Abstract
A basic understanding of evolving 3D technology enables the echocardiographer to master the new skills necessary to acquire, manipulate, and interpret 3D datasets. Single button activation of specific 3D imaging modes for both TEE and transthoracic echocardiography (TTE) matrix array probes include (a) live, (b) zoom, (c) full volume (FV), and (d) color Doppler FV. Evaluation of regional LV wall motion by RT 3D TEE is based on a change in LV chamber subvolume over time from altered segmental myocardial contractility. Unlike standard 2D TEE, there is no direct measurement of myocardial thickening or displacement of individual segments.
Keywords: Echocardiographer, Evolving, Manipulate
INTRODUCTION
Ease of acquisition with rapid online display of detailed dynamic three-dimensional (3D) images has allowed real-time (RT) 3D transesophageal echocardiography (TEE) to be increasingly used in the perioperative period to assess cardiac anatomy and function.[1,2,3,4] A moving (dynamic) 3D object is often referred to as four-dimensional (4D), with time considered as the fourth dimension. A basic understanding of evolving 3D technology enables the echocardiographer to master the new skills necessary to acquire, manipulate, and interpret 3D datasets.
THREE-DIMENSIONAL TECHNOLOGY
A fundamental difference between two-dimensional (2D) and 3D echo imaging is how the image is acquired and displayed. Volume scanning is used in RT 3D echocardiography as compared with the standard sector planes in 2D. This requires the use of special ultrasound probes to acquire raw data and integrated ultrasound machine software to process 3D datasets.[1,5]
RT 3D echocardiography uses probes with special matrix array transducers that contain 2500 piezoelectric crystals arranged in fifty rows by fifty columns [Figure 1]. Each crystal can be independently activated (fully sampled), focused and steered to generate an ultrasound beam in the azimuthal (x-y), and the elevational plane (x-z) to cover a 3D pyramidal scanning volume. Currently, there are two commercially available 3D/4D TEE probes, the 5–7 MHz X7-2t (Philips Healthcare, Andover, MA, USA) and the newly released 3–8 MHz 6VT-D (GE Healthcare, Wauwatosa, WI, USA). These TEE probes can also perform all standard 2D functions including M-mode and Doppler (spectral, color, and tissue) modes. The additional useful feature of multiplane modes, biplane (Philips Healthcare), and triplane (GE Healthcare) can simultaneously display independent 2D scanning planes from the same heartbeat.
Figure 1.
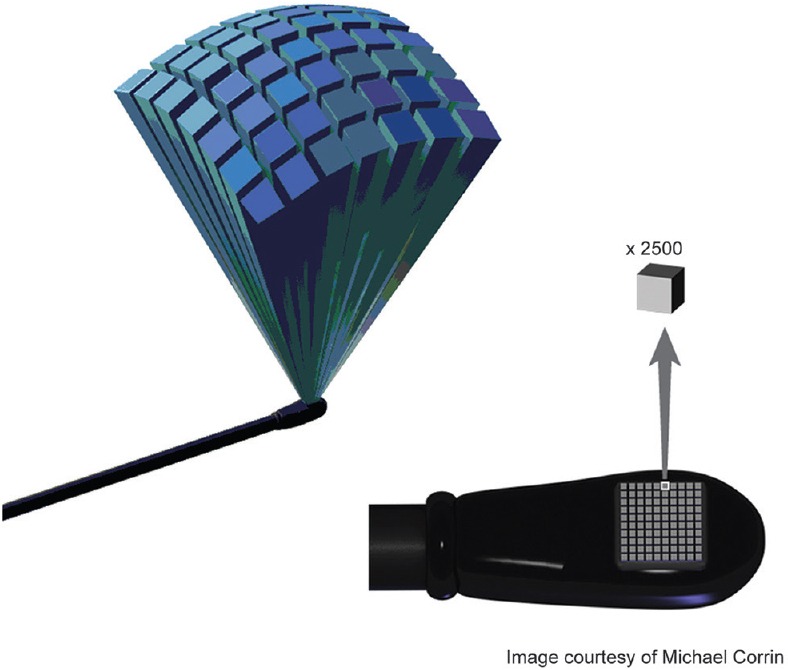
A matrix array transesophageal echocardiography probe is comprised 2500 piezoelectric crystals for which each can be independently activated to volume scan and acquire raw three-dimensional data
THREE-DIMENSIONAL DATASETS
Creation of a 3D ultrasound heart image using volume scanning involves four basic steps [Figure 2]:[1] Data acquisition,[2] data storage,[3] data processing, and[4] image display.
Figure 2.
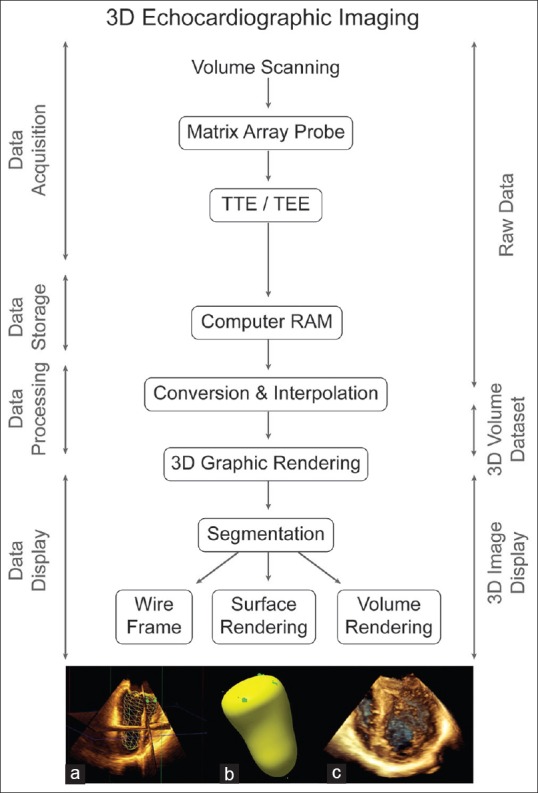
Algorithm three-dimensional imaging. Three-dimensional echocardiographic imaging includes four stages for data handling: acquisition, storage, processing, and display. Three-dimensional datasets can be rendered and displayed as (a) wire frame, (b) surface, or (c) volume as shown here for the left ventricle
Initial 3D data acquisition obtains echo characteristics of many single points within a volume of tissue. The matrix array probe is held immobile while the transducer automatically steers the ultrasound beam to scan a pyramidal volume of tissue in three dimensions[6]
Data storage maintains data flow from the initial raw data acquisition to the next step of data processing. This uses an onboard computer, so offline exportation and reconstruction of the 3D dataset are no longer required
Data processing consists of two integrated steps: Conversion and interpolation that transform the scanned raw data for a specific volume into a 3D dataset used to create a 3D object. This process positions and fills in the gaps between known data points in space to generate a 3D dataset comprised voxels or volume elements [Figure 3]. 3D image resolution now depends on voxel size, with larger voxels resulting in poorer resolution
Graphic rendering is a multistep graphics process that displays the 3D dataset as a 3D graphic object. The initial step, segmentation, separates out the object from surrounding tissue and delineates the 3D surfaces of cardiac tissue. The final image shown results from one of the three increasingly complex graphic rendering techniques: Wireframe, surface, or volume. Currently, all RT 3D imaging modes appear as a volume rendered 3D object with full details of the surface and inner structure.
Figure 3.
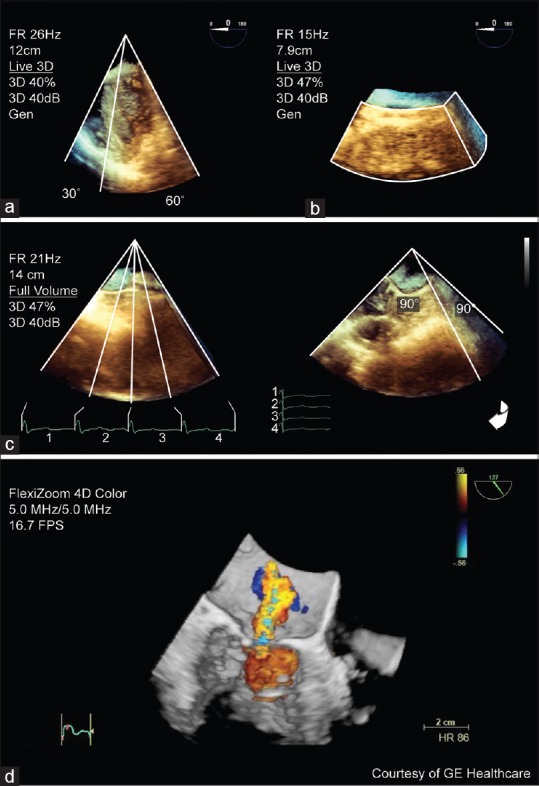
Three-dimensional imaging modes. The different three-dimensional imaging modes include; (a) live (30° × 60°) of a rotated mid-esophageal 4-chamber view; (b) zoom (90° × 90°) of an unrotated enface view of the mitral valve; (c) full volume (90° × 90°) of a mid-esophageal 4-chamber view as four segments joined together and rotated. A mitral regurgitation jet is shown in (d) three-dimensional color Doppler full volume
Despite being composed of voxels, these 3D objects are seen on the screen as pixels of a 2D image. Any rendered 3D object can be freely rotated in the display and shown from any orientation, including the surgeon's perspective. Cropping permits a “virtual dissection” of the 3D object that examines all its details.
THREE-DIMENSIONAL IMAGING MODES
The acquisition of 3D TEE images can occur instantaneously either in (a) RT (live) or (b) gated that is timed to the electrocardiogram (ECG) over multiple heartbeats. Currently, the term “RT” refers to any 3D image that changes on the display as the probe is moved.[2] All forms of ultrasound imaging are limited by the speed of sound (1500 m/s), thus RT 3D TEE has a complex interdependence of temporal resolution, sector size, and image resolution.
Temporal resolution for 3D echo is actually the volume rate (volume/s), though frame rate (FR) still appears on the display. It is limited as there is insufficient time for sound to traverse large volumes of tissue. Spatial resolution is improved by reducing the volume size or increasing the number of scan lines per volume although a higher scan line density reduces temporal resolution. 3D technology is evolving to produce larger 3D volumes in RT with better temporal and spatial resolution.
Single button activation of specific 3D imaging modes for both TEE and transthoracic echocardiography (TTE) matrix array probes include (a) live, (b) zoom, (c) full volume (FV), and (d) color Doppler FV [Figure 4]. Choosing between modes for imaging a specific structure is a balance between selecting spatial resolution (pyramidal image size), temporal resolution (FR) and RT imaging [Table 1].
Figure 4.
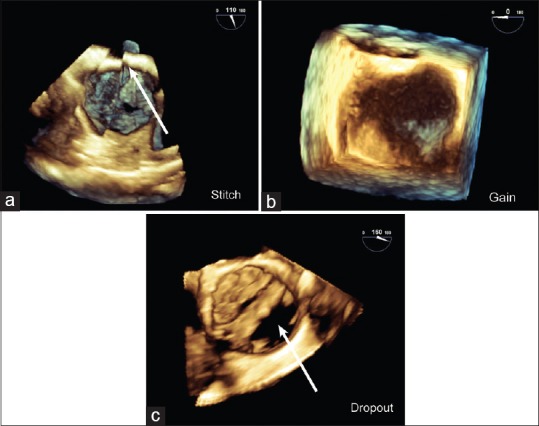
Artifacts. Common artifacts in three-dimensional images include (a) stitch artifact (arrow) between adjacent segments in a full volume en face mitral valve view; (b) overgain appears as brown speckles obscuring the mitral valve in this zoom en face mitral valve view; (c) Dropout from shadowing (arrow) is similar to a two-dimensional image as shown for a calcified bicuspid aortic valve in short-axis using live mode
Table 1.
Three-dimensional imaging modes
| Live | Zoom | Full volume | Color Doppler full volume | |
|---|---|---|---|---|
| Dimensions | 60°×30° by image depth | 20°×20°-100°×100° by variable height | 90°×90° by image depth | 40°×40° by limited height |
| RTa | Yes | Yes | No/yes | No/yes |
| Temporal resolution (FR, Hz) | 20-30 Hz | 5-15 Hz | 20-50 Hz | 15-25 Hz |
| High | Low | High | Mid | |
| Spatial resolution | Mid | High | High | Low |
| Cardiac structures | Any | MV, LAA, IAS | LV, RV, MV | Regurgitant jets, holes |
aThe ability to image in RT depends on ultrasound machine software. IAS: Interatrial septum, LAA: Left atrial appendage, LV: Left ventricle, MV: Mitral valve; RV: Right ventricle, RT: Real time
Live displays in RT, a 3D pyramidal volume with a limited sector size necessitating probe manipulation to image entire structures. Rotating this 3D image quickly checks and allows optimization of general 3D image settings
Zoom displays in RT, a magnified subsection of 3D pyramidal volume that is centered to a specific region of interest (e.g., the mitral valve [MV]). It is kept as small as is reasonable to improve the temporal resolution and image definition
FV is an ECG-gated acquisition of a large 3D pyramidal volume. Individual wedge-shaped subvolumes obtained over consecutive heartbeats (R wave of ECG) are stitched together and synchronized to the same cardiac cycle. Current ultrasound machine software (i.e., 33, Philips Healthcare) displays the FV in RT with the option to determine volume size (elevational and lateral controls) and volume rate (number of heart beats) independently
Color Doppler FV is a gated acquisition of a small 3D pyramidal volume with superimposed 3D representation of color Doppler flow that is displayed nearly instantaneously or in RT. Stitching artifacts are common as acquisition involves multiple subvolumes. Given the amount of information (3D volume and 3D flow), the current technology only creates a small 3D volume at a low FR.
IMAGE PROCESSING
Image optimization
Important determinants of 3D image quality and size during image acquisition are line density and transducer frequency. Line density is the number of scan lines per volume and affects the sector size and spatial resolution. A low line density increases the sector size but with poorer spatial resolution. The quality of the 3D image starts with adjustment of gain and compression to optimize the 2D image. Gain, brightness (increasing whiteness), and smoothing (reducing image coarseness) are adjustable 3D settings in RT or on any stored 3D datasets.
Image artifacts
A stitch artifact is a demarcation line between subvolumes in an FV volume rendered image. It is caused by arrhythmias, electrocautery, and probe movement and may distort anatomical structures potentially affecting analysis [Figure 5]. Excessive gain in a 3D image results in noise that appears as brown speckles and may obscure structures. Dropout is an absence of echoes and appears black on the display. It may result from insufficient gain, shadowing, or ultrasound attenuation. Thin (interatrial septum [IAS]) and distant structures (aortic and pulmonic valves) remain difficult to image by 3D TEE. Most artifacts present in a 2D image will also appear in the 3D image.
Figure 5.
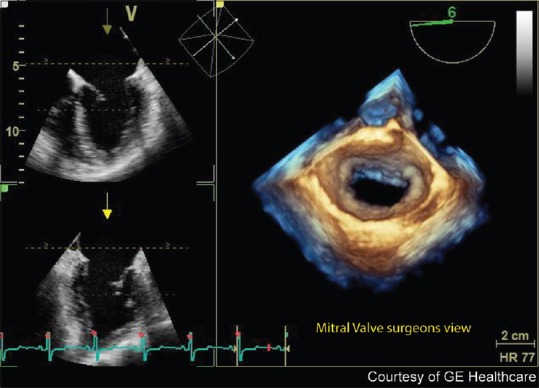
Mitral valve en face view. FlexiZoom (GE Healthcare, Wauwatosa, WI, USA) provides a single button three-dimensional image acquisition, optimization, and en face display of the mitral valve from the surgeon's perspective of the left atrium. This provides a quick, efficient way to assess the mitral valve without the need for manipulation of the three-dimensional dataset
Cropping and orientation
Any 3D volume can be cropped, rotated, and displayed from any perspective. It is now possible to present intraoperative RT 3D TEE images in the surgeon's orientation. The easily recognized aortic valve (AV) is often used as a reference to orient the 3D volume in space. Easier more intuitive cropping tools for live and stored 3D datasets facilitate 3D data interpretation. In addition, there is the opportunity to extract 2D slices from a 3D volume with analytic software (QLAB, Philips Healthcare) or in RT (FlexiSlice, GE Healthcare).
CLINICAL APPLICATIONS
Similar to 2D TEE, structures closest to the TEE probe are easily imaged in 3D,[6] this enables the evaluation of clinical relevant issues in RT [Table 2]. 3D echo has proven superior to 2D echo for the assessment of the left ventricle (LV) (volume, ejection fraction [EF], mass, and dysynchrony) and the MV. Despite recent recommendations for 3D echo image acquisition and display,[2] there remains a lack of a standardized protocol for the use of intraoperative RT 3D TEE in everyday practice. Although 3D image acquisition is rapid, analyzing the 3D datasets takes time, particularly when using analytical software. Time constraints and limitations with simple measurements (area, length) restrict the use of RT 3D TEE to its current role as a complement to 2D TEE studies in the hectic intraoperative environment.
Table 2.
Real-time three-dimensional transesophageal echocardiography adequacy of views and utility
| Structure | 3D viewsa (%) | View | 3D mode | Clinical utilityb |
|---|---|---|---|---|
| Mitral valve | 85-91 | ME 0-120° views±CD | Zoom, FV | Morphology, function, quantify (EROA in MR, MVA in MS) |
| Aortic valve | 18 | ME 60° SAX, 120° LAX±CD | Zoom | AV area, TAVI |
| Tricuspid valve | 11 | ME 0-30° 4C, TG 40°±CD | Zoom | Unstudied |
| Pulmonic valve | - | UE 90° ME 3C 120°±CD | Zoom | TEE limited visualization |
| IAS | 84 | ME 0° view | Zoom, FV | Atrial septal defect |
| Left ventricle | 77 | ME 0-120° views | FV | Volume, mass, EF, dysynchrony |
| Right ventricle | - | ME 0-120° views | FV | Volume, EF |
| LAA | 86 | ME 0°, 45°, 60°, 90° | Live, zoom | Thrombus |
| Left atrium | - | TG view | FV | Volume |
aReference,[6] bReferece.[2] C: Chamber, CD: Color Doppler, EF: Ejection fraction, EROA: Effective regurgitant orifice area, FV: Full volume, IAS: Interatrial septum, LAX: Long-axis, LAA: Left atrial appendage, ME: Mid-esophageal, MR: Mitral regurgitation, MS: Mitral stenosis, MVA: Mitral valve area, SAX: Short-axis, TAVI: Transcatheter aortic valve implantation, TG: Transgastric, UE: Upper esophageal, 3D: Three-dimensional, TEE: Transesophageal echocardiography
Mitral valve
The native or prosthetic MV is easily imaged with excellent spatial resolution by TEE using standard 2D planes and all 3D modes.[7,8] Where previously multiple 2D views were needed to assess MV pathology, the Zoom, and FV modes show the entire MV in a single screen display. The FV acquisition improves temporal resolution for the assessment of MV leaflet motion but may contain stitch artifacts which can complicate image interpretation.
The en face view of the MV in the surgeon's orientation as viewed from the left atrium (LA) with the AV at the top of the image (12 o'clock) and the left atrial appendage (LAA) to the left (9 o'clock) is widely used to display the MV in 3D [Figure 6]. Detailed MV anatomy of the leaflets (posterior scallops, anterior segments), commissures, and subvalvular components (chordae and papillary muscles) can be seen. Manipulation of the 3D image, by rotating and cropping, can better define complex MV pathology involving prolapse, clefts, and perforations.[7]
Figure 6.
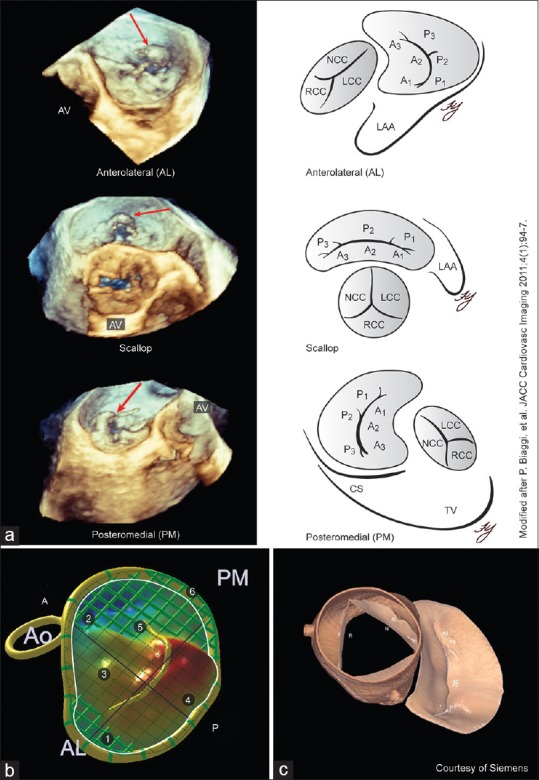
Mitral valve assessment. (a) Angled views obtained from manipulating an enface mitral valve three-dimensional dataset can improve visualization of pathology. The anterolateral, scallop, and posteromedial orientations for the mitral valve demonstrate a flail P2 segment (red arrow) with multiple torn chordae; (b and c) Q Lab Mitral Valve Quantification (Philips Medical Systems, Andover, MA, USA) creates a static three-dimensional model for the mitral valve with multiple measurements ©eSie Valves® (Siemens Medical Solutions, Malvern, PA, USA) analyzes three-dimensional datasets from any vendor to uniquely display a dynamic model of the both the mitral and aortic valves
Rotating the en face view through 360° [Figure 7] provides additional perspectives of the MV from “angled views;” anterolateral commissure, posterior scallops, and posteromedial commissure views.[9] Systematic use of these views is less time-consuming than off-line processing and enables accurate RT identification of prolapsed segments involving both commissures and the posterior leaflet.
Figure 7.
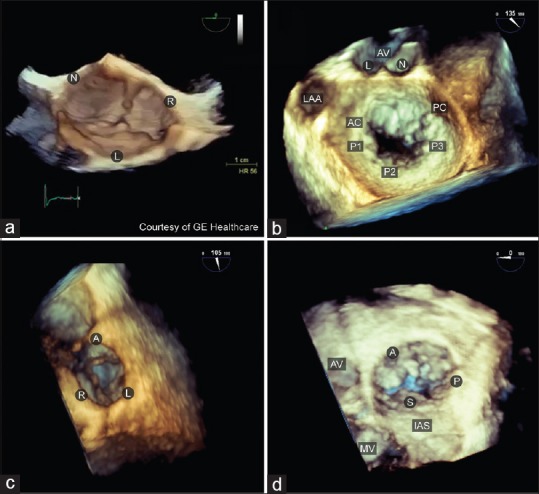
Native valves three--dimensional orientation and display. (a) The aortic valve is viewed from the ascending aorta with the right coronary cusp located at the 6 o'clock position; (b) mitral valve is viewed from the left atrium with the aortic valve located at the 12 o'clock position. The anterior mitral valve leaflet is at the top and the posterior leaflet below; (c) pulmonic valve is viewed from the pulmonary artery with the anterior leaflet is located at the 12 o'clock position. (d) tricuspid valve is viewed from the right atrium with the septal leaflet located at the 6 o'clock position
The off-line construction of dynamic or static 3D MV models [Figure 7] using proprietary software (Philips QLAB MVQ, TomTec 4D MV Assessment© and Siemens eSie Valves®) yields detailed quantitative information about the MV.[10] The preoperative use of 3D TEE may provide surgeons with additional information about MV pathology to guide individual patient management.
3D color Doppler assessment of the MV is limited by a small sector size, stitch artifacts, and poor temporal resolution which can complicate interpretation of pathological flow. Despite these limitations, there is better insight into the variable geometry of the mitral regurgitation (MR) jet from different MV pathologies. The more accurate measurement of an irregular--shaped proximal isovelocity area and vena contracta (VC) width may better quantify MR severity.[11]
Aortic valve
Live, zoom, or FV 3D en face AV views from the aorta or left ventricular outflow tract (LVOT) are orientated with the right coronary cusp positioned inferiorly at 6 o'clock [Figure 8]. However, echo dropout from the normal thin pliable and heavily calcified cusps makes the AV difficult to image by RT 3D TEE, although 3D AV modeling may overcome this.[5] 3D echo can better determine native AV area by planimetry as it identifies the smallest orifice or by the continuity equation with a more accurate measure of LVOT area.[1] TEE has proven a useful adjunct during transcatheter AV implantation for positioning and in assessing function.[12]
Figure 8.
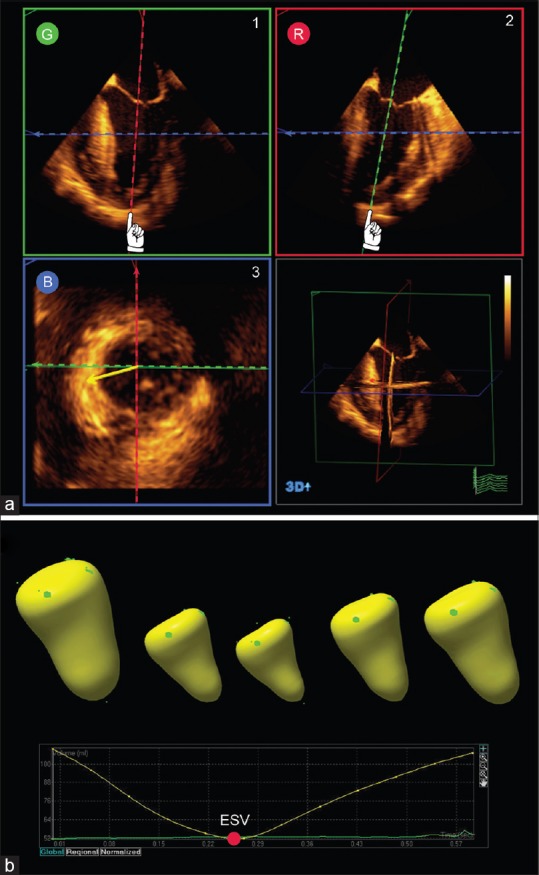
Left ventricular assessment. (a) Biplane analysis uses multiplanar reconstruction (G, green; R, red; B, blue) to align the planes through the left ventricle apex; (b) a semi-automated algorithm generates a surface rendered endocardial cast that calculates the left ventricle volume for each frame in the cardiac cycle. Ejection fraction is determined from the end-diastolic and end-systolic volumes
Tricuspid valve
3D TEE imaging of the normal TV is difficult due to echo dropout from its anterior location and thin leaflets. 2D or 3D transthoracic or epicardial echo can better view anterior cardiac structures. Zoom (mid-esophageal [ME] right ventricular outflow tract view) or FV (ME 4-chamber [4C]) modes can image the entire TV. Incorporation of part of the AV aids image orientation. The recommended orientation for the TV is with the IAS positioned inferiorly (at 6 o'clock) when viewed from either the RA or RV [Figure 8]. This is similar to the surgeon's orientation through a right atriotomy which positions the MV at the bottom of the display, the AV at 9 o'clock and individual TV leaflets: Anterior (9 o'clock), posterior (3 o'clock), and septal (6 o'clock).
Measurement of the TV orifice area by planimetry in rheumatic tricuspid stenosis requires off-line cropping through the smallest TV orifice using analytic software. RT 3D TEE color Doppler more accurately measures the irregular-shaped cross-sectional area of the VC of a tricuspid regurgitant (TR) jet which can improve the accuracy of TR assessment.[11]
Pulmonic valve
The PV is the most anterior with the thinnest cusps of all cardiac valves making it barely visible with 2D or 3D TEE. 2D or RT 3D epicardial echo should be considered for the intraoperative assessment of the PV in selected cases. 3D acquisition using zoom or FV modes begins in the UE arch short-axis (SAX) view which brings the PV closest to the probe. Recommended orientation for the PV is viewed from the pulmonary artery with the anterior leaflet located at the 12 o'clock position [Figure 8].
Quantification of pulmonic insufficiency (PI) can be estimated using RT 3D TTE color Doppler by measuring the regurgitant jet VC area that, when multiplied by the velocity-time integral of the PI jet estimates the regurgitant volume.
Left and right ventricles
Left ventricular volume, global and regional wall motion, mass and synchronicity can be accurately assessed using 3D echo.[2] Off-line measurement of the crescent-shaped RV volume and function can be determined using special analytical software from FV datasets of the RV.[13] The accuracy of 3D TTE in measuring LV and RV volume is comparable to 2D TTE, MRI, and CT techniques.[2] FV acquisition is the only 3D imaging modality that captures the entire LV at sufficient FR (25 Hz) for dynamic assessment of LV volume using two methods: 3D-guided biplanes or direct volumetric analysis.
The 3D-guided biplane method more easily positions two perpendicular 2D planes to accurately cut the LV along its long axis at the true apex, creating ideal ME 4C and 2C 2D views [Figure 9]. The LV volume, EF, and mass are calculated by applying the modified Simpson's biplane method of the disks to the end-systolic and end-diastolic frames. This method minimizes foreshortening of the LV using TEE but still relies on geometric assumptions.
Figure 9.
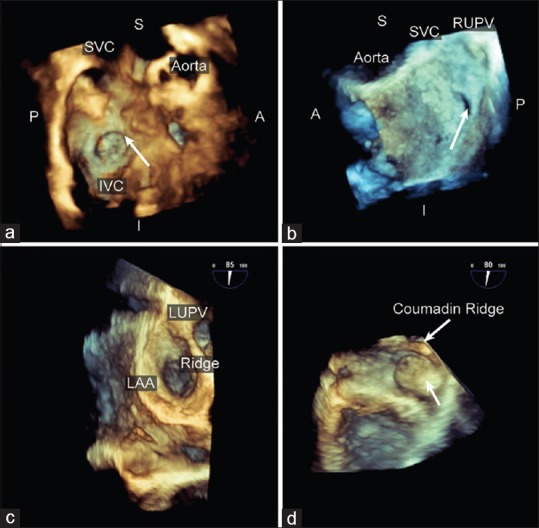
Interatrial septum and left atrial appendage. The intra-atrial septum can be interrogated using a full volume dataset orientated to be viewed from the (a) the right atrium with the superior vena cava at 11 o'clock or (b) the right atrium with the right upper pulmonary vein at 1 o'clock. Shown is a patient with a patent foramen ovale compared with the surgical findings; (c) The left atrial appendage is presented en face from the right atrium perspective with the left upper pulmonary vein located superiorly; (d) the left atrial appendage thrombus (arrow) is shown in this live longitudinal view of the left atrial appendage
Direct volumetric analysis measures LV volume throughout the cardiac cycle from a surface rendered cast of the LV cavity [Figure 9]. The end-diastolic volume and end-systolic volume are measured, and the stroke volume and EF are calculated. This method more accurately quantifies LV volumes, particularly in patients with an abnormal ventricular shape or regional wall motion abnormalities. This in part relates to better alignment through the cardiac apex, inclusion of more endocardial surface during analysis and the lack of geometric shape assumption.
Evaluation of regional LV wall motion by RT 3D TEE is based on a change in LV chamber subvolume over time from altered segmental myocardial contractility. Unlike standard 2D TEE, there is no direct measurement of myocardial thickening or displacement of individual segments.
Interatrial septum
The IAS is well imaged using 3D TEE either with zoom or FV modes.[14] The IAS can be orientated to be shown from the LA or RA to demonstrate common pathology such as a patent foramen ovale or atrial septal defect (ASD). RT 3D TEE better defines the shape and the spatial relations of an ASD with the surrounding structures such as the AV and great vessels. The use of TEE to guide the placement of percutaneous device closure of holes in the heart including not only ASD but also ventricular septal defect and paravalvular leaks of prosthetic valves has been well described.[15]
Left atrial appendage
The LAA has a pyramidal shape and can be imaged in all RT 3D TEE imaging modes. Although 2D TEE is considered the gold standard for the assessment of LAA thrombus, RT 3D TEE provides a higher specificity in defining LAA pathology than 2D TEE. The use of RT 3D TTE provides similar accuracy but lower specificity than TEE in ruling out LAA thrombus.[2]
Aorta
3D TEE can image the entire aorta, excluding the blind spot, to assess for aortic pathology.[16] Details of complex aortic root pathology including aortic aneurysm, aortic dissection, pseudoaneurysm of the intravalvular fibrosa and sinus of valsalva aneurysm can be well imaged. Better topographic definition of the atheromatous disease can be obtained using 3D TEE and epiaortic scanning.
Masses
The location and attachment of intracardiac masses can be accurately assessed using RT 3D TEE to facilitate surgical planning.[17] Measurement of any mass size is performed in 2D or 3D with analytical software.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
TAKE HOME MESSAGES
This enterprising review from a great author will intrigue the readers to know from her on the subject. She describes the principles of 3D TEE technology and its clinical applications for all the four valves, chambers and the aorta. The author describes that real time 3D TEE for regional wall motion evaluation, unlike that in 2D TEE, there is no direct measurement of myocardial thickening or displacement of individual segments, but that is it based on a change in LV chamber sub-volume over time.
REFERENCES
- 1.Houck RC, Cooke JE, Gill EA. Live 3D echocardiography: A replacement for traditional 2D echocardiography? AJR Am J Roentgenol. 2006;187:1092–106. doi: 10.2214/AJR.04.0857. [DOI] [PubMed] [Google Scholar]
- 2.Lang RM, Badano LP, Tsang W, Adams DH, Agricola E, Buck T, et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J Am Soc Echocardiogr. 2012;25:3–46. doi: 10.1016/j.echo.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Vegas A, Meineri M. Core review: Three-dimensional transesophageal echocardiography is a major advance for intraoperative clinical management of patients undergoing cardiac surgery: A core review. Anesth Analg. 2010;110:1548–73. doi: 10.1213/ANE.0b013e3181d41be7. [DOI] [PubMed] [Google Scholar]
- 4.Vegas A, Meineri M, Jerath A. New York: Springer; 2012. Real-Time Three-Dimensional Transesophageal Echocardiography. A Step-by-Step Guide. [Google Scholar]
- 5.Salgo IS. Three-dimensional echocardiographic technology. Cardiol Clin. 2007;25:231–9. doi: 10.1016/j.ccl.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Sugeng L, Shernan SK, Salgo IS, Weinert L, Shook D, Raman J, et al. Live 3-dimensional transesophageal echocardiography initial experience using the fully-sampled matrix array probe. J Am Coll Cardiol. 2008;52:446–9. doi: 10.1016/j.jacc.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 7.Salcedo EE, Quaife RA, Seres T, Carroll JD. A framework for systematic characterization of the mitral valve by real-time three-dimensional transesophageal echocardiography. J Am Soc Echocardiogr. 2009;22:1087–99. doi: 10.1016/j.echo.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Sugeng L, Shernan SK, Weinert L, Shook D, Raman J, Jeevanandam V, et al. Real-time three-dimensional transesophageal echocardiography in valve disease: Comparison with surgical findings and evaluation of prosthetic valves. J Am Soc Echocardiogr. 2008;21:1347–54. doi: 10.1016/j.echo.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Biaggi P, Gruner C, Jedrzkiewicz S, Karski J, Meineri M, Vegas A, et al. Assessment of mitral valve prolapse by 3D TEE angled views are key. JACC Cardiovasc Imaging. 2011;4:94–7. doi: 10.1016/j.jcmg.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Ionasec RI, Voigt I, Georgescu B, Wang Y, Houle H, Vega-Higuera F, et al. Patient-specific modeling and quantification of the aortic and mitral valves from 4-D cardiac CT and TEE. IEEE Trans Med Imaging. 2010;29:1636–51. doi: 10.1109/TMI.2010.2048756. [DOI] [PubMed] [Google Scholar]
- 11.Sugeng L, Weinert L, Lang RM. Real-time 3-dimensional color Doppler flow of mitral and tricuspid regurgitation: Feasibility and initial quantitative comparison with 2-dimensional methods. J Am Soc Echocardiogr. 2007;20:1050–7. doi: 10.1016/j.echo.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 12.Jayasuriya C, Moss RR, Munt B. Transcatheter aortic valve implantation in aortic stenosis: The role of echocardiography. J Am Soc Echocardiogr. 2011;24:15–27. doi: 10.1016/j.echo.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Horton KD, Meece RW, Hill JC. Assessment of the right ventricle by echocardiography: A primer for cardiac sonographers. J Am Soc Echocardiogr. 2009;22:776–92. doi: 10.1016/j.echo.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 14.Saric M, Perk G, Purgess JR, Kronzon I. Imaging atrial septal defects by real-time three-dimensional transesophageal echocardiography: Step-by-step approach. J Am Soc Echocardiogr. 2010;23:1128–35. doi: 10.1016/j.echo.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Perk G, Lang RM, Garcia-Fernandez MA, Lodato J, Sugeng L, Lopez J, et al. Use of real time three-dimensional transesophageal echocardiography in intracardiac catheter based interventions. J Am Soc Echocardiogr. 2009;22:865–82. doi: 10.1016/j.echo.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 16.Evangelista A, Aguilar R, Cuellar H, Thomas M, Laynez A, Rodríguez-Palomares J, et al. Usefulness of real-time three-dimensional transoesophageal echocardiography in the assessment of chronic aortic dissection. Eur J Echocardiogr. 2011;12:272–7. doi: 10.1093/ejechocard/jeq191. [DOI] [PubMed] [Google Scholar]
- 17.Müller S, Feuchtner G, Bonatti J, Müller L, Laufer G, Hiemetzberger R, et al. Value of transesophageal 3D echocardiography as an adjunct to conventional 2D imaging in preoperative evaluation of cardiac masses. Echocardiography. 2008;25:624–31. doi: 10.1111/j.1540-8175.2008.00664.x. [DOI] [PubMed] [Google Scholar]


