Abstract
Background
C. pseudotuberculosis is an important animal pathogen that causes substantial economical loss in sheep and goat farming. Zoonotic infections in humans are rare, but when they occur they are often severe and difficult to treat. One of the most studied proteins from this bacterium, the secreted protein CP40 is being developed as a promising vaccine candidate and has been characterized as a serine protease. In this study we have investigated if CP40 is an endoglycosidase rather than a protease.
Results
CP40 does not show any protease activity and contains an EndoS-like family 18 of glycoside hydrolase (chitinase) motif. It hydrolyzes biantennary glycans on both human and ovine IgGs. CP40 is not a general chitinase and cannot hydrolyze bisecting GlcNAc.
Conclusion
Taken together we present solid evidence for re-annotating CP40 as an EndoS-like endoglycosidase. Redefining the activity of this enzyme will facilitate subsequent studies that could give further insight into immune evasion mechanisms underlying corynebacterial infections in animals and humans.
Keywords: CP40, EndoS, IgG, Corynebacterium pseudotuberculosis, Glycosidase, Chitinase, endo-β-N-acetylglucosaminidase
Background
Glycoproteins are essential for most biological pathways in eukaryotes and play important roles in the immune system of mammals [1, 2]. Glycan chains are important for maintenance of the structure of glycoproteins and their biological function. Alterations in glycosylation pattern on glycoproteins involved in the immune system are associated with disorders such as autoimmunity and cancer [3, 4]. Immunoglobulin G (IgG) is the most abundant immunoglobulin in serum and plays a fundamental role in the adaptive immune response. The hinge and CH2 regions in IgG are involved in binding to both IgG-Fc receptors (FcγR) and the complement factor C1q (3). There is a conserved N-linked glycosylation site on asparagine 297 in the CH2 domain of Fc of human IgG [5]. This N-297 glycosylation is essential for fine tuning IgG effector functions such as binding to FcγRs and complement activation [6–8].
Glycosidases are enzymes that catalyze hydrolysis of glycosidic linkages in glycan chains. There are two kinds of glycosidases: exoglycosidases that cleave terminal carbohydrates from glycan structures, and endoglycosidases that hydrolyze linkages within the glycan chains. Numerous glycosidases from bacterial pathogens with activity on mammalian glycoproteins have been identified and characterized [9]. Some of these glycosidases are used for glycan engineering and glycan analysis of glycoproteins, or as tools for glycobiology research and drug development [10]. Endoglycosidases F1, F2 and F3 are important glycosidases from Elizabethkingia meningoseptica (formerly Flavobacterium meningosepticum) have been used as standard tools for glycoprotein characterization and glycoengineering. These endo-β-N-acetylglucosaminidases hydrolyze β-1,4-N-acetyl-D-glucosamine linkages in the chitobiose core of N-glycans in glycoproteins [11]. Although PNGase F is an amidase and not a glycosidase, it is often used in this context as an important tool able to release most eukaryotic N-glycans by catalyzing the hydrolysis of the amide bond between the reducing end N-acetylglucosamine (GlcNAc) and the asparagine residue of the glycoprotein [10, 11].
EndoS is a 108 kDa endo-β-N-acetylglucosaminidase belonging to family 18 of glycoside hydrolases (GH18) (CAZy, 2016; http://www.cazy.org). It is secreted by the strictly human pathogen Streptococcus pyogenes [12] and cleaves the β-1,4 linkage between the two GlcNAcs in the chitobiose core of the N-linked glycan of IgG [13]. IgG is the preferred substrate for EndoS, and this enzyme hydrolyzes the N-linked glycan only on native, but not denatured IgG [14]. This hydrolysis of IgG glycans most likely contributes to immune evasion by S. pyogenes during colonization and infection [15, 16]. In addition, based on the specificity of EndoS, purified enzyme has been used with success to remove complex N-linked oligosaccharide structures from the Fc region both in vitro and in vivo [16–23].
Corynebacterium pseudotuberculosis causes ovine caseous lymphadenitis (CLA), an infectious and contagious disease in sheep and goats. CLA is characterized by formation of abscesses in superficial lymph nodes and lesions in subcutaneous tissues. The necrotic lesions can also develop internally in spleen, kidneys, lungs, and liver. These infections can reduce meat, wool, and milk production, and are a major cause of economic loss in small ruminant farming [24]. C. pseudotuberculosis is mainly considered an animal pathogen, but can occasionally cause lymphadenitis in humans [25]. In 1994, Walker and colleagues identified a 40 kDa protein secreted by this bacterium and subsequently showed that vaccination of sheep with this protein provides a high level of protection against ovine caseous lymphadenitis CLA [26]. It has been shown that this 40 kDa protein, denoted CP40, is one of the predominant antigens recognized at day 7 of infection by C. pseudotuberculosis in sheep [26]. Subsequent biochemical analysis of recombinantly expressed CP40 including gelatin zymography and inhibition of enzymatic activity using protease inhibitors, have suggested that it has serine protease activity [27], but no further studies of the enzymatic activity have been conducted.
Since there are sequence similarities between CP40 and endoglycosidases such as EndoS, we hypothesized that the annotation of CP40 as a protease is incorrect, and that this major antigen of C. pseudotuberculosis is rather an endoglycosidase with putative activity on host glycoproteins. This hypothesis is also supported by recent a comparative genomics study on C. ulcerans [28], but no experimental evidence has been presented to date.
Methods
Bacterial isolates and growth conditions
The C. pseudotuberculosis type strain DSM-20689 (also ATCC 19410 and NCTC 3450) was originally recovered from infected lymphoid tissue from a sheep (DSMZ, Braunschweig, Germany). Bacteria were cultured in brain heart infusion (BHI) medium (Oxoid, Hampshire, England) supplemented with 0.5 % glucose with aeration at 37 °C for 24 h. Escherichia coli chemically competent strains Top10 (Invitrogen, Hämeenlinna, Finland) and BL21 (DE3) pLysE (Life Technologies, Carlsbad, CA) were propagated on luria broth (LB) agar. For selection of E. coli Top10, 100 μg ml−1 carbenicillin was added to medium and for E. coli BL21 in addition of 100 μg ml−1 carbenicillin, 34 μg ml−1 chloramphenicol was added. E. coli strains were propagated in LB overnight at 37 °C with aeration. Transformation was carried out according to manufacturer’s instructions.
Recombinant expression of CP40 and sequencing of the cp40 gene
Genomic DNA of C. pseudotuberculosis DSM-20689 was extracted using innuPREP Bacteria DNA Kit (Analytikjena Biometra, Göttingen, Germany). The coding sequence of cp40 was amplified by PCR using the oligonucleotide primers 5′-TGT-AGC-CAT-GGG-CGA-GTC-TGC-AAC-CTT-3′ and 5′-GAA-AGG-AAA-ACT-GGA-TCC-TCT-AGA-ACC-AGT-TGG-3′ (The restriction sites for NcoI and BamHI are italic). The 1140 bp PCR product was digested with the restriction enzymes NcoI and BamHI (Thermo Fisher Scientific, New York, NY), and ligated into pMAL-c5X-His vector (New England Biolabs, Berkeley, California) using DNA ligase T4 (Thermo Fisher Scientific). The pMAL-c5X-His vector encodes both Maltose-binding protein (MBP) and a 6xhistidine (His) tags Generated pMAL-c5X-His-cp40 plasmid was transformed to E. coli Top10 chemically competent cells. Plasmids with correct insert were transformed into the E. coli expression strain BL21 (DE3) pLysE. Expression of MBP-cp40-His was induced by 0.1 mM isopropyl-β-D-1-thiogalactopyranoside (IPTG) (VWR International, Radnor, PA) for 2 h at 37 °C. The protein CP40 was purified from the pellet of harvested bacteria according to manufacturer’s protocol using ProteoSpin™ Inclusion Body Protein Isolation Maxi Kit (Norgen Biotek CORP. Thorold, Canada). Purified CP40 protein was dialyzed against PBS at 4 °C.
The cp40 gene in DSM-20689 was sequenced by Sanger sequencing of overlapping PCR products using the Lightrun sequencing service at GATC Biotech (Konstanz, Germany).
N-glycan hydrolysis assay
To analyze the activity of CP40 on N-glycans 0.5 μg of recombinant CP40 was incubated with 1 μg of human IgG and incubated at 37 °C for 2 h. As a control 0.5 μg of MBP and PNGase F also were incubated with IgG in the same condition. The reaction was analyzed by 4–12 % stain-free SDS-PAGE (Bio-Rad Laboratories, Hercules, CA). Proteins were transferred to a PVDF membrane (Bio-Rad Laboratories). Lectin blot was carried out using a Trans-Blot Turbo Transfer system (Bio-Rad Laboratories). The membranes were blocked in lectin buffer (10 mM Hepes, 0.15 M NaCl, 0.1 % Tween 20, 0.01 mM MnCl2 and 0.1 mM CaCl2, pH 7.5) for 20 min and incubated with 2 μg ml−1 of fluorescein labeled LCA (Lens culinaris agglutinin) (Vector Laboratories, Burlingame, CA) in lectin buffer for 45 min. The membranes were washed three times with lectin buffer and visualized using a Chemidoc XRS (Bio-Rad Laboratories). In addition 1 μg ovine, equine, bovine and caprine IgG were incubated with 0.5 μg of recombinant CP40 or EndoS independently at 37 °C for 2 h. In parallel 1 μg of all subclasses of human IgG (Sigma-Aldrich, St. Louis, MO) (Calbiotech, CA, for IgG2) independently were incubated with 0.5 μg CP40 in PBS at 37 °C overnight. The reactions were analyzed by 4–12 % stain-free SDS-PAGE and LCA blot as described above.
Analysis of glycosidase activity of native CP40 in bacterial culture
C. pseudotuberculosis DSM-20689 was grown on blood agar plates at 37 °C. Several colonies were inoculated in 100 ml BHI medium and incubated at 37 °C. After 4 h, 500 μl human plasma was added to the culture medium. Supernatant (1 ml) was collected from the bacterial culture after 8, 10, 12 and 24 h. IgG was purified from the samples using Ab spin trap (GE Healthcare, Little Chalfont, UK) and analyzed by 4–12 % stain-free SDS-PAGE and LCA blot as described above. As a control, 1 μg untreated human IgG was run on the gel. The optical density of the bacterial culture was measured at 600 nm at each time point.
Chitinase assay and substrate specificity of CP40
The fluorogenic substrate 4-methylumbelliferyl-N-acetyl-β-D-glucosaminide (4MU-GlcNAc; 0.2 mM) (Sigma–Aldrich) was incubated with 2 μg of recombinant CP40 in a total volume of 100 μl PBS. As controls, 0.3 mU of a chitinase from Streptomyces griseus (Sigma-Aldrich), 2 μg EndoSd or 2 μg MBP were included. All reactions were incubated for 1.5 h in a black 96-well plate (Thermo Fisher Scientific) at 37 °C. Addition of 100 μl of 0.1 M glycine (pH 10) stopped the reactions. The assays were carried out using 4 replicates for each reaction. Absorbance at 355/460 nm was measured using spectrophotometer and data are shown as mean ± SD. Different response in absorbance were analyzed statistically using an One-Way ANOVA followed up by Tukey’s multiple comparisons test, where differences were considered significant if p < 0.05.
In order to check the substrate specificity of the enzyme, 0.5 μg of recombinantly expressed CP40 was incubated with 1 μg of different glycoproteins such as IgA (Calbiotech), IgE, IgD, IgM, Fetuin, α-1-acid glycoprotein (AGP), and human lactoferrin (hLF) (all Sigma–Aldrich) for 2 h at 37 °C. All samples were incubated with PNGase F under the same conditions as a control. All reactions were analyzed by stain-free SDS-PAGE (Bio-Rad Laboratories). In parallel, 1 μg of bovine, caprine, and equine IgG (Sigma-Aldrich) were incubated with 0.5 μg CP40 or EndoS overnight at 37 °C. In addition 1 μg of all subclasses of human IgG (Sigma-Aldrich, Calbiotech for IgG2) were independently incubated with 0.5 μg CP40 in PBS at 37 °C overnight. The results were analyzed by SDS-PAGE and LCA blot.
Sequence analysis and similarity tree
The sequence of CP40 from C. pseudotuberculosis DSM-20689 was compared with EndoS and different bacterial endoglycosidases and serine proteases. Similarity tree and CP40 active site comparison were generated using the ClustalW alignment in the MacVector software suite (version 15.0.3 (34)) (MacVector, Apex, NC). A reconstruction of a phylogenetic tree was performed using the neighbor-joining systematic method with uncorrected p-values. To validate the tree, bootstrapping with 10,000 replications was used.
Gelatin zymography
To prepare zymogram, 0.1 % gelatin was embedded in 10 % SDS-polyacrylamide gel. Recombinant CP40 (1 μg) or supernatant of Pseudomonas aeruginosa as a control were loaded on gel and run under non-reducing conditions. The zymogram was washed with 2.5 % Triton X-100 (Sigma-Aldrich) for 1 h and incubated in an enzyme assay buffer (50 mM Tris– HCl (pH 7.5), 5 mM CaCl2, 200 mM NaCl, 1 μM ZnCl2) overnight at 37 °C. The gel was stained in Coomassie brilliant blue and then destained with 30 % methanol and 10 % acetic acid.
Analysis of released IgG glycans by HPLC
N-glycans were released from 50 μg of human and ovine IgG by incubation with 2 μg PNGase F, 2 μg EndoS or 5 μg CP40 overnight at 37 °C. Glycans were fluorescently labeled with 2-AB (2-aminobenzamide) (Sigma-Aldrich) and excess labeling reagents removed by normal phase PhyTips (PhyNexus, San Jose, CA). HILIC separation of 2-AB labeled glycans was performed on an Infinity 1260 HPLC System equipped with an Advanced Bio Glycan Map column (2.1 mm × 150 mm 2.7 μm) and a fluorescent detector (all Agilent Technologies, CA). The column temperature was kept at 60 °C and the flow rate set to 0.5 ml min−1 using 100 mM ammonium formate (pH 4.5) against acetonitrile with ammonium formate increasing from 15 to 25 % from 0 to 5 min, then 25–36 % from 5 to 35 min. Fluorescence detection was achieved using excitation and emission wavelengths of 330 nm and 420 nm respectively. The released IgG N-glycan chromatograms has been well established and assigned [29]. In order to analyze sequence, composition and linkage specificities of the glycans, exoglycosidase digestion arrays were performed (Prozyme, Hayward, CA). Non-reducing end glycan residues were specifically removed using 1 U ml−1 ABS (Arthrobacter ureafaciens sialidase) to remove terminal sialic acid. Terminal galactose and GlcNAc were removed using 0.5 U ml−1 BTG (bovine testes β-galactosidase) and 4 U ml−1 GUH (Streptococcus pneumoniae hexosaminidase) respectively. To remove core fucose, 1 U ml−1 BKF (Bovine kidney fucosidase) was used.
Results
CP40 is similar to EndoS-like enzymes
CP40 has been described as a serine protease, but when we searched the protein databases, the protein did not show any significant sequence similarity with any known proteases except for nearly identical corynebacterial proteins that most likely are annotated based on the original characterization of CP40 (data not shown). Instead, CP40 shows similarity to bacterial endoglycosidases and contains a putative glycoside hydrolase family 18 (GH18) domain. We sequenced the cp40 gene in the DSM-20689 used in this study, and this revealed that the coding sequence only differs in one nucleotide leading to one amino acid substation (proline instead of histidine at position 105) compared to cp40 in WA1030 used in the original characterization of the enzyme [27].
We aligned the sequence of CP40 with a number of bacterial endoglycosidases as well as serine protease sequences. The reconstructed phylogentic tree clearly shows that CP40 clusters with the endoglycosidases rather than the proteases (Fig. 1, panel a). Furthermore, CP40 forms a distinct subgroup with the most similar enzyme, EndoE from Enterococcus faecalis (Fig. 1, panel a) [30].
Fig. 1.
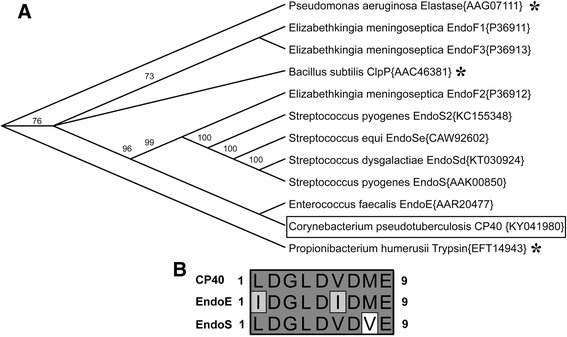
CP40 is similar to bacterial endoglycosidases. a. The amino acid sequence of CP40 was aligned with a number of endoglycosidases and serine proteases from different bacterial species. CP40 is enclosed by a rectangle. A phylogenetic tree was reconstructed using the neighbor-joining method, validated by bootstrapping (10,000 replications) generating a consensus tree. Numbers on branching points shows the percentage of resampling trees supporting the consensus tree. GenBank accession numbers of the proteins are indicated in front of the name of each enzyme. The asterisks indicate the serine proteases. b. The putative family 18 glycosyl hydrolase active site of CP40 was aligned with the verified active sites of EndoE from E. faecalis and EndoS from S. pyogenes
GH18 enzymes contain a conserved consensus sequence motif (LIVMFY)-(DN)-G-(LIVMF)-(DN)-(LIVMF)-(DN)-X-E, where the terminal glutamic acid is essential for enzymatic activity [31, 32]. When aligning a putative GH18 active site in CP40 with the most similar enzymes EndoE and EndoS, it becomes clear that CP40 has a perfect GH18 motif that differs only in one or two amino acids as compared to the active site of EndoS and EndoE, respectively (Fig. 1, panel b). The active sites of both EndoS and EndoE have been confirmed by site-directed mutagenesis, and interestingly both enzymes have activity on the N-linked glycans in IgG Fc [13, 30]. Taken together this analysis clearly indicates that CP40 could be a GH18 enzyme and we addressed this hypothesis experimentally by testing for general chitinase activity, or more specific glycan hydrolyzing activity on host glycoproteins.
CP40 and C. pseudotuberculosis have glycosidase activity on IgG
Based on the clear relationship between CP40 and the known IgG glycan hydrolases EndoS and EndoE, we speculated that IgG could be a substrate also for CP40. As CP40 is secreted [26], we cultured C. pseudotuberculosis DSM-20689 in medium supplemented with 0.5 % human plasma. After 8,10,12 and 24 h of culture, IgG was purified from the culture supernatant and analyzed by SDS-PAGE and LCA blot (Fig. 2, panels a and b). After 24 h when the bacteria were in late exponential phase, a clear mass shift of IgG and a lack of LCA signal could be observed. However, the LCA signals seemed to gradually diminish from the 10 h sample to be completely gone in the 24 h sample. This indicates that C. pseudotuberculosis does secrete a glycoside hydrolase. To test if this activity is indeed mediated by CP40, we recombinantly expressed the enzyme as a maltose-binding protein (MBP)-fusion protein in E. coli and incubated the enzyme with pooled polyclonal human IgG. Glycan hydrolyzing activity was subsequently analyzed by SDS-PAGE and LCA blot. MBP alone, and PNGase F were used as controls. A mobility shift of the IgG heavy chain on SDS-PAGE and a corresponding lack of signal on the LCA blot (LCA recognizes α-linked mannose) were observed when IgG was incubated with CP40 and PNGase F, but not for MBP and the buffer control (Fig. 3, panel a).
Fig. 2.
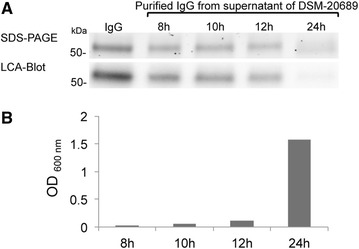
IgG N-glycan hydrolyzing activity can be detected during growth of C. pseudotuberculosis. a. C. pseudotuberculosis was grown in 100 ml BHI. After 4 h, 500 μl of human plasma was added to the culture. 1 ml samples were taken from the bacterial culture after 8, 10,12, 24 h. IgG was purified from the culture and IgG glycan hydrolysis was analyzed by SDS-PAGE and LCA blot. b. The OD of bacterial culture was measured at 600 nm at the indicated time point
Fig. 3.
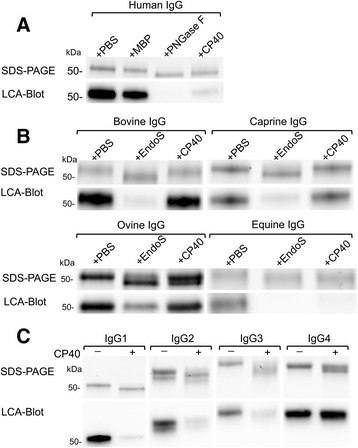
CP40 hydrolyzes N-linked glycans on human and animal IgG. a. Recombinant CP40 (0.5 μg) was incubated with to 1 μg of human. As controls, 0.5 μg of MBP and PNGases F were incubated with human IgG. b. ovine, equine, bovine and caprine IgG (1 μg) were incubated with 0.5 μg of CP40 and EndoS. c. All subclasses of human IgG (1 μg) were incubated with 0.5 μg of recombinant CP40. All of the reactions were at 37 °C for 2 h and IgG glycan hydrolyzing was analyzed by SDS-PAGE and LCA blot
Since C. pseudotuberculosis is primarily an animal pathogen, we investigated whether animal IgGs are substrates for CP40. While EndoS was able to hydrolyze the glycan chain on all tested animal IgGs, as judged by a ~3 kDa shift of the heavy chain in combination with a loss of LCA signal, CP40 was only able to fully hydrolyze equine IgG and partially hydrolyze ovine IgG, but showed no activity on bovine or caprine IgG (Fig. 3, panel b). To further evaluate the glycan hydrolyzing activity of CP40, we incubated CP40 with the four subclasses of human IgG. This revealed that CP40 has activity versus all human IgG subclasses, albeit the hydrolysis of IgG4 was incomplete (Fig. 3, panel c).
CP40 is not a general chitinase or broad spectrum endoglycosidase
In order to investigate if the observed activity of CP40 on IgG could be attributed to a general endochitinase activity, CP40 was incubated with the well-characterized fluorogenic chitinase substrate 4 MB-GlcNAc. We have recently established that the related enzyme EndoS and its homolog EndoSd are not general endochitinases [33]. Therefore EndoSd was used as a negative control, while a verified chitinase from Streptomyces griseus was used as a positive control. This revealed that there is no detectable activity of CP40 on 4 MB-GlcNAc, clearly indicating that CP40 is not a general endochitinase (Fig. 4, panel a).
Fig. 4.
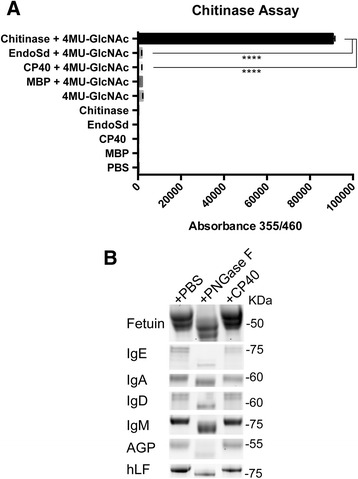
CP40 is not a general chitinase and does not show glycosidase activity on several glycoproteins. a. CP40, EndoSd, MBP and a chitinase from S. griseus were incubated with the fluorescent substrate 4MU-GlcNAc for 1 h and fluorescence was measured at 355/460 nm. The experiments were performed in five replicates. Data are presented as mean ± S.D. The absorbance was analyzed statistically by One-way Anova followed up by Tukey’s multiple comparisons test. ****P < 0.0001. b. Recombinant CP40 (0.5 μg) was incubated with fetuin, IgE, IgA, IgD, IgM, AGP, hLF for 2 h at 37 °C and glycan hydrolyzing activity were analyzed by SDS-PAGE
To elucidate if CP40 has a broader activity on N-linked glycans on glycoproteins, the enzyme was tested for activity against the purified glycoproteins IgA, IgE, IgD, IgM, fetuin, α1-acid glycoprotein (AGP), and human lactoferrin (hLF). As a positive control PNGase F, known to hydrolyze all mammalian N-linked glycans, was used. After incubation, samples were analyzed SDS-PAGE to assess possible size shifts. This revealed that no obvious size shifts could be observed when glycoproteins were incubated with CP40, in sharp contrast to PNGase F, where clear size shifts could be observed (Fig. 4, panel b). These results indicate that CP40 has no major activity on the glycoproteins tested here.
CP40 is not a protease
During the initial characterization of CP40, a recombinant protein with a thrombin cleavable tag was used. Based on gelatin zymography of the thrombin cleaved recombinantly expressed CP40 and culture supernatants from C. pseudotuberculosis strain WA1030 and the use of specific protease inhibitors, it was concluded that CP40 is a serine protease [27]. We attempted to repeat this experiment using recombinantly expressed CP40 and essentially the same gelatin zymography method. This revealed that there was no detectable clearing zone around CP40 in the zymogram, while there was a distinct clearing zone in the positive control corresponding to an elastase in Pseudomonas aeruginosa culture supernatant (Fig. 5).
Fig. 5.
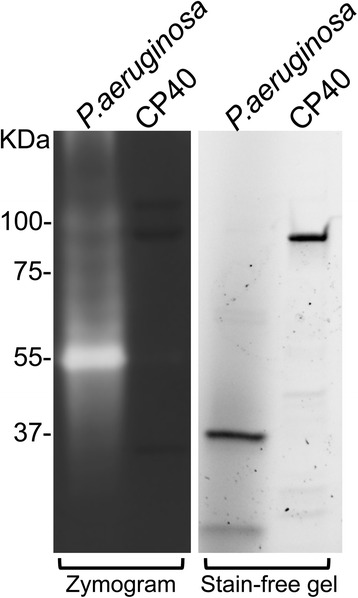
CP40 is not a protease. Recombinant CP40 (1 μg) was loaded on a zymogram (0.1 % gelatin SDS-PAGE) and stain free gel in parallel. Supernatant of P. aeruginosa was used as a positive control
CP40 hydrolyzes biantennary, but not bisecting glycans on IgG
In order to elucidate the glycoform preferences of CP40 in more detail, we turned to HPLC analysis of released glycans. CP40, EndoS, or PNGase, were used to release glycans from human and ovine IgGs (Since C. pseudotuberculosis causes CLA mostly in sheep), and glycans were subsequently fluorescently labeled and analyzed using a glycan dedicated hydrophilic interaction liquid chromatography (HILIC) system. Comparison of the resulting chromatograms from CP40 and EndoS indicate that the glycan cleavage site of CP40 is identical to that of EndoS (Fig. 6). Furthermore, the chromatograms of CP40 generated glycans indicate that this enzyme can hydrolyze all biantennary glycoforms from human and ovine IgGs with the notable exception of structures containing a bisecting GlcNAc (Fig. 6).
Fig. 6.
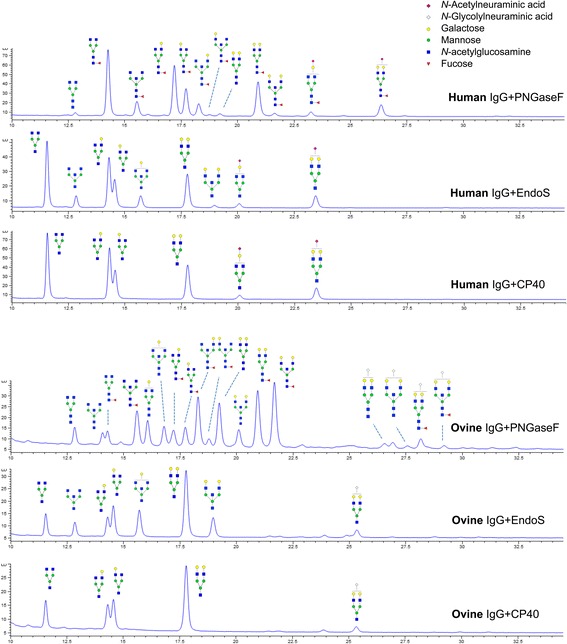
CP40 hydrolyzes biantennary, but not bisecting glycans on IgG glycans from human and ovine IgGs. Glycans were released from human and ovine IgG by PNGase F, EndoS and CP40 respectively, glycans were with 2AB and analyzed by HILIC. The structures annotated according to the digested glycan using a series of defined exoglycosidase. The released biantennary glycans from IgG and the cleavage site of the glycan chain are shown. Monosaccharide identities are shown to the right in the middle and conform to the standard colors and shapes as devised by the Consortium for Functional Glycomic (www.functionalglycomics.org)
Discussion
CLA in sheep caused by C. pseudotuberculosis is a globally important disease that has proven to be difficult to control [34]. Control of the disease is of substantial commercial interest since CLA causes economical losses due to reduced productivity in the dairy, meat and leather industries [24]. Since the vaccines against the different virulence factors of the bacteria such as phospholipase D (PLD) toxin do not induce strong immunity to CLA, researchers are looking for new protective and immunogenic antigens [35]. There are also difficulties associated with using current commercial vaccines due to their limited efficacy as well as regulations in some countries [35]. Towards that end, CP40 is highly interesting since it has been established as a major antigen expressed during infection and animals seroconvert to this protein. Most importantly, CP40 immunization generates protective immunity in both sheep and mice [26, 36, 37]. CP40 has undoubtedly been established in the literature as one the most important secreted proteins from C. pseudotuberculosis. Even though is it not essential for vaccine strategies to completely understand the function of the antigen, molecular characterization can strengthen the basis for targeting the antigen, and most importantly, can increase the understanding of the basic biology of the infective agent.
We have during a number of years been specializing in the characterization of bacterial endoglycosidases with activity of host glycoproteins. During that CP40 has again and again showed up as an odd bird when doing searches with one of our characterized endoglycosidases (data not shown). Furthermore, recent whole genome sequencing projects of different corynebacteria have started to question the annotation of CP40 as a protease and instead link it to endoglycosidase activity [28, 38]. This in combination with our bioinformatic analysis of CP40 strongly suggested that this protein is not a protease, but an endoglycosidase belonging to the GH18 family. Since the protein is fairly small, the likelihood of an additional protease domain is very low. However, since annotation of function based on bioinformatics can be quite ambiguous, clear experimental evidence was needed in order to annotate a completely new function to CP40. When attempting to reproduce the original finding that CP40 has activity against gelatin in zymography [27], we were not able to see any protease activity of the protein (Fig. 5). Size shift of IgG on SDS-PAGE and lack of signal on LCA blot during bacterial growth, suggests that C. pseudotuberculosis express IgG glycan hydrolyzing activity (Fig. 2). This could be attributed to expression of CP40, but we cannot formally exclude that the bacteria express additional enzymes with activity on IgG. Our interpretation is that CP40 is not a protease, and that the previously detected serine protease activity is most likely due to remaining thrombin in the recombinant CP40 preparation, and an unrelated serine protease activity in the culture medium of the C. pseudotuberculosis.
Bacterial endoglycosidases has been extensively used as tools in glycobiology research, but have been somewhat overlooked in pathogenesis research [10]. However, since glycobiology of the immune system, or glycoimmunology, is getting more attention, bacterial modification of glycoproteins beyond mere nutrient acquisition is indeed relevant to study in detail [9]. The activity of GH18 enzymes ranges from chitinase activity that hydrolyzes basically any polymer of GlcNAc, to more specific enzymes that hydrolyses the chitobiose core in certain glycoforms on any glycoprotein, and enzymes that are both glycoform and protein specific as exemplified by EndoS, EndoS2, EndoE, and EndoSd [12, 30, 33, 39]. Based on the similarities to the verified IgG glycan hydrolases EndoE and EndoS, we first analyzed if CP40 could hydrolyze glycans in IgG from different sources, and in the case of human IgG, also of different subclasses (Fig. 3). This revealed that bovine and caprine IgG treated with CP40 were intact, while equine IgG was complete hydrolyzed. All animal IgGs used in this study contain N-glycosylneuraminic acid (NGNA) at the termini of the IgG glycan chain [40]. Furthermore, the total content of carbohydrate in caprine IgG is much lower than human and ovine IgG. The amount of core fucosylated, terminally galactosylated and bisecting oligosaccharides on caprine IgG is also very low compared to human and ovine IgG [40]. Equine IgG has no detectable bisecting GlcNAc on the N-glycan chain and bovine IgG has a very low amount of bisecting glycans on IgG [40].
As mentioned above, many GH18 enzymes have activity on chitin alone, or in the chitobiose core of N-linked glycans, irrespective of the protein backbone. We therefore tested if CP40 had activity on the widely used endochitinase substrate 4MU-GlcNAc and compared to verified chitinases. The results clearly showed that CP40 is not a general chitinase (Fig. 4, panel a).
CP40 seemed to have some preference for IgG, and there were no detectable activity on a number of other glycoproteins harboring N-linked glycan, including some of the other immunoglobulin isotypes (IgA, IgD, and IgA). However, GH18 enzymes can also display preferences for certain glycoforms, as exemplified by EndoF1–3 and EndoS/EndoS2 [41, 42]. We therefore turned to HPLC analysis of released glycans that has the potential elucidate the glycoform specificity in more detail. HPLC chromatograms of CP40 released glycans from human and ovine IgGs showed that this enzyme could only hydrolyze biantennary structures and not bisecting GlcNAc (Fig. 6). This glycoform selectivity closely resembles that of the EndoS homolog EndoSd from S. dysgalactiae [33]. Ovine IgG contains bisecting GlcNAc and high mannose structures compared to human IgG; it also has terminal NGNA instead of N-acetylneuraminic acid (NANA) that is found in human IgG [40]. Previous studies have suggested that carbohydrate binding domains are important for releasing bisecting structures by EndoS, since EndoS lacking carbohydrate binding domain releases bisecting GlcNAc slower than other glycoforms [43]. Since CP40 is a much smaller enzyme than EndoS and lacking putative carbohydrate binding domains, this could potentially explain the lack of activity against IgG glycans with bisecting GlcNAc.
Conclusions
We believe that we have presented very strong evidence for that CP40 is a not a serine protease, but rather an endoglycosidase with activity on immunologically important host proteins. Furthermore, during the final stages of preparation of this manuscript, a review about virulence factors of C. ulcerans identified the CP40 homolog in this species as a GH18 endoglycosidase with similarity to the α domain of EndoE from E. faecalis [44]. Similar endoglycosidases in other bacterial pathogens have been suggested to be involved in evasion of the adaptive immune response, but we have not direct evidence for that CP40 hydrolyzes IgG glycans in vivo and contributes to immune evasion. However, a solid body of work done on CP40 by other research groups shows that CP40 is indeed expressed in vivo, and that immunity towards this protein provides protection against infection. This makes our molecular characterization of this protein relevant, and may aid in the continued development of vaccines and antimicrobial strategies to combat infections with C. pseudotuberculosis.
Acknowledgements
None.
Funding
This work was supported by grants to MC from the Swedish Research Council (project 2012–1875), the Royal Physiographic Society in Lund, the Foundations of Åke Wiberg, Alfred Österlund, Gyllenstierna-Krapperup, Torsten Söderberg, King Gustaf V’s 80 years fund, and Hansa Medical AB. The funders had no role in preparation of the manuscript or in the decision to publish.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author upon request. The phylogenetic tree presented in Fig. 1 has been deposited in TreeBase (http://purl.org/phylo/treebase/phylows/study/TB2:S20068). The sequence of cp40 in DSM-20689 has been submitted to Genbank under accession number KY041980.
Authors’ contributions
AS and MC conceived and designed the study. AS performed the experiments and drafted the paper. AS and AN analyzed the data. All authors contributed to manuscript preparation and approved the final paper.
Competing interests
We have read and understood the BMC Microbiology policy on declaration of interests and declare that we have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Abbreviations
- 2-AB
2-aminobenzamide
- 4MU-GlcNAc
4-methylumbelliferyl-N-acetyl-β-D-glucosaminide
- ABS
Arthrobacter ureafaciens sialidase
- AGP
α-1-acid glycoprotein
- BKF
Bovine kidney fucosidase
- BTG
Bovine testes β-galactosidase
- C. pseudotuberculosis
Corynebacterium pseudotuberculosis
- E. coli
Escherichia coli
- E. faecalis
Enterococcus faecalis
- FcγR
IgG-Fc receptor
- GH18
glycoside hydrolase family 18
- GlcNAc
N-acetylglucosamine
- GUH
Streptococcus pneumoniae hexosaminidase
- HILIC
Hydrophilic interaction liquid chromatography
- hLF
Human lactoferrin
- Ig
Immunoglobulin
- IPTG
Isopropyl-β-D-1-thiogalactopyranoside
- LB
Luria broth
- LCA
Lens culinaris agglutinin
- MBP
Maltose-binding protein
- NANA
N-acetylneuraminic acid
- NGNA
N-glycosylneuraminic acid
- PLD
phospholipase D
Contributor Information
Azadeh Shadnezhad, Email: azadeh.shadnezhad@med.lu.se.
Andreas Naegeli, Email: andreas.nageli@med.lu.se.
Mattias Collin, Email: mattias.collin@med.lu.se.
References
- 1.Dalziel M, Crispin M, Scanlan CN, Zitzmann N, Dwek RA. Emerging principles for the therapeutic exploitation of glycosylation. Science. 2014;343:1235681. doi: 10.1126/science.1235681. [DOI] [PubMed] [Google Scholar]
- 2.Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the immune system. Science. 2001;291:2370–6. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 3.Gornik O, Pavić T, Lauc G. Alternative glycosylation modulates function of IgG and other proteins - implications on evolution and disease. Biochim Biophys Acta. 1820;2012:1318–26. doi: 10.1016/j.bbagen.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Kim YJ, Varki A. Perspectives on the significance of altered glycosylation of glycoproteins in cancer. Glycoconj J. 1997;14:569–76. doi: 10.1023/A:1018580324971. [DOI] [PubMed] [Google Scholar]
- 5.Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution. Biochemistry. 1981;20:2361–70. doi: 10.1021/bi00512a001. [DOI] [PubMed] [Google Scholar]
- 6.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 7.Lux A, Yu X, Scanlan CN, Nimmerjahn F. Impact of immune complex size and glycosylation on IgG binding to human FcγRs. J Immunol. 2013;190:4315–23. doi: 10.4049/jimmunol.1200501. [DOI] [PubMed] [Google Scholar]
- 8.Subedi GP, Barb AW. The structural role of antibody N-glycosylation in receptor interactions. Structure. 2015;23:1573–83. doi: 10.1016/j.str.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garbe J, Collin M. Bacterial hydrolysis of host glycoproteins - powerful protein modification and efficient nutrient acquisition. J Innate Immun. 2012;4:121–31. doi: 10.1159/000334775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sjögren J, Collin M. Bacterial glycosidases in pathogenesis and glycoengineering. Future Microbiol. 2013;9:1039–51. doi: 10.2217/fmb.14.71. [DOI] [PubMed] [Google Scholar]
- 11.Plummer TH, Tarentino AL. Purification of the oligosaccharide-cleaving enzymes of Flavobacterium meningosepticum. Glycobiology. 1991;1:257–63. doi: 10.1093/glycob/1.3.257. [DOI] [PubMed] [Google Scholar]
- 12.Collin M, Olsén A. EndoS, a novel secreted protein from Streptococcus pyogenes with endoglycosidase activity on human IgG. EMBO J. 2001;20:3046–55. doi: 10.1093/emboj/20.12.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allhorn M, Olsén A, Collin M. EndoS from Streptococcus pyogenes is hydrolyzed by the cysteine proteinase SpeB and requires glutamic acid 235 and tryptophans for IgG glycan-hydrolyzing activity. BMC Microbiol. 2008;8:3. doi: 10.1186/1471-2180-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collin M, Olsén A. Effect of SpeB and EndoS from Streptococcus pyogenes on human immunoglobulins. Infect Immun. 2001;69:7187–9. doi: 10.1128/IAI.69.11.7187-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sjögren J, Okumura CYM, Collin M, Nizet V, Hollands A. Study of the IgG endoglycosidase EndoS in group A streptococcal phagocyte resistance and virulence. BMC Microbiol. 2011;11:120. doi: 10.1186/1471-2180-11-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collin M, Svensson MD, Sjöholm AG, Jensenius JC, Sjöbring U, Olsén A. EndoS and SpeB from Streptococcus pyogenes inhibit immunoglobulin-mediated opsonophagocytosis. Infect Immun. 2002;70:6646–51. doi: 10.1128/IAI.70.12.6646-6651.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lood C, Allhorn M, Lood R, Gullstrand B, Olin AI, Ronnblom L, et al. IgG glycan hydrolysis by endoglycosidase S diminishes the proinflammatory properties of immune complexes from patients with systemic lupus erythematosus: a possible new treatment? Arthritis Rheum. 2012;64:2698–706. doi: 10.1002/art.34454. [DOI] [PubMed] [Google Scholar]
- 18.Benkhoucha M, Molnarfi N, Santiago-Raber M-L, Weber MS, Merkler D, Collin M, et al. IgG glycan hydrolysis by EndoS inhibits experimental autoimmune encephalomyelitis. J Neuroinflammation. 2012;9:209. doi: 10.1186/1742-2094-9-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang R, Otten MA, Hellmark T, Collin M, Björck L, Zhao M-H, et al. Successful treatment of experimental glomerulonephritis with IdeS and EndoS, IgG-degrading streptococcal enzymes. Nephrol Dial Transplant. 2010;25:2479–86. doi: 10.1093/ndt/gfq115. [DOI] [PubMed] [Google Scholar]
- 20.van Timmeren MM, van der Veen BS, Stegeman CA, Petersen AH, Hellmark T, Collin M, et al. IgG glycan hydrolysis attenuates ANCA-mediated glomerulonephritis. J Am Soc Nephrol. 2010;21:1103–14. doi: 10.1681/ASN.2009090984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allhorn M, Briceno JG, Baudino L, Lood C, Olsson ML, Izui S, et al. The IgG-specific endoglycosidase EndoS inhibits both cellular and complement-mediated autoimmune hemolysis. Blood. 2010;115:5080–8. doi: 10.1182/blood-2009-08-239020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albert H, Collin M, Dudziak D, Ravetch JV, Nimmerjahn F. In vivo enzymatic modulation of IgG glycosylation inhibits autoimmune disease in an IgG subclass-dependent manner. Proc Natl Acad Sci U S A. 2008;105:15005–9. doi: 10.1073/pnas.0808248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collin M, Shannon O, Björck L. IgG glycan hydrolysis by a bacterial enzyme as a therapy against autoimmune conditions. Proc Natl Acad Sci U S A. 2008;105:4265–70. doi: 10.1073/pnas.0711271105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seyffert N, Guimarães AS, Pacheco LGC, Portela RW, Bastos BL, Dorella FA, et al. High seroprevalence of caseous lymphadenitis in Brazilian goat herds revealed by Corynebacterium pseudotuberculosis secreted proteins-based ELISA. Res Vet Sci. 2010;88:50–5. doi: 10.1016/j.rvsc.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Trost E, Ott L, Schneider J, Schröder J, Jaenicke S, Goesmann A, et al. The complete genome sequence of Corynebacterium pseudotuberculosis FRC41 isolated from a 12-year-old girl with necrotizing lymphadenitis reveals insights into gene- regulatory networks contributing to virulence. BMC Genomics. 2010;11:728. doi: 10.1186/1471-2164-11-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker J, Jackson HJ, Eggelton DG, Meeusen E, Wilson MJ, Brandon MR. Identification of a novel antigen from Corynebacterium pseudotuberculosis that protects sheep against caseous lymphadenitis. Infect Immun. 1994;62:2562–7. doi: 10.1128/iai.62.6.2562-2567.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson MJ, Brandon MR, Walker J. Molecular and biochemical characterization of a protective 40-kilodalton antigen from Corynebacterium pseudotuberculosis. Infect Immun. 1995;63:206–11. doi: 10.1128/iai.63.1.206-211.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trost E, Al-Dilaimi A, Papavasiliou P, Schneider J, Viehoever P, Burkovski A, et al. Comparative analysis of two complete Corynebacterium ulcerans genomes and detection of candidate virulence factors. BMC Genomics. 2011;12:383. doi: 10.1186/1471-2164-12-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pučić M, Knezevic A, Vidic J, Adamczyk B, Novokmet M, Polasek O, et al. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol Cell Proteomics. 2011;10:M111.010090. doi: 10.1074/mcp.M111.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collin M, Fischetti VA. A novel secreted endoglycosidase from Enterococcus faecalis with activity on human immunoglobulin G and ribonuclease B. J Biol Chem. 2004;279:22558–70. doi: 10.1074/jbc.M402156200. [DOI] [PubMed] [Google Scholar]
- 31.Henrissat B, Davies G. Structural and sequence-based classification of glycoside hydrolases. Curr Opin Struct Biol. 1997;7:637–44. doi: 10.1016/S0959-440X(97)80072-3. [DOI] [PubMed] [Google Scholar]
- 32.Karlsson M, Stenlid J. Evolution of family 18 glycoside hydrolases: diversity, domain structures and phylogenetic relationships. J Mol Microbiol Biotechnol. 2008;16:208–23. doi: 10.1159/000151220. [DOI] [PubMed] [Google Scholar]
- 33.Shadnezhad A, Naegeli A, Sjögren J, Adamczyk B, Leo F, Allhorn M, et al. EndoSd: an IgG glycan hydrolyzing enzyme in Streptococcus dysgalactiae subspecies dysgalactiae. Future Microbiol. 2016;11:721–36. doi: 10.2217/fmb.16.14. [DOI] [PubMed] [Google Scholar]
- 34.Baird GJ, Fontaine MC. Corynebacterium pseudotuberculosis and its role in ovine caseous lymphadenitis. J Comp Pathol. 2007;137:179–210. doi: 10.1016/j.jcpa.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Dorella FA, Pacheco LG, Seyffert N, Portela RW, Meyer R, Miyoshi A, et al. Antigens of Corynebacterium pseudotuberculosis and prospects for vaccine development. Expert Rev Vaccines. 2009;8:205–13. doi: 10.1586/14760584.8.2.205. [DOI] [PubMed] [Google Scholar]
- 36.Droppa-Almeida D, Vivas WLP, Silva KKO, Rezende AFS, Simionatto S, Meyer R, et al. Recombinant CP40 from Corynebacterium pseudotuberculosis confers protection in mice after challenge with a virulent strain. Vaccine. 2016;34:1091–6. doi: 10.1016/j.vaccine.2015.12.064. [DOI] [PubMed] [Google Scholar]
- 37.Silva JW, Droppa-Almeida D, Borsuk S, Azevedo V, Portela RW, Miyoshi A, et al. Corynebacterium pseudotuberculosis cp09 mutant and cp40 recombinant protein partially protect mice against caseous lymphadenitis. BMC Vet Res. 2014;10:965. doi: 10.1186/s12917-014-0304-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rückert C, Eimer J, Winkler A, Tauch A. Complete genome sequence of the type strain Corynebacterium mustelae DSM 45274, isolated from various tissues of a male ferret with lethal sepsis. Genome Announc. 2015;3:e01012–5. doi: 10.1128/genomeA.01012-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sjögren J, Struwe WB, Cosgrave EFJ, Rudd PM, Stervander M, Allhorn M, et al. EndoS2 is a unique and conserved enzyme of serotype M49 group A Streptococcus that hydrolyses N-linked glycans on IgG and α1-acid glycoprotein. Biochem J. 2013;455:107–18. doi: 10.1042/BJ20130126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raju TS, Briggs JB, Borge SM, Jones AJS. Species-specific variation in glycosylation of IgG: evidence for the species-specific sialylation and branch-specific galactosylation and importance for engineering recombinant glycoprotein therapeutics. Glycobiology. 2000;10:477–86. doi: 10.1093/glycob/10.5.477. [DOI] [PubMed] [Google Scholar]
- 41.Sjögren J, Cosgrave EFJ, Allhorn M, Nordgren M, Björk S, Olsson F, et al. EndoS and EndoS2 hydrolyze Fc-glycans on therapeutic antibodies with different glycoform selectivity and can be used for rapid quantification of high-mannose glycans. Glycobiology. 2015;25:1053–63. doi: 10.1093/glycob/cwv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trimble RB, Tarentino AL. Identification of distinct endoglycosidase (endo) activities in Flavobacterium meningosepticum: endo F1, endo F2, and endo F3. Endo F1 and endo H hydrolyze only high mannose and hybrid glycans. J Biol Chem. 1991;266:1646–51. [PubMed] [Google Scholar]
- 43.Dixon EV, Claridge JK, Harvey DJ, Baruah K, Yu X, Vesiljevic S, et al. Fragments of bacterial endoglycosidase s and immunoglobulin G reveal subdomains of each that contribute to deglycosylation. J Biol Chem. 2014;289:13876–89. doi: 10.1074/jbc.M113.532812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hacker E, Antunes CA, Mattos-Guaraldi AL, Burkovski A, Tauch A. Corynebacterium ulcerans, an emerging human pathogen. Future Microbiol. 2016;11:1191–208. doi: 10.2217/fmb-2016-0085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author upon request. The phylogenetic tree presented in Fig. 1 has been deposited in TreeBase (http://purl.org/phylo/treebase/phylows/study/TB2:S20068). The sequence of cp40 in DSM-20689 has been submitted to Genbank under accession number KY041980.


