Abstract
Background
Human Epidermal Growth Factor (Her-2/neu) has strong therapeutic implications in certain cancers like breast and gastric cancer. Literature on its frequency in colorectal cancer is scarce. In this study, we have investigated the frequency of Her-2/neu expression in colorectal adenocarcinomas and its association with various clinicopathological variables.
Methods
A total of 95 patients who underwent colonoscopic biopsy or colectomy were studied after Institutional Ethical Approval. Hematoxylin & eosin (H&E) staining was performed on all the tissue sections. Expression of Her-2/neu was investigated by immunohistochemistry using α-Her-2 antibody. In order to quantify Her-2/neu expression, three criterias were applied that includes the pattern of staining, intensity of staining and percentage of tumor cells stained. Furthermore, its association was seen with various clinicopathological variables including age, gender, histopathological type, grade and stage of the tumor. Data was entered and analyzed using SPSS version 21. A p-value of < 0.05 was considered as significant.
Results
From the total of 95 cases, 75 (78.9 %) cases showed Her-2/neu expression. Pattern of Her-2/neu staining was significantly associated with the grade of colorectal cancer depicting cytoplasmic Her-2/neu expression higher in low grade (50 %) while membranous Her-2/neu expression more in high grade colorectal cancer (45 %) (P-value = 0.030). Pattern of Her-2/neu staining was also significantly associated with the type of colorectal cancer representing membranous Her-2/neu expression to be more common in mucinous type (38.5 %) while cytoplasmic Her-2/neu expression to be more frequent in non mucinous type (42.7 %) of colorectal cancer (p-value = 0.024).
We observed a significant association between percentage of cells stained & tumor type, with score 3+ maximum in non mucinous type of colorectal cancer (p-value = 0.006).
Conclusion
Her2/neu is considerably expressed in colorectal adenocarcinoma in Pakistani population. Our findings indicate a significant strong association of cytoplasmic Her-2/neu expression with low grades and membranous Her-2/neu expression with high grades of colorectal cancer. These findings add to the body of information & may help in conducting clinical trials in future to explore its therapeutic significance as well.
Keywords: Her-2/neu, Colorectal adenocarcinoma, Immunohistochemistry, Trastuzumab
Background
Gastrointestinal malignancies are amongst the major oncological problems worldwide. Colorectal cancer is the 3rd most common cancer across the globe [1]. A rise in incidence and mortality rates of colorectal cancers are illustrated in Asia [2]. In Pakistan, a low but rising incidence of colorectal cancer at young age with advanced stages is being consistently observed in the recent past [3]. Rising incidence and high mortality of colorectal cancer demands for a need of newer therapeutic targets to improve the survival of the patients.
Human Epidermal Growth Factor (Her-2/neu) is a proto-oncogene located on chromosome 17q21 that encodes ErbB-2 [4]. Over-expression of Her-2/neu has been notably associated with increased cellular survival, increased proliferation and decreased apoptotic potential of cells leading to malignant transformation and maintenance of the associated malignancy [5]. Presence of Her-2/neu is strongly implicated in certain cancers including breast & stomach. Trastuzumab, a monoclonal antibody against Her-2/neu, has shown a good prognosis in Her-2/neu positive breast cancer patients [6]. Recently, α-Her-2 targeted therapy has also been approved for metastatic gastric adenocarcinomas [7, 8].
A very scarce data is available particularly from our region to indicate expression of Her-2/neu in patients with colorectal adenocarcinomas. Overexpression of Her-2/neu in colorectal cancer shows a wide range of variability between 0-84 % in different studies [9].
Her-2/neu expresses itself in either membranous as well as cytoplasmic forms with different clinical implications in different cancers. For instance, cytoplasmic expression of Her-2/neu in breast cancer does occur but it has been regarded as irrelevant since the monoclonal antibodies approved for its treatment, targets only membranous forms [9]. In contrast, several studies reported that Her-2/neu expression to be membranous as well as cytoplasmic in colorectal adenocarcinoma [10, 11]. Moreover, literature supports that cytoplasmic Her-2/neu expression in colorectal carcinoma could be associated with survival prognosis [9]. The definitive cause of cytoplasmic expression of Her-2/neu still remains unclear but upregulation of promoter binding proteins leading to an increase in Her-2/neu production provides some evidence for the identity of cytoplasmic expression of Her-2/neu in colorectal cancer [9]. Use of α-Her-2 therapy in the treatment of colorectal cancer patients has been less extensively investigated. A clinical trial conducted showed a low positivity (8 %) of Her-2/neu expression but these patients responded to Trastuzumab therapy [12].
However, and alarmingly, Her-2/neu testing in colorectal adenocarcinoma has not gained its popularity in most parts of the world including South Asia region. Moreover, a very few studies have been published from this region regarding Her-2/neu expression in colorectal adenocarcinoma [13–15]. Therefore, in this study we attempted to know the expression of Her-2/neu in colorectal tumors in our population which might prove in future a useful prognostic factor and worthy therapeutic target for colorectal carcinoma.
Methods
Recruitment of patients and ethical approval
The study was conducted at the Dow Diagnostic Research and Reference Laboratory (DDRRL), Dow University of Health Sciences (DUHS), Karachi after ethical approval of Institutional Review Board during August 2014 to February 2016. A total of 95 patients with colorectal carcinoma who underwent colorectal cancer biopsy at National Institute of Liver and Gastrointestinal Diseases (NILGID) and colectomy at surgical ward of DUHS were recruited in the study after their informed consent. Clinico-pathological parameters of the patients were then recorded.
Processing of tissues and microscopy
Subsequent to biopsy/colectomy, specimens were transferred to the Histology section in 10 % neutral buffer formalin. Samples were examined for gross features and paraffin blocks were prepared for subsequent staining and microscopy. Tissue sections, each measuring 3 to 4 μm in thickness were cut from the paraffin blocks and processed for H&E staining. Based on microscopic examination of the H&E stained slides, clinico-pathological parameters were recorded including tumor type, tumor grade amongst others. Histopathological grading of tumors was performed according to the World Health Organization (WHO) criteria as grade I (well differentiated), grade II (moderately differentiated) and grade III (poorly differentiated). Pathological staging of colectomy cases were recorded as per the 7th edition of the American Joint Committee on Cancer Staging (stage I to stage IV) [16].
Immunohistochemistry and scoring of Her-2/neu over-expression
In order to investigate expression of Her-2/neu in colorectal adenocarcinoma, conventional immunhohistochemistry protocol was subjected on all cases. Sections were deparaffinized, dehydrated and antigen retrieval was performed. Further, the sections were incubated with the mouse primary monoclonal antibody against Her-2/neu (clone CB-11, dilution 1:65, Cellmarque) for 1 h in the moisturisation chamber. Secondary antibody (Hi-Def detection system) (Cellmarque) containing solution A as amplifier and solution B as polymer was used. For all read-outs, staining was controlled by using known Her-2/neu over-expressing breast carcinoma tissues.
Her-2/neu stained slides were independently evaluated by two experienced pathologists. In order to quantify/score Her-2/neu expression, three criterias were followed [17]:
Pattern of Her-2/neu expression: Membranous/cytoplasmic/membranous + cytoplasmic
Intensity of staining: Weak/moderate/strong
- Percentage of cells stained:
- 10-40 % cells stained as score 1+
- 40-70 % cells stained as score 2+
- >70 % cells stained as score 3+
Statistical analysis
Data was recorded for different variables including age and gender of the patients, histopathological type, grade, stage of the tumor and Her-2/neu expression and analysed using SPSS version 21. In order to investigate association of Her-2/neu expression in colorectal adenocarcinomas with other categorical variables, Chi-square or Fisher’s exact test were applied. A p-value < 0.05 was considered as significant.
Results
Clinico-pathological parameters of colorectal cancer patients
The study included a total of 95 cases of colorectal adenocarcinoma. Mean age of the patients recruited in this study was 46 years (range22-85 years). Of all the patients studied, a total of 51 (53.7 %) were males while 44 (46.3 %) were females. A total of 74 (77.9 %) were biopsy specimens while 21 (22.1 %) were colectomy specimens. Overall, a total of 14 (14.7 %) patients had grade I, 59 (62.1 %) had grade II and 22 (23.2 %) had grade III tumors. Histologically, 13 (13.7 %) tumors were mucinous and 82 (86.3 %) were non mucinous variants. Pathological staging could only be performed on colectomy samples (n = 21). A total of 9 (42.9 %) cases were of stage IIA, 1 (4.8 %) case of stage IIB, 2 (9.5 %) cases of stage IIIA, 3 (14.3 %) cases of stage IIIB & 6 (28.6 %) cases of stage IIIC.
Her-2/neu Immunoreactivity in Colorectal Cancer
Of the total 95 colorectal cancer tissues, 75 (78.9 %) showed Her-2/neu staining. Out of the total 75 positive cases, 20 (26.6 %) showed membranous Her-2/neu expression, 36 (48 %) cases showed cytoplasmic expression and 19 (25.3 %) cases showed membranous + cytoplasmic expression.
Of the total 75 positive cases, 19 (25.3 %) cases showed weak intensity, 35 (46.6 %) showed moderate intensity & 21 (28 %) showed strong intensity of Her-2/neu staining.
Of the total 75 positive cases, 23 (30.6 %) were scored as 1+, 18 (24 %) cases were scored as 2+ & 34 (45.3 %) were scored as 3 + .
A statistically significant difference was seen between pattern of Her-2/neu staining & grades of colorectal cancer (p-value = 0.038) (Fig. 1). Membranous Her-2/neu staining was maximum in high grade while cytoplasmic staining was more frequent in low grade colorectal cancer. Moreover, we observed that the frequency of membranous Her-2/neu over expression increased with grades of tumor while cytoplasmic over expression decreased from low to high grade colorectal cancer. A statistically significant association was observed between pattern of Her-2/neu staining & type of colorectal cancer depicting membranous Her-2/neu staining more common in mucinous while cytoplasmic more frequent in non mucinous type of colorectal cancer (p-value = 0.024). No significant difference was noted between pattern of Her-2/neu staining with age of the patient & stage of the tumor (Table 1).
Fig. 1.
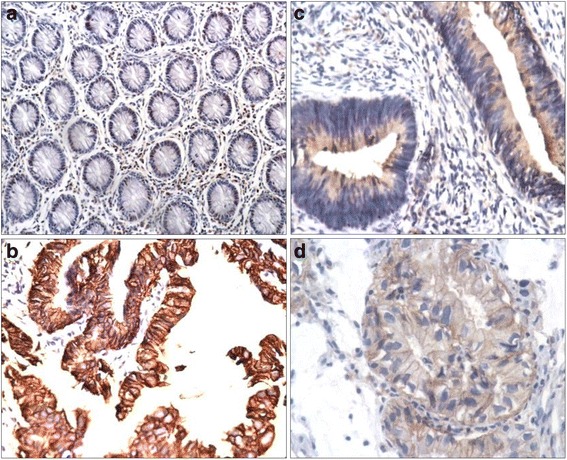
a Control (normal colonic mucosa). b Membranous expression of Her-2/neu in colorectal adenocarcinoma. c Cytoplasmic expression of Her-2/neu in colorectal adenocarcinoma. d Membranous + cytoplasmic expression of Her-2/neu in colorectal adenocarcinoma
Table 1.
Pattern of Her-2 staining in colorectal cancer
| Pattern of Her-2 staining n(%) | p-value | ||||||
|---|---|---|---|---|---|---|---|
| Negative | Memb | Cyto | M + C | ||||
| Gender | F | 44 | 7 (15.9) | 4 (9.1) | 21 (47.7) | 12 (27.3) | 0.013* |
| M | 51 | 13 (25.5) | 16 (31.4) | 15 (29.4) | 7 (13.7) | ||
| Age | ≤60 year | 70 | 15 (21.4) | 16 (22.9) | 24 (34.3) | 15 (21.4) | 0.666* |
| >60 year | 25 | 5 (20.0) | 4 (16.0) | 12 (48.0) | 4 (16.0) | ||
| Grade | I | 14 | 3 (21.4) | 1 (7.1) | 7 (50.0) | 3 (21.8) | 0.038** |
| II | 59 | 11 (18.6) | 9 (15.3) | 25 (42.4) | 14 (23.7) | ||
| III | 22 | 6 (27.3) | 10 (45.5) | 4 (18.2) | 2 (9.1) | ||
| Type | Mucinous | 13 | 5 (38.5) | 5 (38.5) | 1 (7.7) | 2 (15.4) | 0.024** |
| Nonmucinous | 82 | 15 (18.3) | 15 (18.3) | 35 (42.7) | 17 (20.7) | ||
| pTNM | IIA | 9 | 3 (37.5) | 2 (50.0) | 1 (80.0) | 0 | 0.116** |
| IIB | 1 | 1 (100) | 0 | 0 | 0 | ||
| IIIA | 2 | 2 (100) | 0 | 0 | 0 | ||
| IIIB | 3 | 1 (33.3) | 1 (33.3) | 1 (33.3) | 0 | ||
| IIIC | 6 | 1 (16.7) | 2 (33.3) | 0 | 3 (50.0) | ||
*Pearson Chi square, ** Fisher’s exact, level of significance at < 0.05
(Age ≤ 60 = age less than or equal to 60 years)
There was no significant association between intensity of Her-2/neu staining with clinicopathological variables (Table 2).
Table 2.
Intensity of Her-2 staining in colorectal cancer
| Intensity of Her-2 staining n(%) | p-value | ||||||
|---|---|---|---|---|---|---|---|
| Negative | Weak | Mod | Strong | ||||
| Gender | F | 44 | 7 (15.9) | 9 (20.5) | 20 (45.5) | 8 (18.2) | 0.353* |
| M | 51 | 13 (25.5) | 10 (19,6) | 15 (29.4) | 13 (25.5) | ||
| Age | ≤60 year | 70 | 15 (21.4) | 12 (17.1) | 25 (35.7) | 18 (25.7) | 0.438* |
| >60 year | 25 | 5 (20.0) | 8 (32.0) | 8 (32.0) | 4 (16.0) | ||
| Grade | I | 14 | 3 (21.4) | 4 (28.6) | 4 (28.6) | 3 (21.4) | 0.514* |
| II | 59 | 11 (18.6) | 9 (15.3) | 23 (39.0) | 16 (27.1) | ||
| III | 22 | 6 (27.3) | 6 (27.3) | 8 (36.4) | 2 (9.1) | ||
| Type | Mucinous | 13 | 5 (38.5) | 3 (23.1) | 4 (30.8) | 1 (7.7) | 0.294** |
| Nonmucinous | 82 | 15 (18.3) | 16 (19.5) | 31 (37.8) | 20 (24.4) | ||
| pTNM | IIA | 9 | 3 (33.3) | 3 (33.3) | 3 (33.3) | 0 | 0.267** |
| IIB | 1 | 1 (100) | 0 | 0 | 0 | ||
| IIIA | 2 | 2 (100) | 0 | 0 | 0 | ||
| IIIB | 3 | 1 (33.3) | 1 (33.3) | 1 (33.3) | 0 | ||
| IIIC | 6 | 1 (16.7) | 1 (6.7) | 1 (16.7) | 3 (50.0) | ||
*Pearson Chi square, ** Fisher’s exact, level of significance at < 0.05
(Age ≤ 60 = age less than or equal to 60 years)
We observed a significant association between percentage of cells stained & tumor type, with score 3+ maximum in non mucinous type of colorectal cancer (p-value = 0.006 (Table 3)).
Table 3.
Percentage of Her-2 staining in colorectal cancer
| Her-2 score n (%) | p-value | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | ||||
| Gender | F | 44 | 7 (15.9) | 10 (22.7) | 10 (22.7) | 17 (38.6) | 0.592* |
| M | 51 | 13 (25.5) | 13 (25.5) | 8 (15.7) | 17 (33.3) | ||
| Age | ≤60 year | 70 | 15 (21.4) | 14 (20.0) | 15 (21.4) | 26 (37.1) | 0.408* |
| >60 year | 25 | 5 (20.0) | 9 (36.0) | 3 (12.0) | 8 (32.0) | ||
| Grade | I | 14 | 3 (21.4) | 4 (28.6) | 1 (7.1) | 6 (4.9) | 0.627** |
| II | 59 | 11 (18.6) | 12 (20.3) | 13 (22.0) | 23 (39.0) | ||
| III | 22 | 6 (27.3) | 7 (31.8) | 4 (18.2) | 5 (22.7) | ||
| Type | Mucinous | 13 | 5 (38.5) | 5 (38.5) | 3 (23.1)) | 0 | 0.006** |
| Nonmucinous | 82 | 15 (18.3) | 18 (22.0) | 15 (18.3) | 34 (41.5) | ||
| pTNM | IIA | 9 | 3 (33.3) | 4 (44.4) | 2 (22.2) | 0 | 0.028** |
| IIB | 1 | 1 (100) | 0 | 0 | 0 | ||
| IIIA | 2 | 2 (100) | 0 | 0 | 0 | ||
| IIIB | 3 | 1 (33.3) | 0 | 2 (66.7) | 0 | ||
| IIIC | 6 | 1 (16.7) | 1 (16.7) | 0 | 4 (66.7) | ||
*Pearson Chi square, ** Fisher’s exact, level of significance at < 0.05
(Age ≤ 60 = age less than or equal to 60 years)
Discussion
In this study our focus was to evaluate Her-2/neu expression in colorectal adencarcinoma and to correlate it with various clinicopathological variables. Various research groups have investigated the expression patterns of Her-2/neu in colorectal carcinomas with a variability ranging from 0 to 84 % [9]. Average membranous expression of Her-2/neu in colorectal cancer is about 5 % while cytoplasmic expression ranges between 0-66 % with an average of 30 % [9]. These variable data could be attributed to several factors including use of different antibodies, different sample size, and use of non-uniform scoring system for interpretation of results amongst others [9]. We have highlighted some of the studies done in the past which observed correlation between Her-2/neu expression in colorectal cancer & grade and stage of tumor using different antibodies (Table 4). Hence, to avoid the inconsistency in results and for better reporting of Her-2/neu expression in colorectal carcinoma a standardized protocol is urgently in need. In breast cancer, Her-2/neu scoring follows Hercep test where membranous expression of Her-2/neu is evaluated by a cumulative score based on intensity of reactivity, complete or incomplete staining & percentage of cells stained [18]. In gastric cancer, Hofmann Validation scoring is recommended for membranous expression of Her-2/neu [19]. Whereas in colorectal cancer, a validated scoring has not been established yet. However, there are few studies which followed the three criterias related to Her-2/neu staining pattern, intensity and percentage of cells stained [15, 17, 20].
Table 4.
Correlation of Her-2+/neu cases of colorectal carcinoma with tumor grade & stage
| Author | Antibody used | Samples (n) | Her-2 + ve (%) | Grade | Stage | ||
|---|---|---|---|---|---|---|---|
| M | C | M + C | |||||
| Seo et al. [26] | Monoclonal 4B5 | 365 | 6 | - | - | NA | NA |
| Gill et al. [15] | Monoclonal RTU-CB11 | 40 | - | 57.5 | 7.5 | II/III | III/IV |
| Li et al. [27] | Hercep test Kit | 317 | 15.4 | - | - | NS | III |
| Anwar et al. [13] | Monoclonal PY1248 | 100 | 42 | - | - | I/II | NS |
| Kruszweski et al. [21] | Polyclonal A0485 | 202 | 26.7 | 66.3 | - | NA | NA |
| Kafi et al. [17] | Polyclonal K5204 | 69 | - | 27 | 14 | I | NA |
| Tavangar et al. [28] | Monoclonal | 55 | - | - | 21.8 | II/III | III |
M membranous, C cytoplasmic, M + C membranous + cytoplasmic expression, NA not associated, NS not stated in the manuscript
Our study observed majority of colorectal cancers with cytoplasmic expression of Her-2/neu (48 %) which corresponds to the studies which followed all the three parameters of Her-2/neu evaluation [15, 17, 20, 21]. In our series we found 26.6 % positivity for membranous Her-2/neu staining. On contrary to this, two studies from the same region observed higher percentage of membranous expression of Her-2/neu in colorectal carcinomas, 74.1 % (n = 31) and 42 % (n = 100) respectively [13, 22, 23]. The reason for the difference in the rate might be due to different scoring protocols where they took into account only the membranous expression of Her-2/neu staining and did not consider the cytoplasmic expression. In the present study, we found a significant association between pattern of Her-2/neu staining & grade of colorectal cancer which is in accordance with one of the study done in the same region but to note here is that they compared the significance of Her-2/neu expression in different grades amongst different types of gastrointestinal carcinomas (gastric, small intestine and colorectal carcinomas) and did not primarily correlate the Her-2/neu expression within different grades of colorectal carcinoma. Similarly, the other study done in our area reported a significant membranous Her-2/neu expression in low grade colorectal carcinoma [13]. On contrary to this we noted membranous Her-2/neu expression to be significantly associated with high grade colorectal cancer. As mentioned earlier that these disagreements might be due to difference in scoring protocol, where they observed only membranous staining.
A study from West, conducted on 121 colorectal cancer cell lines for Her-2/neu expression, observed 63.5 % cases with cytoplasmic expression and a significant association with low grade colorectal carcinoma [24]. Their finding was similar to ours with respect to association with grade of colorectal cancer. While, study from Iran & India found significant association of cytoplasmic Her-2 expression with high grade (100 %). This discrepancy might be due to the fact that they had only two cases classified under high grade cancer [15, 20, 24].
A very few studies attempted to study the intensity & percentage of Her-2/neu staining where as similar to our study they also depicted insignificant findings [15, 17, 20]. These studies suggest that in future focus should be made on the pattern of Her-2/neu staining in colorectal carcinomas. Membranous Her-2/neu expression in colorectal cancer could be a future extracellular therapeutic target by Trastuzumab. An intracellular kinase inhibitor, Lapatinib has been recently approved for Trastuzumab resistant breast cancer patients [25]. Assuming the role of cytoplasmic Her-2/neu expression in pathogenesis of colorectal carcinoma, Lapatinib could be advancement in the treatment of colorectal cancer patients.
One of the limitation of our study was small sample size of colectomy cases (n = 21) to investigate the association between pTNM & Her-2/neu expression. Although, a significant association is seen between percentage of cells stained with Her-2/neu & stage of tumor, this might have low statistical power. Nevertheless, our study is an extensive effort done in South Asia region which observed all the three parameters of Her-2/neu expression in colorectal carcinoma.
Conclusion
Our study concludes a high expression of Her-2/neu in colorectal cancer in our population. A significant strong association of cytoplasmic Her-2/neu expression with low grades whereas membranous Her-2/neu expression with high grade colorectal cancer. These findings may help in conducting clinical trials in future to explore its therapeutic significance as well.
Acknowledgement
We thank Prof. Dr. Shaheen Sharafat, Director, DDRRL for her support during conducting the laboratory procedures at DDRRL.
Funding
Self finance by the corresponding author.
Availability of data and material
We state that data will not be shared as it is not permitted under the policy of DUHS. The data that support the findings of this study are available from DDRRL, DUHS. But restrictions apply to the availability of these data, which were used under the license for the current study & so are not publicly available. Data are however available from the authors upon reasonable request & with permission of DDRRL, DUHS.
Authors’ contributions
AS & TM planned the study. AS, TM, AK & AQ designed the manuscript. AK & SA performed colonoscopic biopsies. AS performed laboratory procedures under the guidance of TM. AS wrote the paper. All the authors reviewed & approved the manuscript.
Authors’ information
AS & TM are histopathologists. TM is the professor & dean of Basic Medical Sciences, Dow University of Health Sciences. AQ is assistant professor at DUHS & AvH Postdoctoral Fellow (Germany). AK & SA are consultant gastroenterologists & assistant professors at DUHS.
Competing interest
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval & consent to participate
The Institutional Review Board of Dow University of Health Sciences has approved the study. Reference number: IRB-480/DUHS/14.
Abbreviations
- DDRRL
Dow Diagnostic Research and Reference Laboratory
- DUHS
Dow University of Health Sciences
- H&E
Hematoxylin & eosin
- Her-2
Human Epidermal Growth Factor Receptor
- NILGID
National Institute of Liver & Gastrointestinal Diseases
- WHO
World Health Organization
Contributor Information
Asma Shabbir, Email: drasma52@gmail.com.
Talat Mirza, Email: talat.mirza@duhs.edu.pk.
Abdullah Bin Khalid, Email: abdullah.khalid@duhs.edu.pk.
Muhammad Asif Qureshi, email2asifqureshi@gmail.com.
Sadaf Ahmed Asim, Email: doc.sadaf.ahmed@gmail.com.
References
- 1.Cancer IAfRo. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. World Health Organization. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed 9 Oct 2014.
- 2.Ng SC, Wong SH. Colorectal cancer screening in Asia. Br Med Bull. 2013;105(1):29–42. doi: 10.1093/bmb/lds040. [DOI] [PubMed] [Google Scholar]
- 3.Zahir MN, Azhar EM, Rafiq S, Ghias K, Shabbir-Moosajee M. Clinical features and outcome of sporadic colorectal carcinoma in young patients: a cross-sectional analysis from a developing country. ISRN Oncol. 2014;2014:461570. doi: 10.1155/2014/461570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.New A, Whitney-Miller CL, Hicks DG. HER2 Testing in Gastric and Esophageal Adenocarcinoma: Emerging Therapeutic Options and Diagnostic Challenges. Connection. 2010;47-51. [DOI] [PubMed]
- 5.Ung L, Chua TC, Merrett ND. Targeting HER2 amplifications in gastric cancer. Gastrointestinal Cancer Targets Ther. 2014;4:11–22. [Google Scholar]
- 6.Bang Y-J. Advances in the management of HER2-positive advanced gastric and gastroesophageal junction cancer. J Clin Gastroenterol. 2012;46(8):637–48. doi: 10.1097/MCG.0b013e3182557307. [DOI] [PubMed] [Google Scholar]
- 7.Song Y, Huang J, Wang JW. Relationship between HER2/neu gene amplification and protein expression and prognosis in patients with advanced gastric carcinoma. Chin J Cancer. 2010;29(1):76–81. doi: 10.5732/cjc.009.10377. [DOI] [PubMed] [Google Scholar]
- 8.Boku N. HER2-positive gastric cancer. Gastric Cancer. 2014;17(1):1–12. doi: 10.1007/s10120-013-0252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blok EJ, Kuppen PJ, van Leeuwen JE, Sier CF. Cytoplasmic overexpression of HER2: a key factor in colorectal cancer. Clin Med Insights Oncol. 2013;7:41. doi: 10.4137/CMO.S10811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demirbaş S, Sücüllü I, Yildirim S, Celenk T. Influence of the c-erb B-2, nm23, bcl-2 and p53 protein markers on colorectal cancer. Turk J Gastroenterol. 2006;17(1):13–9. [PubMed] [Google Scholar]
- 11.Jesus EC, Matos D, Artigiani R, Waitzberg AF, Goldenberg A, Saad SS. Assessment of staging, prognosis and mortality of colorectal cancer by tumor markers: receptor erbB-2 and cadherins. Acta Cir Bras. 2005;20(6):422–7. doi: 10.1590/S0102-86502005000600005. [DOI] [PubMed] [Google Scholar]
- 12.Ramanathan RK, Hwang JJ, Zamboni WC, Sinicrope FA, Safran H, Wong MK, et al. Low Overexpression of HER-2/Neu in Advanced Colorectal Cancer Limits the Usefulness of Trastuzumab (Herceptin®) and Irinotecan as Therapy. A Phase II Trial#. Cancer Invest. 2004;22(6):858–65. doi: 10.1081/CNV-200039645. [DOI] [PubMed] [Google Scholar]
- 13.Anwar S, Nagi A, Naseem N, Saqib M, Sami W. Clinicopathological pattern and HER 2/neu status in patients presenting with different histological grades of colorectal carcinomas. Basic Appl Pathol. 2010;3(1):21–6. doi: 10.1111/j.1755-9294.2009.01069.x. [DOI] [Google Scholar]
- 14.Farzand S, Siddique T, Saba K, Bukhari MH. Frequency of HER2/neu overexpression in adenocarcinoma of the gastrointestinal system. World J Gastroenterol. 2014;20(19):5889. doi: 10.3748/wjg.v20.i19.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill MK, Manjari M, Jain K, Kaur T. Expression of Her-2/neu in colon carcinoma and its correlation with the histological grades and the lymph nodes status. JCDR. 2011;5(8):1564–8. [Google Scholar]
- 16.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 17.Kafi SG, Lari S, Nassiri G. HER2/neu expression in colon adenocarcinoma and its correlation with clinicopathologic variables. IJBMS. 2006;9(1):64. [Google Scholar]
- 18.Wolff AC, Hammond MEH, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131(1):18–43. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann M, Stoss O, Shi D, Büttner R, Van De Vijver M, Kim W, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52(7):797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 20.Al-Temimi SMA. HER-2/neu expression in colorectal carcinoma and its correlation with pathological parameters by immunohistochemistery. Med J Babylon. 2014;8:2. doi: 1812-156X-8-2.
- 21.Kruszewski WJ, Rzepko R, Ciesielski M, Szefel J, Zieliński J, Szajewski M, et al. Expression of HER2 in colorectal cancer does not correlate with prognosis. Dis Markers. 2010;29(5):207–12. doi: 10.1155/2010/109063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKay J, Loane J, Ross V, Ameyaw M, Murray G, Cassidy J, et al. c-erbB-2 is not a major factor in the development of colorectal cancer. Br J Cancer. 2002;86(4):568–73. doi: 10.1038/sj.bjc.6600127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farzand S, Siddique T, Saba K, Bukhari MH. Frequency of HER2/neu overexpression in adenocarcinoma of the gastrointestinal system. World J Gastroenterol. 2014;20(19):5889–96. doi: 10.3748/wjg.v20.i19.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Half E, Broaddus R, Danenberg KD, Danenberg PV, Ayers GD, Sinicrope FA. HER‐2 receptor expression, localization, and activation in colorectal cancer cell lines and human tumors. Int J Cancer. 2004;108(4):540–8. doi: 10.1002/ijc.11599. [DOI] [PubMed] [Google Scholar]
- 25.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 26.Seo AN, Kwak Y, Kim D-W, Kang S-B, Choe G, Kim WH, et al. HER2 status in colorectal cancer: its clinical significance and the relationship between HER2 gene amplification and expression. PLoS One. 2014;9(5):e98528. doi: 10.1371/journal.pone.0098528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, Wang D, Li J, Chen P. Clinicopathological and prognostic significance of HER-2/neu and VEGF expression in colon carcinomas. BMC Cancer. 2011;11(1):277. doi: 10.1186/1471-2407-11-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tavangar SM, Shariftabrizi A, Soroush AR. Her–2/neu over-expression correlates with more advanced disease in Iranian colorectal cancer patients. Med Sci Monitor Basic Res. 2005;11(3):CR123–6. [PubMed] [Google Scholar]


