significantly more of these cells than controls after challenge with SIVmac239 (p=0.01) (Fig. 6A, right panel). To further interrogate a trend in the relationship between Th17 cell frequency and viral load observed analysis that had not reached significance in the GEE analysis, linear mixed effects regression was used. Animal ID and time point post-infection were included as random effects to account for the impact of inter-individual variability and time since infection on log plasma RNA viral load, respectively. Each 1% increase in Th17 cell frequency was found to reduce plasma RNA viral load by 0.1 logs, resulting in a highly significant (p<0.0001) relationship between the frequency of Th17 cells and log plasma RNA viral load (Fig. 6B). Additionally, Th17 cell frequencies were found to be positively associated with the proliferation marker, Ki67, on CD4 (Fig. S6A) but not CD8-positive (Fig. S6B) T cells.
Fig. 6. Animals exposed to SIVmac1A11 in utero have increased Th17 cells associated with reduced viral loads post-infection.
(A) (left panel) Representative cytogram of cryopreserved CD4+ T cells producing the cytokine IL-17 (Th17) after stimulation with PMA and ionomycin and (right panel) the frequencies of IL-17+ (Th17) CD4+ T cells post-infection. (B) The association between the frequency of Th17 cells and viral load for all time points post-infection. Slope was calculated by linear mixed effects regression, including animal ID and time point post-infection as random effects. P-value was calculated by Likelihood Ratio Test. Individual animals are coded by color and shape.
SIV-specific T cell responses
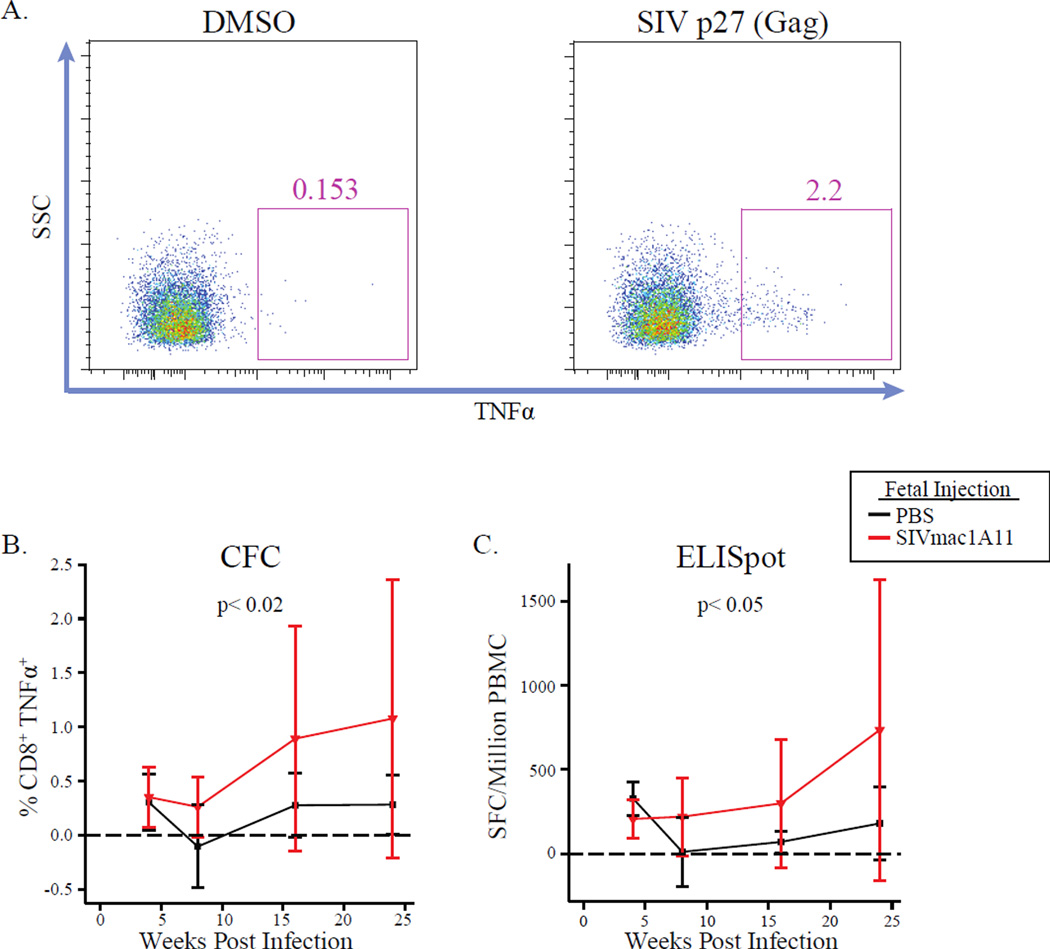
To measure SIV-specific cellular responses, PBMCs were cultured with a peptide pool spanning the SIV Gag protein p27 and responses were assessed by cytokine flow cytometry (CFC) (Fig. 7A) or an IFNγ-capture ELISpot assay. Compared to controls, animals exposed to SIV in utero had significantly higher antigen-specific production of TNF-α from CD8+ T cells by CFC (p<0.02) (Fig. 7B) and more IFNγ-producing cells by ELISpot (p<0.05) (Fig. 7C) over the course of infection. Although the variance was high because not every animal responded to these stimulations, the results were consistent within a given animal and increased over the course of infection in both assays. However, the GEE model did not detect a significant association between these responses and viral load. Therefore, although animals exposed to SIVmac1A11 in utero mounted greater antigen-specific responses, it is unclear that these responses had an impact on the observed differences in viral load.
Fig. 7. Animals exposed to SIVmac1A11 in utero have increased antigen-specific immune responses post-infection.
(A) Representative cytograms of cryopreserved CD4+ T cells producing the cytokine TNFα after stimulation with DMSO (negative control, left panel) or an SIV Gag (p27) peptide pool (right panel). (B and C) The frequencies of SIV-specific responses measured by (B) cytokine flow cytometry (CFC) or (C) ELISpot post-infection. P-values were calculated by GEE.
Measures of T cell exhaustion
Expression of the negative regulators, PD-1 and Tim-3, was measured on splenic CD4+ and CD8+ T cells at the time of tissue harvest. Rhesus monkeys exposed to SIVmac1A11 in utero were found to have significantly fewer CD8+ T cells expressing PD-1 than did control animals (p<0.04) (Fig. S7A) with an even more substantial difference found in the fraction of PD-1 expressing central memory cells (p<0.002) (Fig. S7B). Due to limited cell numbers we were not able to measure the expression of these proteins at any time point other than the time of tissue harvest and thus these were not included in the GEE analyses.
Evidence of persistent SIVmac1A11 infection postnatally
Previous reports of live attenuated SIV vaccines (LAVs) have described the persistence of LAV replication in lymph nodes, associated with a more robust antigen-specific T cell response in lymph node cells and lower levels of viral replication after pathogenic challenge (33). To investigate whether exposure of rhesus macaques to SIVmac1A11 in utero had a similar effect, cryopreserved inguinal lymph node cell suspensions from animals in Group B2 were screened for the presence of SIV RNA and DNA at 14 weeks of age (prior to pathogenic SIV challenge). Two of 4 animals had detectable levels of SIV RNA and 3 of 4 animals had detectable levels of SIV DNA at this time point (Table. S1).
Linear regression was used to determine if the measured lymph node viral loads at week 14 of postnatal life were associated with any of the measured immunological and clinical parameters described above. Given that only two animals had measurable levels of SIV RNA in lymph node samples, these associations could only be assessed by viral DNA measurements. To increase the stringency of this analysis, we asked if the association between lymph node viral load and any outcome variable was statistically significant at three or more measurements of the outcome variable. No significant associations were found.
Discussion
We report here a preliminary study in nonhuman primates that asks the question: might exposure to nonpathogenic SIV in utero have a determinative impact on the response to challenge with pathogenic SIV after birth? Given prior data examining such in utero antigenic exposure in mice, nonhuman primates, and humans, it was anticipated that non-responsiveness to SIV antigens might ensue, e.g., because of clonal deletion, the induction of antigen-specific regulatory cells, and/or the generation of antigen-specific anergy. If so, and should an adaptive immune response normally be protective against SIV infection and/or disease progression, monkeys challenged after birth with pathogenic SIV would predictably fare poorly; by contrast, and should infection and/or disease progression be driven by the effects of chronic immune activation, then such nonresponsiveness to SIV might provide a measure of protection instead. The latter possibility is supported by a recent study in macaques that indicated relevant findings with a vaccination regimen engineered to produce antigen-specific tolerance to SIV protected monkeys from multiple infectious challenges with a pathogenic SIV isolate (34).
Parameters of altered responsiveness after in utero exposure
The results of the current study support the hypothesis that exposure to SIV antigens during the late first trimester in utero has an effect on the response of the newborn to subsequent challenge with pathogenic SIV. Most remarkably, circulating peak plasma RNA viral loads as well as set point viral loads were 1 log lower in the SIVmac1A11-exposed infants when compared to sham controls. This difference in viral load was not clearly related to the generation of antiviral T or B cell effector responses, as was described by Otsyula et al. (28), where it was shown that fetal or neonatal infection with SIVmac1A11 induced a small but detectable CTL response in cells from SIVmac1A11-exposed animals stimulated in vitro. However, Otsyula et al. were only able to study a small number of animals (n=3 injected in utero) for a relatively short period of time (12 weeks post-infection) and were not able to further characterize the immune responses that may have contributed to the differences in viral loads. In the study presented here, strong IFN-γ (Th1-like) responses and higher antibody titers against SIV were (with the exception of one monkey) neither associated with nor predictive of the lower viral loads observed in the SIVmac1A11 exposed animals. Exposure to SIVmac1A11 in utero was also not associated with any demonstrable measure of antigen-specific “tolerance” to SIV, e.g., as assayed by responses to SIV before or after challenge in the presence or absence of CD25+ Tregs. Rather, and of a total of 192 parameters of clinical health and immune phenotype and function that were measured, only four general categories were associated with plasma viral load: the fraction of CD8+ T cells expressing the transcription factor FoxP3, the peripheral blood measurements of four clinical parameters (CD4:CD8 T cell ratio, monocyte abundance, ALT, and serum cholesterol levels), the fraction of CD4+ T cells producing IL-2, and different cell subsets expressing the proliferation marker, Ki-67. In particular, animals exposed to SIVmac1A11 in utero and challenged with SIVmac239 after birth were much more likely to generate multiple CD4+ and CD8+ T cell phenotypes with an intermediate level of expression of Ki-67, and the appearance of such cells was associated with differences observed in viral load between treatment groups.
These observations raise the possibility that exposure of the fetal immune system to antigen results in the generation of responses that are not yet clearly defined and that are, at the very least, different than those expected in adults. Classical adaptive immune responses (e.g., Th1 effector cells producing IFN-γ or SIV-specific antibodies) might be generated but are not able to contain viral replication and spread under these circumstances. On the other hand, it is intriguing that SIVmac1A11-exposed monkeys showed a high representation of CD4+ T cells with intermediate expression levels of Ki-67 after pathogenic challenge and that the frequency of such cells was predictive of the observed lower peak and set point viral loads found in the group. Sieg et al. (35) have reported that cells from a subset of HIV-infected patients have an inability to upregulate Ki-67 and progress through the cell cycle after T cell receptor stimulation. Defects in IL-2 signaling were proposed to play a role, but the exact mechanism remains unclear (35). The Ki-67int phenotype observed in this study may represent a similar population of unresponsive cells and it is intriguing that we also measured reduced frequencies of IL-2-producing CD4+ T cells. Another possibility is that Ki-67int cells are arrested in one stage of the cell cycle (36), or in a state of anergy, as previously described in the setting of “aborted activation” (37).
Had tolerance been induced in this study, we might have also predicted an increase in peripheral Tregs in animals exposed to SIVmac1A11 in utero. While this study did not identify a role for total CD4+CD127lowFoxP3+ Tregs, there were observed associations between CD8+ T cells with a central memory (both CD95+CD28+ and CD45RA−CD27+) phenotype that expressed FoxP3 and low levels of CD127 and viral load after four weeks post-infection. Although not functionally analyzed in the present study, CD8+ T cells expressing FoxP3 have been previously reported to possess potent suppressor activities (29) and, of note, noncytolytic MHC1b/E-restricted CD8+ T regulatory cells appear to be playing a role in the protective responses against SIV observed after oral feeding of inactivated SIV with lactobacillus (34). Thus, these cells could have been involved in dampening cellular immune responses and thereby enabling viral replication and spread. It is also possible that high levels of such cells may have been induced in response to increasing viral loads found at peak viremia. Our GEE analysis, however, showed no association between the antigen-specific immune responses and viral load, although our power was limited by the small number of animals in our study.
CD4+ T cells and other cells producing the cytokines IL-17 and IL-22 are important for the maintenance of gut barrier integrity (7,38–43). Studies in rhesus macaques have revealed that the levels of Th17 and Treg cells, and the relative ratio between these two cell populations, can distinguish pathogenic from non-pathogenic SIV infection and may also predict disease progression (7,39,44). In this light, the increased number of Th17 cells observed in this study could be important for maintenance of mucosal epithelial integrity. While not directly measured here, we have previously proposed a model of chronic inflammation whereby the loss of Th17 cells, especially in the gastrointestinal tract, compromises gut barrier integrity and can allow luminal contents, including microbial products, into the lamina propria or potentially into the bloodstream (39). It has been proposed that this breakdown sets off a cycle of inflammation where intestinal immune cells respond to microbial insult by producing pro-inflammatory cytokines (such as type I interferons) (45–49). Th17 cell frequency was found to be significantly inversely related to viral load in this study, supporting the hypothesis that the maintenance of Th17 cells may have had a positive impact on disease progression. To further clarify the role of cells producing IL-17 and/or IL-22 in the context of in utero exposure to virus, future studies are aimed at also measuring parameters of microbial translocation in treated animals and controls.
Due to the limited availability of tissue samples from these animals, we were only able to interrogate four animals for the presence of virus in lymph nodes at 14 weeks after birth (approximately 29 weeks after injection in utero), giving us little power to detect associations between the presence of virus in the lymph nodes and any of the outcome variables measured. In addition, SIV-specific T cell responses in these lymph nodes were low and indistinguishable between groups, and we cannot rule out the possibility that the persistence of SIVmac1A11 was able to prime a lymph node antiviral response capable of reducing the viral load after challenge (33). It is worth noting, however, that Fukazawa et al. found that the presence of active replication (as measured by the presence of viral RNA) from a live attenuated vaccination had the most dramatic impact on viral control after challenge, with levels of viral RNA that were orders of magnitude higher than those observed in this study.
Unexpectedly, 13 of 14 animals in this study remained uninfected despite multiple oral challenges with SIVmac239. Although oral infection with SIVmac239 has been described previously (50), to our knowledge this has not been attempted before in juvenile macaques. We chose this challenge virus due to its homology with the available antigens for in vitro tolerance assays (e.g., AT-2). While it is possible that the oral challenges may have induced tolerance to SIV, we observed no impact of the number of oral challenges on viral load or on the magnitude of the antigen-specific responses. Additionally, it is possible that the administration of SIV antigens between weeks 4 and 12 postnatal age could have hindered our ability to observe the direct impact of in utero exposure on the outcome of disease after challenge. Further experiments without oral challenges with SIVmac239 or neonatal immunization are currently underway to address each of these possibilities.
Exposure of the human fetus to HIV and other chronic infectious agents
It is interesting to consider the observations of this study in the context of mother-to-child transmission of HIV. It will be important to know to what extent exposure of the fetus and/or newborn to HIV alters the developing immune system and the subsequent ability to either clear HIV in the face of effective antiretroviral therapy and/or to fend off the virus upon subsequent exposure. This line of inquiry might illuminate a long-standing question in HIV biology: why is it that so few fetuses are infected with the virus in utero? Might it be the case, for instance, that a strong adaptive immune response is engendered (by the mother and/or the fetus) upon exposure in utero, resulting in the clearance of the virus before birth? Alternatively, might it be that altered responsiveness to HIV in utero results in less immune activation and slower rates of viral replication and spread, allowing the fetus to eliminate infected cells and to replace them with uninfected counterparts instead?
Cohorts of exposed-uninfected (EU) children have been reported for a variety of other infectious diseases as well (51). In studies of filarial infections during pregnancy, children born to infected mothers with a high worm burden had reduced cellular responses to filarial antigens and were at higher risk of filarial infection during childhood (52). Similarly, studies of children born to mothers that had placental malaria infection during pregnancy have demonstrated increased frequencies of Tregs associated with decreased malaria-specific cytokine production by T cells (53). HIV EU children have been shown to have detectable levels of T cell responses to HIV antigens after in vitro stimulation (54,55). These data were corroborated in a study by Legrand et al. where researchers found a population of antigen-specific T cells in umbilical cord blood from HIV EU children (56). Importantly, this study also found that removal of CD4+CD25+ Tregs resulted in a significant increase in antigen-specific CD4+ and CD8+ T cell responses, revealing the presence of a circulating population of Tregs that suppressed HIV-specific immune responses (56).
These data suggest that prenatal exposure can lead to the induction of tolerance to infectious pathogens in neonates. However, it remains unclear how immunologic tolerance to HIV may influence the outcome of subsequent HIV infection. Perhaps the outcome would be similar to the lack of disease progression observed in SIV infection in natural host species. While other mechanisms, such as the low levels of CCR5-expressing CD4+ T cells (57,58), certainly play a role in the lack of disease progression in SM infection, the control of immune activation during infection is strongly associated with a lack of disease progression in SIV-infected SM and AGM and has also been noted in the context of HIV infection (59–61). To date, human cohort studies on the long-term impact of in utero exposure to HIV are lacking.
The study described here was designed to be exploratory in nature and thus was composed of a relatively small number of animals (3–4 per group), limiting statistical power to detect small differences in some outcomes between the groups. Additionally, the young age of the animals provided some challenges in detecting SIV-specific responses, both related to the magnitude of the responses, and because the quantity of PBMCs was limited since small volumes of blood were collected at each time point baased on body weight. Despite this we believe that these studies, while preliminary, provide a highly relevant and interesting avenue of research to pursue.
Implications for vaccine development
Although these studies, like those of Andrieu and colleagues (34,62), should be viewed as preliminary, it is striking that maneuvers usually associated with the generation of a tolerogenic immune response are in each case associated with partial or complete protection against pathogenic SIV challenge. If our findings can be confirmed and extended, then one might imagine vaccines that induce such a tolerogenic response in people at risk for infection. Similar vaccines might be useful against other chronic infectious agents (e.g., tuberculosis, malaria, helminthic worms) in which, like HIV, pathology and spread of the infectious agent is often associated with an activated immune system. Not least, further work on tolerogenic vaccines might find application in the prevention or treatment of inflammatory diseases of an autoimmune nature.
Materials and Methods
Rhesus monkeys
All animal procedures conformed to the requirements of the Animal Welfare Act, and protocols were approved prior to implementation by the Institutional Animal Care and Use Committee at the University of California, Davis. Female rhesus macaques (n=14) negative for the MHC alleles Mamu-A*01 and Mamu-A*02 as well as for Mamu alleles associated with “elite control” of SIV viremia (e.g., Mamu-B*08 and Mamu-B*17) (63) were time-mated with males who were heterozygous for Mamu-A*01 and Mamu-A*02. Animals were identified as pregnant by ultrasound, using established methods (64). Activities related to animal care were performed according to California National Primate Research Center (CNPRC) standard operating procedures. Newborns were delivered by cesarean section at term (160 ± 2 days gestation; term 165 ± 10 days) using standardized protocols, then nursery-reared through 3 months postnatal age. Infant health, food intake, and body weights were recorded daily in the nursery and then on a regular basis when moved into juvenile housing and according to established protocols. Blood samples (~3–6 ml, dependent on age) were collected from a peripheral vessel to monitor complete blood counts (CBCs), clinical chemistry panels, and viral loads post-inoculation. At defined time points an aliquot (1–3 ml) was also used for immunologic assays. An outline of the study is shown in Fig. 1A.
SIV antigens
Lentiviral vectors expressing the ectodomain of the SIV envelope protein (gp140) were produced using plasmids by the Penn Vector Core, University of Pennsylvania, Philadelphia, PA. A second plasmid containing a gene insert for SIV gp140 was used for naked DNA injections (SIV DNA). In vitro assays were stimulated with whole, AT-2 inactivated SIVmac239 or microvesicle control (MV), (Biological Products Core, AIDS and Cancer Virus Program, Leidos Biomedical Research, Inc., Frederick National Laboratory), or SIVmac239 15-mer peptide pools corresponding to p27 (Gag) or gp120 (Env) proteins (NIH AIDS Reagent Resource Program).
Immunizations
In the late first trimester, eight fetuses were injected under ultrasound guidance using an intraperitoneal approach with nonpathogenic SIVmac1A11 (100 TCID50), and established methods. Controls were administered PBS. Beginning at four weeks postnatal age four infants that had received SIVmac1A11 prenatally and three sham controls were immunized with SIV plasmid DNA (1.4 mg) IM. At 8 and 12 weeks postnatal age they were administered the LV-Env (LV#1, LV#2) (~107 infectious units) (Fig. 1A). A summary of the treatment groups is shown in Fig. 1B.
Virus preparations
All virus stocks were obtained through the CNPRC Immunology and Pathogen Detection Resources Core, prepared and titered by endpoint dilution in CEMX174 cells according to standard protocols (65), and stored frozen at ≤ −135°C until use. Aliquots were thawed immediately prior to inoculation.
Virus challenge
At 16 weeks postnatal age all animals were challenged orally with 1 ml of SIVmac239 (105 TCID50). Only one animal (Group B2) had detectable plasma viral loads post-infection. Consequently, all remaining animals were injected IV with SIVmac239 (100 TCID50) at a range of 37–57 weeks.
Sample preparation
Freshly isolated peripheral blood was spun at 300g in a benchtop centrifuge to separate cells from plasma. The plasma fraction was removed, spun again at 500g to pellet any contaminating cells, placed in aliquots, and frozen at ≤−80°C. PBMCs were isolated from the cellular fraction by diluting samples 1:2 in PBS, layered onto Ficoll-Hypaque (Sigma), and centrifuged at 800g for 20 minutes. The leukocyte layer was removed by pipette, diluted in PBS containing 2% fetal bovine serum (FBS), and cells pelletted by centrifugation at 350g for 5 minutes. Cells were washed twice with PBS containing 2% FBS and resuspended in fresh RPMI 1640 supplemented with 10% FBS, 2 nM L-glutamine, and 100 U/ml penicillin and streptomycin (R10), and left overnight at 4°C.
Cytokine flow cytometry
Description included in supplementary materials.
ELISpot
ELISpot plates (Millipore) were washed with PBS and coated with anti-IFNγ capture antibody (Clone GZ-4, Mabtech, 16 µg/ml) for one hour at room temperature. Plates were washed four times with PBS and blocked with R10 media for one hour at 37°C and 5% CO2. AT-2 SIV (10 µg capsid/ml), MV (dose matched to total protein content of AT-2 antigen), p27 peptides (10 µg/ml), or gp120 peptides (10 µg/ml) were added in triplicate wells, along with the co-stimulatory antibodies, CD28 (BD Biosciences, 4 µg/ml) and CD49d (BD Biosciences, 4 µg/ml). 1×105 cells/well in R10 medium were added and plates were incubated at 37°C and 5% CO2 for 16–18 hours. A mixture of PMA (20 ng/ml) and ionomycin (1 µg/ml) was used as a positive control. Plates were then washed twice in PBS, washed two more times in PBS with 0.05% Tween-20 (PBST), and incubated with a biotinylated anti-IFNγ secondary antibody (Clone 7B6-1, Mabtech, 1 µg/ml) and incubated for one hour at 37°C and 5% CO2. Plates were next washed twice in PBST and incubated with streptavidin-Alkaline Phosphatase for one hour at room temperature, washed with PBST and soaked in a bath of PBST for one hour at room temperature. PBST was then removed and spots were developed with Vector Blue substrate in the dark for 5–15 minutes, after which the reaction was stopped by rinsing plates with water. When plates were dry, spots were counted using an S5 Analyser (CTL, LLC, Shaker Heights, OH). Results were reported as spot forming cells (SFC) per million PBMCs after subtraction from background (DMSO only wells).
Phenotypic analysis of lymphocyte populations
Freshly-isolated PBMCs (5×105 cells) were surface stained with the viability dye, Aqua Amine Reactive Dye (Invitrogen), as well as monoclonal antibodies directed against CD3 (Clone SP 34-2, BD Biosciences), CD4 (NIH-Nonhuman Primate Reagent Resource Program), CD8 (Clone 3B5, Invitrogen), CD25 (Clone M-A251, BD Biosciences or Clone 4E3, Miltenyi), CD95 (Clone DX2, BD Biosciences) or CD45RA (Clone 2H4, Beckman Coulter), CD27 (Clone M-T271, BD Biosciences) or CD28 (Clone CD28.2, BD Biosciences), and CD127 (Clone hIL-7R-M21, BD Biosciences) for 20 minutes at room temperature, fixed and then permeabilized with Affymetrix FoxP3 Fix/Perm Buffers (Affymetrix) per the manufacturer’s instructions. Permeabilized cells were then stained intracellularly for Ki-67 (Clone B56, BD Biosciences) and FoxP3 (Clone PCH101, Affymetrix) for 30 minutes at 4°C, washed in PBS-2% FBS and analyzed by flow cytometry. A minimum of 150,000 events was acquired on a BD LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star, Inc.).
Treg depletion assays
Treg cells were depleted from PBMC cultures using anti-CD25-labeled paramagnetic microbeads and MS columns, according to the manufacturer’s instructions (Miltenyi Biotec).
Proliferation assays
Description included in supplementary materials.
Antibody production
Antigen-specific antibody production was measured by ELISA; 96-well ELISA plates (Nunc, Inc.) were coated overnight with AT-2 SIV (1 µg capsid/ml) or PBS. Plates were then washed three times with PBS containing 0.05% Tween (PBST) and blocked with blocking buffer (PBS containing 2.5% BSA) for one hour at room temperature. Frozen plasma samples were thawed and eight 4-fold serial dilutions were made in blocking buffer. 100 µl of diluted plasma was then added to duplicate wells and incubated at room temperature for two hours. Wells were washed three times in PBST and incubated with an anti-nonhuman primate IgG secondary antibody conjugated to horseradish peroxidase (HRP) (12.5 µg/ml, Rockland Immunochemicals). Plates were then incubated for one hour at room temperature and developed using a TMB substrate kit (BD Biosciences), and the reaction stopped with dilute sulfuric acid (2N). Plates were read at 450 nm on an ELISA plate reader and at 690 nm for the background. Midpoint titers were calculated from sigmoidal dilution curves using Prism Software (Graph Pad, Inc.).
Viral Load
Description included in supplementary materials.
Statistical analyses
t-tests (results of which appear in Fig. 2) were performed using GraphPad Prism version 5.00 (GraphPad Software, San Diego California USA, www.graphpad.com). Longitudinal analyses and associations with viral load were generated using R software (http://CRAN.R-project.org) with a GEE package (http://cran.r-project.org/web/packages/gee/index.html). Linear regression models were generated in R using ln commands. Linear mixed effects regression was generated in R using lmer commands. P-values for lmer analysis were generated using the Likelihood Ratio Test by generating two lmer models with log plasma RNA viral load as an outcome and including the random effects of animal ID and time point post-infection with or without the fixed effect of Th17 cell frequency. The two models were then compared using ANOVA to assess how Th17 cell frequency helps to explain the variability in plasma RNA viral load.
Predictors of viral load
Regression analyses were used to examine potential associations of log transformed plasma viral load measurements with a panel of 192 clinical and immunologic parameters. Measurements were taken from each animal at multiple time points post-infection; because measurements from the same animal are more likely to be correlated than measurements taken from different animals, we utilized GEE techniques to conduct repeated measures regression analyses. GEE has the effect of adjusting standard errors to account for both within-animal and between-animal variability, while averaging over all animals to generate mean estimates of the outcomes. We limited the analyses to time points following the viral load set point (four weeks post-infection), the time at which viral load measurements were observed to increase and diverge between the two groups. As this analysis was exploratory, adjustments were not made for multiple comparisons.
Supplementary Material
Acknowledgments
We would like to thank Nancy Hills of the UCSF Clinical and Translational Sciences Institute for help with the statistical analyses and BJ Bosche for technical assistance with cell-associated viral load analysis for residual SIV 1A11.
Funding: This work was supported NIH grants OD000329, R01 AI090677, and R01 AI084109 (to J.M.M.), Contract No. HHSN261200800001E (to JDL) from the National Cancer Institute, the NHLBI Center for Fetal Gene Transfer for Heart, Lung, and Blood Diseases (HL085794, to A.T.), the California National Primate Research Center base-operating grant (OD011107), the UCSF Clinical and Translational Research Institute (UL1 RR024131), and the Harvey V. Berneking Living Trust. JMM is a recipient of the NIH Director’s Pioneer Award Program, part of the NIH Roadmap for Medical Research, through grant DPI OD00329.
Footnotes
Supplementary Materials
Materials and Methods
Fig. S1. Animals exposed to SIVmac1A11 in utero show no difference in the levels of Tregs post-infection.
Fig. S2. CD8+ T cell subsets expressing FoxP3 but not different between groups.
Fig. S3. Clinical measurements associated with viral load but not different between groups.
Fig. S4. Animals exposed to SIVmac1A11 in utero have lower levels of IL-2 producing CD4 cells post-infection.
Fig. S5. Animals exposed to SIVmac1A11 in utero have reduced humoral responses post-infection.
Fig. S6. Th17 cells are directly correlated with CD4 but not CD8 proliferation at multiple time points post-infection.
Fig. S7. Splenocytes from animals exposed to SIVmac1A11 in utero have lower expression of exhaustion markers on CD8+ T cells.
Table S1. Residual SIVmac1A11 found in lymph nodes postnatally.
Author contributions: DHOC, AT, and JMM designed the experiment. CARB, LS, DL, SW performed the assays. AT performed the injections, animal-related procedures, and harvested tissues. CARB, LS, DHOC, JDL, AT, and JMM consulted on the manuscript preparation. CARB, LS, and JMM wrote the paper.
Competing interests: No conflicts of interest.
References and Notes
- 1.Fauci AS, Marovich MA, Dieffenbach CW, Hunter E, Buchbinder SP. Immunology. Immune activation with HIV vaccines. Science. 2014;344:49–51. doi: 10.1126/science.1250672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCune JM. The dynamics of CD4+ T-cell depletion in HIV disease. Nature. 2001;410:974–979. doi: 10.1038/35073648. [DOI] [PubMed] [Google Scholar]
- 3.Okoye AA, Picker LJ. CD4(+) T-cell depletion in HIV infection: Mechanisms of immunological failure. Immunol Rev. 2013;254:54–64. doi: 10.1111/imr.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Autran B, Descours B, Bacchus C. Immune control of HIV-1 reservoirs. Curr Opin HIV AIDS. 2013;8:204–210. doi: 10.1097/COH.0b013e32835fe6d2. [DOI] [PubMed] [Google Scholar]
- 5.Klatt NR, Chomont N, Douek DC, Deeks SG. Immune activation and HIV persistence: Implications for curative approaches to HIV infection. Immunol Rev. 2013;254:326–342. doi: 10.1111/imr.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chahroudi A, Bosinger SE, Vanderford TH, Paiardini M, Silvestri G. Natural SIV hosts: Showing AIDS the door. Science. 2012;335:1188–1193. doi: 10.1126/science.1217550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Favre D, Lederer S, Kanwar B, Ma ZM, Proll S, Kasakow Z, Mold J, Swainson L, Barbour JD, Baskin CR, Palermo R, Pandrea I, Miller CJ, Katze MG, McCune JM. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lederer S, Favre D, Walters KA, Proll S, Kanwar B, Kasakow Z, Baskin CR, Palermo R, McCune JM, Katze MG. Transcriptional profiling in pathogenic and non-pathogenic SIV infections reveals significant distinctions in kinetics and tissue compartmentalization. PLoS Pathog. 2009;5:e1000296. doi: 10.1371/journal.ppat.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandrea IV, Gautam R, Ribeiro RM, Brenchley JM, Butler IF, Pattison M, Rasmussen T, Marx PA, Silvestri G, Lackner AA, Perelson AS, Douek DC, Veazey RS, Apetrei C. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J Immunol. 2007;179:3035–3046. doi: 10.4049/jimmunol.179.5.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silvestri G. AIDS pathogenesis: A tale of two monkeys. J Med Primatol. 2008;37(Suppl 2):6–12. doi: 10.1111/j.1600-0684.2008.00328.x. [DOI] [PubMed] [Google Scholar]
- 11.Naylor PH, Hadden JW. T cell targeted immune enhancement yields effective T cell adjuvants. Int Immunopharmacol. 2003;3:1205–1215. doi: 10.1016/S1567-5769(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 12.Lambert PH, Liu M, Siegrist CA. Can successful vaccines teach us how to induce efficient protective immune responses? Nat Med. 2005;11:S54–S62. doi: 10.1038/nm1216. [DOI] [PubMed] [Google Scholar]
- 13.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M, Lifson JD, Nelson JA, Jarvis MA, Picker LJ. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A. 1998;95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 16.Persaud D, Gay H, Ziemniak C, Chen YH, Piatak M, Chun TW, Strain M, Richman D, Luzuriaga K. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013;369:1828–1835. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sáez-Cirión A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, Potard V, Versmisse P, Melard A, Prazuck T, Descours B, Guergnon J, Viard JP, Boufassa F, Lambotte O, Goujard C, Meyer L, Costagliola D, Venet A, Pancino G, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI study. PLoS Pathog. 2013;9:e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta N, Culina S, Meslier Y, Dimitrov J, Arnoult C, Delignat S, Gangadharan B, Lecerf M, Justesen S, Gouilleux-Gruart V, Salomon BL, Scott DW, Kaveri SV, Mallone R, Lacroix-Desmazes S. Regulation of immune responses to protein therapeutics by transplacental induction of T cell tolerance. Sci Transl Med. 2015;7:275ra21. doi: 10.1126/scitranslmed.aaa1957. [DOI] [PubMed] [Google Scholar]
- 19.Mold JE, McCune JM. Immunological tolerance during fetal development: From mouse to man. Adv Immunol. 2012;115:73–111. doi: 10.1016/B978-0-12-394299-9.00003-5. [DOI] [PubMed] [Google Scholar]
- 20.Rachid R, Umetsu DT. Immunological mechanisms for desensitization and tolerance in food allergy. Semin Immunopathol. 2012;34:689–702. doi: 10.1007/s00281-012-0333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shamji MH, Durham SR. Mechanisms of immunotherapy to aeroallergens. Clin Exp Allergy. 2011;41:1235–1246. doi: 10.1111/j.1365-2222.2011.03804.x. [DOI] [PubMed] [Google Scholar]
- 22.Lehman DA, Farquhar C. Biological mechanisms of vertical human immunodeficiency virus (HIV-1) transmission. Reviews in Medical Virology. 2007;17:381–403. doi: 10.1002/rmv.543. [DOI] [PubMed] [Google Scholar]
- 23.Scarlatti G. Paediatric HIV infection. The Lancet. 1996;348:863–868. doi: 10.1016/S0140-6736(95)11030-5. [DOI] [PubMed] [Google Scholar]
- 24.Mold JE, Venkatasubrahmanyam S, Burt TD, Michaëlsson J, Rivera JM, Galkina SA, Weinberg K, Stoddart CA, McCune JM. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mold JE, Michaëlsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee T-H, Nixon DF, McCune JM. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krow-Lucal ER, Kim CC, Burt TD, McCune JM. Distinct functional programming of human fetal and adult monocytes. Blood. 2014;123:1897–1904. doi: 10.1182/blood-2013-11-536094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batchelder CA, Duru N, Lee CI, Baker CA, Swainson L, McCune JM, Tarantal AF. Myeloid-Lymphoid ontogeny in the rhesus monkey (Macaca mulatta) Anat Rec (Hoboken) 2014;297:1392–1406. doi: 10.1002/ar.22943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otsyula MG, Miller CJ, Tarantal AF, Marthas ML, Greene TP, Collins JR, van Rompay KK, McChesney MB. Fetal or neonatal infection with attenuated simian immunodeficiency virus results in protective immunity against oral challenge with pathogenic SIVmac251. Virology. 1996;222:275–278. doi: 10.1006/viro.1996.0420. [DOI] [PubMed] [Google Scholar]
- 29.Robb RJ, Lineburg KE, Kuns RD, Wilson YA, Raffelt NC, Olver SD, Varelias A, Alexander KA, Teal BE, Sparwasser T, Hammerling GJ, Markey KA, Koyama M, Clouston AD, Engwerda CR, Hill GR, MacDonald KP. Identification and expansion of highly suppressive CD8(+)Foxp3(+) regulatory T cells after experimental allogeneic bone marrow transplantation. Blood. 2012;119:5898–5908. doi: 10.1182/blood-2011-12-396119. [DOI] [PubMed] [Google Scholar]
- 30.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 31.Sakhdari A, Mujib S, Vali B, Yue FY, MacParland S, Clayton K, Jones RB, Liu J, Lee EY, Benko E, Kovacs C, Gommerman J, Kaul R, Ostrowski MA. Tim-3 negatively regulates cytotoxicity in exhausted CD8+ T cells in HIV infection. PLoS One. 2012;7:e40146. doi: 10.1371/journal.pone.0040146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang JY, Zhang Z, Wang X, Fu JL, Yao J, Jiao Y, Chen L, Zhang H, Wei J, Jin L, Shi M, Gao GF, Wu H, Wang FS. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 2007;109:4671–4678. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
- 33.Fukazawa Y, Park H, Cameron MJ, Lefebvre F, Lum R, Coombes N, Mahyari E, Hagen SI, Bae JY, Reyes MD, Swanson T, Legasse AW, Sylwester A, Hansen SG, Smith AT, Stafova P, Shoemaker R, Li Y, Oswald K, Axthelm MK, et al. Lymph node T cell responses predict the efficacy of live attenuated SIV vaccines. Nat Med. 2012;18:1673–1681. doi: 10.1038/nm.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu W, Chen S, Lai C, Guo W, Fu L, Andrieu JM. Induction of CD8(+) regulatory T cells protects macaques against SIV challenge. Cell Rep. 2012;2:1736–1746. doi: 10.1016/j.celrep.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Sieg SF, Bazdar DA, Lederman MM. Impaired TCR-mediated induction of Ki67 by naive CD4+ T cells is only occasionally corrected by exogenous IL-2 in HIV-1 infection. J Immunol. 2003;171:5208–5214. doi: 10.4049/jimmunol.171.10.5208. [DOI] [PubMed] [Google Scholar]
- 36.Tsurusawa M, Fujimoto T. Cell cycle progression and phenotypic modification of Ki67 antigen-negative G1- and G2-phase cells in phorbol ester-treated Molt-4 human leukemia cells. Cytometry. 1995;20:146–153. doi: 10.1002/cyto.990200207. [DOI] [PubMed] [Google Scholar]
- 37.Knoechel B, Lohr J, Zhu S, Wong L, Hu D, Ausubel L, Abbas AK. Functional and molecular comparison of anergic and regulatory T lymphocytes. J Immunol. 2006;176:6473–6483. doi: 10.4049/jimmunol.176.11.6473. [DOI] [PubMed] [Google Scholar]
- 38.Cecchinato V, Franchini G. Th17 cells in pathogenic simian immunodeficiency virus infection of macaques. Curr Opin HIV AIDS. 2010;5:141–145. doi: 10.1097/COH.0b013e32833653ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, Barbour JD, Lowe MM, Jayawardene A, Aweeka F, Huang Y, Douek DC, Brenchley JM, Martin JN, Hecht FM, Deeks SG, McCune JM. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2:32ra36. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanwar B, Favre D, McCune JM. Th17 and regulatory T cells: Implications for AIDS pathogenesis. Curr Opin HIV AIDS. 2010;5:151–157. doi: 10.1097/COH.0b013e328335c0c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klatt NR, Estes JD, Sun X, Ortiz AM, Barber JS, Harris LD, Cervasi B, Yokomizo LK, Pan L, Vinton CL, Tabb B, Canary LA, Dang Q, Hirsch VM, Alter G, Belkaid Y, Lifson JD, Silvestri G, Milner JD, Paiardini M, et al. Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal Immunol. 2012;5:646–657. doi: 10.1038/mi.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reeves RK, Rajakumar PA, Evans TI, Connole M, Gillis J, Wong FE, Kuzmichev YV, Carville A, Johnson RP. Gut inflammation and indoleamine deoxygenase inhibit IL-17 production and promote cytotoxic potential in NKp44+ mucosal NK cells during SIV infection. Blood. 2011;118:3321–3330. doi: 10.1182/blood-2011-04-347260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu H, Wang X, Liu DX, Moroney-Rasmussen T, Lackner AA, Veazey RS. IL-17-producing innate lymphoid cells are restricted to mucosal tissues and are depleted in SIV-infected macaques. Mucosal Immunol. 2012;5:658–669. doi: 10.1038/mi.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hartigan-O'Connor DJ, Abel K, Van Rompay KK, Kanwar B, McCune JM. SIV replication in the infected rhesus macaque is limited by the size of the preexisting Th17 cell compartment. Sci Transl Med. 2012;4:136ra69. doi: 10.1126/scitranslmed.3003941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ancuta P, Monteiro P, Sekaly RP. Th17 lineage commitment and HIV-1 pathogenesis. Curr Opin HIV AIDS. 2010;5:158–165. doi: 10.1097/COH.0b013e3283364733. [DOI] [PubMed] [Google Scholar]
- 46.Elhed A, Unutmaz D. Th17 cells and HIV infection. Curr Opin HIV AIDS. 2010;5:146–150. doi: 10.1097/COH.0b013e32833647a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hunt PW. Th17, gut, and HIV: Therapeutic implications. Curr Opin HIV AIDS. 2010;5:189–193. doi: 10.1097/COH.0b013e32833647d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim CH. Migration and function of Th17 cells. Inflamm Allergy Drug Targets. 2009;8:221–228. doi: 10.2174/187152809788681001. [DOI] [PubMed] [Google Scholar]
- 49.Paiardini M. Th17 cells in natural SIV hosts. Curr Opin HIV AIDS. 2010;5:166–172. doi: 10.1097/COH.0b013e328335c161. [DOI] [PubMed] [Google Scholar]
- 50.Suh YS, Park KS, Sauermann U, Kim KS, Ahn SS, Franz M, Schulte R, Wilfingseder D, Stoiber H, Uberla K, Hunsmann G, Stahl-Hennig C, Sung YC. Prolonged survival of vaccinated macaques after oral SIVmac239 challenge regardless of viremia control in the chronic phase. Vaccine. 2008;26:6690–6698. doi: 10.1016/j.vaccine.2008.07.055. [DOI] [PubMed] [Google Scholar]
- 51.Dauby N, Goetghebuer T, Kollmann TR, Levy J, Marchant A. Uninfected but not unaffected: Chronic maternal infections during pregnancy, fetal immunity, and susceptibility to postnatal infections. Lancet Infect Dis. 2012;12:330–340. doi: 10.1016/S1473-3099(11)70341-3. [DOI] [PubMed] [Google Scholar]
- 52.Malhotra I, Mungai PL, Wamachi AN, Tisch D, Kioko JM, Ouma JH, Muchiri E, Kazura JW, King CL. Prenatal T cell immunity to Wuchereria bancrofti and its effect on filarial immunity and infection susceptibility during childhood. J Infect Dis. 2006;193:1005–1013. doi: 10.1086/500472. [DOI] [PubMed] [Google Scholar]
- 53.Mackroth MS, Malhotra I, Mungai P, Koech D, Muchiri E, King CL. Human cord blood CD4+CD25hi regulatory T cells suppress prenatally acquired T cell responses to Plasmodium falciparum antigens. J Immunol. 2011;186:2780–2791. doi: 10.4049/jimmunol.1001188. [DOI] [PubMed] [Google Scholar]
- 54.Kuhn L, Meddows-Taylor S, Gray G, Tiemessen C. Human immunodeficiency virus (HIV)-specific cellular immune responses in newborns exposed to HIV in utero. Clin Infect Dis. 2002;34:267–276. doi: 10.1086/338153. [DOI] [PubMed] [Google Scholar]
- 55.Rich KC, Siegel JN, Jennings C, Rydman RJ, Landay AL. Function and phenotype of immature CD4+ lymphocytes in healthy infants and early lymphocyte activation in uninfected infants of human immunodeficiency virus-infected mothers. Clin Diagn Lab Immunol. 1997;4:358–361. doi: 10.1128/cdli.4.3.358-361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Legrand FA, Nixon DF, Loo CP, Ono E, Chapman JM, Miyamoto M, Diaz RS, Santos AMN, Succi RCM, Abadi J, Rosenberg MG, de Moraes-Pinto MI, Kallas EG. Strong HIV-1-specific T cell responses in HIV-1-exposed uninfected infants and neonates revealed after regulatory T cell removal. PLoS One. 2006;1:e102. doi: 10.1371/journal.pone.0000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McClure HM, Anderson DC, Fultz PN, Ansari AA, Jehuda-Cohen T, Villinger F, Klumpp SA, Switzer W, Lockwood E, Brodie A. Maternal transmission of SIVsmm in rhesus macaques. J Med Primatol. 1991;20:182–187. [PubMed] [Google Scholar]
- 58.Pandrea I, Parrish NF, Raehtz K, Gaufin T, Barbian HJ, Ma D, Kristoff J, Gautam R, Zhong F, Haret-Richter GS, Trichel A, Shaw GM, Hahn BH, Apetrei C. Mucosal simian immunodeficiency virus transmission in african green monkeys: Susceptibility to infection is proportional to target cell availability at mucosal sites. J Virol. 2012;86:4158–4168. doi: 10.1128/JVI.07141-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, Page-Shafer K, Hsue P, Emu B, Krone M, Lampiris H, Douek D, Martin JN, Deeks SG. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ipp H, Zemlin AE, Erasmus RT, Glashoff RH. Role of inflammation in HIV-1 disease progression and prognosis. Crit Rev Clin Lab Sci. 2014;51:98–111. doi: 10.3109/10408363.2013.865702. [DOI] [PubMed] [Google Scholar]
- 61.Katlama C, Deeks SG, Autran B, Martinez-Picado J, van Lunzen J, Rouzioux C, Miller M, Vella S, Schmitz JE, Ahlers J, Richman DD, Sekaly RP. Barriers to a cure for HIV: New ways to target and eradicate HIV-1 reservoirs. Lancet. 2013;381:2109–2117. doi: 10.1016/S0140-6736(13)60104-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andrieu JM, Chen S, Lai C, Guo W, Lu W. Mucosal SIV vaccines comprising inactivated virus particles and bacterial adjuvants induce CD8(+) T-regulatory cells that suppress SIV-positive CD4(+) T-cell activation and prevent SIV infection in the macaque model. Front Immunol. 2014;5:297. doi: 10.3389/fimmu.2014.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loffredo JT, Maxwell J, Qi Y, Glidden CE, Borchardt GJ, Soma T, Bean AT, Beal DR, Wilson NA, Rehrauer WM, Lifson JD, Carrington M, Watkins DI. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J Virol. 2007;81:8827–8832. doi: 10.1128/JVI.00895-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tarantal AF. The Laboratory Primate. chap. 20. San Diego, CA: Elsevier Academic Press; 2005. Ultrasound imaging in rhesus (Macaca mulatta) and long-tailed (Macaca fascicularis) macaques: Reproductive and Research Applications. [Google Scholar]
- 65.Marthas ML, Ramos RA, Lohman BL, Van Rompay KK, Unger RE, Miller CJ, Banapour B, Pedersen NC, Luciw PA. Viral determinants of simian immunodeficiency virus (SIV) virulence in rhesus macaques assessed by using attenuated and pathogenic molecular clones of SIVmac. J Virol. 1993;67:6047–6055. doi: 10.1128/jvi.67.10.6047-6055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hansen SG, Piatak M, Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, Gilliam AN, Xu G, Whizin N, Burwitz BJ, Planer SL, Turner JM, Legasse AW, Axthelm MK, Nelson JA, Früh K, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502:100–104. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.