Highlight
Characterization of a novel β-barrel protein in Arabidopsis identifies its role in leaf senescence by mediating the transport of chloroplast breakdown products across the outer mitochondrial membrane for their recycling.
Keywords: Arabidopsis, At3g27930, β-barrel protein, membrane transport, mitochondria, OM47, senescence, VDAC.
Abstract
In plant cells, mitochondria are major providers of energy and building blocks for growth and development as well as abiotic and biotic stress responses. They are encircled by two lipid membranes containing proteins that control mitochondrial function through the import of macromolecules and metabolites. Characterization of a novel β-barrel protein, OUTER MEMBRANE PROTEIN 47 (OM47), unique to the green lineage and related to the voltage-dependent anion channel (VDAC) protein family, showed that OM47 can complement a VDAC mutant in yeast. Mutation of OM47 in Arabidopsis thaliana by T-DNA insertion had no effect on the import of proteins, such as the β-barrel proteins translocase of the outer membrane 40 (TOM40) or sorting and assembly machinery 50 (SAM50), into mitochondria. Molecular and physiological analyses revealed a delay in chlorophyll breakdown, higher levels of starch, and a delay in the induction of senescence marker genes in the mutant lines. While there was a reduction of >90% in OM47 protein in mitochondria isolated from 3-week-old om47 mutants, in mitochondria isolated from 8-week-old plants OM47 levels were similar to that of the wild type. This recovery was achieved by an up-regulation of OM47 transcript abundance in the mutants. Combined, these results highlight a role in leaf senescence for this plant-specific β-barrel protein, probably mediating the recovery and recycling of chloroplast breakdown products by transporting metabolic intermediates into and out of mitochondria.
Introduction
Eukaryotic cells are characterized by the presence of membrane-bound compartments, known as organelles, which carry out various functions and contain a specific subset of the cellular proteome. Mitochondria and plastids are endosymbiotic organelles in that their origin can be attributed to a symbiotic event occurring ~2 billion and 1 billion to 500 million years ago, respectively (Gould et al., 2008; Lane and Martin, 2010). All organelles require the transport of various metabolites and compounds across the membrane(s), and thus transmembrane transport proteins are intensively studied in cell biology. Mitochondria and plastids are somewhat different from other organelles in that they contain two or more membrane structures. Both mitochondria and plastids possess outer and inner membranes; chloroplasts also harbour thylakoid membranes within their stroma. While the diverse array of proteins involved in primary metabolism and electron transport in plastids (more specifically chloroplasts) and mitochondria have been identified and intensively studied, the protein components of the outer membrane of both these organelles remain less well understood. For both mitochondria and chloroplasts, the outer membrane defines the organelle’s boundary and thus its interaction with the rest of the cell. These membranes are considered semi-permeable because they contain β-barrel-type proteins that facilitate the passage of molecules and metabolites of up to ~5kDa (Homble et al., 2012).
Homologous β-barrel proteins are also present in Gram-negative bacteria. They are comprised of a number of β-sheets, ranging from eight to 24 (Fairman et al., 2011), that form a pore which further allows the passage of molecules through a membrane. Interestingly, ~50 β-barrel proteins are known to be encoded by many bacterial genomes (Wimley, 2003); and it is therefore not surprising that both chloroplasts and mitochondria have retained some of these β-barrel proteins after endosymbiosis occurred. For instance, in Arabidopsis thaliana, the few known β-barrel proteins in chloroplasts are: the three outer envelope proteins 21, 24, and 37 (OEP 21, OEP 24, and OEP37) that act as anion or cation transporters; the translocon outer envelope membrane complex 75 (TOC75), of which there are multiple isoforms involved in protein import; and the dimeric β-barrel endoplasmic reticulum to chloroplast lipid transfer protein trigalactosyldiacylglycerol 4 (TGD4) (Simm et al., 2013; Oh and Hwang, 2015). Mitochondria generally contain three types of β-barrel proteins. These include four isoforms of the voltage-dependent anion channel (VDAC), which are generally involved in metabolite exchange (Robert et al., 2012; Tateda et al., 2011); the two isoforms of the translocase of the outer mitochondrial membrane 40 (TOM40), the primary channel by which almost all proteins enter the mitochondrion (Murcha et al., 2015); and the two isoforms of the sorting and assembly machinery 50 (SAM50) protein that is specifically involved in the insertion of β-barrel proteins into the outer membrane (Murcha et al., 2015). These are the three types of β-barrel protein common in most but not all eukaryotes. Exception or additions to this general picture include MDM10 (mitochondrial distribution and morphology protein), which is the most recently characterized mitochondrial β-barrel protein and to date only reported in fungi. It is involved in the assembly of the outer membrane protein complexes and a constituent of the endoplasmic reticulum–mitochondria encounter structure (ERMES) complex, best characterized in yeast (Meisinger et al., 2004; Flinner et al., 2013). Trypanosomids appear to lack a TOM40 orthologue and use an alternative β-barrel protein, called archaic translocase of the outer mitochondrial membrane (Pusnik et al., 2012).
The three types of mitochondrial β-barrel proteins outlined above have been studied in plants to varying degrees. The most extensively studied family is the VDAC family, the most abundant protein in the outer membrane. In Arabidopsis, the four genes encoding VDAC have varied and important functions with respect to plant development such as pollen development (Tateda et al., 2011), regulation of respiration during seed germination at low temperatures (Yang et al., 2011), and in the plant stress response (Homble et al., 2012; Takahashi and Tateda, 2013). There are a limited number of studies with regards to the other plant mitochondrial β-barrel proteins TOM40 and SAM50, both involved in protein transport. While two genes code for TOM40 in Arabidopsis, the major pore-forming translocase responsible for the import of the majority of the mitochondrial proteome, only one is thought to play a major role, as deletion results in an embryo lethal phenotype. SAM50 is responsible for the insertion of β-barrel proteins into the outer membrane (Lister et al., 2007; Duncan et al., 2013).
A biochemical characterization of the mitochondrial outer membrane proteome from Arabidopsis revealed 42 proteins with functions putatively associated with mitochondrial morphology and lipid synthesis, as well as a number of others for which the function remains unknown based on sequence comparisons alone (Duncan et al., 2011). Although this study added 27 novel proteins, thus more than doubling the confirmed number of mitochondrial outer membrane proteins, it is likely that more proteins with related functions are present in the mitochondrial outer membrane. In comparison, there are >100 proteins predicted from a variety of sources of data to be part of the mitochondrial outer membrane in yeast (Zahedi et al., 2006).
Here we report the characterization of a novel plant mitochondrial β-barrel protein, previously identified by biochemical characterization (Duncan et al., 2011). Combining phenotypical, physiological, biochemical, and molecular analyses, we reveal that this 47kDa outer membrane protein, named OM47, plays a role in plant development. The om47 knockout plants display a delay in leaf senescence, leading to a prolonged life span and enhanced vegetative growth.
Materials and methods
Plant material and growth conditions
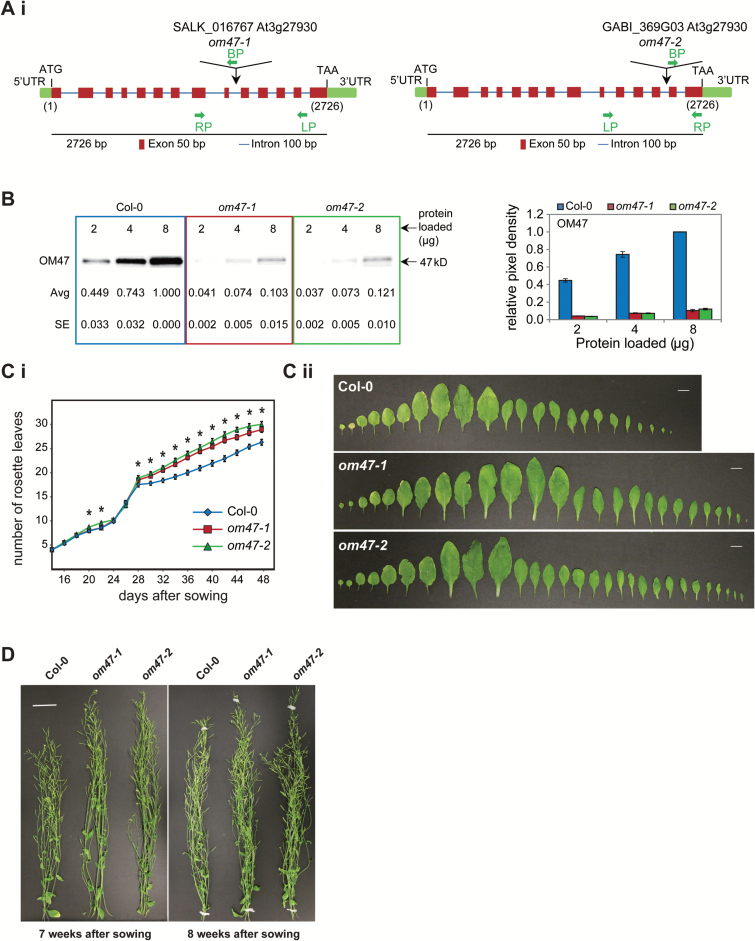
Arabidopsis thaliana Columbia (Col-0) plants were used as the wild type in this study. Homozygous T-DNA insertion lines for om47-1 (SALK_016767; http://abrc.osu.edu/) and om47-2 (GABI_369G03; http://www.gabi-kat.de/) were obtained and genotyped by PCR (Fig. 3Ai; Supplementary Table S1 at JXB online). The sites of insertions were confirmed by Sanger sequencing of the amplified PCR products (Supplementary Fig. S1).
Fig. 3.
Confirmation and characterization of T-DNA insertional knock-out lines for Arabidopsis thaliana OM47. (A) Diagram illustrating the positions of the T-DNA inserts in (i) om47-1 (SALK_016767) and (ii) om47-2 (GABI_369G03) lines of the A. thaliana OM47 (At3g27930) gene. Also shown is the length (bp), UTRs (untranslated regions), ATGs (start codon), TAAs (stop codon), exons (red squares), and introns (blue lines) of the OM47 (At3g27930) gene (see Supplementary Fig. S1 for the exact site of T-DNA inserts). LP, RP, and BP represent the primers used for screening the T-DNA insertions (see Supplementary Table S1 for specific primer sequences). (B) SDS–PAGE (left panel) and quantified relative pixel density (right panel) showing OM47 proteins in mitochondria isolated from 2-week-old A. thaliana wild type (Col-0), om47-1 (SALK_016767), and om47-2 (GABI_369G03). The amounts of mitochondrial protein loaded and apparent molecular mass of the protein detected (47kDa) are indicated. Serial dilutions of mitochondrial proteins were used to ensure linearity of detection, and immunodetection was carried out in biological triplicate, with numbers giving averages (avg) and SEs. (C) (i) The two om47 mutant lines had an increased number of leaves from ~8 d after sowing when compared with the wild type (Col-0) which was maintained through later stages of development. (ii) A series of developmental stages of all leaves from the 7-week-old wild type and om47 mutants reveals the increased number of leaves in the two mutant lines. In addition, the oldest leaves of the wild type show an earlier onset in senescence when compared with the mutants. (D) At late developmental stages (7 and 8 weeks after sowing), both om47 mutant lines had a higher inflorescence than the wild type.
Phenotypic analysis was carried out according to Boyes et al. (2001) on plants grown on either soil or Murashige and Skoog (MS) media. For plate-based phenotyping, seeds were surfaced-sterilized with 70% (v/v) ethanol and germinated on MS medium containing 4.3g l–1 (w/v) MS powder, 0.5g l–1 MES, and 1ml l–1 Gamborg B5 vitamins (pH 5.7) with varying concentrations of sucrose. Plates were stratified for 48h at 4 °C before being transferred to growth conditions. Root length of 50 plants per line per condition was measured at day 6, day 10, and day 14, respectively. Analysis of root length was carried out using Image J. For soil-based phenotyping, all lines were grown in a randomized design on soil mix consisting of grade 2 vermiculite, perlite, and soil in a 1:1:3 ratio. All growth conditions were at 22 °C/22 °C (day/night), 65% relative humidity, 120 μmol m−2 s−1 photosynthetic photon flux density (PPFD) and 16h/8h (day/night) photoperiod unless specified otherwise. All measurements were carried out in biological triplicate.
Bioinformatic analysis
Protein domains in the OM47 predicted protein were identified using the Conserved Domains database (NCBI CDD; http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) (Marchler-Bauer et al., 2013). The β-strands were predicted using the transmembrane strands and topology of the β-barrel outer membrane protein prediction web server (PRED TMBB; http://bioinformatics.biol.uoa.gr/PRED-TMBB/) based on a hidden Markov model (Bagos et al., 2004). The predicted amino acid sequences encoding a variety known of β-barrel proteins were retrieved from the Arabidopsis Information Resource (TAIR; http://www.arabidopsis.org/) TAIR10 genome release. The corresponding yeast sequences were obtained from GenBank using a text search.
Phylogenetic analysis was carried out using MEGA 5.2.2 (Tamura et al., 2011). The full-length protein alignments were first generated using the Clustal option, and the phylogenetic tree was created using the maximum likelihood tree method and the Jones–Thornton–Taylor model after 1000 replications.
In silico developmental expression analysis
Heatmaps illustrating developmental expression analysis were generated in silico in the Partek genomics suite (version 6.6) using GC-RMA normalized publicly available microarray data from the AtGenExpress developmental set (Schmid et al., 2005) and the Arabidopsis germination data set, as described previously (Narsai et al., 2011). The heatmap illustrating expression levels for At3g27930 in A. thaliana at different stages of plant development were generated using the development tool in GENEVESTIGATOR (ID: 256851_at) and the web-browser data mining interface for Affymetrix GeneChip data (https://genevestigator.com) (Hruz et al., 2008). The transcript abundance patterns of genes encoding β-barrel proteins during senescence was normalized separately using the highest value set to 1 for each gene and data taken from Breeze et al. (2011).
Yeast transformation for functional complementation
Functional complementation of a VDAC protein by OM47 was carried out using ScVDAC1(–), a yeast mutant strain lacking VDAC1 protein (por) (YNL055c; MATα, his3Δ1; leu2Δ0; lys2Δ0; ura3Δ0; UNL055c::kanMX4) (Lee et al., 1998), and a wild-type yeast strain (BY4742; MATα, his3Δ1; leu2Δ0; lys2Δ0; ura3Δ0) according to the experimental design previously described (Lee et al., 2009).
The full-length coding sequence of OM47 was cloned into pDONR201 using Gateway technology and recombined into the yeast expression vector pAG426GPD (Alberti et al., 2007) under the constitutive promoter GPD and the URA3 selectable marker, and transformed into YNL055c. As a positive control, YNL055c was also cloned into pAG426GPD and transformed into the yeast VDAC (ScVDAC) deletion strain.
Transformed strains (YNL055c+ScVDAC and YNL055c+OM47) along with BY4742 and untransformed YNL055c were plated on synthetic defined (SD) media (6.7g l–1 yeast nitrogen base without amino acids, 0.6g l–1 amino acid dropout powder) at 2% (w/v) glucose for 3 d, or 2.5% (v/v) glycerol for 5 d at 30 °C.
Dark-induced senescence assay
Two types of dark-induced senescence assays were carried out. On attached leaves, 3-week-old Col-0 and om47 mutants had leaves 5, 6, and 7 covered with aluminium pouches for a number of days, 3 d or 8 d as indicated; with control treated lines grown without aluminium pouches (Weaver and Amasino, 2001). For detached leaf dark-induced senescence assay treatments, 4-week-old Col-0 and om47 mutants had leaves 5, 6, and 7 excised, covered in aluminium foil, and placed inside foil-wrapped Petri dishes for 3 d, with Whatman 3MM paper moistened with water to prevent drying.
Gas exchange
Fully expanded leaves were enclosed into a 1cm reach chamber (Li-COR, 6400-15) attached to a Li-COR portable photosynthesis system (BioScientfic Ltd) according to Li et al. (2014) with modifications. Gas exchange parameters including net photosynthetic CO2 fixation rate, stomatal conductance, and transpiration were measured at 23 °C with a PPFD of 400 μmol m−2 s−1 (using the Li-COR 6400-18A Red, Green, Blue Light Source) and CO2 concentration of 400 μmol mol−1. The rate of mitochondrial respiration in the dark was determined by measuring the net photosynthetic CO2 fixation rate at 0 μmol m−2 s−1 PPFD.
Chlorophyll analysis and fluorescence
Three covered or non-covered leaves per plant and five plants for each genotype were snap-frozen in liquid nitrogen and ground well using a TissueLyser II (QIAGEN). A 50mg aliquot of crushed leaf material was incubated with 1.5ml of 80% pre-chilled acetone for 2min and thoroughly mixed for 5min. Samples were cleared by centrifugation at 14 000rpm for 1min and the supernatant was kept in new pre-chilled tubes. The above procedure was repeated for the remaining samples and all supernatants were mixed together. Chlorophyll was then quantified at 663nm and 646nm with a spectrophotometer (BMG, ClarioSTAR) as previously described (Lichtenthaler and Wellburn, 1983).
Chlorophyll fluorescence in F v/F m (maximum quantum yield of PSII) was determined as previously described (Rossel et al., 2006). After a short dark acclimation, plants were pulsed with 120 µmol m−2 s−1 of actinic light using the IMAGING-PAM M-series Chlorophyll Fluorescence System (Walz).
Starch analysis and histochemical staining
Starch was extracted and analysed with the help of a plate reader (BMG, ClarioSTAR) using the NAD(P)H-linked assay (Sanjaya et al., 2011). All samples were analysed in biological triplicate. Changes in the absorbance were determined by performing endpoint assays before and after the addition of each enzyme.
The histochemical staining of leaf starch was carried out as described previously (Zhang et al., 2012). Briefly, leaves were incubated in 75% (v/v) ethanol and 25% (v/v) acetic acid for 48h to remove the chlorophyll, followed by three washes in MilliQ water. Staining was achieved by further incubation in 50% (v/v) Lugol solution (Sigma-Aldrich) for 5min. Plant tissue was then destained for 90min in MilliQ water. Leaves were collected at the start (ZT0) and end (ZT16) of the light period. Attached senescent leaves 5, 6, and 7 or detached dark-induced senescent leaves 5, 6 7, 8, and 9 of each plant were collected, and three plants per genotype in each treatment were sampled.
Reactive oxygen species (ROS) in the form of O2 − were determined as previously described (Sedigheh et al., 2011). Leaves were stained using a 600 μM solution of nitroblue tetrazolium (NBT) for 3h and incubated for 24h in 80% ethanol, twice, in order to remove the chlorophyll. Five leaves per plant and three plants per genotype were sampled.
Isolation of mitochondria
For the isolation of mitochondria from water-cultured plants, mutant and Col-0 seeds were surfaced-sterilized with 70% (v/v) ethanol and grown in half-strength MS medium containing 2.15g l–1 (w/v) MS powder, 0.53g of Gamborg’s B5 salts, 1% (w/v) sucrose, 50 μg ml–1 cefotaxime, and 2mM MES/KOH, pH 5.7, with continuous shaking. Plants were grown for 2 weeks and mitochondria isolated as described previously (Lister et al., 2007). For the isolation of mitochondria from soil-grown plants, mutant and Col-0 seeds were sown directly onto soil and stratified for 48h at 4 °C before being transferred to 16h light/8h dark growth conditions. Plants were grown for 3, 4, 7, and 8 weeks, and mitochondria were isolated as described previously (Murcha and Whelan, 2015).
Immunodetection
Mitochondrial protein was separated by SDS–PAGE (Bio-Rad, Sydney), transferred to Hybond-C extra nitrocellulose (Bio-Rad, Sydney, Australia), and immunodetected as described previously (Wang et al., 2012) (ST2). To ensure linearity of detection, 2, 4, and 8 µg of mitochondrial proteins were loaded. The intensity of the cross-reacting bands was quantitated using Image Lab™ software (Bio-Rad). The pixel densities were expressed relative to the wild type (Col-0, 2 µg), where the pixel density value was set to 1. Three biological replicates were performed and the averages determined. Significant changes were determined using Student’s t-test, with an asterisk indicating a significant difference with a P-value ≤0.05. Antibodies used were raised against Ndufs4 (Meyer et al., 2009), NDB2 (Soole and Smith, 2015), AOX (Elthon et al., 1989), Porin (Lister et al., 2004), Tim9, Tim50 (Wang et al., 2012), TOM40, RISP (Duncan et al., 2011), SAM50, Erv1 (Carrie et al., 2010), COXII, cytochrome c, ATP β-subunit (Agrisera), and KDSB (Duncan et al., 2011). To generate antibodies against OM47 and ELM1 (At5g22350), cDNA of OM47 and ELM1 (amino acids 101–200) was cloned into pDONR201 and then recombined into pDEST17 using Gateway® technology (Invitrogen). The recombinant protein was expressed in Escherichia coli strain BL21 (DE3) pLys (Stratagene, La Jolla, CA, USA), and purified by denaturing immobilized metal affinity chromatography (IMAC) using the Profinia protein purification system (Bio-Rad). Purified protein was confirmed by MS prior to inoculation into rabbits according to the standard protocol of Cooper and Paterson (2009).
RNA isolation and digital quantitative RT–PCR
Total RNA from ~60mg of frozen homogenized A. thaliana tissue was isolated using the RNeasy Plant mini kit (Qiagen, Sydney, Australia) according to the manufacturer’s instructions with modifications. The RNA was eluted in molecular grade DNase- and RNase-free water and analysed on agarose gels prior to reverse transcription and PCR. The cDNA was synthesized by reverse transcription of the total RNA using the iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions. Subsequently, absolute transcript abundances were assayed using the QX200 Droplet Digital System using EvaGreen ddPCR Supermix (Bio-Rad, Gladesville, NSW, Australia) in biological triplicates. Significant changes were determined using Student’s t-test, with an asterisk indicating a significant difference with a P-value ≤0.05. Primers used for ddPCR are listed in Supplementary Table S1.
To ensure that the correct leaf was chosen from each plant, leaf number and position were marked after emergence, and tracked on a daily basis to ensure that the correct leaf age was chosen.
Results
Bioinformatic analysis reveals OM47 as a Porin3 β-barrel protein
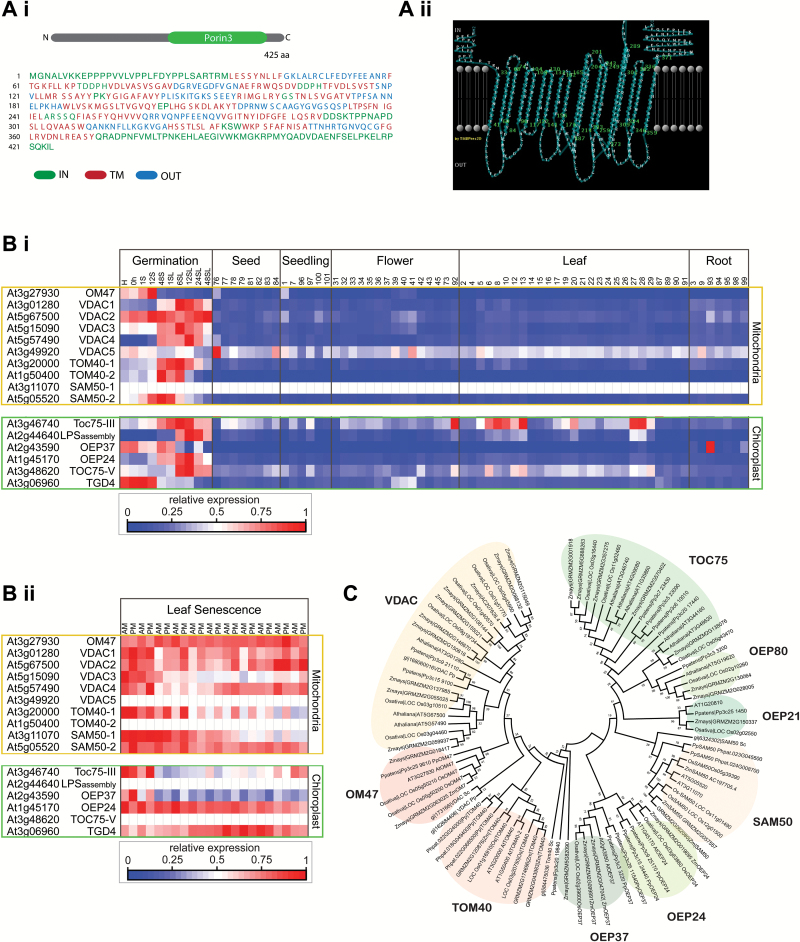
The amino acid sequence of OM47 protein, corresponding to the gene locus At3g27930, was retrieved from The Arabidopsis Information Resource (TAIR10, http://www.arabidopsis.org/). A search for conserved domains using the CDD revealed that OM47 contains the highly conserved mitochondrial outer membrane channel-forming Porin3 family motif (CD07303) within amino acids 183–361 (Fig. 1A) (Marchler-Bauer et al., 2013). The β-strands of the OM47 protein were identified using PRED-TMBB (http://bioinformatics.biol.uoa.gr/PRED-TMBB/) based on a hidden Markov model (Bagos et al., 2004). (Fig. 1A).
Fig. 1.
Bioinformatics analysis of OM47 reveals a β-barrel outer mitochondrial membrane protein. (A) The sequence of OM47 was retrieved from The Arabidopsis Information Resource (TAIR10, http://www.arabidopsis.org/). OM47 is predicted to belong to the Porin3 superfamily of proteins. (i) Sequences corresponding to loops outside of the membrane are indicated in blue and loops inside the membrane are indicated in green. Sequences corresponding to 18 putative β-strands (amino acids: 33–41, 60–68, 74–84, 96–104, 109–117, 122–130, 133–141, 156–162, 165–173, 18–201, 204–214, 233–243, 249–259, 273–289, 301–309, 324–332, 336–346, and 359–371) are indicated in red. Diagrammatic representation is shown in (Aii). (B) Heatmaps of expression analysis for nuclear-encoded genes encoding mitochondrial and chloroplastic β-barrel proteins (i) across plant development in various organs and (ii) across senescence in leaf tissue. Data are shown relative to maximum expression for each gene. Two different heat maps are shown as the senescence array data transcriptome was carried out on a different platform from the Affymetrix developmental series, and thus had to be normalized individually. (C) Phylogenetic analysis of OM47 along with mitochondrial and chloroplastic β-barrel proteins from Arabidopsis thaliana (At), Physcomitrella patens (Pp), Oryza sativa (Os), Zea mays (Zm), and Saccharomyces cerevisiae (Sc) using MEGA5.2.2. Mitochondrial proteins are shaded in orange and chloroplastic proteins are shaded in green. VDAC, voltage-dependent anion channel; TOM40, translocase of the outer mitochondrial membrane; SAM50, sorting and assembly machinery; TOC, translocon at the outer envelope membrane of chloroplasts; OEP, outer envelope protein.
Transcript expression analysis of OM47, as well as other genes encoding mitochondrial outer β-barrel membrane proteins (i.e. VDAC, TOM40, and SAM50) and chloroplast outer envelope β-barrel proteins (i.e. TOC75, OEP, and TGD) was applied to identify any putative roles these proteins may have during plant development. OM47 transcript abundance was relatively low throughout most developmental stages; however, it was slightly higher during the early stages of germination and throughout leaf senescence (Fig. 1Bi, Bii).
The amino acid sequences of OM47, VDAC, TOM40, SAM50, OEP37, OEP24, OEP21, OEP80, and TOC75 were retrieved from Arabidopsis, Physcomitrella patens (Pp), Zea mays (Zm), and Oryza sativa (Os) databases using Phytozome 9.0, along with Saccharomyces cerevisiae (Sc) VDAC, SAM50, and TOM40 to construct a bootstrapped consensus tree using MEGA 5.2.2 (Tamura et al., 2011). Phylogenetic analysis reveals that orthologues of OM47 and VDAC branch as sister clades from TOM40, with both probably being derived from a common ancestor (Fig. 1C). Although OM47 is related to both VDAC and TOM40, it represents its own distinct clade that is already present in the early land plant P. patens and is conserved in higher plants.
Arabidopsis thaliana OM47 can functionally replace the VDAC protein in a yeast (Saccharomyces cerevisiae) vdac mutant
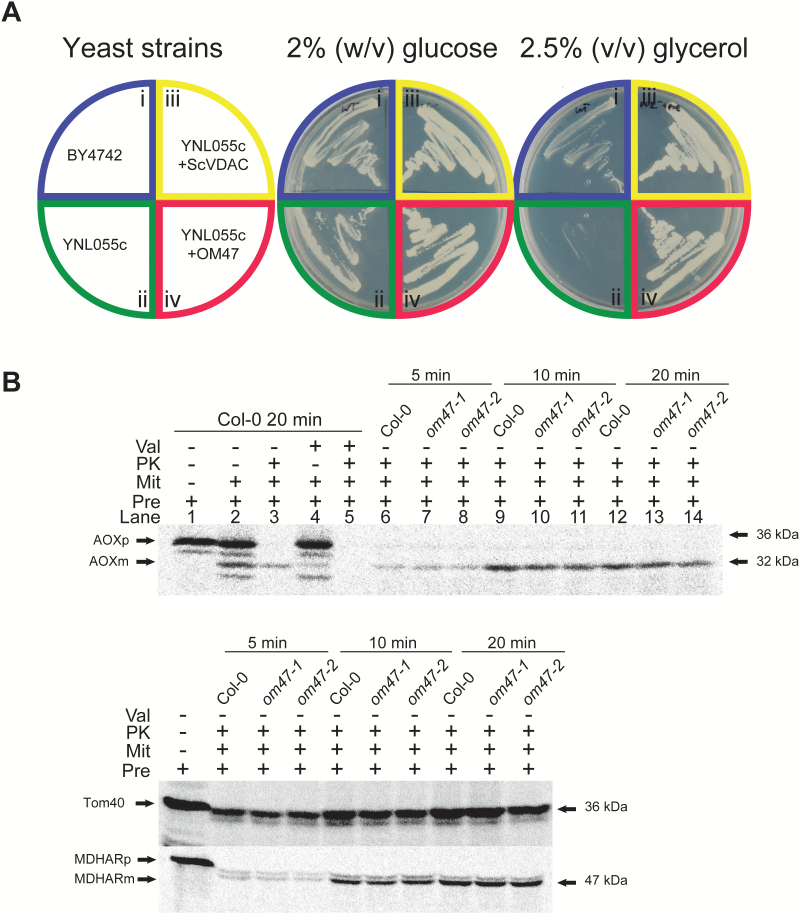
As OM47 contains the Porin3 superfamily domain and shares the highest identity and similarity scores with VDAC proteins, it was tested whether OM47 can functionally replace the VDAC protein. A complementation experiment was carried out as described previously using a yeast strain (ΔYNL055c) lacking the VDAC protein (Lee et al., 2009). As anticipated, strains of the wild type (BY4742), ΔYNL055c, ΔYNL055c complemented with the yeast wild-type VDAC gene (ΔYNL055c+ScVDAC), or complemented with OM47 (ΔYNL055c+OM47), all grew well on media with 2% (w/v) glucose (Fig. 2A). Although the BY4742 strain grew well on media with 2.5% glycerol, ΔYNL055c did not grow on the glycerol medium (Fig. 2A) as reported previously (Lee et al., 2009). The ΔYNL055c yeast strain transformed with an OM47-containing construct was able to remain viable on media containing glycerol as the sole source of carbon, as observed with the ΔYNL055c strain transformed with ScVDAC (Fig. 2A), suggesting that OM47 can functionally complement yeast VDAC. In vitro protein import assays using mitochondria isolated from wild-type plants and om47 mutants (see below for detailed characterization) did not display any effects on protein import ability (Fig. 2B). The rate of import of the alternative oxidase (AOX) via the general import pathway and the dual targeted protein monodehydroascorbate reductase (MDHAR) displayed no change in protein import as determined by the amount of mature protein in mitochondria isolated from om47 plants compared with mitochondria isolated from wild-type Arabidopsis plants. Import of TOM40 via the SAM pathway as determined by the amount of proteinase K (PK)-protected protein similarly showed no difference (Fig. 2B). From this, it was concluded that OM47 does not play a role in protein import unlike the other two β-barrel proteins TOM40 and SAM50. Taken together with the ability of OM47 to complement a vdac mutant from yeast, this suggests a likely involvement of OM47 in the transport of small molecules or metabolites.
Fig. 2.
Arabidopsis thaliana OM47 can functionally replace yeast VDAC. (A) Complementation assay of the yeast (Saccharomyces cerevisiae) ScVDAC protein with OM47. The yeast VDAC deletion strain ΔYNL055c was transformed with the OM47 and ScVDAC. The ΔYNL055c+OM47 complemented strain grew on the medium with glycerol, suggesting that OM47 can functionally replace the ScVDAC protein. ΔYNL055c+ScVDAC was used as a positive control. (B) In vitro import assays of precursor proteins into isolated mitochondria from 2-week-old wild-type and om47 plants. Upper panel: to verify the import assay, the precursor for alternative oxidase (AOXp) was imported into mitochondria isolated from wild-type plants, in the presence and absence of valinomycin, that dissipates the membrane potential across the inner membrane required for protein import into or across the inner membrane. A protease-protected product (mature AOX, AOXm) was detected (lane 3) that was not present in the presence of valinomycin (lane 5). No difference in the amount of protein import between mitochondria isolated from wild-type plants (Col-0) and om47 mutants could be detected over 20min. Lower panel: the TOM40 and monohydroascorbate reductase (MDAR) precursor proteins were used instead of AOX precursor as in lanes 1 and 6–14 in the upper panel. Val, valinomycin; PK, protease K; Mit, mitochondria, Pre, precursor protein.
Confirmation and characterization of T-DNA insertional knock-out lines for Arabidopsis thaliana OM47
Two independent T-DNA insertion lines for OM47 (At3g27930) were characterized. PCR screening and genomic DNA sequencing of these lines revealed that both om47-1 and om47-2 have inserts within the intron regions (Fig. 3Ai, 3Aii; Supplementary Fig. S1). To confirm protein abundance, a polyclonal antibody was raised against the full-length OM47 protein. SDS–PAGE and immunodetection on total mitochondrial protein isolated from 3-week-old wild-type (Col-0) and om47 mutant plants revealed an ~90% decrease in OM47 protein abundance in the two T-DNA lines (Fig. 3B), showing that the T-DNA inserts in the introns effectively reduced the amount of the OM47 protein.
Phenotypic analysis of om47 mutant lines grown on MS media, supplemented with or without 3% (w/v) sucrose, was carried out in accordance with parameters as described previously (Boyes et al., 2001). For lines grown on both types of MS media, no significant difference in phenotype was observed (Supplementary Fig. S2A, B). Soil-based phenotyping initiated at 14 d indicated that all lines reached stage 1.04 (four rosette leaves) simultaneously (Supplementary Fig. S2C). Col-0 and om47-1 reached the first flower open stage (6.0) at day 28; om47-2 was significantly (P≤0.05) delayed by 2 d (day 30) (Supplementary Fig. S2C). The significantly (P≤0.05) greater number of leaves in both mutant lines indicates an increased leaf production before phase transition (Fig. 3C). Furthermore, although om47-2 initially showed on average a significantly lower inflorescence height compared with Col-0 and om47-1 up until day 31 (Supplementary Fig. S2D), a subsequent increase in inflorescence growth of om47-2 resulted in a higher inflorescence in both om47-1 and om47-2, from day 50 when compared with the wild type (Fig. 3D; Supplementary Fig. S2D, E). Finally, a significant extension (P≤0.05) of the total flowering time by ~4 d and increased maximum rosette radius was observed in both mutant lines (Supplementary Fig. S2C–E).
Arabidopsis thaliana om47 mutants display an extended growth period phenotype that prolongs photo-assimilation and starch accumulation
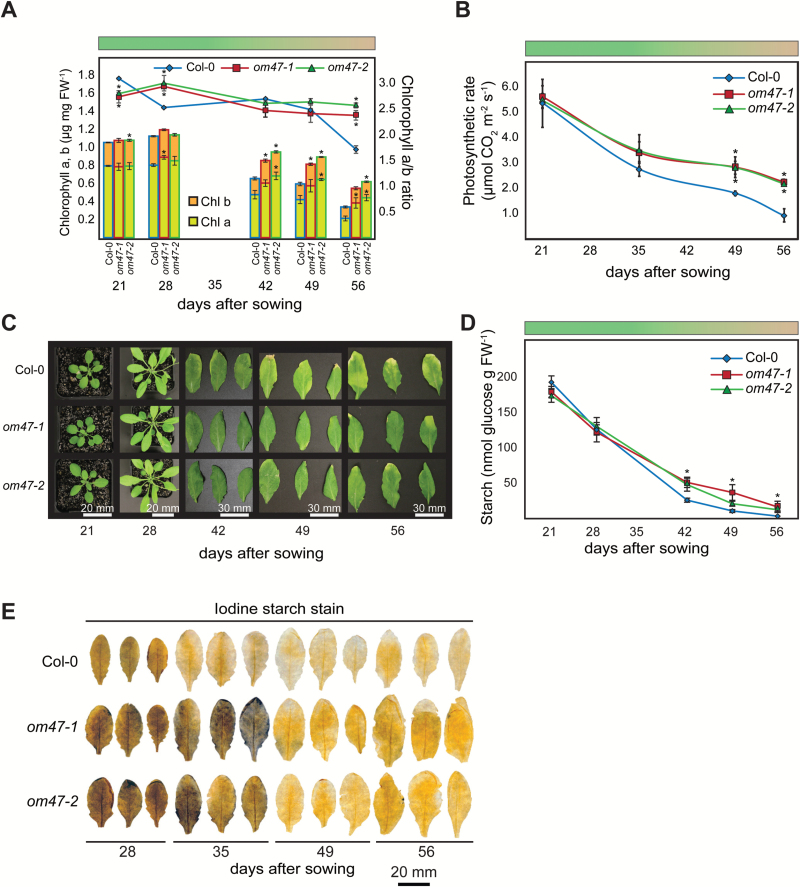
As om47 lines consistently exhibited 3–4 additional rosette leaves compared with the wild type, close biochemical and physiological monitoring of leaf development across the mid (21 d post-germination) to late (56 d post-germination) developmental stages was carried out. There was a decrease in chlorophyll after day 28 in the wild type and both om47 lines, but the decline in the om47 mutants was less pronounced (Fig. 4A) Furthermore, leaf gas exchange measurements were carried out in wild-type and om47 leaves. The photosynthetic rate in om47 mutants was comparable with that of the wild type at 21 d (Fig. 4B). From then on, there was a steady reduction during ageing that paralleled chlorophyll concentrations (Fig. 4A, B). As with the chlorophyll concentration, the reduction in photosynthetic rate was significantly (P<0.05) less in om47 mutants compared with wild-type plants, with a 1.5-fold difference at 49 d and 2-fold by 56 d (Fig. 4B). In agreement with these data, senescence seemed to occur earlier for wild-type than for om47 mutant plants (Fig. 4C). The similarity of this trend was observed in other physiological parameters as well, including transpiration, stomatal conductance, and dark respiration (Supplementary Fig. S3). Additionally, starch concentration was quantified in wild-type and om47 mutant plants at the end of the light period throughout development. Not surprisingly, starch concentration in the wild type and om47 mutants was reduced as leaf ageing progressed (Fig. 4D, E). However, starch concentration in both om47 mutant plants was significantly (P≤0.05) greater than in the wild type at the three latest points (42, 49, and 56 d).
Fig. 4.
Arabidopsis thaliana om47 mutants display a stay-green phenotype during senescence that prolongs photoassimilation and starch accumulation. (A) The chlorophyll, Chl a, and Chl b concentrations and the Chl a/b ratio of the A. thaliana wild type (Col-0) and om47 mutants over 56 d under standard (16/8h photoperiod) growth conditions. The coloured bar represents the stage of senescence throughout the experiment (n=5; data are given as averages; error bars represent ±SE). *Significant differences (P≤0.05) compared with the wild type at each time point within treatments as determined by Student’s t-test. (B) Photosynthetic rate of A. thaliana wild type (Col-0) and om47 mutants over 56 d under standard (16/8h photoperiod) growth conditions. The coloured bar represents the stage of senescence throughout the experiment (n = 4; data are given as averages; error bars represent ±SE). *Significant differences (P≤0.05) compared with the wild type as determined by Student’s t-test. (C) Representative rosette and leaf images of A. thaliana wild type (Col-0) and om47 mutants over 56 d under standard (16/8h photoperiod) growth conditions. The bar indicates the scale. (D) Starch concentration and (E) iodine starch stains of the A. thaliana wild type (Columbia-0) and om47 mutants over 56 d under standard (16/8h photoperiod) growth conditions at the end of the light period (EOLP, ZT16). The coloured bar represents the stage of senescence throughout the experiment (n=5; data are given as averages; error bars represent ±SE). *Significant differences (P≤0.05) compared with the wild type as determined by Student’s t-test. The bar indicates scale.
Arabidopsis thaliana om47 mutants delay chlorophyll breakdown during dark-induced senescence
To investigate further the function of OM47 during development, dark-induced senescence experiments were carried out. Individual leaves from 3-week-old wild-type (Col-0) and om47 mutants were covered with aluminium foil for a total of 8 d to trigger dark-induced senescence (Fig. 5). Furthermore, individual leaves were excised from 4-week-old Col-0 and om47 mutants, placed in Petri dishes with moist blotting paper, and covered for a total of 3 d to trigger dark-induced senescence (Fig. 6).
Fig. 5.
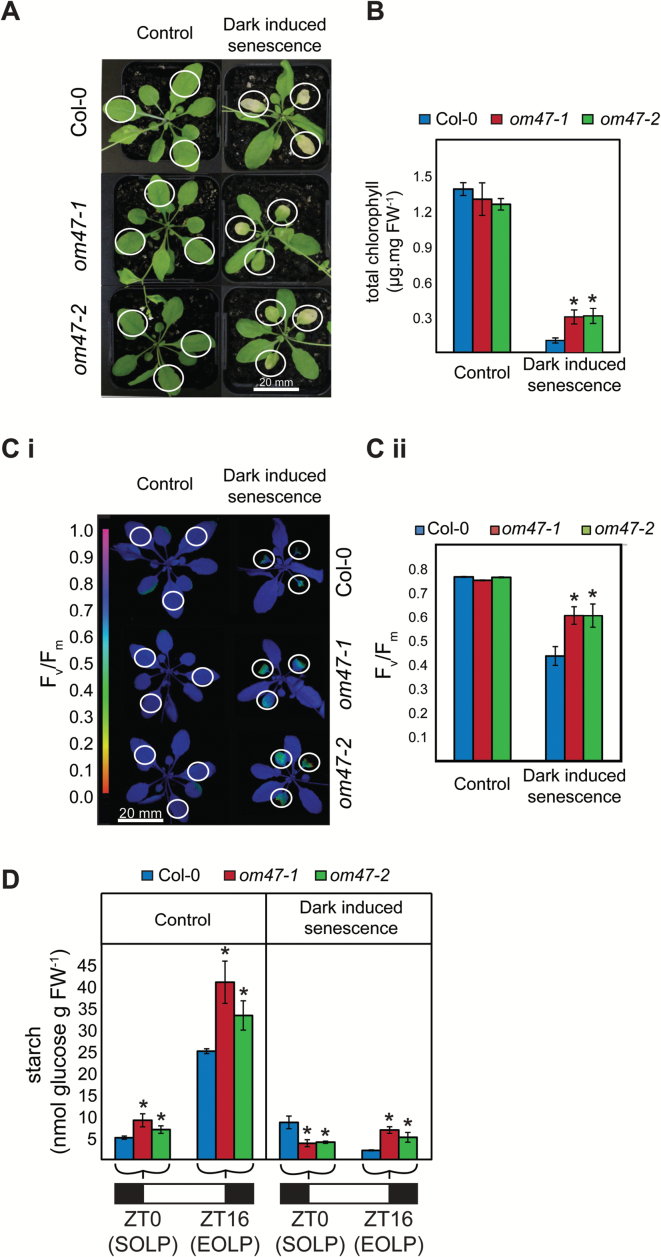
Phenotypes in Arabidopsis thaliana om47 mutants prolong chlorophyll breakdown during (attached leaf) dark-induced senescence. (A) Representative rosette images for 29-day-old A. thaliana wild type (Col-0) and om47 mutants under controlled (16h light/8h dark photoperiod) growth conditions and 8 d of dark-induced senescence (24h dark) conditions. Only leaves 5, 6, and 7 were either dark acclimated or used as controls as indicated by circles (see also Ci). The bar indicates scale. (B) Chlorophyll concentration of emerging leaves 5, 6, and 7 (indicated by circles in A) from 29-day-old A. thaliana wild type (Col-0) and om47 mutants under control (16h light/8h dark photoperiod) and after 8 d of dark-induced senescence (24h dark photoperiod) conditions (n=5; data are given as averages; error bars represent ±SE). *Significant differences (P≤0.05) compared with the wild type at each time point within treatments as determined by Student’s t-test. (C) PSII maximum efficiency (F v/F m) images (i) and quantifications (ii) illustrating photosynthetic capacity of three covered or uncovered leaves (indicated by circles) from 29-day-old A. thaliana wild type (Columbia-0, Col-0) and om47 mutants under control (16h light/8h dark photoperiod) and 8 d of dark-induced senescence (24h dark photoperiod) conditions. (n=4; data are given as averages; error bars represent ±SE). *Significant differences (P≤0.05) compared with the wild type in each treatment as determined by Student’s t-test. (D) Starch concentration of three uncovered leaves in controlled (16h light/8h dark photoperiod) growth conditions and three covered leaves in 8 d of dark acclimation (24h dark photoperiod; attached leaf) from 29-day-old A. thaliana wild type (Columbia-0, Col-0) and om47 mutants grown under the standard (16/8h photoperiod) growth conditions at the start of the light period (SOLP, ZT0) and at the end of the light period (EOLP, ZT16). Measurements were carried out in biological triplicates and data are given as averages for pools of five plants per genotype; error bars represent ±SE. *Significant differences (P≤0.05) compared with the wild type at each time point within treatments as determined by Student’s t-test.
Fig. 6.
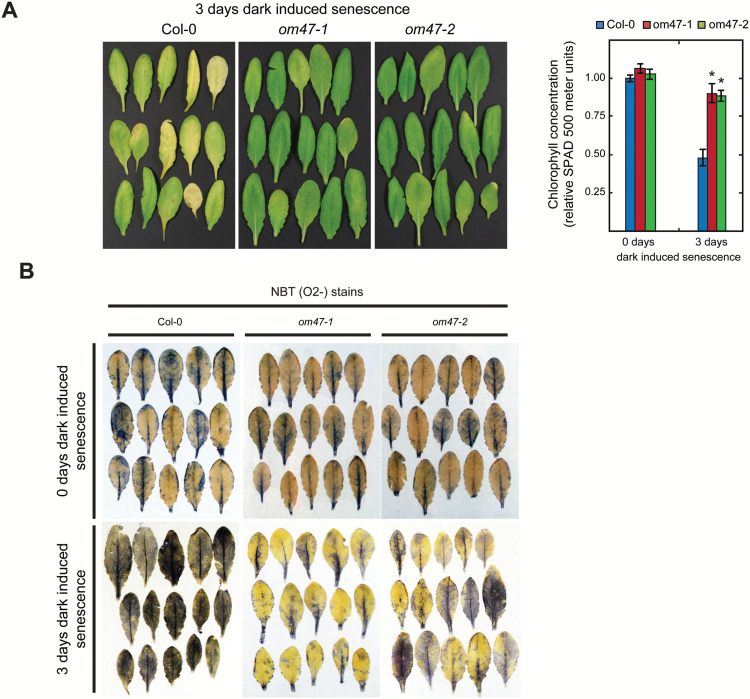
Arabidopsis thaliana om47 mutants display a stay-green phenotype, and have altered reactive oxygen species accumulation in a dark-induced senescence assay on detached leaves. (A) Individual leaves from A. thaliana wild type (Col-0) and om47 mutants detached from 4-week-old plants and covered for 3 d to induce the senescence process. The right-hand panel shows relative senescence-related chlorophyll breakdown of A. thaliana wild type (Col-0) and om47 mutants before (4-week-old plants, 0 d of dark-induced senescence) and after (3 d of dark-induced senescence) detachment from 4-week-old plants as quantified by using the SPAD 500 chlorophyll meter. Values are relative to the wild type at 0 d of dark-induced senescence. Leaves were detached and placed inside lightproof plates. Five leaves per plant and three plants per genotype were sampled (n=3; data are given as averages; error bars represent ±SE). *Significant differences (P≤0.05) compared with the wild type at each growth stage as determined by Student’s t-test. (B) Reactive oxygen species in the form of O2 − from individual leaves of A. thaliana wild type (Col-0) and om47 mutants before (4-week-old plants, 0 d of dark-induced senescence) and after (3 d of dark-induced senescence) detachment from 4-week-old plants.
Consistent with the higher chlorophyll concentration observed during development (Fig. 4) in the om47 lines, chlorophyll breakdown during dark-induced senescence was reduced in om47-1 and om47-2 compared with the wild type after 8 d of dark-induced senescence treatment in attached leaves (Fig. 5A, B). Following treatment, the chlorophyll concentrations in the covered leaves of om47 mutants were significantly (~2.5 times, P≤0.05) greater than in the wild type (Fig. 5B) and displayed higher photosynthetic efficiency (F v/F m) (Fig. 5C). In addition, wild-type F v/F m was reduced from 0.75 to 0.45, while it decreased for om47 mutants to only 0.6 (Fig. 5Cii). The starch concentration in om47 mutants was significantly greater than in Col-0 at the end of the light period (EOLP) in both control and dark-induced senescence (Fig. 5D) (P≤0.05).
The chlorophyll concentration was measured non-destructively (using a SPAD 500 chlorophyll meter) in detached leaves before (day 0) and after 3 d of acclimation to darkness. Following dark acclimation, chlorophyll concentration dropped by 50% in wild-type leaves while it was reduced by only 10% in the two om47 mutant plants (Fig. 6A). During dark-induced senescence, large quantities of ROS such as superoxide, hydrogen peroxide, hydroxyl radicals, and singlet oxygen are produced (Sedigheh et al., 2011). Cellular ROS production (as superoxide anions, O2 −) in leaves of the wild type and om47 lines was visualized when stained with NBT. Before dark acclimation, there were no observable differences in steady-state levels of ROS in either Col-0 or om47 plants (Fig. 6B). Following the 3 d dark treatment of detached leaves, production of ROS in om47 plants was less than the level seen in Col-0 leaves, indicating a lower level of stress-induced ROS accumulation in om47 mutants (Fig. 6B).
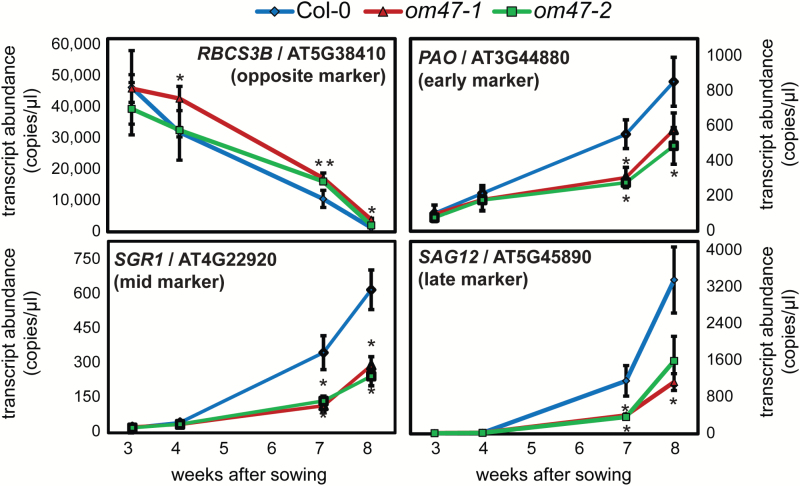
To determine whether the differential regulation of the chlorophyll concentration between the wild type and the om47 mutants was linked to the process of senescence, the transcript abundance of typical senescence marker genes was evaluated (Brouwer et al., 2014). As expected, transcript abundance of the gene encoding the small subunit of 1, 5-ribulose bisphosphate carboxylase oxygenase (At5g38410; RBCS3B) decreased in abundance from 3-week-old leaves to 8-week-old leaves of both wild-type and om47 plants (Fig. 7). There was no significant difference in transcript abundance for RBCS3B in both om47 mutant lines compared with the wild type when plants were growing for 3 weeks. Although the transcript abundance of RBCS3B showed a consistent decline in all three genotypes with age, it was significantly higher than in the wild type in the om47-1 line at 4 weeks, and for both om47 lines at 7 and 8 weeks (Fig. 7). Furthermore, expression analyses of other marker genes for senescence, such as PHEOPHORBIDE A OXYGENASE (PAO, early senescence marker), STAY-GREEN 1 (SGR1, mid senescence marker), and SENESCENCE-ASSOCIATED GENE 12 (late senescence marker) (Breeze et al., 2011; Thomas and Ougham, 2014), all increased in expression over time, indicating that the developmental process of senescence had been initiated (Fig. 7). For each of these senescence marker genes, the transcript abundance was, however, significantly higher (P<0.05) in wild-type plants compared with the om47 mutants at 7 and 8 weeks (Fig. 7). These expression patterns support the observed delayed senescence phenotype of the two om47 mutants as already observed by the physiological and biochemical characterizations.
Fig. 7.
Digital RT–PCR of senescence marker genes in Arabidopsis thaliana om47 mutants during natural senescence. Quantification of transcript abundance by digital droplet RT–PCR for RUBISCO SMALL SUBUNIT 3B (RBCS3B; At5g38410), (PHEOPHORBIDE A OXYGENASE (PAO; At3g44840), STAY-GREEN 1 (SGR1; At4g22920), and SENESCENCE-ASSOCIATED GENE 12 (SAG12, At5g45890) from leaves of A. thaliana wild-type (Columbia-0, Col-0) and om47 mutants at 21, 28, 49, and 56 d after germination. The decrease in abundance of the transcript of RBCS3B (declining under senescence) was used as a senescence-repressed marker, while PAO (early senescence marker), SGR1 (middle senescence marker), and SAG12 (late senescence marker) were included as senescence-induced markers (n=3; data are given as averages; error bars represent ±SE). *Significant differences (P≤0.05) compared with the wild type at each time point within treatments as determined by Student’s t-test.
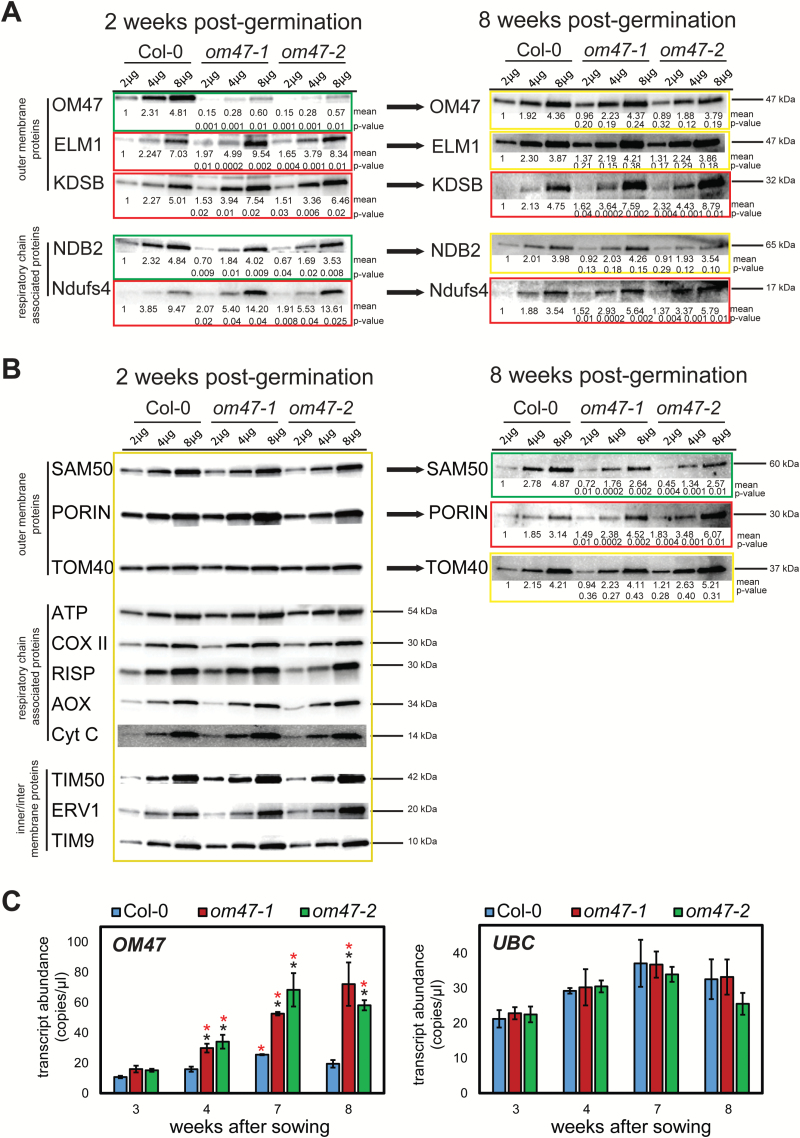
To investigate further the stay-green phenotype in om47 mutants, mitochondria were purified from 2-week-old om47 mutants and wild-type plants, and immunodetection analysis was carried out using a number of antibodies to known mitochondrial proteins (Fig. 8). As previously shown (Fig. 3), OM47 protein was reduced by ~85–90% in abundance in om47 mutants compared with the wild type (Col-0) (Fig. 8A). NDB2 [an alternative external NAD(P)H dehydrogenase located on the mitochondrial inner membrane] also showed an ~25% lower abundance in om47 plants compared with the wild type. Three proteins that were examined were significantly more abundant in om47 mutants: the outer mitochondrial membrane protein ELM1 (ELONGATED MITOCHONDRIA 1, located in the mitochondrial outer membrane, At5g22350) (Arimura et al., 2008), a subunit of complex I, Ndufs4 (NADH:UBIQUINONE OXIDOREDUCTASE FE-S PROTEIN4, At5g67590) (Meyer et al., 2009), and KDSB (CMP-KDO SYNTHETASE, At1g53000) (Duncan et al., 2011) (Fig. 8A). A variety of other mitochondrial proteins, including the β-barrel protein family members Porin, TOM40, and SAM50 (Duncan et al., 2011), did not change significantly in abundance between the wild type and mutants (Fig. 8B) in mitochondria isolated from 2-week-old plants.
Fig. 8.
Analyses of the abundance of mitochondrial proteins. Immunological analyses of protein isolated from mitochondria of 2-week-old and 8-week-old Arabidopsis thaliana wild type (Columbia-0, Col-0) and om47 mutants probed with various antibodies raised to mitochondrial proteins. (A) Proteins that were found to change significantly in abundance between mitochondria isolated from 2-week-old wild-type plants and om47 mutants are shown. (B) Analyses of the proteins that did not significantly change in abundance between mitochondria isolated from 2-week-old wild-type plants and om47 mutants are shown. Yellow boxes indicate no significant difference, while red and green boxes indicate a significant increase and decrease, respectively, in abundance in mitochondria isolated from the wild type and om47 mutants. *Significant differences (P-value shown) compared with the wild type at each time point within treatments as determined by Student’s t-test. The apparent molecular mass is indicated. (C) Digital droplet RT–PCR transcript abundance of OM47 from leaves of A. thaliana wild type (Columbia-0, Col-0) and om47 mutants at 3, 4, 7, and 8 weeks after germination (n=3; data are given as averages; error bars represent ±SE). *Significant differences (P≤0.05) of each line compared with samples at 3 weeks as determined by Student’s t-test. Ubiquitin (At5g25760) was used as a control to ensure equal RNA input and for normalization of transcript abundance.
Mitochondria were also isolated from 8-week-old plants and the yield was noticeably reduced on a fresh weight basis compared with 2-week-old samples (data not shown). Similar immunodetection analyses using the antibodies to proteins that displayed differences in abundance from mitochondria isolated from 2-week-old plants revealed that by this time, the abundance of OM47 was in fact equal in all three genotypes (Fig. 8A). The decrease in the abundance of NDB2 was also no longer seen for the 8-week-old plants (Fig. 8A). Analysis of other outer membrane β-barrel proteins revealed that PORIN increased in abundance (P<0.01), while SAM50 was significantly decreased in abundance (P<0.01) in the om47 samples compared with the wild type. There was no difference in the abundance of TOM40 (Fig. 8B).
In order to investigate the underlying mechanism involved in the significant increase in OM47 protein in the om47-1 and om47-2 lines at 8 weeks, transcript abundance of OM47 was examined in 3-, 4-, 7-, and 8-week-old leaf tissue. A clear increase in transcript abundance of OM47 in the om47 lines was observed after the 3 week time point, with a 2- to 4-fold higher expression seen compared with the wild type (Fig. 8C). These increases in transcript abundance were also paralleled on the protein level (Supplementary Fig. S4). Thus, a developmental compensation occurs to increase the abundance of OM47 protein, which was at 10–15% of the wild type in mitochondria isolated from 2- to 4-week old om47 mutants, to wild-type levels at 8 weeks.
Discussion
In this study, the role of a novel plant-specific β-barrel protein, OM47, was investigated. Based on the observed ability to complement a vdac mutant in yeast, and the fact the absence of OM47 had no apparent effect on the import of precursor proteins into isolated mitochondria, it was concluded that OM47 plays a role in the transport of metabolites, akin to VDAC. This is consistent with OM47 branching from the VDAC lineage at least 400 million years ago based on the presence of an OM47 orthologue in P. patens (Schaefer and Zryd, 2001). Two independent T-DNA lines obtained for the gene (At3g27930) encoding OM47 display an ~90% reduction in the amount of OM47 protein from mitochondria isolated from 2-week-old seedlings. Subsequent analyses of the physiological consequences revealed an apparent delayed senescence in both attached and detached leaves upon dark-induced senescence in the two om47 mutants. This delay in senescence was evidenced by a significantly attenuated up-regulation of typical senescence-induced marker genes (SAG12, PAO, and SGR1), a delay in chlorophyll breakdown, and a subsequent maintenance of a higher F v/F m value, that resulted in a greater amount of starch being present after the various treatments.
The decreased abundance of OM47 in the two T-DNA insertion lines at 2 weeks was also accompanied by an increase in the abundance of three other mitochondrial proteins, namely ELM1, KDSB (Duncan et al., 2011), and Ndufs4, a subunit of complex I (Meyer et al., 2009). A decrease in the abundance of an alternative NAD(P)H dehydrogenase, NDB2, was observed (Elhafez et al., 2006). Unexpectedly at week 8, the amount of OM47 protein in mitochondria isolated from om47 mutants was the same as observed in mitochondria isolated from wild-type plants. This recovery of OM47 protein was achieved by a >3-fold increase in transcript abundance of OM47 in the om47 lines compared with the wild type. This surprising up-regulation, likely to be a compensatory mechanism, strongly reinforces the idea that OM47 plays a major role during leaf senescence. Furthermore, the recovery of OM47 to wild-type levels was paralleled by similar increases in ELM1 and NDB2, that were at wild-type levels at week 8, although KSDB and the complex I subunit, Ndufs4, remained elevated. Importantly, the recovery in OM47 protein abundance was accompanied by changes in other outer mitochondrial membrane β-barrel proteins, namely SAM50 decreased and VDAC (Porin) increased. Thus, overall there was a complex series of events occurring, whereby the factors driving the recovery of the abundance of OM47 protein, based on elevated transcript abundance, also impacted the abundance of other proteins. Note that alterations in the abundance of the other proteins, especially the outer membrane proteins, may be occurring at a post-transcriptional level, as previously it has been documented that changes in the abundance of other membrane proteins or proteins associated with protein import into mitochondria occurred without any changes in transcript abundance of the genes encoding these proteins (Howell et al., 2006; Lister et al., 2007; Narsai et al., 2011; Law et al., 2012).
The decrease in the abundance of OM47 in the mutant lines can be compensated for by elevating transcript abundance, probably because the presence of both T-DNA insertions in an intron allows for the production of an authentic OM47 transcript after splicing. However, this compensation was not sufficient or fast enough to restore normal function as a distinct delayed senescence phenotype could be observed in the om47 mutants. The observation of this altered phenotype in dark-induced senescence assays was consistent with the increase in the number of rosette leaves in the om47 mutants. Essentially the om47 plants showed an extended vegetative growth period, producing more leaves and increased biomass before bolting. The most visible sign of this delayed senescence was the delay in chlorophyll breakdown, evidenced in both om47 mutants. It has been established in a number of plant systems that mitochondria persist long after chloroplasts are undergoing disassembly (Keech et al., 2007). This suggests a central role for mitochondria to support cell energy functions, to ensure all nutrients and molecules are recovered during senescence before cell death. As such it would be expected that the transport of various metabolites through the outer membrane would be essential to execute the programmed process of senescence. Given the uniqueness of leaf senescence to plants, it may not be surprising that a protein involved in facilitating metabolites into and out of mitochondria may have specialized in the plant lineage.
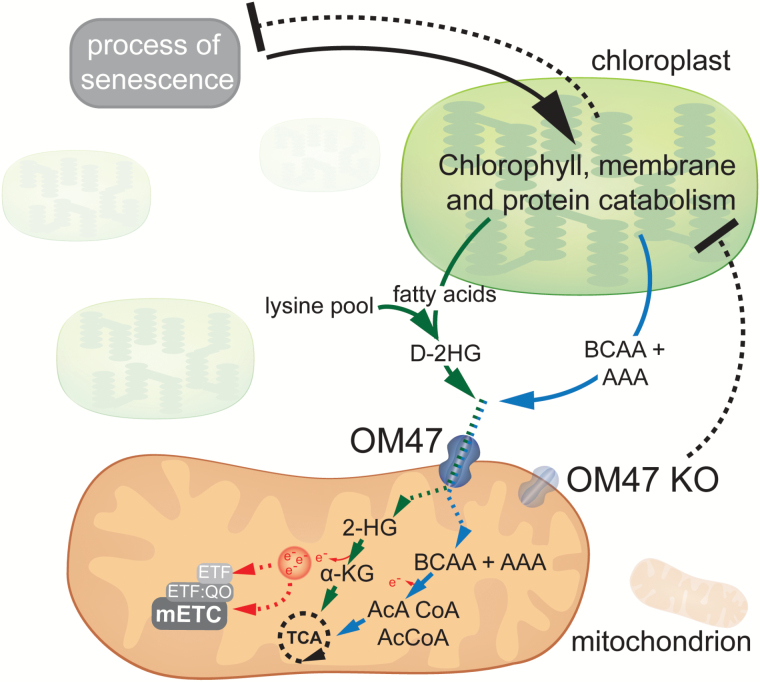
The specific metabolites that may be transported by OM47 are not yet defined. VDAC proteins are reported to be able to transport molecules ranging up to 4000Da (Colombini, 2012). OM47 might be transporting a number of metabolites and, in its absence, the metabolic readjustments during senescence might be retarded, to result in metabolic feedback on the process of senescence, leading to a delayed breakdown in chlorophyll and disassembly of chloroplasts. Also, OM47 could be specifically involved in the breakdown of chlorophyll. From current knowledge, there are two types of specific metabolites for which OM47 might mediate transport (Fig. 9). The first option is that OM47 is involved in transporting 2-hydroxyglutarate (2-HG), a metabolite that can derive from lysine catabolism as well as from fatty acid degradation (Araujo et al., 2010; Engqvist et al., 2011). Once imported into the mitochondrion, 2-HG is oxidized to α-ketoglutarate, which can then enter the tricarboxylic acid (TCA) cycle. Such reactions also lead to the production of reducing equivalents that could provide electrons to the mitochondrial electron transport chain (mETC), which in turn generates ATP to support the senescence-associated catabolic processes. The second option is that OM47 participates in channelling branched chain amino acids (BCAAs) and aromatic amino acids (AAAs), mostly arising from chloroplast degradation, into the mitochondrion. Indeed, it has been reported that BCAAs and AAAs can also provide reducing equivalents to the mETC via the electron transfer flavoprotein complex/ubiquinone oxide reductase under senescence and starvation situations (Ishizaki et al., 2006; Araujo et al., 2010; Peng et al., 2015). Furthermore, this reaction produces acetoacetyl- and acetyl-CoA, which can feed into the TCA cycle and further support metabolic reactions (Fig. 9). Although such metabolic schemes are legitimate, we cannot currently rule out other types of metabolites being transported via OM47. Regardless, it is likely that OM47 channels pivotal metabolites, and that in its absence the metabolic readjustments during leaf senescence are retarded. This in turn results in a metabolic feedback on the process of senescence, as shown by the delayed breakdown in chlorophyll and disassembly of chloroplasts (Fig. 9). It is worth noting that these processes may lead to altered signalling as a result of altered metabolite pools. The observed changes in starch levels in the om47 mutants may affect sugar signalling from organelles (Lastdrager et al., 2014). The retarded breakdown of chlorophyll may lead to an accumulation of intermediates of chlorophyll breakdown. As various haem or pigment biosynthetic intermediates are known to have signalling properties (Chan et al., 2016), it is not unexpected that any alterations in chlorophyll breakdown may have implication for signalling.
Fig. 9.
Model describing the putative role of OM47 and its effect on the process of senescence as observed through the use of T-DNA insertion lines. Chloroplast disassembly leads to the release of BCAAs, AAAs, and 2-HG, which in turn can provide reducing equivalents and carbon skeletons to support mitochondrial functions (see Discussion for details). Plain lines/arrows represent known molecular mechanisms while dotted lines/arrows indicate putative mechanisms. AAA, aromatic amino acid; BCAA, branched chain amino acid; 2-HG, 2-hydroxyglutarate; AcCoA, acetyl-CoA; AcACoA, acetoacetyl-CoA; α-KG, α -ketoglutarate; mETC, mitochondrial electron transport chain; TCA, tricarboxylic acid cyle.
In summary, the transport of molecules into and out of mitochondria may have both direct and indirect effects on the overall metabolic homeostasis during leaf senescence. If perturbed, as in the om47 mutants, this results in the delay of the whole process.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. T-DNA insertion position as determined by sequencing for the T-DNA lines used in this study.
Figure S2. Plate-based development stages of Arabidopsis thaliana om47 mutants.
Figure S3. Arabidopsis thaliana om47 mutants have enhanced gas exchange parameters during senescence.
Figure S4. Relative protein abundance of OM47 in mitochondria isolated from om47-2 mutant plants at different developmental stages.
Table S1. List of primers used in this study.
Table S2. List of antibodies used in this study.
Acknowledgements
The research was funded by the ARC Centre of Excellence in Plant Energy Biology (CE140100008). The Swedish research council ‘VetenskapsRådet’ (grant: 621-2014-4688), the Carl Tryggers Stiftelse, as well as the Kempe Foundation are also acknowledged for their financial support (SL, OK). JW designed the research, LL, SKJ, JR, OB, and OD performed research, LL, SKJ, JR, OB, YW, SL, OD, MWM, RN, JW, analysed data, and LL, SKJ, OD, JR, SL, OK, OB, MWM, and JW interpreted the results as well as contributed to the preparation, writing, and reviewing of the manuscript.
References
- Alberti S, Gitler AD, Lindquist S. 2007. A suite of Gateway cloning vectors for high-throughput genetic analysis in Saccharomyces cerevisiae. Yeast 24, 913–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo WL, Ishizaki K, Nunes-Nesi A, et al. 2010. Identification of the 2-hydroxyglutarate and isovaleryl-CoA dehydrogenases as alternative electron donors linking lysine catabolism to the electron transport chain of Arabidopsis mitochondria. The Plant Cell 22, 1549–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura S, Fujimoto M, Doniwa Y, Kadoya N, Nakazono M, Sakamoto W, Tsutsumi N. 2008. Arabidopsis ELONGATED MITOCHONDRIA1 is required for localization of DYNAMIN-RELATED PROTEIN3A to mitochondrial fission sites. The Plant Cell 20, 1555–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagos PG, Liakopoulos TD, Spyropoulos IC, Hamodrakas SJ. 2004. PRED-TMBB: a web server for predicting the topology of beta-barrel outer membrane proteins. Nucleic Acids Research 32, W400–W404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Gorlach J. 2001. Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. The Plant Cell 13, 1499–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeze E, Harrison E, McHattie S, et al. 2011. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. The Plant Cell 23, 873–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer B, Gardestrom P, Keech O. 2014. In response to partial plant shading, the lack of phytochrome A does not directly induce leaf senescence but alters the fine-tuning of chlorophyll biosynthesis. Journal of Experimental Botany 65, 4037–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrie C, Murcha MW, Whelan J. 2010. An in silico analysis of the mitochondrial protein import apparatus of plants. BMC Plant Biology 10, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KX, Phua SY, Crisp P, McQuinn R, Pogson BJ. 2016. Learning the languages of the chloroplast: retrograde signaling and beyond. Annual Review of Plant Biology 67, 25–53. [DOI] [PubMed] [Google Scholar]
- Colombini M. 2012. VDAC structure, selectivity, and dynamics. Biochimica et Biophysica Acta 1818, 1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper HM, Paterson Y. 2009. Production of polyclonal antisera. Current Protocols in Neuroscience 48, 5.5.1–5.5.10. [DOI] [PubMed] [Google Scholar]
- Duncan O, Murcha MW, Whelan J. 2013. Unique components of the plant mitochondrial protein import apparatus. Biochimica et Biophysica Acta 1833, 304–313. [DOI] [PubMed] [Google Scholar]
- Duncan O, Taylor NL, Carrie C, Eubel H, Kubiszewski-Jakubiak S, Zhang B, Narsai R, Millar AH, Whelan J. 2011. Multiple lines of evidence localize signaling, morphology, and lipid biosynthesis machinery to the mitochondrial outer membrane of Arabidopsis. Plant Physiology 157, 1093–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhafez D, Murcha MW, Clifton R, Soole KL, Day DA, Whelan J. 2006. Characterization of mitochondrial alternative NAD(P)H dehydrogenases in Arabidopsis: intraorganelle location and expression. Plant and Cell Physiology 47, 43–54. [DOI] [PubMed] [Google Scholar]
- Elthon TE, Nickels RL, McIntosh L. 1989. Monoclonal antibodies to the alternative oxidase of higher plant mitochondria. Plant Physiology 89, 1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engqvist MKM, Kuhn A, Wienstroer J, Weber K, Jansen EEW, Jakobs C, Weber APM, Maurino VG. 2011. Plant d-2-Hydroxyglutarate Dehydrogenase Participates in the Catabolism of Lysine Especially during Senescence. Journal of Biological Chemistry 286, 11382–11390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairman JW, Noinaj N, Buchanan SK. 2011. The structural biology of beta-barrel membrane proteins: a summary of recent reports. Current Opinion in Structural Biology 21, 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinner N, Ellenrieder L, Stiller SB, Becker T, Schleiff E, Mirus O. 2013. Mdm10 is an ancient eukaryotic porin co-occurring with the ERMES complex. Biochimica et Biophysica Acta 1833, 3314–3325. [DOI] [PubMed] [Google Scholar]
- Gould SB, Waller RF, McFadden GI. 2008. Plastid evolution. Annual Review of Plant Biology 59, 491–517. [DOI] [PubMed] [Google Scholar]
- Homble F, Krammer EM, Prevost M. 2012. Plant VDAC: facts and speculations. Biochimica et Biophysica Acta 1818, 1486–1501. [DOI] [PubMed] [Google Scholar]
- Howell KA, Millar AH, Whelan J. 2006. Ordered assembly of mitochondria during rice germination begins with pro-mitochondrial structures rich in components of the protein import apparatus. Plant Molecular Biology 60, 201–223. [DOI] [PubMed] [Google Scholar]
- Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P. 2008. Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Advances in Bioinformatics 2008, 420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki K, Schauer N, Larson TR, Graham IA, Fernie AR, Leaver CJ. 2006. The mitochondrial electron transfer flavoprotein complex is essential for survival of Arabidopsis in extended darkness. The Plant Journal 47, 751–760. [DOI] [PubMed] [Google Scholar]
- Keech O, Pesquet E, Ahad A, Askne A, Nordvall D, Vodnala SM, Tuominen H, Hurry V, Dizengremel P, Gardestrom P. 2007. The different fates of mitochondria and chloroplasts during dark-induced senescence in Arabidopsis leaves. Plant, Cell and Environment 30, 1523–1534. [DOI] [PubMed] [Google Scholar]
- Lane N, Martin W. 2010. The energetics of genome complexity. Nature 467, 929–934. [DOI] [PubMed] [Google Scholar]
- Lastdrager J, Hanson J, Smeekens S. 2014. Sugar signals and the control of plant growth and development. Journal of Experimental Botany 65, 799–807. [DOI] [PubMed] [Google Scholar]
- Law SR, Narsai R, Taylor NL, Delannoy E, Carrie C, Giraud E, Millar AH, Small I, Whelan J. 2012. Nucleotide and RNA metabolism prime translational initiation in the earliest events of mitochondrial biogenesis during Arabidopsis germination. Plant Physiology 158, 1610–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, Xu X, Blachly-Dyson E, Forte M, Colombini M. 1998. The role of yeast VDAC genes on the permeability of the mitochondrial outer membrane. Journal of Membrane Biology 161, 173–181. [DOI] [PubMed] [Google Scholar]
- Lee SM, Hoang MH, Han HJ, Kim HS, Lee K, Kim KE, Kim DH, Lee SY, Chung WS. 2009. Pathogen inducible voltage-dependent anion channel (AtVDAC) isoforms are localized to mitochondria membrane in Arabidopsis. Molecules and Cells 27, 321–327. [DOI] [PubMed] [Google Scholar]
- Li X, Han L, Zhao Y, You Z, Dong H, Zhang C. 2014. Hpa1 harpin needs nitroxyl terminus to promote vegetative growth and leaf photosynthesis in Arabidopsis. Journal of Biosciences 39, 127–137. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK, Wellburn AR. 1983. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochemical Society Transactions 11, 591–592. [Google Scholar]
- Lister R, Carrie C, Duncan O, Ho LH, Howell KA, Murcha MW, Whelan J. 2007. Functional definition of outer membrane proteins involved in preprotein import into mitochondria. The Plant Cell 19, 3739–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A, Zheng C, Chitsaz F, et al. 2013. CDD: conserved domains and protein three-dimensional structure. Nucleic Acids Research 41, D348–D352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisinger C, Rissler M, Chacinska A, et al. 2004. The mitochondrial morphology protein Mdm10 functions in assembly of the preprotein translocase of the outer membrane. Developmental Cell 7, 61–71. [DOI] [PubMed] [Google Scholar]
- Meyer EH, Tomaz T, Carroll AJ, Estavillo G, Delannoy E, Tanz SK, Small ID, Pogson BJ, Millar AH. 2009. Remodeled respiration in ndufs4 with low phosphorylation efficiency suppresses Arabidopsis germination and growth and alters control of metabolism at night. Plant Physiology 151, 603–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murcha MW, Narsai R, Devenish J, Kubiszewski-Jakubiak S, Whelan J. 2015. MPIC: a mitochondrial protein import components database for plant and non-plant species. Plant and Cell Physiology 56, e10. [DOI] [PubMed] [Google Scholar]
- Murcha MW, Whelan J. 2015. Isolation of intact mitochondria from the model plant species Arabidopsis thaliana and Oryza sativa. In: Whelan J, Murcha WM, eds. Plant mitochondria: methods and protocols. New York: Springer, 1–12. [DOI] [PubMed] [Google Scholar]
- Narsai R, Law SR, Carrie C, Xu L, Whelan J. 2011. In-depth temporal transcriptome profiling reveals a crucial developmental switch with roles for RNA processing and organelle metabolism that are essential for germination in Arabidopsis. Plant Physiology 157, 1342–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh YJ, Hwang I. 2015. Targeting and biogenesis of transporters and channels in chloroplast envelope membranes: unsolved questions. Cell Calcium 58, 122–130. [DOI] [PubMed] [Google Scholar]
- Peng C, Uygun S, Shiu SH, Last RL. 2015. The impact of the branched-chain ketoacid dehydrogenase complex on amino acid homeostasis in Arabidopsis. Plant Physiology 169, 1807–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusnik M, Mani J, Schmidt O, Niemann M, Oeljeklaus S, Schnarwiler F, Warscheid B, Lithgow T, Meisinger C, Schneider A. 2012. An essential novel component of the noncanonical mitochondrial outer membrane protein import system of trypanosomatids. Molecular Biology of the Cell 23, 3420–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert N, d’Erfurth I, Marmagne A, et al. 2012. Voltage-dependent-anion-channels (VDACs) in Arabidopsis have a dual localization in the cell but show a distinct role in mitochondria. Plant Molecular Biology 78, 431–446. [DOI] [PubMed] [Google Scholar]
- Rossel JB, Walter PB, Hendrickson L, Chow WS, Poole A, Mullineaux PM, Pogson BJ. 2006. A mutation affecting ASCORBATE PEROXIDASE 2 gene expression reveals a link between responses to high light and drought tolerance. Plant, Cell and Environment 29, 269–281. [DOI] [PubMed] [Google Scholar]
- Sanjaya, Durrett TP, Weise SE, Benning C. 2011. Increasing the energy density of vegetative tissues by diverting carbon from starch to oil biosynthesis in transgenic Arabidopsis. Plant Biotechnology Journal 9, 874–883. [DOI] [PubMed] [Google Scholar]
- Schaefer DG, Zryd JP. 2001. The moss Physcomitrella patens, now and then. Plant Physiology 127, 1430–1438. [PMC free article] [PubMed] [Google Scholar]
- Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Scholkopf B, Weigel D, Lohmann JU. 2005. A gene expression map of Arabidopsis thaliana development. Nature Genetics 37, 501–506. [DOI] [PubMed] [Google Scholar]
- Sedigheh HG, Mortazavian M, Norouzian D, Atyabi M, Akbarzadeh A, Hasanpoor K, Ghorbani M. 2011. Oxidative stress and leaf senescence. BMC Research Notes 4, 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simm S, Papasotiriou DG, Ibrahim M, Leisegang MS, Muller B, Schorge T, Karas M, Mirus O, Sommer MS, Schleiff E. 2013. Defining the core proteome of the chloroplast envelope membranes. Frontiers in Plant Science 4, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soole KL, Smith CA. 2015. Analysis of Type II NAD(P)H dehydrogenases. Methods in Molecular Biology 1305, 151–164. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Tateda C. 2013. The functions of voltage-dependent anion channels in plants. Apoptosis 18, 917–924. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28, 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateda C, Watanabe K, Kusano T, Takahashi Y. 2011. Molecular and genetic characterization of the gene family encoding the voltage-dependent anion channel in Arabidopsis. Journal of Experimental Botany 62, 4773–4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H, Ougham H. 2014. The stay-green trait. Journal of Experimental Botany 65, 3889–3900. [DOI] [PubMed] [Google Scholar]
- Wang Y, Carrie C, Giraud E, Elhafez D, Narsai R, Duncan O, Whelan J, Murcha MW. 2012. Dual location of the mitochondrial preprotein transporters B14.7 and Tim23-2 in complex I and the TIM17:23 complex in Arabidopsis links mitochondrial activity and biogenesis. The Plant Cell 24, 2675–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver LM, Amasino RM. 2001. Senescence is induced in individually darkened Arabidopsis leaves, but inhibited in whole darkened plants. Plant Physiology 127, 876–886. [PMC free article] [PubMed] [Google Scholar]
- Wimley WC. 2003. The versatile beta-barrel membrane protein. Current Opinion in Structural Biology 13, 404–411. [DOI] [PubMed] [Google Scholar]
- Yang XY, Chen ZW, Xu T, Qu Z, Pan XD, Qin XH, Ren DT, Liu GQ. 2011. Arabidopsis kinesin KP1 specifically interacts with VDAC3, a mitochondrial protein, and regulates respiration during seed germination at low temperature. The Plant Cell 23, 1093–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedi RP, Sickmann A, Boehm AM, Winkler C, Zufall N, Schonfisch B, Guiard B, Pfanner N, Meisinger C. 2006. Proteomic analysis of the yeast mitochondrial outer membrane reveals accumulation of a subclass of preproteins. Molecular Biology of the Cell 17, 1436–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Carrie C, Ivanova A, et al. 2012. LETM proteins play a role in the accumulation of mitochondrially encoded proteins in Arabidopsis thaliana and AtLETM2 displays parent of origin effects. Journal of Biological Chemistry 287, 41757–41773. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.