Abstract
Transesophageal echocardiography (TEE) can be used to identify risk factors such as aortic atherosclerosis[2] before any sort of surgical manipulations involving aorta and its related structures. TEE has become an important noninvasive tool to diagnose acute thoracic aortic pathologies. TEE evaluation of endoleaks helps early detection and immediate corrective interventions. TEE is an invaluable imaging modality in the management of aortic pathology. TEE has to a large extent improved the patient outcomes.
Keywords: Bedside, Mortality, Proximal
INTRODUCTION
Atherosclerosis of the ascending aorta (AA) and aortic arch is recognized as one of the important predictors of postoperative stroke after cardiac surgery. Aortic dissection and rupture are emergent situations requiring a rapid and accurate diagnosis to reduce the morbidity and mortality.[1] Transesophageal echocardiography (TEE) can be used to identify risk factors such as aortic atherosclerosis[2] before any sort of surgical manipulations involving aorta and its related structures. TEE has become an important noninvasive tool to diagnose acute thoracic aortic pathologies.
Epiaortic scanning (EAS)[3] is ideal in high-risk patients to identify the sites of severe atherosclerosis on the distal AA and the proximal aortic arch. A novel TEE technique, so-called TEE A-view, by van Zaane et al.,[4] overcomes the “blind spot” of TEE. This allows bedside imaging of the distal AA, the aortic arch, and cerebral branch vessels. With this additional TEE information, appropriate modifications of the surgical techniques can be made to reduce morbidity and mortality.
ANATOMY OF THORACIC AORTA
The thoracic aorta is divided into four segments surgically: Aortic root, AA, aortic arch, and descending thoracic aorta. Aortic root consists of the aortic annulus, aortic cusps, sinuses of valsalva, and sinotubular junction (STJ). The diameter of the aorta varies between 33 and 35 mm with a wall thickness of about 2 mm. The AA with a length of about 7–11 cm originates from the aortic annulus at the level of STJ crossing beneath the main pulmonary artery, then courses in an anterior and rightward direction over the origin of the right pulmonary artery, which terminates and continues as the aortic arch at the origin of the innominate artery. The aortic arch courses in a posterior and leftward direction giving rise to the innominate (brachiocephalic), left common carotid (LCC), and left subclavian arteries (LSAs). The underlying trachea makes it difficult to visualize the distal AA and the proximal aortic arch.
The descending thoracic aorta begins distal to the LSA at the level of the ligamentum arteriosum and courses anteriorly and caudally toward the diaphragmatic hiatus. At the site of the ligamentum arteriosum is a relatively fixed narrowing called the aortic isthmus which is the most common site of traumatic decelerating injury. The aorta courses in a smooth curved fashion from the anterolateral side of the 4th thoracic vertebra to the anterior surface of the 11th vertebral body. At distal level, the descending thoracic aorta lies directly posterior to the esophagus [Figure 1].
Figure 1.
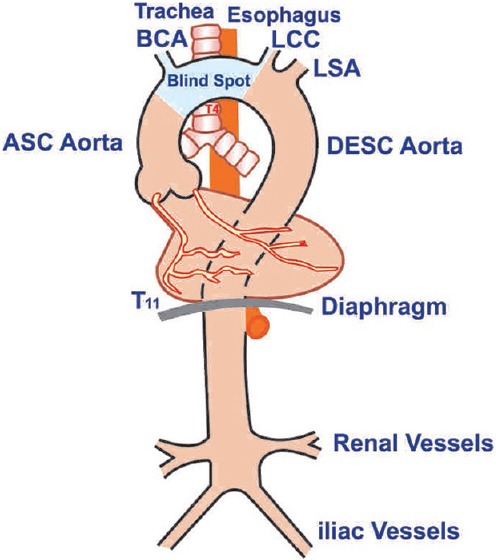
Anatomical course of the aorta from the aortic root down to its bifurcation (illustrations by Srinivasa Holla)
ECHOCARDIOGRAPHIC EVALUATION OF THE THORACIC AORTA
Primarily insertion of the TEE probe must be performed gently with due consideration to abandon the procedure if resistance is encountered, more so in patients with suspected aortic pathology.
As per the SCA/ASE guidelines, the following imaging planes are recommended for imaging the thoracic aorta.[5] Mid-esophageal (ME) aortic valve (AV) long-axis view, ME ascending (ASC) aortic short-axis (SAX) and long-axis (LAX) view, descending aortic short- and long-axis views, upper esophageal (UE) aortic arch long- and SAX views. The techniques of imaging have been dealt with in the earlier chapter.
ME AV LAX view [Figure 2] is important in evaluating the AV and the proximal AA. Measurements can be made of the left ventricular outflow tract, the aortic annulus, sinuses of valsalva, STJ, and AA if valve replacement and/or root reconstruction is being planned.
Figure 2.
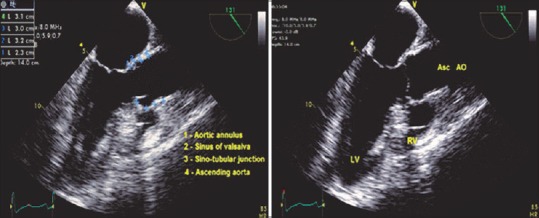
Mid-esophageal AV LAX view - measurements across root. LV: Left ventricle, RV: Right ventricle, ASC AO: Ascending aorta
ME ASC aortic SAX and LAX views [Figure 3] are crucial to exclude dissections in the aorta. Artifacts are commonly encountered within the AA in this view, hence the priority to distinguish artifacts from true dissections to prevent needless, expensive interventions.
Figure 3.
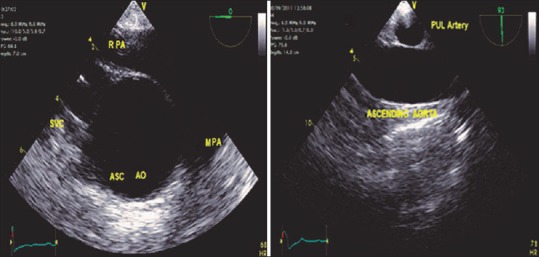
Mid-esophageal ASC AO SAX, LAX views. RPA: Right pulmonary artery, MPA: Main pulmonary artery, SVC: Superior vena cava, ASC AO: Ascending aorta, SAX: Short-axis, LAX: Long-axis
At the mid-esophageal level from the four-chamber view, rotating the probe in a counterclockwise direction or to the left posteriorly and optimizing the depth to 4–6 cm result in descending aortic SAX view. Increasing the multiplane angle to 90°, the descending aortic LAX view is obtained. This is an important view to evaluate many diagnostic issues such as the presence of an atheromatous plaque, dissection, aneurysm, left pleural effusion, and position of intra-aortic balloon [Figures 4 and 5].
Figure 4.
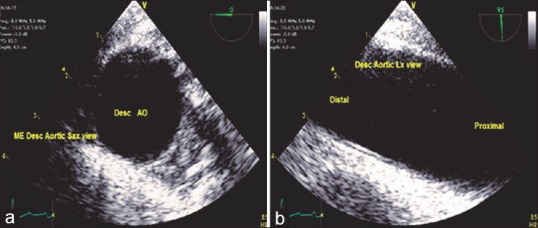
(a) Descending aortic short-axis view, (b) long-axis view - proximal, distal end
Figure 5.
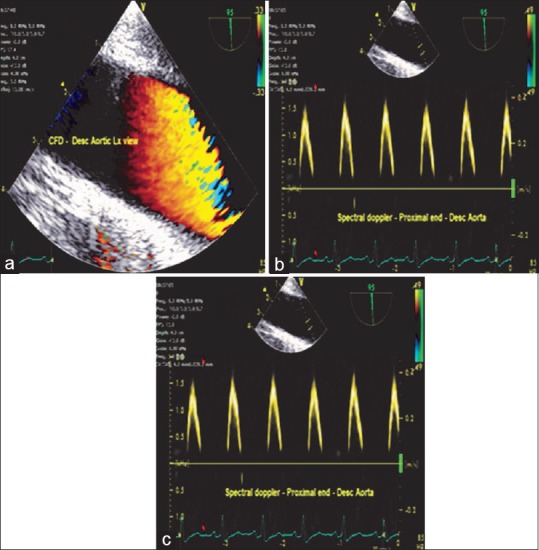
(a) Descending aortic long-axis view-color flow Doppler, (b) Spectral Doppler at the proximal and (c) distal end of the aorta depicted as above and below the baseline, respectively, indicating the flow toward and away from the transducer
The presence of a pathological lesion in the descending aortic view necessitates additional procedures such as TEE A-view and/or EAS of the distal AA and aortic arch, especially in the presence of an atheromatous plaque to alter the surgical plan.
A disadvantage of EAS is, however, that it can only be applied during surgery after sternotomy and cannot be used in closed chest procedures (transcatheter aortic valve implantation [TAVI]) or preoperative screening. Since the guidelines do not describe the views of this innovative technique, a short explanation is necessary.
TRANSESOPHAGEAL ECHOCARDIOGRAPHY A-VIEW METHOD
A recently introduced modification of conventional TEE, known as the A-view method, has proven capable of inspecting the distal AA and aortic arch. Assessment of the distal AA sign TEE is disturbed by the interposition of the air-filled trachea between the esophagus and the AA, the so-called “blind spot.”[6] Recently, the A-view (aortic view) method,[7] a modification of conventional TEE, was introduced to overcome this limitation. The A-view method uses an intratracheal balloon (the A-view catheter developed by Stroke2prevent [Figure 6a], Hilversum, the Netherlands) filled with saline to replace the air in the distal trachea and left main bronchus [Figure 6b]. It, therefore, becomes possible to assess the distal AA and aortic arch for the presence and severity of atherosclerosis before cardiac surgery, which provides the surgeon with 30–45 min of additional planning time. Figure 6c shows the TEE A-view distal AA long-axis view with a mobile plaque and Figure 6d shows the Diagrammatic representation of the image. Since visualization will only lead to an improved outcome, if the information is translated to an adjusted surgical approach, further clinical implementation and training is ongoing, but preliminary clinical outcome data are promising.[8]
Figure 6.
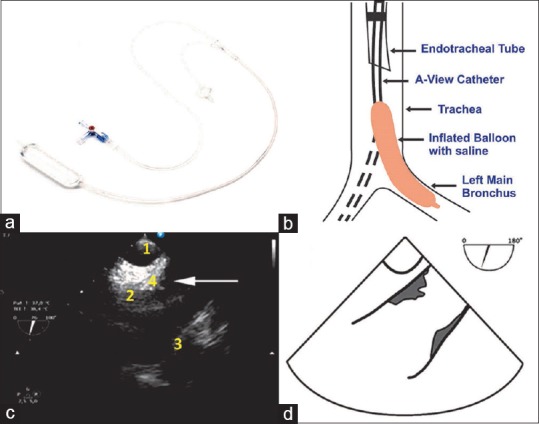
(a) The A-view catheter. (b) The A-view catheter in the trachea and left main bronchus. (c) The A-view distal ascending aorta long-axis view. An image of the trachea and the ascending aorta as imaged with the A-view method with plaque formation on the posterior and anterior wall (1: A-view catheter in the trachea, 2: The posterior wall of the distal ascending aorta, 3: The anterior wall of the distal ascending aorta, 4: A mobile protruding atheroma indicated by the arrow). (d) Diagrammatic representation of the image
A-view catheter
A-view catheter in situ.
Withdrawing the TEE probe and maintaining the descending aortic SAX view on the screen, the aorta changes its appearance from circular to longitudinal at the level of the aortic arch, UE aortic arch LAX view, and increasing the multiplane angle to 90°, the UE aortic arch SAX view is obtained. The diagnostic issues related to this view include recognition of a patent ductus arteriosus, spectral Doppler assessment of the pulmonary artery, and presence of atheromatous plaques in the aortic arch. The origins of the left subclavian, LCC, and brachiocephalic arteries can be visualized frequently by minimal rotation of the TEE probe [Figure 7].
Figure 7.
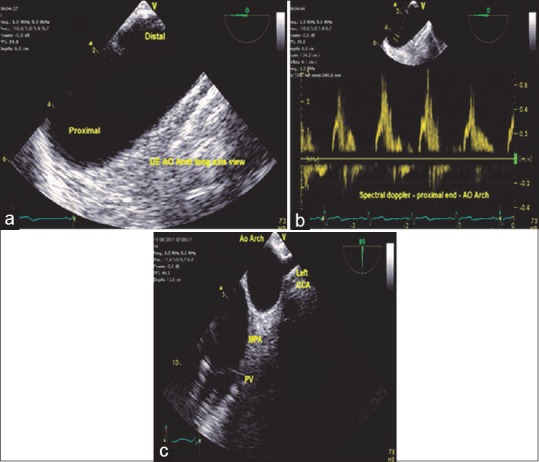
(a) Upper esophageal aortic arch long-axis view, (b) spectral Doppler-aortic arch - proximal end, (c) upper esophageal aortic arch short axis view - PV: Pulmonary valve, MPA: Main pulmonary artery, Left CCA: Left common carotid artery, AO: Aortic arch
THORACIC AORTIC DISEASES
The primary diseases of the aorta are atherosclerosis, aortic aneurysm, and aortic dissection.
Atherosclerosis
Atherosclerosis of the AA and aortic arch is an important predictor of stroke after cardiac surgery occurring in 1%–6% of the patients.[9] The best treatment option is prevention. TEE should be used to identify aortic atherosclerosis before anticipated surgical manipulations. Identification of significant disease by TEE permits alterations in the surgical procedure, such as adopting femoral arterial cannulation, altering the site of aortic cross-clamp, changing the type of cannulae used for extracorporeal circulation, off-pump coronary artery bypass coronary artery surgery with no touch technique of the aorta, using fibrillatory or hypothermic circulatory arrest, alteration of the site of graft anastomoses, and avoiding antegrade cardioplegia.[10]
Lesions of the AA and arch have been identified as risk factors for stroke, peripheral embolization, perioperative stroke, as well as neuropsychological dysfunction after open heart surgery. Atheroemboli, thromboemboli, and plaque thickness >4 mm correlate with significant embolic risk. Although TEE evaluation of the lesions is sensitive and specific, one of the important limitations is an inadequate visualization of distal AA and proximal aortic arch because of the interposed air-filled trachea. TEE A-view overcomes this problem and gives adequate atherosclerotic information before surgery or during closed chest procedures such as TAVIs.
EAS or TEE A-view imaging should be performed in high-risk patients to further delineate the sites of severe atherosclerosis so that surgical modifications can be made to reduce the morbidity and mortality.
Royse et al.[11] divided the thoracic aorta into six zones in relation to the areas of surgical manipulations which simplified the indications for EAS. Zones 1–3 are the proximal, mid, and distal AA. Zones 1 and 2 are the sites of incision for AV replacement, proximal coronary graft anastomosis, and antegrade cardioplegia cannulation. Zone 3 is the site of aortic cross-clamp, Zone 4 includes the proximal aortic arch which is the site of aortic cannula placement, Zones 5 and 6 include distal aortic arch and proximal descending aorta, respectively, and are not manipulated during cardiac surgery. Atheromatous embolism can occur during aortic cannulation or due to the presence of an intra-aortic balloon. In the study of Royse et al.,[11] Zone 3 was imaged by TT only in 58% of the cases. Manual surgical palpation detects only 50% of those atheromas identified by EAS[11] and might even be dangerous by dislodging the atheroma.[12] When moderate to severe atheroma is identified in Zones 5 and 6 by TEE, the incidence of atheroma in Zones 1–4 is very high. Hence, EAS is recommended. If Zones 1, 2, 3, 5, and 6 are negative, then EAS of Zones 3 and 4 is not recommended in this publication.
A recent meta-analysis by Van Zaane et al.[13] studied the diagnostic accuracy of TEE for estimating AA atherosclerosis. The sensitivity and specificity were 21% (95% confidence interval 13%–32%) and 99% (96%–99%), respectively. Because of the low sensitivity of TEE for the detection of AA atherosclerosis, a negative test result requires verification by additional tests such as TEE A-view and EAS. In case of a positive test result, AA atherosclerosis can be considered as present, and less manipulative strategies might be indicated.
Katz et al.[14] recommended a five-point grading system for aortic atheroma. Patients with a mobile atheroma had a 45% incidence of stroke, while the incidence of stroke was only 5% with Grade 3 atheroma [Figures 8 and 9].
Figure 8.
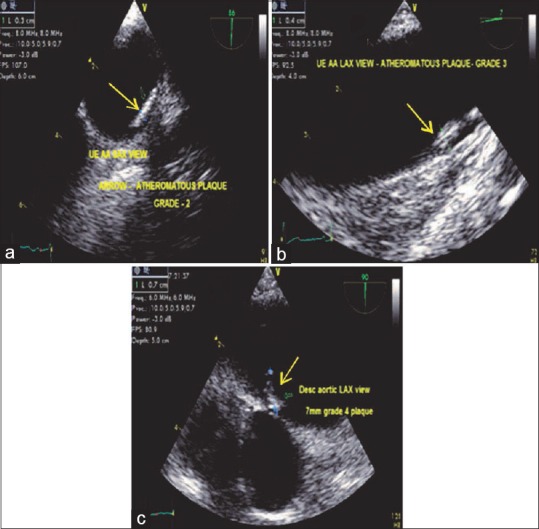
(a) Upper esophageal aortic arch axis view demonstrating (arrow) a Grade 2 plaque. (b) Upper esophageal aortic arch long-axis view with a (arrow) Grade 3 plaque. (c) Descending aortic long-axis view with a (arrow) Grade 4 plaque
Figure 9.
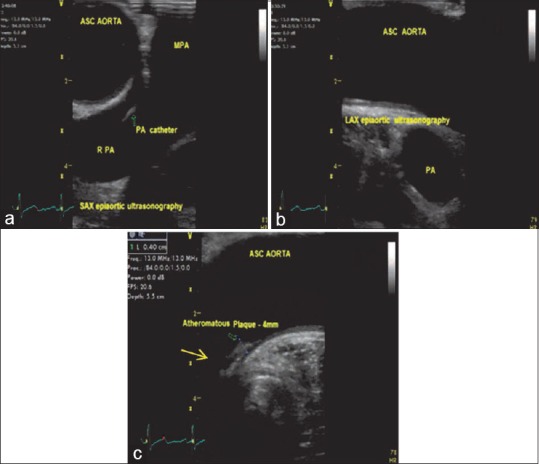
(a) Short-axis epiaortic scanning demonstrating the ascending aorta, main pulmonary artery, right pulmonary artery, pulmonary artery catheter in the right pulmonary artery. (b) Long-axis epiaortic scanning demonstrating the ascending aorta and the pulmonary artery. (c) Long-axis epiaortic scanning demonstrating an atheromatous 4 mm plaque (arrow) on the wall of the ascending aorta
Grading of aortic atheroma
| Grade | Description | Percentage of incidence of stroke (%) | |
|---|---|---|---|
| 1 | Normal aorta | 0 | |
| 2 | Extensive intimal thickening <3 mm | 0 | |
| 3 | Protrudes <5 mm into aortic lumen | 5 | |
| 4 | Protrudes >5 mm into aortic lumen | 10.5 | |
| 5 | Mobile atheroma | 46.5 | |
In comparison to EAS, TEE has been found to be superior for plaque identification in the descending aorta and arch but has a poor predictive value in detecting disease in the AA. A combination of techniques, using EAS, A-view catheter technique and TEE, is recommended in patients at high risk for disease in the distal AA.
Aortic aneurysms
An aneurysm is defined as dilation of aorta more than 50% of normal aortic diameter involving all the three layers of the vessel wall. The aorta is considered to be dilated if its diameter varies between 3.7 cm and 5.0 cm, aneurysmal if the diameter is more than 5.0 cm and dissected if it presents with an intimal flap. An aortic aneurysm is either saccular or fusiform. A fusiform aneurysm has a uniform shape with a symmetrical dilatation that involves the entire circumference of the aortic wall whiles saccular aneurysm is a localized outpouching of the aortic wall.
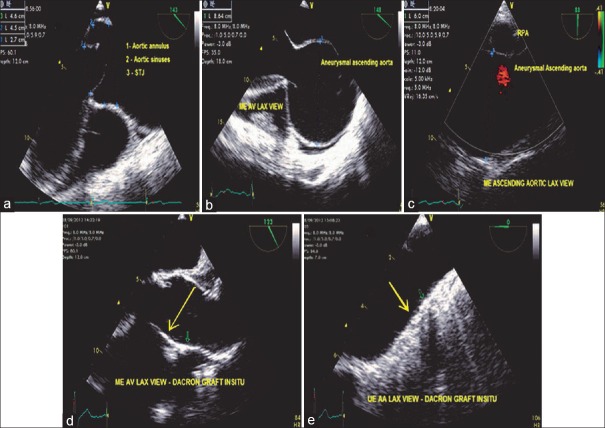
Aneurysms are primarily diseases of aging as a result of degeneration and atherosclerosis. Aging leads to intimal thickening, lipid deposition, and calcification resulting in dilation and weakening of the aortic wall. Connective tissue disorders such as Marfan syndrome, Ehlers-Danlos syndrome trauma, and hypertension are some of the other causes of aneurysms. Thoracic aortic aneurysms are divided into three groups depending on location: AA, aortic arch, and descending thoracic aneurysms or thoracoabdominal aneurysms [Figure 10].
Figure 10.
(a) Mid-esophageal aortic valve long-axis view demonstrating measurements of a dilated aortic root. (b) The same view with an aneurysmal ascending aorta. (c) Mid-esophageal ascending aortic long-axis view with an aneurysmal ascending aorta. (d) Mid-esophageal aortic valve long-axis view demonstrating a Dacron graft in situ (arrow) at the aortic arch level. (e) upper esophageal aortic valve long axis view demonstrating a Dacron graft in situ (arrow)
Thoracoabdominal aneurysms are grouped into four types based on the Crawford classification.[15]
Thoracoabdominal aneurysms [Figure 11]
Figure 11.
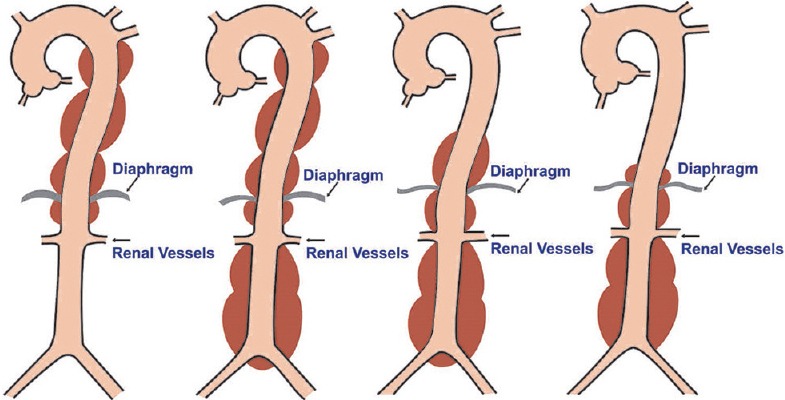
Types of thoracoabdominal aneurysms (illustrations by Srinivasa Holla)
Type 1: Involves the descending aorta just distal to the origin of LSA to the abdominal aorta above the renal arteries
Type 2: Involves the descending aorta terminating distal to the renal arteries
Type 3: Involves the distal half of the descending aorta and the abdominal aorta to its bifurcation
Type 4: Involves the abdominal aorta to its bifurcation.
As per the recommendations by the Society of Thoracic Surgeons, aneurysms with a diameter twice that of the normal aorta need to be surgically corrected.[16] Surgery is indicated if the sinuses are more than 40 mm, AA is more than 50 mm with aortopathy, and more than 60 mm without aortopathy. Patients with connective tissue disorders, strong family history of aortic aneurysms, and symptomatic patients should be considered for early operative therapy because of increased risk of dissection or rupture. Another alternative for complex cases is the hybrid approach in which an open surgical technique is combined with endovascular aortic repair which has the benefit of complete repair simultaneously reducing the risk of open technique.
TEE can be used to define the size, location, and extent of the aortic aneurysm as well as the presence of a hematoma or thrombus. TEE may also be used to evaluate the patency aortic arch branches to confirm the presence of organ malperfusion. It is an important monitoring tool of cardiac function during aortic aneurysm surgeries.
Aortic dissection
Aortic dissection is formed by an intimal tear which is contained by the media leading to the development of a true and false lumen. The false lumen may extend into branches of the aorta in the chest or abdomen, causing malperfusion, ischemia, or occlusion with resultant complications. The dissection can also progress proximally involving the aortic sinus, AV leading to aortic insufficiency, and may also involve the coronary arteries resulting in ischemic events. Aortic dissection is the most common cause of death involving the aorta. The mortality rate is extremely high at the rate of 1%/h–3%/h[17] during the first 48 h. Early and rapid diagnosis is essential to reduce mortality.
TEE has 98% sensitivity and 100% specificity[18] for diagnosing dissections, hence it is an ideal imaging modality in unstable patients because of its accuracy, low cost, minimal invasiveness, eliminates the problems associated with a short time. One of the major limitations of TEE is the inability to visualize the distal AA and the proximal aortic arch which can overcome by the usage of A-view catheter at bedside or EAS during surgery.
The DeBakey et al. classification[19] and the Stanford classification[20] systems are used to describe aortic dissections.
DISSECTIONS [FIGURE 12]
Figure 12.
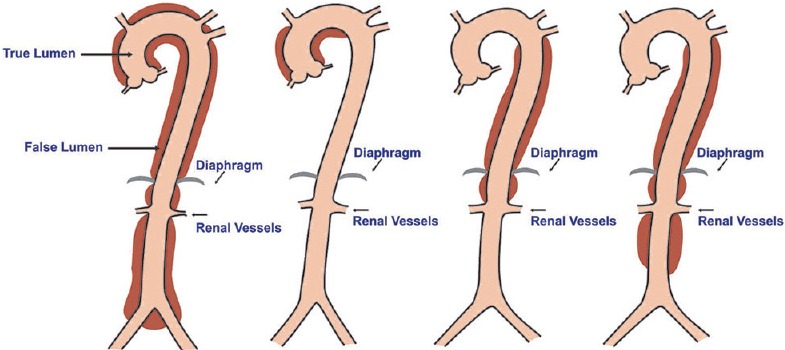
Types of aortic dissection (illustrations by Srinivasa Holla)
DeBakey classification
Type 1: Originates in the AA extending distally up to the aortic bifurcation
Type 2: Originates in the AA up to the brachiocephalic trunk
Type 3a: Originates distal to the LSA terminating just above the diaphragm
Type 3b: Originates distal to the LSA up to the aortic bifurcation [Figure 12].
The Stanford classification is divided into two types: Type A involving the AA (DeBakey Type 1 and 2), Type B the AA is spared (DeBakey Type 3a and 3b). This classification is simple and clinically important. Type A includes all types of dissections involving the AA no matter where the intimal tear is located and how far the dissection. Hence, surgery is recommended for Type A dissections. Mortality rates for medical and surgical management are similar for Type B dissections.
Patients with Marfan syndrome are at an increased risk of aortic dissection. An aortic ratio (AO) <1.3 indicates a low-risk group.[21] The AO ratio is the sinus of valsalva diameter divided by the predicted sinus diameter for a given age and body surface area, for patients above 40 years - predicted sinus dimension (cm) = 1.92 + J 0.74 × BSA (m2). Patients with an aortic root diameter of 50 mm undergoing coronary revascularization are at increased of aortic dissection.
Aortic dissections can be evaluated by TEE using the standard views mentioned earlier. The presence of an intimal flap is the most important evidence of an aortic dissection. The presence of an intimal flap, true and false lumen, and forward systolic flow in the true lumen are sensitive features of dissection.[22] Other findings are complete thrombosis of the false lumen, central displacement of intimal calcification, and separation of intimal layers form the thrombus[23] [Figure 13].
Figure 13.
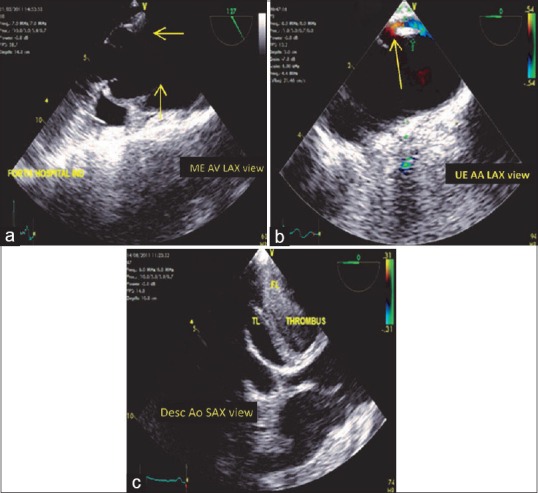
(a) Mid-esophageal aortic valve long-axis view demonstrating the intimal flap arising at the sinotubular junction (arrows). (b) Upper esophageal aortic arch long-axis view (arrow) indicating the presence of an intimal tear in the aortic wall. (c) Stanford Type B dissection with the intimal flap at the level of descending aorta. TL: True lumen, FL: False lumen
ENTRY AND EXIT SITES
TEE is a valuable imaging modality to locate the intimal tear site which is of extreme importance to determine the type of dissection and the success of surgical repair. Resection of the primary site decreases the incidence of complications and redo surgeries. Intimal tear occurs in the AA, 1–3 cm above the right or left sinus of valsalva in about 70% of the cases and at the site of ligamentum arteriosum in about 30% of the cases.[24] In the ascending and aortic arch, the dissection plane is along the greater curvature, but in the descending aorta, it is located lateral to the true lumen.
TEE plays an important role in the identification of the true and false lumen. True lumen expanded during systole and compressed during diastole. M-mode placed through the dissection helps in identifying the systolic expansion of the true lumen. True lumen is smaller than the false lumen,[25] exhibits a forward systolic flow pattern, has a less echogenic thin layer compared to the false lumen which has a bright echogenic layer. Thickening of the aortic wall more than 15mm is considered to be a sign of dissection,[26] indicating massive thrombosis of the false lumen making it difficult to identify the intimal flap [Figure 14].
Figure 14.
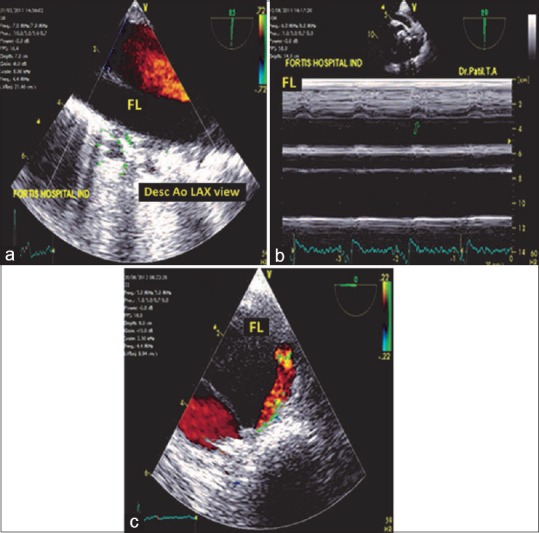
(a) Descending aortic long-axis view with the systolic color flow Doppler in the true lumen. FL: False lumen. (b) M-mode echocardiography (arrow) indicating the systolic expansion of the true lumen toward the false lumen. (c) Descending aortic short axis view indicating the flow of blood by color flow Doppler from the true lumen to the false lumen in the late systolic phase. Note the tracer (red) on the electrocardiogram at the end of systole
It is important to examine the ME AV LAX view carefully to detect proximal AA dissections. It is in this view a Swan-Ganz catheter appears as an artifact. Hence, it is important to differentiate an artifact from a true dissection, lest the patient may be subjected to a needless expensive surgical procedure thereby increasing the morbidity. A linear artifact of the AA can be distinguished from an intimal flap by its indistinct borders, lack of rapid oscillatory movements, extension of the artifact through the aortic wall as a straight line,[27] and the presence of color flow on both sides of the artifact.
Artifacts can also occur in the descending aorta. These are mirror image artifacts caused by a highly reflective aorta which occur at twice the distance from the transducer appearing as a double-lumen aorta [Figure 15].
Figure 15.
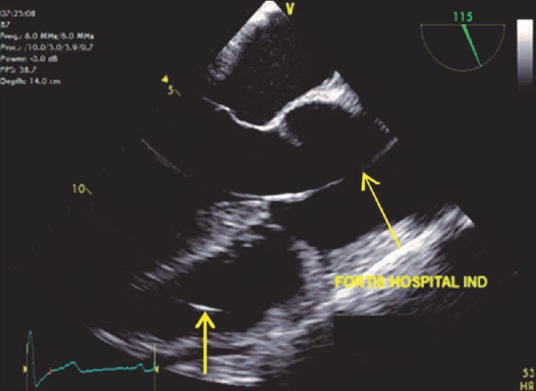
Mid-esophageal aortic valve long-axis view demonstrating an artifact (red arrow) in the ascending aorta due to the presence of a pulmonary artery catheter in the right ventricle (yellow arrow)
COMPLICATIONS
Complications associated with dissections are aortic insufficiency occurring in about 50%–70% of the cases, coronary dissections in 10%–20%[18] of the cases, pleural and pericardial effusion, and global left ventricular dysfunction. TEE can be used to determine the cause of aortic insufficiency in the presence of dissection in the ME AV SAX view. LV dysfunction may be secondary to ischemia following coronary dissection or due to severe aortic insufficiency presenting in about 10%–15% of the cases. Pericardial effusion is due to transudation of fluid from the wall of the false lumen into the pericardial cavity.[28] Another routine finding is the presence of left pleural effusion causing a hemothorax [Figure 16].
Figure 16.
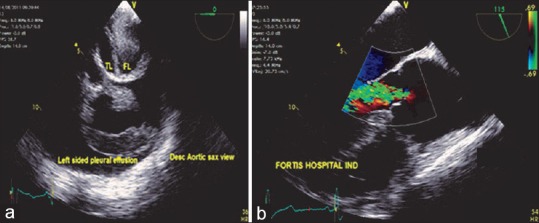
(a) Descending aortic short-axis view demonstrating the intimal flap with massive left sided pleural effusion, TL: True lumen, FL: False lumen. (b) Mid-esophageal aortic valve long-axis view demonstrating severe aortic insufficiency following the Stanford Type A dissection
INTRAMURAL HEMATOMA
Intramural hematomas are characterized by thickened aortic walls without an intimal flap involving the ascending or the descending aorta, probably due to rupture of the vasa vasorum leading to hemorrhagic vessel wall. Hematomas involving the AA need emergent surgical therapy, while those involving the descending aorta can be managed medically. Intramural hematomas as described by Mohr-Kahaly et al.[22] are characterized by intimal tear Figure 17.
Figure 17.
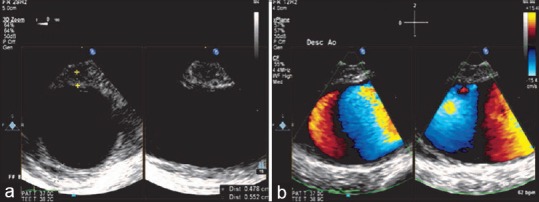
(a) Three-dimensional (zoom) image of the descending aortic short-axis view showing a 5 mm intramural hematoma in the wall of the descending aorta. (b) X-plane color Doppler imaging of the descending aorta showing the intramural hematoma
GIANT PENETRATING ULCER
This disease occurs in elderly patients with hypertension, hyperlipidemia, and atherosclerotic disease. It commonly affects the descending aorta in the form of an ulcer, which at a later stage may result in an aneurysm formation.
COARCTATION OF AORTA
This is a congenital narrowing of the aorta at the level of the aortic isthmus. It can be preductal, ductal, or postductal. This condition is commonly associated with a bicuspid AV and a patent ductus arteriosus. Upper esophageal aortic arch shot and LAX views are the recommended views for evaluating this condition by TEE. Findings include narrowing of the aorta distal to LSA and turbulent flow on the color flow Doppler. Transthoracic echocardiography is more favorable due to its anatomical location [Figure 18].
Figure 18.
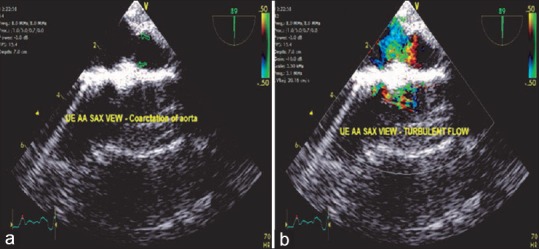
(a) Upper esophageal aortic arch short-axis view demonstrating the narrowing (arrow) of the aorta below the level of left subclavian artery. (b) Turbulent flow across the narrowed segment
TRAUMATIC AORTIC DISEASE
This disease is associated with a high mortality rate of about 30%, deceleration injury being the most common cause[29] and aortic isthmus being the most common site of injury apart from descending aorta, aortic arch, and abdominal aorta. TEE with its specificity of 100% remains the imaging modality of choice.
Three types of lesions are seen – sub adventitial aortic rupture, aortic intimal tear, mediastinal hematoma.[30] The subadventitial rupture can be partial or complete (transection) with a thickened highly mobile flap, absence of tear, similar blood flow velocities on both sides of the flap, mosaic color flow Doppler in the area of disruption, and presence of mediastinal hematomas. Mediastinal hematomas appear as an increasing space between the TEE probe and the aortic wall and a bright echogenic space between the aortic wall and the pleura [Figure 19].
Figure 19.
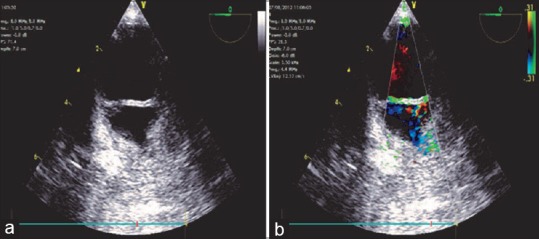
(a) Descending aortic short-axis view demonstrating the thickened medial flap with an asymmetrical aortic contour indicating complete subadventitial rupture of the aorta. (b) Similar blood flow velocities with a mosaic appearance on both sides of the disruption are seen
TRANSESOPHAGEAL ECHOCARDIOGRAPHY ASSESSMENT OF ENDOVASCULAR REPAIR OF AORTIC ANEURYSMS
Endovascular repair is gaining popularity as an alternative to open surgical repair of aortic aneurysms, thereby minimizing the risk of surgery. TEE is an invaluable tool for identification of aortic pathology, confirmation of placement of the guide wire within the true lumen, helps in stent graft positioning, detecting endoleaks, and evaluating the cardiac function.
The main aim of stent deployment in aneurysms is to exclude the aneurysmal sac so as to prevent further dilation. While in dissections, the aim would be to exclude the intimal tear preventing its evolution.
An endoleak is a common complication after endovascular repair of the aorta. It is characterized by persistent blood flow within the aneurysmal sac occurring in about 20% of the patient.[31] Endoleaks are classified into four types.[32] They may be primary endoleaks usually detected within 30 days or secondary endoleaks occurring after 30 days.
Most of the endoleaks can be detected using color flow Doppler. Another echocardiographic sign of endoleak is the development of spontaneous echo contrast within the aneurysmal sac after deployment of the stent. If contrast is being used swirling of contrast within the sac indicates the presence of endoleak, a static contrast indicates the absence of endoleak. TEE evaluation of endoleaks helps early detection and immediate corrective interventions [Figures 20 and 21].
Figure 20.
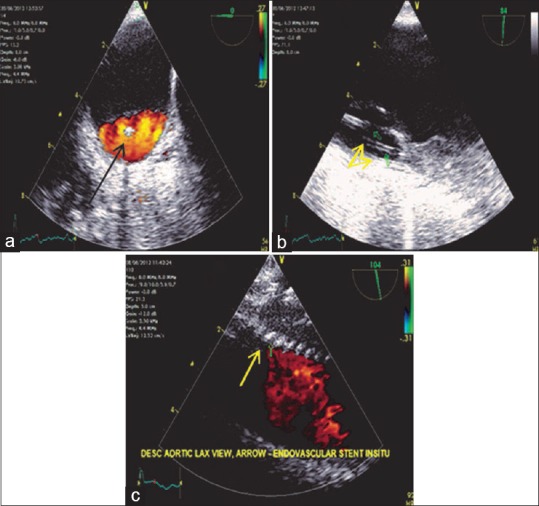
(a) Descending aortic short-axis view demonstrating color flow Doppler in the true lumen, arrow (black) pointing toward the guide wire in the center. (b) Descending aortic long-axis view demonstrating the true lumen with the two guide wires (yellow arrows) in situ. (c) Descending aortic long-axis view demonstrating (arrow) the Medtronic talent endovascular stent in situ
Figure 21.
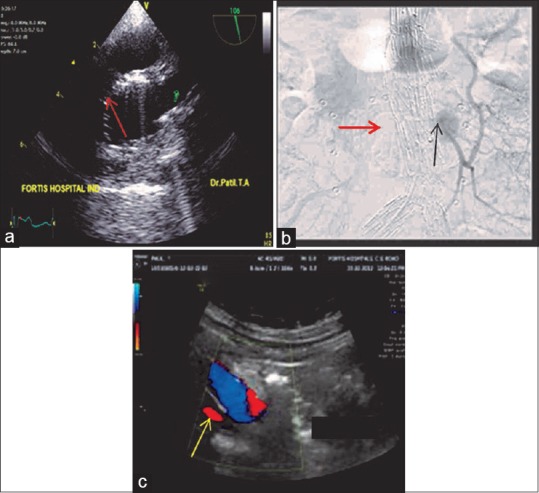
(a) Upper esophageal aortic arch short-axis view demonstrating the (red arrow) endovascular stent in the aortic arch proximal to the origin of the left subclavian artery. (b) Aortography demonstrating a Type 2A endoleak (black arrow) into the aneurysmal sac from the inferior mesenteric artery. Red arrow is indicating the endovascular stent. (c) Transabdominal ultrasound color flow Doppler demonstrating a Type 4 (yellow arrow) endoleak
SUMMARY
TEE is an invaluable imaging modality in the management of aortic pathology. TEE has to a large extent improved the patient outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Classification of endoleaks
| Type | Description |
|---|---|
| Attachment site leak | Proximal leak |
| Distal leak | |
| Liliac occlude | |
| Branch leak | To and fro flow into the aneurysmal sac |
| Complex flow from more branch vessels into the sac | |
| Graft defect | Mid graft hole |
| Junctional leak or graft disconnection | |
| Failure from suture holes | |
| Graft wall porosity |
TAKE HOME MESSAGES
In this detailed review of “TEE evaluation of the thoracic Aorta”, the authors describe based on anatomy of the thoracic aorta, the echo evaluation in six different TEE planes, and the importance of the epiaortic scanning (EUS) of the distal ascending aorta (AA) and aortic arch especially in the case of an atheromatous plaque which changes the surgical plan. The article also describes the six zones in EUS, the aortic aneurysms and aortic dissections with entry and exit sites. Coarctation of aorta and traumatic aortic diseases with EVAR is also described.
REFERENCES
- 1.Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: Increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114:2611–8. doi: 10.1161/CIRCULATIONAHA.106.630400. [DOI] [PubMed] [Google Scholar]
- 2.Nihoyannopoulos P, Joshi J, Athanasopoulos G, Oakley CM. Detection of atherosclerotic lesions in the aorta by transesophageal echocardiography. Am J Cardiol. 1993;71:1208–12. doi: 10.1016/0002-9149(93)90647-u. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberger P, Shernan SK, Löffler M, Shekar PS, Fox JA, Tuli JK, et al. The influence of epiaortic ultrasonography on intraoperative surgical management in 6051 cardiac surgical patients. Ann Thorac Surg. 2008;85:548–53. doi: 10.1016/j.athoracsur.2007.08.061. [DOI] [PubMed] [Google Scholar]
- 4.van Zaane B, Nierich AP, Buhre WF, Brandon Bravo Bruinsma GJ, Moons KG. Resolving the blind spot of transoesophageal echocardiography: A new diagnostic device for visualizing the ascending aorta in cardiac surgery. Br J Anaesth. 2007;98:434–41. doi: 10.1093/bja/aem009. [DOI] [PubMed] [Google Scholar]
- 5.Shanewise JS, Cheung AT, Aronson S, Stewart WJ, Weiss RL, Mark JB, et al. ASE/SCA guidelines for performing acomprehensive intraoperative multiplane transesophageal echocardiography examination: Recommendations of the American Society of Echocardiography Council for Intraoperative Echocardiography and the Society of Cardiovascular Anesthesiologists Task Force for Certification in Perioperative Transesophageal Echocardiography. Anesth Analg. 1999;89:870–84. doi: 10.1097/00000539-199910000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Konstadt SN, Reich DL, Quintana C, Levy M. The ascending aorta: How much does transesophageal echocardiography see? Anesth Analg. 1994;78:240–4. doi: 10.1213/00000539-199402000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Nierich AP, van Zaane B, Buhre WF, Coddens J, Spanjersberg AJ, Moons KG. Visualization of the distal ascending aorta with A-Mode transesophageal echocardiography. J Cardiothorac Vasc Anesth. 2008;22:766–73. doi: 10.1053/j.jvca.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Nierich A. Clinical outcome after pre-incision assessment of aorticatherosclerosis by A-View echocardiography in 5,886 elective cardiac surgerypatients. J Cardiothorac Vasc Anesth. 2011;25:S1. [Google Scholar]
- 9.Bucerius J, Gummert JF, Borger MA, Walther T, Doll N, Onnasch JF, et al. Stroke after cardiac surgery: A risk factor analysis of 16,184 consecutive adult patients. Ann Thorac Surg. 2003;75:472–8. doi: 10.1016/s0003-4975(02)04370-9. [DOI] [PubMed] [Google Scholar]
- 10.Ribakove GH, Katz ES, Galloway AC, Grossi EA, Esposito RA, Baumann FG, et al. Surgical implications of transesophageal echocardiography to grade the atheromatous aortic arch. Ann Thorac Surg. 1992;53:758–61. doi: 10.1016/0003-4975(92)91431-8. [DOI] [PubMed] [Google Scholar]
- 11.Royse C, Royse A, Blake D, Grigg L. Screening the thoracic aorta for atheroma: A comparison of manual palpation, transesophageal and epiaortic ultrasonography. Ann Thorac Cardiovasc Surg. 1998;4:347–50. [PubMed] [Google Scholar]
- 12.Sylivris S, Calafiore P, Matalanis G, Rosalion A, Yuen HP, Buxton BF, et al. The intraoperative assessment of ascending aortic atheroma: Epiaortic imaging is superior to both transesophageal echocardiography and direct palpation. J Cardiothorac Vasc Anesth. 1997;11:704–7. doi: 10.1016/s1053-0770(97)90161-0. [DOI] [PubMed] [Google Scholar]
- 13.Van Zaane B, Zuithoff NP, Reitsma JB, Bax L, Nierich AP, Moons KG. Meta-analysis of the diagnostic accuracy of transesophageal echocardiography for assessment of atherosclerosis in the ascending aorta in patients undergoing cardiac surgery. Acta Anaesthesiol Scand. 2008;52:1179–87. doi: 10.1111/j.1399-6576.2008.01694.x. [DOI] [PubMed] [Google Scholar]
- 14.Katz ES, Tunick PA, Rusinek H, Ribakove G, Spencer FC, Kronzon I. Protruding aortic atheromas predict stroke in elderly patients undergoing cardiopulmonary bypass: Experience with intraoperative transesophageal echocardiography. J Am Coll Cardiol. 1992;20:70–7. doi: 10.1016/0735-1097(92)90139-e. [DOI] [PubMed] [Google Scholar]
- 15.Svensson LG, Kouchoukos ES. Aortic dissection and aortic aneurysm surgery: Clinical observations, experimental investigations, and statistical analyses. Part II. Curr Probl Surg. 1992;29:915–1057. doi: 10.1016/0011-3840(92)90019-y. [DOI] [PubMed] [Google Scholar]
- 16.Svensson LG, Kouchoukos NT, Miller DC, Bavaria JE, Coselli JS, Curi MA, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg. 2008;85(1 Suppl):S1–41. doi: 10.1016/j.athoracsur.2007.10.099. [DOI] [PubMed] [Google Scholar]
- 17.Nienaber CA, Spielman RP, Von Kodolitsch Y, Siglow V, Piepho A, Jaup T, et al. Diagnosis of thoracic aortic dissection: Magnetic resonance imagery versus transesophageal echocardiography. Circulation. 1992;85:434–47. doi: 10.1161/01.cir.85.2.434. [DOI] [PubMed] [Google Scholar]
- 18.Ballal RS, Nanda NC, Gatewood R, D'Arcy B, Samdarshi TE, Holman WL, et al. Usefulness of transesophageal echocardiography in assessment of aortic dissection. Circulation. 1991;84:1903–14. doi: 10.1161/01.cir.84.5.1903. [DOI] [PubMed] [Google Scholar]
- 19.DeBakey ME, Henly WS, Cooley DA, Morris GC, Jr, Crawford ES, Beall AC., Jr Surgical management of dissecting aneurysms of the aorta. J Thorac Cardiovasc Surg. 1965;49:130–49. [PubMed] [Google Scholar]
- 20.Daily PO, Trueblood HW, Stinson EB, Wuerflein RD, Shumway NE. Management of acute aortic dissections. Ann Thorac Surg. 1970;10:237–47. doi: 10.1016/s0003-4975(10)65594-4. [DOI] [PubMed] [Google Scholar]
- 21.Legget ME, Unger TA, O'sullivan EB. Management of acute aortic dissections. Ann Thorac Surg. 1970;10:237–47. doi: 10.1016/s0003-4975(10)65594-4. [DOI] [PubMed] [Google Scholar]
- 22.Mohr-Kahaly S, Erbel R, Kearney P, Puth M, Meyer J. Aortic intramural hemorrhage visualized by transesophageal echocardiography: Findings and prognostic implications. J Am Coll Cardiol. 1994;23:658–64. doi: 10.1016/0735-1097(94)90751-x. [DOI] [PubMed] [Google Scholar]
- 23.Erbel R, Engberding R, Daniel W, Roelandt J, Visser C, Rennollet H. Echocardiography in diagnosis of aortic dissection. Lancet. 1989;1:457–61. doi: 10.1016/s0140-6736(89)91364-0. [DOI] [PubMed] [Google Scholar]
- 24.Erbel R, Mohr-Kahaly S, Rennollet H, Brunier J, Drexler M, Wittlich N, et al. Diagnosis of aortic dissection: The value of transesophageal echocardiography. Thorac Cardiovasc Surg. 1987;35:126–33. doi: 10.1055/s-2007-1020273. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto S, Kumada T, Osakada G, Kubo S, Tokunaga S, Tamaki S, et al. Assessment of transesophageal Doppler echography in dissecting aortic aneurysm. J Am Coll Cardiol. 1989;14:1253–62. doi: 10.1016/0735-1097(89)90424-5. [DOI] [PubMed] [Google Scholar]
- 26.Iliceto S, Nanda NC, Rizzon P, Hsuing MC, Goyal RG, Amico A, et al. Color Doppler evaluation of aortic dissection. Circulation. 1987;75:748–55. doi: 10.1161/01.cir.75.4.748. [DOI] [PubMed] [Google Scholar]
- 27.Appelbe AF, Walker PG, Yeoh JK, Bonitatibus A, Yoganathan AP, Martin RP. Clinical significance and origin of artifacts in transesophageal echocardiography of the thoracic aorta. J Am Coll Cardiol. 1993;21:754–60. doi: 10.1016/0735-1097(93)90109-e. [DOI] [PubMed] [Google Scholar]
- 28.Khan IA, Nair CK. Clinical, diagnostic, and management perspectives of aortic dissection. Chest. 2002;122:311–28. doi: 10.1378/chest.122.1.311. [DOI] [PubMed] [Google Scholar]
- 29.Wall MJ Jr, Hirshberg A, LeMaire SA, Holcomb J, Mattox K. Thoracic aortic and thoracic vascular injuries. Surg Clin North Am. 2001;81:1375–93. doi: 10.1016/s0039-6109(01)80013-x. [DOI] [PubMed] [Google Scholar]
- 30.Vignon P, Gueret P, edrinne JM, Lagrange P, Semba R, Mitchell S, et al. Transluminal Placement of endovascular stent-graftsfor the treatment of descending thoracic aortic aneurysms. N Engl J Med. 1994;331:1729–34. doi: 10.1056/NEJM199412293312601. [DOI] [PubMed] [Google Scholar]
- 31.van Marrewijk C, Buth J, Harris PL, Norgren L, Nevelsteen A, Wyatt MG. Significance of endoleaks after endovascular repair of abdominal aortic aneurysms: The EUROSTAR experience. J Vasc Surg. 2002;35:461–73. doi: 10.1067/mva.2002.118823. [DOI] [PubMed] [Google Scholar]
- 32.Veith FJ, Baum RA, Ohki T, Amor M, Adiseshiah M, Blankensteijn JD, et al. Nature and significance of endoleaks and endotension: Summary of opinions expressed at an international conference. J Vasc Surg. 2002;35:1029–35. doi: 10.1067/mva.2002.123095. [DOI] [PubMed] [Google Scholar]