Abstract
Background
There are clinical situations (CS) in which the use of somatostatin analogs (SSAs) in patients with neuroendocrine tumors (NET) is controversial due to lack of evidence. A Delphi study was conducted to develop common treatment guidelines for these CS, based on clinical practice and expert opinion of Spanish oncologists.
Methods
A scientific committee identified 5 CS with a common core (c-c) [non-functioning NET, not susceptible of surgery/locoregional therapy, Ki67 < 10 % (except for CS5: >10 %), ECOG ≤ 2], and controversy regarding use of SSAs, and prepared a Delphi questionnaire of 48 treatment statements. Statements were rated on a 1 (completely disagree) to 9 (completely agree) scale. Responses were grouped by tertiles: 1–3: Disagreement, 4–6: Neutral, 7–9: Agreement. Consensus was reached when the responses of ≥2/3 participants were located in the same tertile as the median value of all reported responses for that statement.
Results
Sixty five (81.2 %) of 80 invited oncologists with experience in the management of NETs answered a first round of the questionnaire and 57 (87.7 %) of those 65 answered a second round (mean age 43.5 years; 53.8 % women; median time of experience 9 years). Consensus was obtained in 42 (36 agreement and 6 disagreement) of the 48 statements (87.5 %). Regarding CS1 (Enteropancreatic NET, c-c, non-progressive in the last 3–6 months), overall, SSA treatment is recommended (a wait and see approach is anecdotal and reserved for fragile patients or with low tumor load or ki-67 < 2 %); CS2 (Pancreatic NET, c-c), overall, SSA monotherapy is recommended, except when high tumor load or tumor progression exists, where combination therapy would be considered; CS3 [Gastroenteropancreatic (GEP)-NET, c-c, in treatment with anti-proliferative dose of SSA and progressing], overall, SSA maintenance is recommended at the time of progression, with or without adding molecular targeted drugs; CS4 (GEP-NET, c-c, and negative octreoscan®), SSA in monotherapy is only considered in low-risk patients (low tumor load and Ki-67 < 5 %); CS5 [GEP-NET, c-c (ki67 > 10 %), and positive octreoscan®], monotherapy with SSA is mainly considered in patients with comorbidities.
Conclusion
Several recommendations regarding use of SSAs in controversial NET CS were reached in consensus and might be considered as treatment guideline.
Keywords: Neuroendocrine tumors, NET, Gastroenteropancreatic NETs, Somatostatin analogue, SSA, Delphi study
Background
Neuroendocrine tumors (NETs) are neoplasms that originate from the peripheral neuroendocrine cell system and lungs, and are most frequently located in the gastroenteropancreatic (GEP) system [1]. GEP-NETs may present as hormonally functioning or nonfunctioning tumors and have distinct clinical features based on their site of origin [2]. The age-adjusted incidence of GEP-NETs in the United States was 3.65/100,000/year between 2003 and 2007 [3], while data from Europe (United Kingdom) regarding gastrointestinal NETs showed an age-adjusted incidence of 1.32 and 1.33 in males and females, respectively, between 2000 and 2006 [4]. Overall, the age-adjusted incidence of NETs has increased 3.65-fold in the United States and up to 4.8-fold in the United Kingdom over the past four decades. Regarding life expectancy, the median survival of registered GEP-NETs in Spain was shown to be 12 years (75 % at 5 years) [5].
Somatostatin analogues (SSAs) have been mainly used in functioning tumors to improve the symptoms of carcinoid syndrome or symptoms of other functional NETs [6, 7]; however, their antiproliferative properties are beneficial in both functioning and non-functioning tumors [8–10]. The randomized, double-blind, placebo-controlled PROMID study [11], was the first study reporting an antiproliferative effect of the SSA octreotide long-acting repeatable (LAR) in patients with metastatic G1 midgut NETs, prolonging time to tumor progression as compared to placebo in patients with functionally active and inactive tumors. Recently, the randomized, double-blind, placebo-controlled CLARINET study [12], conducted in patients with advanced grade 1 or 2 (Ki67 < 10 %) NETs originating in the pancreas, midgut or hindgut, or of unknown origin, showed that treatment with the SSA lanreotide significantly prolonged progression-free survival (PFS) compared with placebo, regardless of the hepatic tumor burden. The antitumor effects of SSAs can be direct, via the interaction with somatostatin receptors, or indirect, by a complex mechanism leading to immune system modulation, apoptosis induction, and angiogenesis inhibition [13].
Clinical guidelines can help in the management of NET patients [14–21]. However, there are still clinical situations (CS) in which the use of SSAs is controversial due to lack of evidence. It is recognized that clinical experience and expert opinion could help establish recommendations for the management of these situations and thus, the NETPraxis program was created.
The NETPraxis program aimed to develop common treatment guidance for controversial CS regarding the use of SSAs, based on clinical practice experience and expert opinion of Spanish oncologists.
Methods
A scientific committee of 6 Spanish oncologists with experience in the management of NETs identified 5 CS with a common core [non-functioning NET, not susceptible of surgery/locoregional therapy, Ki67 < 10 % (except for CS5: Ki67 > 10 %), ECOG ≤ 2], and controversy regarding pharmacologic treatment with SSAs: CS1 (Enteropancreatic NET, common core, non-progressive in the last 3–6 months), wait and see or SSA?; CS2 (Pancreatic NET, common core), initial SSA, molecular targeted drugs (MTD) or chemotherapy?; CS3 [GEP-NET, common core, in treatment with anti-proliferative dose of SSA and progressing], maintain SSA?; CS4 (GEP-NET, common core and negative octreoscan®), initial SSA?; CS5 [GEP-NET, common core (ki67 > 10 %), initial SSA?.
Supported by related bibliography, these 5 CS were discussed in 13 local meetings among a total of 66 Spanish oncologists, including the members of the scientific committee. Based on the results of the discussions, the scientific committee prepared a Delphi questionnaire of 48 statements regarding treatment with SSAs, divided into blocks for each of the 5 CS (see tables in the Results section for the whole list of statements; the references used to discuss each of the 5 CS in the local meetings are contiguous to the corresponding CS in the tables). The Delphi method is a widely accepted technique for reaching a consensus among a panel of experts [22]. The experts respond anonymously to at least two rounds of a questionnaire and are provided with a summary of all responses after each round [23]. The experts may then revise their earlier responses in light of those by other members.
Eighty Spanish oncologists with proven experience in the treatment of NETs (>5 years), including those participating in the meetings, were invited to answer the first round of Delphi questionnaire. From October to November 2015, 65 (81.2 %) of the 80 oncologists anonymously answered the questionnaire online (mean age: 43.5 ± 7.8 years; women; 53.8 %; median years of experience in NETs [p25-p75]: 9 [6–15]). Participants were asked to rate each statement on a scale from 1 to 9 (1 = “completely disagree”; 9 = “completely agree”). Responses were grouped by tertiles: 1–3: Disagreement, 4–6: Neutral, 7–9: Agreement. Consensus on a statement was reached when the responses of ≥2/3 participants (≥66.6 %) were located in the same tertile as the median value of all the reported responses for that statement.
A second round of the Delphi questionnaire was performed containing only the statements for which consensus had not been reached in the first round and statements with a neutral consensus, along with a summary of the responses for those statements in the first round. Only the 65 oncologists who had responded the questionnaire in the first round were invited to answer the second round of the Delphi. From December 2015 to January 2016, 57 (87.7 %) of the 65 oncologists completed the second round of the questionnaire (mean age: 42.8 ± 7.3 years; women; 56.1 %; median years of experience in NETs [p25-p75]: 9 [6–14.5]).
Statistics
The median and interquartile range (p25-p75) of the answers to every item of the questionnaire were calculated.
Cronbach’s alpha (Cα) was used to measure the internal consistency of the questionnaire. Cα can range between 0 and 1, from lower to greater reliability, with values above 0.7 considered acceptable [24]. Intra-class correlation coefficient (ri) was used to assess inter-rater reliability, which is considered as poor for ri values <0.40, fair for ri: 0.40–0.59, good for ri: 0.60–0.74, and excellent for ri: 0.75–1.0 [25]. The correlation between the two rounds of the questionnaire was measured by the Spearman coefficient (rs), which is considered as non-existent when rs: 0–0.25, weak when rs: 0.26–0.50, moderate to strong when rs: 0.51–0.75 and strong to very strong when rs: 0.76–1 [26]. The Kappa index (k) was calculated to estimate the qualitative agreement between the rounds having into account the three answer groups (1–3, 4–6 and 7–9). Coherence is poor or inexistent when Kappa index is < 0.20, weak from 0.21 to 0.40, moderate from 0.41 to 0.60, good from 0.61 to 0.80 and very good from 0.81 to 1 [27]. All these values were calculated for the overall questionnaire and for each block. Statistical significance was considered when p <0.05.
The variation coefficient (VC) of the questionnaire was calculated for every round, along with the delta or relative increase in the second round above the first (VCsecond-VCfirst/VCfirst). When delta is <10 %, there is no large variability between the rounds, and thus, there is no need for another round.
Results
The questionnaire had good internal consistency (Cα value >0.7 for the total questionnaire and each of the blocks) and inter-rater reliability (ri value ≥0.7 for the total questionnaire and each of the blocks) (Table 1).
Table 1.
Internal consistency (Cα) and inter-rater reliability (ri) of the Delphi questionnaire
| Cα | p-value | ri | p-value | |
|---|---|---|---|---|
| TOTAL (48 items) | 0.821 | <0.001 | 0.808 | <0.001 |
| CS1 (9 items) | 0.868 | <0.001 | 0.792 | <0.001 |
| CS2 (16 items) | 0.936 | <0.001 | 0.891 | <0.001 |
| CS3 (9 items) | 0.764 | <0.001 | 0.722 | <0.001 |
| CS4 (6 items) | 0.705 | <0.001 | 0.673 | <0.001 |
| CS5 (8 items) | 0.912 | <0.001 | 0.885 | <0.001 |
Bold data are the total
CS Clinical situation, Cα Cronbach’s alpha, r i Intra-class correlation coefficient
Between the two rounds of the questionnaire, quantitative correlation, as assessed by the Spearman coefficient, was acceptable, with values from moderate (rs > 0.5–0.75) to strong (rs > 0.76–1) for the questions that were asked in both rounds (25) and for every block. Qualitative agreement was also acceptable, with kappa values from moderate (k > 0.4–0.6) to good (k > 0.6–0.8) for the total 25 questions and for every block.
The VC in the first round was 0.38 ± 0.09 and in the second 0.41 ± 0.09, which means a delta increase of 7.9 %; i.e. lower than 10 %, and thus, a third round was not necessary.
Overall, consensus was obtained in 42 (36 agreement and 6 disagreement) of the 48 statements (87.5 %) (Tables 2, 3, 4, 5, and 6). In the first round, there was consensus in 23 (47.9 %) statements (21 agreement and 2 disagreement) and non-consensus in 25 (52.1 %). Out of these 25, in the second round, there was consensus in 19 (15 agreement and 4 disagreement) and non-consensus in 6. The results observed in each CS are discussed in the next section.
Table 2.
Items regarding CS1: Patient with non-functioning enteropancreatic NET, non-susceptible to surgery or to loco-regional treatment, and with Ki-67 < 10 %, ECOG ≤2, NON-PROGRESSIVE in the last 3–6 months: Wait and see vs. SSA treatment? [11, 12, 16, 30, 32, 62]
| Previous rounda | Median (p25-p75) | Median range | Participants in median range n (%) | Result | |
|---|---|---|---|---|---|
| 1. Treatment is initiated with SSAs in most of this type of patients, since available evidence shows that even in patients with stable disease, treatment initiation significantly lengthens the time to progression. | 8 (8–9) | 7–9 | 58 (89.2) | C - A | |
| 2. In the absence of other risk factors (younger age [<60–65 years], important comorbidities or high Ki67), wait and see may be considered in patients with low tumor load (hepatic ≤25 %). | 30.8 %: 4–6 | 7 (4–8) | 7–9 | 38 (66.7) | C - A |
| 3. In the absence of other risk factors (high Ki67 or extra-hepatic disease), wait and see may be considered in fragile patients (with important comorbidities/elderly [>75 years]). | 8 (6–9) | 7–9 | 47 (72.3) | C - A | |
| 4. In the absence of other risk factors (younger age [<60–65 years], important comorbidities or extra-hepatic disease) wait and see may be considered in patients with low Ki-67 (<2 %). | 32.3 %: 4–6 | 7 (6–8) | 7–9 | 42 (73.7) | C - A |
| 5. Overall, wait and see is not considered in patients with Ki-67 > 5 %. | 8 (7–9) | 7–9 | 50 (76.9) | C - A | |
| 6. Tumor localization (pancreatic or non-pancreatic) is not a key criterion when deciding between wait and see or initiate treatment with SSAs. | 63.1 %: 7–9 | 8 (7–8) | 7–9 | 48 (84.2) | C - A |
| 7. Based on available evidence, lanreotide is the SSA of choice in the treatment of patients with NET of pancreatic origin and Ki67 > 2 % and <10 %. | 8 (7–9) | 7–9 | 54 (83.1) | C - A | |
| 8. Based on available evidence, octreotide is the SSA of choice in the treatment of patients with NET of pancreatic origin and Ki67 > 2 % and <10 %. | 40 %: 4–6 | 2 (2–3) | 1–3 | 45 (78.9) | C - D |
| 9. Based on available evidence, SSAs are the treatment of choice in patients with NET of digestive origin and Ki-67 < 2 %. | 8 (7–9) | 7–9 | 53 (81.5) | C - A |
CS Clinical situation, SSAs Somatostatin analogs, NET Neuroendocrine tumor, C consensus, NC non-consensus, A Agreement, D Disagreement
aOnly applies to statements with 2 rounds; participant % in median range: median range
Table 3.
Items regarding CS2: Patient with non-functioning PANCREATIC NET, non-susceptible to surgery or to loco-regional treatment, and with Ki-67 < 10 %, ECOG ≤2: Treatment initiation with SSA, molecular targeted drugs or chemotherapy? [12, 36, 37, 63, 64]
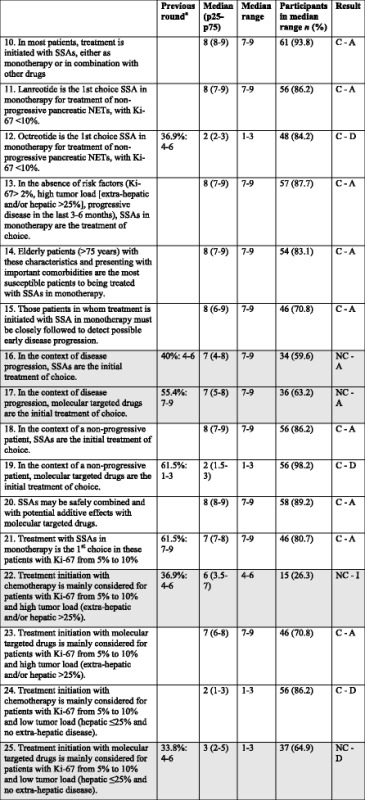
CS Clinical situation, SSAs Somatostatin analogs, NET Neuroendocrine tumor, C consensus, NC non-consensus, A Agreement, D Disagreement, I Indeterminate
aOnly applies to statements with 2 rounds; participant % in median range: median range
Shadowed boxes: non-consensus
Table 4.
Items regarding CS3: Patient with non-functioning GEP-NET, with Ki-67 < 10 %, ECOG ≤2, in treatment with anti-proliferative dose of SSA and PROGRESSING: Is SSA treatment maintained? [13, 37, 40, 43, 44, 65–67]
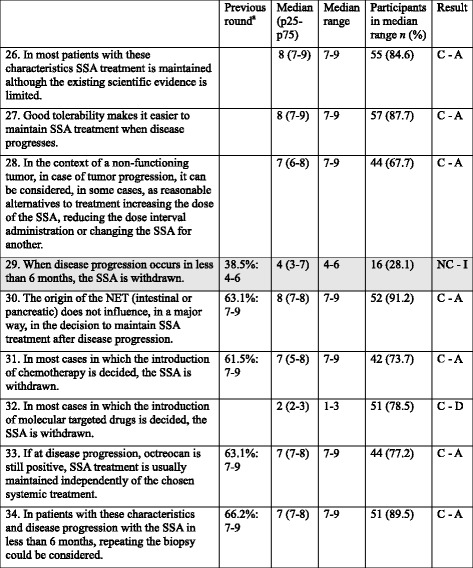
CS Clinical situation, SSAs Somatostatin analogs, GEP Gastroenteropancreatic, NET Neuroendocrine tumor, C consensus, NC non-consensus, A Agreement, D Disagreement, I Indeterminate
aOnly applies to statements with 2 rounds; participant % in median range: median range
Shadowed boxes: non-consensus
Table 5.
Items regarding CS4: Patient with non-functioning GEP-NET, non-susceptible to surgery or to loco-regional treatment, with Ki-67 < 10 %, ECOG ≤2, AND NEGATIVE OCTREOSCAN®: Is SSA treatment initiated? [11, 48, 49, 68]
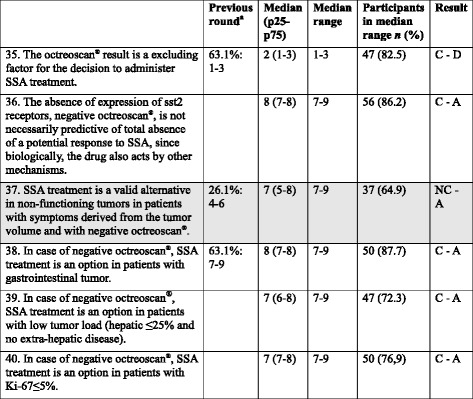
CS Clinical situation, SSAs Somatostatin analogs, GEP Gastroenteropancreatic, NET Neuroendocrine tumor, C consensus, NC non-consensus, A Agreement, D Disagreement, I Indeterminate
aOnly applies to statements with 2 rounds; participant % in median range: median range
Shadowed boxes: non-consensus
Table 6.
Items regarding CS5: Patient with non-functioning GEP-NET, non-susceptible to surgery or to loco-regional treatment, with Ki-67 > 10 %, ECOG ≤2, AND POSITIVE OCTREOSCAN®: Is SSA treatment initiated? [36, 59]
| Previous rounda | Median (p25-p75) | Median range | Participants in median range n (%) | Result | |
|---|---|---|---|---|---|
| 41. The use of SSA in monotherapy in these patients is reasonable in patients with Ki-67 <20 %. | 50.8 %: 7–9 | 7 (7–8) | 7–9 | 48 (84.2) | C - A |
| 42. The use of SSA in monotherapy in these patients is reasonable in case of low tumor load (hepatic ≤25 % and no extra-hepatic disease). | 63.1 %: 7–9 | 8 (7–8) | 7–9 | 50 (87.7) | C - A |
| 43. The use of SSA in monotherapy in these patients is reasonable in case of NETs of gastrointestinal origin. | 52.3 %: 7–9 | 7 (7–8) | 7–9 | 49 (86) | C - A |
| 44. In case of important comorbidities, SSAs are an option in these patients. | 8 (7–9) | 7–9 | 56 (86.2) | C - A | |
| 45. In these patients with Ki-67 from 10 to 20 % and/or high tumor load (extra-hepatic and/or hepatic >25 %) and/or NET of pancreatic origin, treatment is usually initiated with chemotherapy. | 66.2 %: 7–9 | 7 (7–8) | 7–9 | 48 (84.2) | C - A |
| 46. In these patients with Ki-67 from 10 to 20 % and/or high tumor load (extra-hepatic and/or hepatic >25 %) and/or NET of pancreatic origin, treatment is usually initiated with molecular targeted drugs (with the possibility of combination with SSA). | 7 (6–8) | 7–9 | 45 (69.2) | C - A | |
| 47. If discrepancy exists between the degree of cellular proliferation and the octreoscan® results, it is recommended to perform 18FDG-PET-TAC to help making a therapeutic decision. | 53.8 %: 7–9 | 7 (7–8) | 7–9 | 46 (80.7) | C - A |
| 48. If discrepancy exists between the degree of cellular proliferation and the octreoscan® results, a re-biopsy of the growing lesions will be considered. | 64.4 %: 7–9 | 7 (7–8) | 7–9 | 53 (93) | C - A |
CS Clinical situation, SSAs Somatostatin analogs, GEP Gastroenteropancreatic, NET Neuroendocrine tumor, C consensus, NC non-consensus, A Agreement
aOnly applies to statements with 2 rounds; participant % in median range: median range
Discussion
A Delphi study was conducted to establish recommendations for the management of CS with undefined protocols regarding the use of SSAs.
CS1 (Patient with non-functioning enteropancreatic NET, non-susceptible to surgery or to loco-regional treatment, and with Ki-67 < 10 %, ECOG ≤2, NON-PROGRESSIVE in the last 3–6 months: Wait and see vs. SSA treatment?)
Due to their proven benefits and tolerable safety profile [11, 12, 28, 29], participants clearly agreed that this type of patients should be treated with SSAs (even in patients with stable disease) rather than considering a wait and see strategy. In the CLARINET study (in which 95 % of the patients had stable disease at baseline) median PFS was not reached in the lanreotide arm vs. 18.0 months (95 % CI: 12.1, 24.0) in the placebo arm [12]. In the extension study, median PFS for patients receiving lanreotide was 32.8 months (95 % CI: 30.9, 68) [29]. Having stable disease before treatment is one of the factors associated with tumor control with lanreotide in patients with well-differentiated malignant digestive NETs [30]. Additionally, a recent post-hoc analysis of the CLARINET study has shown that lanreotide was associated with improvements in tumour growth rates (% change in volume per month) vs. placebo as early as 12 weeks (treatment difference: −2.9 [95 % CI: −5.1, −0.8]; p = 0.008) [31].
The experts also agreed that SSAs should be the treatment of choice in patients with NET of digestive origin and Ki-67 < 2 %, since almost every patient in the PROMID study and two thirds of those in the CLARINET study had a Ki-67 up to 2 %[11, 12].
However, a wait and see approach could also be considered (although the level of agreement was lower) in patients with low tumor load, elderly patients or with important comorbidities or patients with Ki-67 < 2 % (in absence of other risk factors). No evidence-based data is available with respect to overall survival (OS) to support the early use of SSA vs. wait and see [11, 12, 32], although it is difficult to obtain survival results in patients with slow-growing tumors and long-life expectancy. In any case, participants agreed that wait and see should not be considered if Ki-67 > 5 %.
Currently, there is no clinical data regarding a potential class effect of the two SSAs. Although this issue was not directly addressed in the Delphi questionnaire, the participants agreed with lanreotide, and disagreed with octreotide, being the SSA of choice in the treatment of patients with NET of pancreatic origin and Ki67 > 2 % and <10 % (so far, lanreotide is the only SSA with proven efficacy in pancreatic NETs [12]). In the recent ENETS guidelines update, octreotide is recommended as medical first-line therapy in patients with G1 advanced NETs, originated at midgut, with positive somatostatin receptors and low tumor burden, while lanreotide is recommended as medical first-line therapy in patients with G1/G2 (<10 %) advanced NETs, originated at midgut or pancreas, with positive somatostatin receptors and low or high (>25 %) liver tumor burden [33].
CS2 (Patient with non-functioning PANCREATIC NET, non-susceptible to surgery or to loco-regional treatment, and with Ki-67 < 10 %, ECOG ≤2: Treatment initiation with SSA, MTD or chemotherapy?)
The participants agreed that SSAs monotherapy is the treatment of choice in this patient in absence of high tumor load and in the context of non-progressive disease, mainly due to the ease of use and low adverse effects of these drugs. In this regard, elderly patients with these characteristics and presenting with important comorbidities are considered the most susceptible candidates for SSA monotherapy. Patients receving SSA monotherapy should be closely followed to detect possible early disease progression. The participants agreed that lanreotide should be the SSA of choice in monotherapy in these cases, since it is the only SSA with proven efficacy in pancreatic NETs [33] and patients in the CLARINET study had ki-67 up to 10 % [12].
In the context of disease progression no consensus was reached regarding the initial treatment of choice (although almost 60 % of the participants consider the use of SSA). According to recent ENETS guidelines [33], SSAs may be of value in subgroups of patients with slowly progressive G1 NETs of pancreatic origin, and two prospective randomized trials in metastatic GEP-NETs have shown antiproliferative effects after disease progression [34, 35].
Regarding MTDs, everolimus or sunitinib have shown benefits in two placebo-controlled trials conducted in patients with advanced pancreatic NETs with disease progression [36, 37]. In the study by Raymond et al., patients randomized to sunitinib improved PFS and the objective response rate vs. those receiving placebo [37]. An OS difference favouring sunitinib was observed, but data from the 5 years follow-up was not significant (although crossover might have confounded the results) [38]. In the RADIANT-3 trial, patients randomized to everolimus had longer median PFS than those randomized to placebo [36]. Additionally, the efficacy of everolimus as first-line therapy was confirmed in the subgroup of patients naive to chemotherapy [39].
Consensus agreement was reached on the safe combination and potential additive effects of SSAs with MTDs. This was suggested by the RADIANT-2 study, although it was performed in patients with functioning NETs [40]. In this double-blind, placebo-controlled, phase 3 study comparing octreotide LAR alone with octreotide LAR plus everolimus in patients with advanced, progressive NET with carcinoid symptoms, median PFS were 11.3 and 16.4 months, respectively (p = 0.026), failing to reach the level of pre-specified boundary for significance (p ≤ 0.0246) [40]. However, “informative censoring” might explain this [41], since when investigators determined that progression had occurred the patient receiving octreotide LAR alone was allowed to cross over to the combination group, but if central review failed to confirm this progression, the patient was censored resulting in inflating PFS values in the octreotide LAR alone group.
Regarding patients with Ki-67 5–10 % and low tumor load, the use of chemotherapy as initial treatment was rejected and a tendency towards rejecting the use of MTDs was also observed. This probably suggests that the use of SSAs in these patients is not uncommon. In patients with Ki-67 5-10 % and high tumor load, the participants agreed on initiating treatment with MTDs.
CS3 (Patient with non-functioning GEP-NET, with Ki-67 < 10 %, ECOG ≤2, in treatment with anti-proliferative dose of SSA and PROGRESSING: Is SSA treatment maintained?)
The participants agreed on maintaining SSA in this patient (mainly due to its good tolerability), independently of whether a MTD is added [42, 43]. Most studies have shown a tendency for improved PFS in the combination arm vs. the single MTD arm, without reaching statistical significance. In the RADIANT-2 study (functioning NETs), patients who received everolimus/octreotide LAR had longer median PFS than those who received octreotide LAR alone, regardless of previous SSA exposure [with previous exposure: PFS 14.3 months (95%CI: 12.0–20.1) vs. 11.1 months (95 % CI: 8.4–14.6); without: 25.2 months (95 % CI: 12.0-not reached) vs. 13.6 months (95 % CI: 8.2–22.7)] [44]. A subanalysis of the Phase III sunitinib study conducted in patients with advanced and progressing pancreatic NETs showed improved PFS in patients receiving SSAs as compared to those who did not, but it was not significantly different [45]. Therefore, there was disagreement with statement 32 about withdrawing SSA when introducing a MTD. In contrast, SSAs are usually withdrawn if chemotherapy is started.
Additionally, the study participants consider increasing the dose of the SSA, reducing the dose administration interval or changing one SSA to the other in these patients.
CS4 (Patient with non-functioning GEP-NET, non-susceptible to surgery or to loco-regional treatment, with Ki-67 < 10 %, ECOG ≤2, AND NEGATIVE OCTREOSCAN®: Is SSA treatment initiated?)
The octreoscan® result was rejected as an excluding factor for the decision to administer SSA treatment, since SSAs do not only exert an effect on hormone secretion but have indirect antiproliferative effects, which do not require the expression of all sybtypes of somatostatine receptors [13, 46, 47]. In addition, the octreoscan® may identify only a specific subtype of somatostatin receptor, such as type 2 [48]. In fact, patients with negative octreoscan® have been shown to respond to SSA [49], and patients with negative octreoscan® were responsive in the PROMID study [11].
According to the results of sentences 39 and 40, monotherapy with SSAs is not considered in patients with negative octreoscan®, high tumor load and Ki67 > 5 %. A retrospective study in patients with digestive NETs treated with lanreotide showed that Ki-67 ≤ 5 % and hepatic tumor load ≤25 % were significantly associated with disease stability [30]. However, it must be taken into account that the CLARINET study showed the efficacy of lanreotide in tumors with Ki-67 up to <10 %, and half of patients had >25 % liver involvement and benefited from treatment [12].
Frequently, an octreoscan® result only considers whether an uptake of the radiolabeled SSA has occurred or not; however, the Krenning scale [lower than (grade 1), equal to (grade 2), or greater than (grade 3) normal liver tissue; or higher than normal spleen or kidney uptake (grade 4)] [50]] suggest that results considered as negative could be positive.
CS5 (Patient with non-functioning GEP-NET, non-susceptible to surgery or to loco-regional treatment, with Ki-67>10 %, ECOG ≤2, AND POSITIVE OCTREOSCAN®: Is SSA treatment initiated?)
A higher Ki-67 % is perceived as worse prognosis, and thus, more aggressive treatment is recommended; therefore, in this patient SSA are usually combined with other options.
The participants agreed that when Ki-67 is 10–20 % (i.e., WHO grade 2 [51]), and/or there is high tumor load and/or NET of pancreatic origin, treatment is usually initiated with MTDs (usually combined with SSA) or chemotherapy. In the RADIANT-3 and RADIANT-4 studies, conducted in patients with progressive, advanced, pancreatic (RADIANT-3) or lung or gastrointestinal (RADIANT-4) NETs, including intermediate-grade tumors, everolimus resulted in significantly prolonged PFS vs. placebo [36, 52], and the addition of octreotide to everolimus was previously shown to be well tolerated [42]. Other studies conducted in patients with advanced, pancreatic NETs, including intermediate-grade tumors, have achieved substantial clinical benefits with streptozotocin and 5FU or doxorubicin, capecitabine and temozolomide chemotherapy [53–55] or with oxaliplatin-based chemotherapy [56–58]. In addition, platinum-based chemotherapy resulted in a median survival of 11 months in a study conducted in patients with G3 (Ki-67 > 20 %) gastrointestinal neuroendocrine carcinoma, and survival was significantly better in tumors of pancreatic origin than colon origin [59].
The use of SSA in monotherapy could be considered when Ki67 < 20 %, when there is low tumor load, and in case of important comorbidities, since SSA results mainly in tumor stabilization, and has low toxicity [34, 46, 60]. It also seems reasonable in tumors of gastrointestinal origin, since most of the evidence for chemotherapy and MTDs has been observed in tumors of pancreatic origin (although >50 % of the patients in the everolimus arm in the recent RADIANT-4 study had gastrointestinal NETs [52]).
Additionally, a 18FDG-PET-TAC or a re-biopsy can be performed in case of discrepancy between the degree of cellular proliferation and the octreoscan® results.
The study presented the limitations intrinsic to the subjective nature of the answers. In addition, the number of oncologists invited to participate was reduced (given the low frequency of the disease), and thus, the number of questionnaires evaluated was limited. However, the response rate was high in both rounds of the questionnaire (>80 %), and the results reflect clinical practice in the field of NETs in Spain. Only the questions not reaching consensus in the first round, along with those with neutral consensus, were passed on to the second round in order to shorten the time needed to answer the questionnaire, to reduce participant fatigue [61]. The questionnaire showed great internal consistency and high quantitative and qualitative correlation between the two rounds for the questions that needed the second round, and thus, the answers may be considered reliable.
Conclusion
A series of recommendations based on the clinical practice and opinion of Spanish oncologists with experience in the management of NETs were developed and may be used as treatment guidance for the use of SSAs in controversial NET CS.
Acknowledgements
The authors would like to thank the Panel of Experts for their participation in the Delphi study: Inmaculada Concepción Ales Díaz (Hospital Regional Universitario Carlos Haya), Juan Domingo Alonso Lajara (Hospital Clínico Universitario Virgen de la Arrixaca), María Carmen Alonso López (Hospital General de Albacete), Vicente Alonso Orduña (Hospital Universitario Miguel Servet), Antonio Arrivi García-Ramos (Clínica Rotger), Virginia Arrazubi Arrula (Hospital Universitario de Basurto), Eduardo Batiste-Alentorn Guillén (Hospital Universitario de Vic), Matilde Bolaños Naranjo (Hospital General Juan Ramón Jiménez), Elena María Brozos Vázquez (Hospital Clínico Universitario de Santiago de Compostela), Luís Cabezón Gutiérrez (Hospital de Torrejón), Enrique Cabrera Espinos (Hospital Arnau de Vilanova), Juana María Cano Cano (Hospital General de Ciudad Real), Alberto Carmona Bayonas (Hospital General Universitario Morales Meseguer), Guillermo Crespo Herrero (Hospital Universitario de Burgos), Ricardo Cubedo Cervera (Hospital Universitario Puerta de Hierro Majadahonda), Ana Belén Custodio Carretero (Hospital Universitario La Paz), Juan Cruz de la Cámara Gómez (Hospital Arquitecto Marcide), María Teresa Delgado Ureña (Hospital Universitario San Cecilio), Roberto Pedro Díaz Beveridge (Hospital Universitario y Politécnico La Fe), María Olga Donnay Candil (Hospital Universitario de la Princesa), Emma Dotor Navarro (Corporació Sanitària Parc Taulí), María Rosario Dueñas García (Hospital Universitario Médico Quirúrgico de Jaén), Larraitz Egaña Otaño (Hospital Universitario Donostia), María Pilar Escudero Emperador (Hospital Clínico Universitario Lozano Blesa), Ovidio Fernández Calvo (Hospital Santa María Nai), Julen Fernández Plana (Hospital Universitari Mútua de Terrassa), Teresa Fernández Rodríguez (Hospital Son Llàtzer), Gaspa Ferran Losa (Hospital de Sant Joan Despí Moisès Broggi), Javier Gallego Plazas (Hospital General Universitario de Elche), María Isabel Gallegos Sancho (Hospital General de Segovia), Pilar García Alfonso (Hospital General Universitario Gregorio Marañón), Inés María García Castro (Hospital Universitario de Guadalajara), María Teresa García García (Hospital General Universitario Morales Meseguer), Beatriz García Paredes (Hospital Universitario Clínico San Carlos), Encarnación González Flores (Hospital General Virgen de las Nieves), Raquel Guardeño Sánchez (Institut Català d’Oncologia Girona), Irene Hernández García (Hospital de Navarra), Paula Jiménez Fonseca (Hospital Universitario Central de Asturias), Encarnación Jiménez Orozco (Hospital de Jerez), José Miguel Jurado García (Hospital Universitario San Cecilio), Rosa María Llorente Doménech (Hospital Universitario Doctor Peset), Diego Malon Jiménez (Hospital Universitario de Fuenlabrada), Ray Antonio Manneh Kopp (Hospital Universitario 12 de Octubre), Miguel Marín Vera (Hospital Clínico Universitario Virgen de la Arrixaca), David Marrupe González (Hospital Universitario de Móstoles), Alfonso Martín Carnicero (Hospital San Pedro), José Miguel Martín Martínez (Hospital Universitario de Getafe), Marta Martín Richard (Hospital de la Santa Creu i Sant Pau), Alejandro Martínez Fernández (Hospital del Mar), Begoña Medina Magan (Complejo Hospitalario Torrecárdenas), Javier Medina Martínez (Hospital Virgen de la Salud), Sandra Merino Varela (Hospital Universitari Joan XXIII), Sílvia Muñoz Borrajo (Hospital de Mollet), Jorge Muñoz Luengo (Hospital San Pedro de Alcántara), Luís Miguel Navarro Martín (Hospital Clínico Universitario de Salamanca), Beatriz Nieto Mangudo (Hospital de León), Paola Patricia Pimentel Cáceres (Hospital General Universitario Santa Lucía), Juan José Reina Zoilo (Hospital Universitario Virgen de la Macarena), María Ángeles Rodríguez Jaraiz (Hospital San Pedro de Alcántara), Javier Sastre Valera (Hospital Universitario Clínico San Carlos), Raquel Serrano Blanch (Hospital Universitario Reina Sofía), Diego Soto de Prado Otero (Hospital Clínico Universitario de Valladolid), Alexandre Teule Vega (Institut Català d’Oncologia l’Hospitalet), Silvia Varela Ferreiro (Hospital Universitario Lucus Augusti), María Francisca Vázquez Rivera (Hospital Clínico Universitario de Santiago de Compostela), Ana Lucía Yuste Izquierdo (Hospital General Universitari d’Alacant).
Almudena Pardo, at Ogilvy Healthworld Barcelona, provided writing services.
Funding
Ipsen Pharma Spain provided logistic support for the development of the NETpraxis program. Medical writing services for the drafting of this manuscript were also funded by Ipsen Pharma Spain.
Availability of data and materials
The datasets generated during and/or analysed during the current study (the anwsers [1–9] to all the sentences of the Delphi questionnaire given by the participants in both rounds [first round: 65 participants, 48 sentences; Second round: 57 participants, 25 sentences]) are available from the corresponding author on reasonable request.
Authors’ contributions
IS, AS, JC, CL, RGC and EG designed the Delphi questionnaire, analyzed the data and critically reviewed and approved the manuscript.
Competing interests
JC has participated as advisor or received fee for lectures from Ipsen, Novartis, Pfizer, Adacap and Lexicon. RGC has provided scientific advise to Ipsen, Novartis, Pfizer, Adacap and Lexicon. EG has participated as advisor or received fee for lectures from Ipsen, Novartis, Pfizer, Adacap and Lexicon. CL has participated as advisor, has received fee for lectures or grants from clinical trials from Ipsen, Novartis and Pfizer. IS has participated as advisor or received fee for lectures from Ipsen, Novartis and Pfizer. AS declares no conficts of interest.
Consent for publication
Not applicable
Ethics approval and consent to participate
Not applicable
Abbreviations
- CS
Clinical situations
- Cα
Cronbach’s alpha
- ECOG
Eastern Cooperative Oncology Group
- GEP
Gastroenteropancreatic
- k
Kappa index
- MTD
Molecular targeted drugs
- NET
Neuroendocrine tumor
- ri
Intra-class correlation coefficient
- rs
Spearman coefficient
- SSA
Somatostain analogue
- VC
Variation coefficient
Contributor Information
Isabel Sevilla, Phone: (+34) 951 29 00 00, Email: isevilla02@yahoo.es.
Ángel Segura, Email: segura_ang@gva.es.
Jaume Capdevila, Email: jcapdevila@onco.cat.
Carlos López, Email: clopez@humv.es.
Rocío García-Carbonero, Email: rgcarbonero@gmail.com.
Enrique Grande, Email: egrande@oncologiahrc.com.
References
- 1.Ilett EE, Langer SW, Olsen IH, Federspiel B, Kjaer A, Knigge U. Neuroendocrine carcinomas of the gastroenteropancreatic system: a comprehensive review. Diagnostics (Basel) 2015;5:119–176. doi: 10.3390/diagnostics5020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cives M, Strosberg J. An update on gastroenteropancreatic neuroendocrine tumors. Oncology (Williston Park) 2014;28:749–756. [PubMed] [Google Scholar]
- 3.Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1–18. doi: 10.1016/j.ecl.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Ellis L, Shale MJ, Coleman MP. Carcinoid tumors of the gastrointestinal tract: trends in incidence in England since 1971. Am J Gastroenterol. 2010;105:2563–2569. doi: 10.1038/ajg.2010.341. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Carbonero R, Capdevila J, Crespo-Herrero G, et al. Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): results from the National Cancer Registry of Spain (RGETNE) Ann Oncol. 2010;21:1794–1803. doi: 10.1093/annonc/mdq022. [DOI] [PubMed] [Google Scholar]
- 6.Ruszniewski P, Ish-Shalom S, Wymenga M, et al. Rapid and sustained relief from the symptoms of carcinoid syndrome: results from an open 6-month study of the 28-day prolonged-release formulation of lanreotide. Neuroendocrinology. 2004;80:244–251. doi: 10.1159/000082875. [DOI] [PubMed] [Google Scholar]
- 7.Janson ET. Somatostatin analogs in the treatment of neuroendocrine gastroenteropancreatic and intrathoracic tumors. J Endocrinol Invest. 2005;28:137–140. doi: 10.1007/BF03345356. [DOI] [PubMed] [Google Scholar]
- 8.Culler MD, Oberg K, Arnold R, Krenning EP, Sevilla I, Diaz JA. Somatostatin analogs for the treatment of neuroendocrine tumors. Cancer Metastasis Rev. 2011;30(Suppl 1):9–17. doi: 10.1007/s10555-011-9293-0. [DOI] [PubMed] [Google Scholar]
- 9.Oberg KE. The management of neuroendocrine tumours: current and future medical therapy options. Clin Oncol (R Coll Radiol) 2012;24:282–293. doi: 10.1016/j.clon.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Kos-Kudla B. Treatment of neuroendocrine tumors: new recommendations based on the CLARINET study. Contemp Oncol (Pozn) 2015;19:345–349. doi: 10.5114/wo.2015.56006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 12.Caplin ME, Pavel M, Cwikla JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224–233. doi: 10.1056/NEJMoa1316158. [DOI] [PubMed] [Google Scholar]
- 13.Alonso-Gordoa T, Capdevila J, Grande E. GEP-NETs update: Biotherapy for neuroendocrine tumours. Eur J Endocrinol. 2015;172:R31–R46. doi: 10.1530/EJE-14-0354. [DOI] [PubMed] [Google Scholar]
- 14.Kulke MH, Shah MH, Benson AB, 3rd, et al. Neuroendocrine tumors, version 1.2015. J Natl Compr Canc Netw. 2015;13:78–108. doi: 10.6004/jnccn.2015.0011. [DOI] [PubMed] [Google Scholar]
- 15.Oberg K, Knigge U, Kwekkeboom D, Perren A, Group EGW Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii124–vii130. doi: 10.1093/annonc/mds295. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Carbonero R, JImenez-Fonseca P, Teulé A, Barriuso J, Sevilla I. SEOM clinical guidelines for the diagnosis and treatment of gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) 2014. Clin Transl Oncol. 2014;16:1025–1034. doi: 10.1007/s12094-014-1214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochoa-Carrillo FJ, Alvarado-Cabrero I, Barreto-Zúñiga R, et al. Parámetros de práctica clínica del diagnóstico y tratamiento de los tumores neuroendocrinos gastroenteropancreáticos 2014. Gaceta mexicana de oncología. 2014;13:1–34. [Google Scholar]
- 18.ENETS The 2012 European Neuroendocrine Tumor Society (ENETS) Consensus Guidelines for the Diagnosis and Treatment of Neuroendocrine Tumors. Neuroendocrinology. 2012;95:71–176. doi: 10.1159/000335600. [DOI] [PubMed] [Google Scholar]
- 19.Kvols LK, Brendtro KL, Klimstra DS, et al. The North American Neuroendocrine Tumor Society (NANETS) Guidelines. Pancreas. 2010;39:705–948. doi: 10.1097/MPA.0b013e3181eb7451. [DOI] [PubMed] [Google Scholar]
- 20.Ramage JK, Ahmed A, Ardill J, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs) Gut. 2012;61:6–32. doi: 10.1136/gutjnl-2011-300831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimaldi F, Fazio N, Attanasio R, et al. Italian Association of Clinical Endocrinologists (AME) position statement: a stepwise clinical approach to the diagnosis of gastroenteropancreatic neuroendocrine neoplasms. J Endocrinol Invest. 2014;37:875–909. doi: 10.1007/s40618-014-0119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linstone HA, Turoff M. The Delphi Method Techniques and Applications. Reading: Addison-Wesley Publishing Company; 1975. [Google Scholar]
- 23.Varela-Ruiz M, Díaz-Bravo L, García-Durán R. [Description and uses of the Delphi method for research in the healthcare area]. Inv Ed Med. 2012;1(2):90–5.
- 24.Schmitt N. Uses and abuses of coefficient alpha. Psychological Assessment. 1996;8:350–353. doi: 10.1037/1040-3590.8.4.350. [DOI] [Google Scholar]
- 25.Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess. 1994;6:284–290. doi: 10.1037/1040-3590.6.4.284. [DOI] [Google Scholar]
- 26.Dawson B, Trapp RG. Basic & Clinical Biostatistics (LANGE Basic Science) New York: Lange Medical Books/McGraw- Hill; 2004. [Google Scholar]
- 27.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 28.Khan MS, El-Khouly F, Davies P, Toumpanakis C, Caplin ME. Long-term results of treatment of malignant carcinoid syndrome with prolonged release Lanreotide (Somatuline Autogel) Aliment Pharmacol Ther. 2011;34:235–242. doi: 10.1111/j.1365-2036.2011.04693.x. [DOI] [PubMed] [Google Scholar]
- 29.Caplin ME, Pavel M, Cwikla JB, et al. Anti-tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: the CLARINET open-label extension study. Endocr Relat Cancer. 2016;23:191–199. doi: 10.1530/ERC-15-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palazzo M, Lombard-Bohas C, Cadiot G, et al. Ki67 proliferation index, hepatic tumor load, and pretreatment tumor growth predict the antitumoral efficacy of lanreotide in patients with malignant digestive neuroendocrine tumors. Eur J Gastroenterol Hepatol. 2013;25:232–238. doi: 10.1097/MEG.0b013e328359d1a6. [DOI] [PubMed] [Google Scholar]
- 31.Caplin M, Pavel M, Ruszniewski P, Liyanage N, Massien C, Dromanin C. Tumor Growth Rate (TGR) as an Indicator of Antitumor Activity With Lanreotide Autogel/Depot (LAN) Versus Placebo (Pbo) in Intestinal/Pancreatic NET: Post Hoc Analysis of CLARINET Data. Clin Adv Hematol Oncol. 2016;14(5 Suppl 7):6–7. [PubMed] [Google Scholar]
- 32.Pavel M, Baudin E, Couvelard A, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95:157–176. doi: 10.1159/000335597. [DOI] [PubMed] [Google Scholar]
- 33.Pavel M, O’Toole D, Costa F, et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology. 2016;103:172–185. doi: 10.1159/000443167. [DOI] [PubMed] [Google Scholar]
- 34.Faiss S, Pape UF, Bohmig M, et al. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors--the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol. 2003;21:2689–2696. doi: 10.1200/JCO.2003.12.142. [DOI] [PubMed] [Google Scholar]
- 35.Arnold R, Rinke A, Klose KJ, et al. Octreotide versus octreotide plus interferon-alpha in endocrine gastroenteropancreatic tumors: a randomized trial. Clin Gastroenterol Hepatol. 2005;3:761–771. doi: 10.1016/S1542-3565(05)00481-7. [DOI] [PubMed] [Google Scholar]
- 36.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 38.Raymond E, Niccoli P, Castellano D, et al. Sunitinib (SU) in patients with advanced, progressive pancreatic neuroendocrine tumors (pNET): Final overall survival (OS) results from a phase III randomized study including adjustment for crossover. J Clin Oncol. 2016;34(suppl 4S):309. [Google Scholar]
- 39.Lombard-Bohas C, Yao JC, Hobday T, et al. Impact of prior chemotherapy use on the efficacy of everolimus in patients with advanced pancreatic neuroendocrine tumors: a subgroup analysis of the phase III RADIANT-3 trial. Pancreas. 2015;44:181–189. doi: 10.1097/MPA.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 41.Yao JC, Lagunes DR, Kulke MH. Targeted therapies in neuroendocrine tumors (NET): clinical trial challenges and lessons learned. Oncologist. 2013;18:525–532. doi: 10.1634/theoncologist.2012-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao JC, Lombard-Bohas C, Baudin E, et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J Clin Oncol. 2010;28:69–76. doi: 10.1200/JCO.2009.24.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capdevila J, Sevilla I, Alonso V, et al. Evaluation of the efficacy and safety of lanreotide in combination with targeted therapies in patients with neuroendocrine tumours in clinical practice: a retrospective cross-sectional analysis. BMC Cancer. 2015;15:495. doi: 10.1186/s12885-015-1512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anthony LB, Pavel ME, Hainsworth JD, et al. Impact of previous somatostatin analogue use on the activity of everolimus in patients with advanced neuroendocrine tumors: analysis from the phase III RADIANT-2 trial. Neuroendocrinology. 2015;102:18–25. doi: 10.1159/000381715. [DOI] [PubMed] [Google Scholar]
- 45.Valle J, Faivre S, Raoul J, et al. Phase III trial of sunitinib (SU) versus placebo (PLO) for treatment of pancreatic neuroendocrine tumors (NET): impact of somatostatin analogue (SSA) treatment on progression-free survival (PFS) Ann Oncol. 2010;21:viii264. doi: 10.1093/annonc/mdq420. [DOI] [Google Scholar]
- 46.Butturini G, Bettini R, Missiaglia E, et al. Predictive factors of efficacy of the somatostatin analogue octreotide as first line therapy for advanced pancreatic endocrine carcinoma. Endocr Relat Cancer. 2006;13:1213–1221. doi: 10.1677/erc.1.01200. [DOI] [PubMed] [Google Scholar]
- 47.Susini C, Buscail L. Rationale for the use of somatostatin analogs as antitumor agents. Ann Oncol. 2006;17:1733–1742. doi: 10.1093/annonc/mdl105. [DOI] [PubMed] [Google Scholar]
- 48.Asnacios A, Courbon F, Rochaix P, et al. Indium-111-pentetreotide scintigraphy and somatostatin receptor subtype 2 expression: new prognostic factors for malignant well-differentiated endocrine tumors. J Clin Oncol. 2008;26:963–970. doi: 10.1200/JCO.2007.12.7431. [DOI] [PubMed] [Google Scholar]
- 49.Hillman N, Herranz L, Alvarez C, Martinez Olmos MA, Marco A, Gomez-Pan A. Efficacy of octreotide in the regression of a metastatic carcinoid tumour despite negative imaging with In-111-pentetreotide (Octreoscan) Exp Clin Endocrinol Diabetes. 1998;106:226–230. doi: 10.1055/s-0029-1211980. [DOI] [PubMed] [Google Scholar]
- 50.Kwekkeboom DJ, Teunissen JJ, Bakker WH, et al. Radiolabeled somatostatin analog [177Lu-DOTA0, Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol. 2005;23:2754–2762. doi: 10.1200/JCO.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 51.Rindi G, Arnold R, Bosman FT. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. WHO classification of tumours of the digestive system. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. World Health Organization classification of tumours. 4. Lyon: IARC Press; 2010. [Google Scholar]
- 52.Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387:968–977. doi: 10.1016/S0140-6736(15)00817-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strosberg JR, Fine RL, Choi J, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011;117:268–275. doi: 10.1002/cncr.25425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saif MW, Kaley K, Brennan M, Garcon MC, Rodriguez G, Rodriguez T. A retrospective study of capecitabine/temozolomide (CAPTEM) regimen in the treatment of metastatic pancreatic neuroendocrine tumors (pNETs) after failing previous therapy. JOP. 2013;14:498–501. doi: 10.6092/1590-8577/1589. [DOI] [PubMed] [Google Scholar]
- 55.Abbasi S, Kashashna A, Albaba H. Efficacy of capecitabine and temozolomide combination in well-differentiated neuroendocrine tumors: Jordan experience. Pancreas. 2014;43:1303–1305. doi: 10.1097/MPA.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 56.Dussol AS, Joly MO, Vercherat C, et al. Gemcitabine and oxaliplatin or alkylating agents for neuroendocrine tumors: Comparison of efficacy and search for predictive factors guiding treatment choice. Cancer. 2015;121(19):3428–34 [DOI] [PubMed]
- 57.Ferrarotto R, Testa L, Riechelmann RP, et al. Combination of Capecitabine and Oxaliplatin is an Effective Treatment Option for Advanced Neuroendocrine Tumors. Rare Tumors. 2013;5:e35. doi: 10.4081/rt.2013.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spada F, Antonuzzo L, Marconcini R, et al. Oxaliplatin-based chemotherapy in advanced neuroendocrine tumors: clinical outcomes and preliminary correlation with biological factors. Neuroendocrinology. 2016;103(6):806–14. [DOI] [PubMed]
- 59.Sorbye H, Welin S, Langer SW, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152–160. doi: 10.1093/annonc/mds276. [DOI] [PubMed] [Google Scholar]
- 60.Aparicio T, Ducreux M, Baudin E, et al. Antitumour activity of somatostatin analogues in progressive metastatic neuroendocrine tumours. Eur J Cancer. 2001;37:1014–1019. doi: 10.1016/S0959-8049(01)00073-9. [DOI] [PubMed] [Google Scholar]
- 61.Thangaratinam S, Redman CWE. The Delphi technique. The Obstetrician & Gynaecologist. 2005;7:120–125. doi: 10.1576/toag.7.2.120.27071. [DOI] [Google Scholar]
- 62.Toumpanakis C, Caplin ME. Update on the role of somatostatin analogs for the treatment of patients with gastroenteropancreatic neuroendocrine tumors. Semin Oncol. 2013;40:56–68. doi: 10.1053/j.seminoncol.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 63.Falconi M, Bartsch DK, Eriksson B, et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: well-differentiated pancreatic non-functioning tumors. Neuroendocrinology. 2012;95:120–134. doi: 10.1159/000335587. [DOI] [PubMed] [Google Scholar]
- 64.Costa FP, Gumz B, Pasche B. Selecting patients for cytotoxic therapies in gastroenteropancreatic neuroendocrine tumours. Best Pract Res Clin Gastroenterol. 2012;26:843–854. doi: 10.1016/j.bpg.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol. 2008;26:4311–4318. doi: 10.1200/JCO.2008.16.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salazar R, Reidy-Lagunes D, Yao J. Potential synergies for combined targeted therapy in the treatment of neuroendocrine cancer. Drugs. 2011;71:841–852. doi: 10.2165/11585500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 67.Grande E, Casanovas O, Earl J, et al. sVEGFR2 and circulating tumor cells to predict for the efficacy of pazopanib in neuroendocrine tumors (NETs): PAZONET subgroup analysis. J Clin Oncol. 2013;31(suppl):4140. [Google Scholar]
- 68.van Essen M, Krenning EP, Kam BL, de Jong M, Valkema R, Kwekkeboom DJ. Peptide-receptor radionuclide therapy for endocrine tumors. Nat Rev Endocrinol. 2009;5:382–393. doi: 10.1038/nrendo.2009.105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study (the anwsers [1–9] to all the sentences of the Delphi questionnaire given by the participants in both rounds [first round: 65 participants, 48 sentences; Second round: 57 participants, 25 sentences]) are available from the corresponding author on reasonable request.


