Martin discusses work by Xue and Sokac defining the motor requirements for ring closure during cellularization in Drosophila.
Abstract
Actomyosin rings drive numerous closure processes, but the mechanisms by which they contract are still poorly understood. In this issue, Xue and Sokac (2016. J. Cell Biol. http://dx.doi.org/10.1083/jcb.201608025) show that actomyosin ring closure during Drosophila melanogaster cellularization uses two steps, only one of which involves Myosin-2.
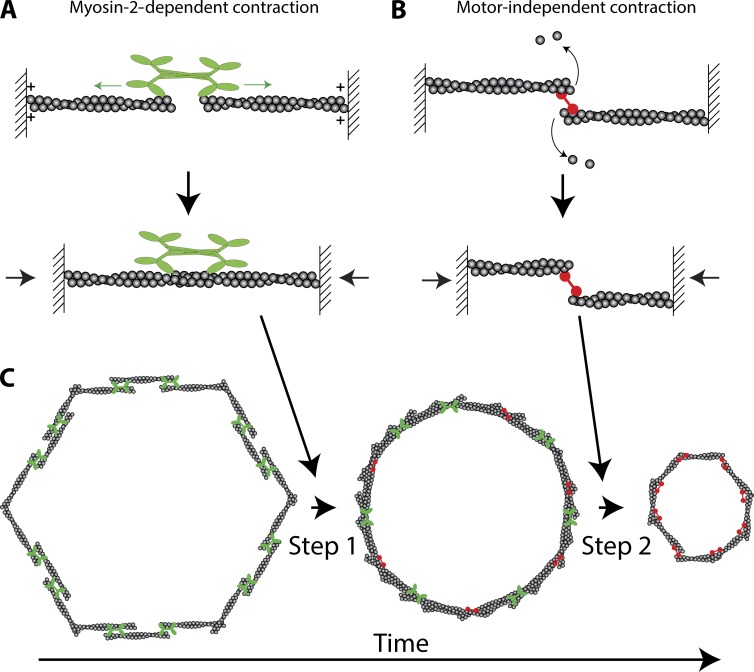
Actomyosin ring closure is important for both unicellular and multicellular events, playing roles in processes ranging from cytokinesis to apoptotic cell extrusion and tissue movement (Schwayer et al., 2016). Actomyosin rings are composed of actin filaments (F-actin), nonmuscle Myosin-2 (Myo-2), and other cytoskeletal proteins that affect F-actin organization and/or stability within the ring. An important and debated question is how actomyosin rings contract. One model is that ring contraction is powered by actin filament sliding driven by the motor activity of Myo-2 (Fig. 1 A; Schwayer et al., 2016). In contrast, a motor-independent model for contraction is that F-actin disassembly coupled with F-actin cross-linking can shrink the ring (Fig. 1 B; Sun et al., 2010; Mendes Pinto et al., 2012). In this issue, Xue and Sokac show that ring closure during Drosophila melanogaster cellularization involves both Myo-2–dependent and –independent mechanisms occurring back to back.
Figure 1.
Two-step model for actomyosin ring closure. (A) Model for Myo-2–dependent contraction. The motor heads of Myo-2 walk (green arrows) toward the barbed or plus ends of actin filaments (plus symbols). Because the forces on opposing heads are balanced, the actin filaments slide together, contracting the structure (black arrows). (B) Model for Myo-2–independent contraction. Two actin filaments held together by a cross-linking protein (red) depolymerize. Depolymerization brings the opposite ends of the actin filaments closer together, resulting in contraction (black arrows). (C) Back-to-back mechanism of ring closure. Step 1 represents the Myo-2–dependent step. Step 2 represents the Myo-2–independent step. Note that Myo-2 (green) and actin cross-linkers (red) are likely present during both steps, but are not included so as to illustrate the distinct steps.
For lovers of actomyosin rings, Drosophila cellularization presents an attractive model. This is because the Drosophila embryo develops as a syncytium: 13 nuclear divisions occur without an accompanying cytokinesis. To make a nascent epithelium these ∼6,000 cortically anchored nuclei are separated from the yolk through the simultaneous closure of ∼6,000 actomyosin rings. The paper by Xue and Sokac (2016) characterizes the closure of these actomyosin rings by analyzing both the kinetics and the molecular requirements for closure, which allowed them to discover a new aspect of actomyosin ring closure.
Drosophila cellularization involves both plasma membrane furrows extending down between cortical nuclei (furrow ingression) and the furrows eventually closing off the nuclei from the yolk after the plasma membrane extends beyond nuclei (ring closure). Because the kinetics of furrow ingression are highly reproducible, Xue and Sokac (2016) used furrow length as an indication of developmental time. By measuring ring perimeter and circularity during ingression, they identified two steps. The first step involves the rounding of the actomyosin ring and a slow reduction in ring perimeter (Fig. 1 C). The authors additionally assessed the levels of cytoskeletal proteins to define the molecular machinery underlying these steps. Based on the protein dynamics observed, they focused on Myo-2. The Myo-2 knockout does not result in viable embryos, so the authors used transgenic fly lines expressing a nonphosphorylatable, and thus inactive, version of the Drosophila myosin regulatory light chain, spaghetti squash. These experiments revealed that the first step in ring closure depends on the presence of functional Myo-2. In contrast, the second step does not require Myo-2. This second step involves a more rapid constriction of the ring perimeter and requires F-actin disassembly. These results suggest the exciting possibility that Myo-2–dependent and –independent mechanisms do not operate in isolation, but can be combined in tandem to “tune” contraction kinetics or enhance robustness to molecular or environmental perturbations (Fig. 1 C).
In addition to using a Myo-2 regulatory light chain mutant that cannot be phosphorylated, Xue and Sokac (2016) also disrupted Myo-2 activity by using mutants in a kinase that phosphorylates Myo-2, Rho kinase. Myo-2 has been shown to have multiple activities: (a) it can function as a motor that translocates F-actin; and (b) it can function as a cross-linker to connect the actin network (Ma et al., 2012; Schwayer et al., 2016). Both these functions involve Myo-2 assembling as oligomers. Myo-2 regulatory light chain phosphorylation regulates both actin-binding activity and the structural assembly of Myo-2 into bipolar filaments (Sellers, 1991), which are important for both Myo-2’s motor and cross-linking activities. Because the perturbations used by Xue and Sokac (2016) disrupt Myo-2 phosphorylation and, thus, likely both Myo-2 motor and cross-linking activities, an outstanding question is whether the Myo-2–dependent step represents an actin filament sliding mechanism (Fig. 1 A).
Another interesting question is what governs the transition from a Myo-2–dependent phase of contraction to an F-actin disassembly mode of contraction. Xue and Sokac (2016) tested the involvement of F-actin disassembly by showing the presence of F-actin turnover by fluorescence recovery after photobleaching in wild-type embryos. In addition, the authors measured F-actin turnover in mutant embryos with reduced levels of Cofilin, a protein known to regulate F-actin disassembly and turnover and previously implicated in ring contractility (Mendes Pinto et al., 2012). Low levels of Cofilin impaired F-actin turnover and constriction in both phases of ring closure and also delayed the transition from one phase to the next. Based on the molecular role of Cofilin, Xue and Sokac (2016) hypothesized that both phases as well as the transition depend on F-actin disassembly. To test this model, they blocked F-actin disassembly with the F-actin–stabilizing drug phalloidin and saw no effect on ring constriction in the first phase of ring closure. The mobile fraction and recovery period of actin in fluorescence recovery after photobleaching experiments did not change over the course of the transition (Xue and Sokac, 2016). However, phalloidin injection slowed ring constriction in the second step. Therefore, it appears that F-actin disassembly happens during both phases, but is functionally important only in the second, rapid constriction phase.
Further, Xue and Sokac (2016) assessed the time of the transition between the two phases of ring closure using Drosophila mutants in which F-actin organization is known to be perturbed. Rather than an activation of F-actin disassembly, Xue and Sokac (2016) showed that both Septin (Peanut in Drosophila) and Anillin are required to promote the switching from the slow first phase to the second, rapid phase of constriction. Because peanut and anillin mutants have F-actin organization defects, it is possible that a reorganization of the F-actin network is important for the transition from slow to fast constriction. Precedent for this idea comes from fission yeast cytokinesis, where the organization of the actin network dramatically changes from a broad band of nodes interconnected by F-actin to a condensed bundle before ring contraction (Vavylonis et al., 2008).
Although the work by Xue and Sokac (2016) tested the contributions of a few cytoskeletal regulators, it did not address the roles of other proteins expressed during ring closure that could also play important roles. Indeed, there are a host of other genes, with colorful Drosophila names, such as slow as molasses (slam), disrupted underground network (dunk), nullo, serendipity-α, and bottleneck, whose zygotic expression is required for proper cellularization (Rose and Wieschaus, 1992; Schejter and Wieschaus, 1993; Lecuit et al., 2002; Zheng et al., 2013; He et al., 2016). These genes are transcriptionally induced and are some of the first genes whose zygotic expression is required for Drosophila embryo development (Merrill et al., 1988). Interestingly, these genes are often expressed during specific periods of cellularization, suggesting that they function as developmental cues that trigger or modulate cellularization and possibly actomyosin ring closure. For example, the bottleneck gene is required for the proper timing of ring constriction and its removal causes premature ring contraction (Schejter and Wieschaus, 1993). Two paralogous genes, serendipity-α and spitting image, are related to Vinculin/α-catenin and are zygotically expressed and maternally loaded, respectively. Both genes regulate F-actin levels during cellularization and are attractive candidates to regulate F-actin organization and/or turnover because they bind directly to F-actin (Zheng et al., 2013).
Another question that arises from this study is how this two-step contraction is regulated at the level of cell signaling. Contractile rings in dividing cells are regulated by the activity of the small GTPase RhoA, which is activated by the guanine nucleotide exchange factor (GEF) Ect2 (Schwayer et al., 2016). slam has been shown to recruit another Rho GEF, RhoGEF2 (PDZ-RhoGEF in mammals), to the contractile ring (Wenzl et al., 2010). Interestingly, increasing or dysregulating RhoA activity by removing a RhoA GTPase activating protein (Rho GAP) results in premature ring constriction, suggesting that RhoA plays a role in timing ring contraction (Mason et al., 2016). An exciting prospect for future studies is to characterize how this host of other genes, and RhoA regulation, affect the distinct steps of ring contraction. Even if some of the Drosophila genes involved in actomyosin ring closure do not have obvious homologues in more complex metazoans, they are modulating a core machine based on RhoA, actin, and Myo-2 that is conserved. Therefore, determining how developmental cues tune actomyosin ring contraction during Drosophila cellularization is a unique and attractive way to understand the mechanisms governing the contraction of all actomyosin rings.
Acknowledgments
The author declares no competing financial interests.
References
- He B., Martin A., and Wieschaus E.. 2016. Flow-dependent myosin recruitment during Drosophila cellularization requires zygotic dunk activity. Development. 143:2417–2430. 10.1242/dev.131334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit T., Samanta R., and Wieschaus E.. 2002. slam encodes a developmental regulator of polarized membrane growth during cleavage of the Drosophila embryo. Dev. Cell. 2:425–436. 10.1016/S1534-5807(02)00141-7 [DOI] [PubMed] [Google Scholar]
- Ma X., Kovács M., Conti M.A., Wang A., Zhang Y., Sellers J.R., and Adelstein R.S.. 2012. Nonmuscle myosin II exerts tension but does not translocate actin in vertebrate cytokinesis. Proc. Natl. Acad. Sci. USA. 109:4509–4514. 10.1073/pnas.1116268109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason F.M., Xie S., Vasquez C.G., Tworoger M., and Martin A.C.. 2016. RhoA GTPase inhibition organizes contraction during epithelial morphogenesis. J. Cell Biol. 214:603–617. 10.1083/jcb.201603077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes Pinto I., Rubinstein B., Kucharavy A., Unruh J.R., and Li R.. 2012. Actin depolymerization drives actomyosin ring contraction during budding yeast cytokinesis. Dev. Cell. 22:1247–1260. 10.1016/j.devcel.2012.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill P.T., Sweeton D., and Wieschaus E.. 1988. Requirements for autosomal gene activity during precellular stages of Drosophila melanogaster. Development. 104:495–509. [DOI] [PubMed] [Google Scholar]
- Rose L.S., and Wieschaus E.. 1992. The Drosophila cellularization gene nullo produces a blastoderm-specific transcript whose levels respond to the nucleocytoplasmic ratio. Genes Dev. 6:1255–1268. 10.1101/gad.6.7.1255 [DOI] [PubMed] [Google Scholar]
- Schejter E.D., and Wieschaus E.. 1993. bottleneck acts as a regulator of the microfilament network governing cellularization of the Drosophila embryo. Cell. 75:373–385. 10.1016/0092-8674(93)80078-S [DOI] [PubMed] [Google Scholar]
- Schwayer C., Sikora M., Slováková J., Kardos R., and Heisenberg C.-P.. 2016. Actin rings of power. Dev. Cell. 37:493–506. 10.1016/j.devcel.2016.05.024 [DOI] [PubMed] [Google Scholar]
- Sellers J.R. 1991. Regulation of cytoplasmic and smooth muscle myosin. Curr. Opin. Cell Biol. 3:98–104. 10.1016/0955-0674(91)90171-T [DOI] [PubMed] [Google Scholar]
- Sun S.X., Walcott S., and Wolgemuth C.W.. 2010. Cytoskeletal cross-linking and bundling in motor-independent contraction. Curr. Biol. 20:R649–R654. 10.1016/j.cub.2010.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavylonis D., Wu J.-Q., Hao S., O’Shaughnessy B., and Pollard T.D.. 2008. Assembly mechanism of the contractile ring for cytokinesis by fission yeast. Science. 319:97–100. 10.1126/science.1151086 [DOI] [PubMed] [Google Scholar]
- Wenzl C., Yan S., Laupsien P., and Grosshans J.. 2010. Localization of RhoGEF2 during Drosophila cellularization is developmentally controlled by slam. Mech. Dev. 127:371–384. 10.1016/j.mod.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Xue Z., and Sokac A.M.. 2016. Back-to-back mechanisms drive actomyosin ring closure during Drosophila embryo cleavage. J. Cell Biol. 10.1083/jcb.201608025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Sepúlveda L.A., Lua R.C., Lichtarge O., Golding I., and Sokac A.M.. 2013. The maternal-to-zygotic transition targets actin to promote robustness during morphogenesis. PLoS Genet. 9:e1003901 10.1371/journal.pgen.1003901 [DOI] [PMC free article] [PubMed] [Google Scholar]