Abstract
Background
Sex chromosomes change more frequently in fish than in mammals or birds. However, certain chromosomes or genes are repeatedly used as sex determinants in different members of the teleostean lineage. East African cichlids are an enigmatic model system in evolutionary biology representing some of the most diverse extant vertebrate adaptive radiations. How sex is determined and if different sex-determining mechanisms contribute to speciation is unknown for almost all of the over 1,500 cichlid species of the Great Lakes. Here, we investigated the genetic basis of sex determination in a cichlid from Lake Tanganyika, Astatotilapia burtoni, a member of the most species-rich cichlid lineage, the haplochromines.
Results
We used RAD-sequencing of crosses for two populations of A. burtoni, a lab strain and fish caught at the south of Lake Tanganyika. Using association mapping and comparative genomics, we confirmed male heterogamety in A. burtoni and identified different sex chromosomes (LG5 and LG18) in the two populations of the same species. LG5, the sex chromosome of the lab strain, is a fusion chromosome in A. burtoni. Wnt4 is located on this chromosome, representing the best candidate identified so far for the master sex-determining gene in our lab strain of A. burtoni.
Conclusions
Cichlids exemplify the high turnover rate of sex chromosomes in fish with two different chromosomes, LG5 and LG18, containing major sex-determining loci in the two populations of A. burtoni examined here. However, they also illustrate that particular chromosomes are more likely to be used as sex chromosomes. Chromosome 5 is such a chromosome, which has evolved several times as a sex chromosome, both in haplochromine cichlids from all Great Lakes and also in other teleost fishes.
Electronic supplementary material
The online version of this article (doi:10.1186/s12864-016-3178-0) contains supplementary material, which is available to authorized users.
Keywords: Sex determination, Cichlid, Wnt4, Sex chromosome, RAD sequencing
Background
Sexual reproduction originated in the last common ancestor of eukaryotes and is almost ubiquitous in the animal kingdom [1]. Surprisingly for such an old trait, the initial triggers of sex determination (SD), the process driving the undifferentiated embryo towards a female or male phenotype, are not conserved. Classically, SD has been divided into genetic sex determination (GSD) and environmental sex determination (ESD, including e.g. temperature and the social environment). The best-studied form of GSD relies on a pair of sex chromosomes with one homolog carrying a master SD locus necessary for the development of one sex and when missing, leading to the development of the opposite sex.
The emergence of a novel SD gene, for example by gene duplication, transposition or allelic diversification, may initiate the formation of a proto-sex chromosome pair from autosomes (reviewed in [2]). Linkage to further genes impacting SD may then lead to the evolution of suppressed recombination, which extends the SD region, followed by the accumulation of deleterious mutations on the homolog with the SD gene due to enhanced genetic drift, background selection, Muller’s ratchet and genetic hitch-hiking (reviewed in [3]). The homolog carrying the SD locus may hence progressively degenerate with the expansion of the non-recombining segment facilitated by linkage disequilibrium and sexually antagonistic selection [4]. Such degenerated, heteromorphic sex chromosomes are exemplified by the mammalian Y chromosome or the avian W; in contrast to this, sex chromosomes in fish, if present at all, are often homomorphic and differentiation is not detectable by karyotyping [2]. Overall, fish show frequent turnover of sex-determining systems [5], which stands in sharp contrast to other vertebrate clades, especially to birds or mammals, where an entire class shares the same sex chromosomal system with sex being determined at fertilization.
So far, sex determination has been studied only in a small fraction of the over 30,000 described teleost species. Cichlid fishes with their outstanding adaptive radiations in the East African Great Lakes [6] offer the ideal evolutionary framework to study the emergence of SD systems. Early data on several species already indicate a high plasticity of SD systems, including male and female heterogamety as well as polygenic systems and even supernumerary B-chromosomes carrying SD loci [7–11]. African cichlids show a rather conserved karyotype allowing a direct comparison between species of chromosomes involved in SD [12]. In the mbuna cichlids from Lake Malawi (LM), an XX-XY system has been mapped to LG7, whereas a ZZ-ZW system in the same clade maps to LG5 [13]. Sex in two cichlid species from Lake Victoria (LV) has been mapped to a different region also on LG5 [8]. In the tilapiine lineage (basal to the lake radiations), which feature male and female heterogametic systems with additional ESD to some extent, sex-associated chromosomes include LGs 1, 3 and 23 [14, 15].
Here we set out to investigate the genetic basis of sex determination in Astatotilapia burtoni, a cichlid that inhabits Lake Tanganyika (LT) and its tributaries. A. burtoni has become an important fish model system especially in behavioral research and neurobiology but also in genetics and genomics [16–24]. This sexually dimorphic species, in which males are larger and more brightly colored than females, belongs to the most derived and species-rich lineage of East African cichlids, the haplochromines, which is the lineage found in all three Great Lakes [25]. We previously showed that our laboratory population of this species has an XX-XY sex chromosomal system [10].
Genomic analyses based on next generation sequencing data have rapidly increased the identification of sex chromosomal systems in different taxa (e.g., [26, 27]). To further disentangle the genetics underlying sex determination, we applied RAD-tag population genomics to offspring of two A. burtoni crosses. Curiously, results showed different sex-associated chromosomes in each of the two populations.
Methods
Crosses
Fish were kept at the aquarium facilities of the Zoological Institute of the University of Basel under standard conditions [23]. A male-female pair of the reference lab strain of A. burtoni (inbred line) as well as a male-female pair of A. burtoni caught at the estuary of the Kalambo river in LT at Chipwa village (Additional file 1: Figure S1) were crossed. Offspring of each cross (two broods each for lab strain and the Chipwa population) were raised until sexing was possible based on the appearance of sex-specific color patterns (~4 months post hatching; near equal sex-ratios, yellow-orange color markings on the anal fin of male fish, so-called egg-spots, and typical male body coloration were clearly visible). Fish were then fin-clipped and kept for an additional three months to confirm sexing by visual gonad inspection after dissection.
The analysis included the two parents of each cross as well as 28 sons and 27 daughters for the lab strain and 20 sons and 20 daughters for the Chipwa cross.
RAD-sequencing
DNA of each individual was extracted from caudal fin tissue with a Qiagen QIAamp DNA mini kit and quantified using a Qubit Fluorometer. RAD-sequencing library preparation was done at the University of Oregon, Eugene, following the method described in [28]. In brief, DNA of each individual fish (500 ng in 50 μl) was digested with SbfI (high-fidelity enzyme, New England Biolabs no. R3642S). Each individual DNA was labeled with a 5-nucleotide barcode adapter ligated to each restriction site during library preparation. Libraries were sequenced on an Illumina HiSeq 2500 using the 100 bp-single-end mode. The software package Stacks (version 1.20, [29, 30]) was used to organize reads into loci and to identify sex-related polymorphisms (SNPs within the loci) using the “RAD-sex” approach [31] as described in the following. First, reads were sorted and filtered using “process_radtags” of Stacks. Next, we ran the reference-free “denovomap.pl” pipeline of Stacks for the lab strain and the Chipwa offspring separately, to generate a catalog of loci present in each population (settings: -m minimum stack depth 0, -M differences when merging stacks into loci 4, and –n differences between loci when building the catalog 2). To identify SNPs associated with sex, we defined males and females each as one population. We ran the “populations” program of Stacks, which calculates F ST values and associated statistics for pairwise comparisons (here males and females) [29]. We ran “populations” separately for the lab strain and wild fish, each with defining minimum number of individuals required for a locus in each population as 1 (-p 1) and minimum stack depth required for individuals at a locus as 3 (-m 3) excluding over-merged loci (-B blacklisted markers, generated with a custom python script [31]). We further included a p-value correction to F ST values (-f p_value). Resulting SNPs with corresponding statistics including F ST and associated p-values of Fisher’s Exact Test implemented in “populations” [29], were exported in text format for further inspection.
Coverage analysis
To identify potential sex-associated RAD loci by differences in coverage in male and female genomes, we used the sequencing reads of the two fathers to construct reference loci with ustacks (-m 3, -M 4). These loci were extracted from the Stacks tsv output and transformed into fasta format. The loci were indexed using Bowtie2 (version 2.2.2, [32]) and then reads of each offspring were aligned against this reference dataset in sensitive local alignment mode of Bowtie2. Lab strain offspring were aligned against the lab strain father reference, Chipwa offspring against the Chipwa father reference. Alignments of each individual in SAM format were sorted, indexed and converted to BAM format using SAMtools (version 1.2, [33]). Next, sequence coverage per file was extracted from BAM files with BEDTools (version 2.25, [34]) using the genomeCoverageBed function. A custom perl script was used to calculate mean coverage per RAD locus for each male and female offspring separately. Subsequently, the mean of all means was calculated for each mapped locus for sons and daughters. Loci with a mean coverage below 1 (spurious reads) and above 500 (likely repeat motifs) were filtered out in R. From the remaining loci, we extracted those with a coverage below 2 in females (spurious reads or reads from wrongly sexed individuals) and above 5 in males as male-only coverage loci. For the lab strain, the resulting 41 loci were manually inspected in the original Stacks catalog for presence and absence in males and females. For loci present in at least 10 males, PCR primers were constructed at the very beginning and end of the 100 bp locus and amplified on male and female DNA by PCR using RedTaq (Sigma Aldrich, Primers and PCR condition see Additional file 2: Table S1). We also inspected loci with a coverage ratio in daughters/sons over 1.5. We mapped the father’s loci on the Nile tilapia genome (standalone BLAST+/2.2.31, blastn, e-value cutoff of 0.001) and recovered LG placement information from the best hit of each locus. We then tested for an over representation of male-only and female-biased loci on LG5 in the lab strain and LG18 in the wild fish (two-tailed Fisher’s exact test).
Comparative genomics
The sequences of RAD loci of interest were compared to the genomes of A. burtoni and O. niloticus (https://blast.ncbi.nlm.nih.gov/Blast.cgi, blastn, default settings) to determine their chromosomal locations. Contig and gene positions were derived from NCBI map viewer. To compare sex-determining regions of different cichlid species on LG5, sequence information was retrieved from (Figure S2 in [7]) and the NCBI SNP archive for [35]. The obtained sequences were blasted against the Nile tilapia genome to locate them.
Gene expression
To investigate male and female expression of genes located on the potential sex chromosome of A. burtoni, filtered RNA-sequencing reads of testis and ovary tissue from [22] were mapped against a non-redundant mRNA dataset of O. niloticus retrieved from the RefSeq RNA GBF file accession number GCF_000188235.2 keeping only the longest isoform for each gene. The reference was indexed with Bowtie2 (version 2.2.2, [32]) and reads were then mapped in local mode (settings --local -D 20 -R 3 -N 1 -L 20 -i S,1,0.50). Resulting alignments were reported in SAM format and sorted, indexed and transformed into count tables (number of mapped reads per transcript per sample) using SAMtools (version 1.2, [33]). Count data were imported in R and differences in gene expression were analyzed using the Bioconductor edgeR package (version 3.12, [36]) with the implemented GLM approach with quasi-likelihood F-tests.
Gene ontology
To investigate GO representation of genes located on Nile tilapia LGs 5, 14 and 18, gene sequences were exported from NCBI, loaded into blast2GO, blasted against nr, mapped and annotated with default settings.
Results
Polymorphic sites support male heterogametic systems in A. burtoni lab strain and Chipwa
For the inbred lab strain cross, we obtained 5,358 polymorphic sites in males and females (located in 2,969 RAD loci). For the wild type Chipwa cross, we obtained 24,185 polymorphic sites (located in 11,633 RAD loci). We inspected the 100 most significant sites for each cross (Additional file 3: Table S2 and Additional file 4: Table S3, ordered by Stacks Fisher’s p-value). In the lab strain, at 52 of these SNPs, father and sons are heterozygous whereas daughters and the mother are homozygous; meaning that only sons inherit specific alleles from the father, as expected from an XX-XY sex-determining system. At most of the remaining loci, mother and father were both heterozygous. In the Chipwa offspring, we found 19 SNPs among the 100 most significant ones in which inheritance patterns also support an XX-XY system. Again, at the other loci, either both parents are heterozygous or the loci represent repetitive motifs.
A fused chromosome is the sex chromosome in the A. burtoni lab strain
For A. burtoni, a rather fragmented reference genome from a female individual is available, which frustrates long-range conserved synteny analyses that are possible with a ‘chromonome’, a chromosome-length genome assembly [37]. Fragmented assemblies also present the problem that small scaffolds are less likely to contain genetic polymorphisms that can be readily assayed without whole genome sequences from many tens of individuals. The only currently available cichlid chromonome assembly is from the Nile tilapia (Oreochromis niloticus), a member of a lineage basal to the cichlids of the Great Lakes. Chromosomal organization is largely conserved among cichlids, although A. burtoni has fewer chromosomes (n = 20) compared to O. niloticus (n = 22) due to two chromosome fusions [38]. One of the fusions comprises O. niloticus LG15 and LG19 [8]. In the second fused chromosome, one of the partners corresponds to LG5 in O. niloticus [38], but the second remains to be identified.
We located all RAD loci of interest (2,969 for the lab strain and 11,633 of the Chipwa fish) in the Nile tilapia genome (Fig. 1) In the lab strain, we detected a strong association between phenotypic sex and the fused chromosome 5 and to some extent chromosome 14 (sharp peak on LG5, weaker peak on LG14 in Fig. 1a). In the Chipwa fish, we did not find the same association, but a peak on LG18 (Fig. 1b). In the lab strain, the highest density of the most significantly associated SNPs corresponds to a ~14 MB region on LG5, located between 6.8 and 20.3 MB, referred to as SD region (Fig. 3, blue box).
Fig. 1.
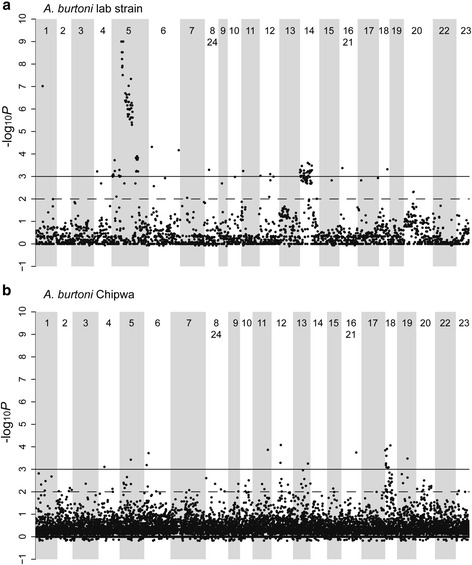
Association of chromosomes with phenotypic sex in a A. burtoni lab strain and b Chipwa wild-caught fish. The plots show the –log10 p-values of genotypes associated with sex plotted against the Nile tilapia chromosomes used as reference genome. Odd-numbered chromosomes have a white background while even-numbered chromosomes have a grey background. The solid line corresponds to a p-value cut off of 0.001, the dashed line to a p-value of 0.01
Fig. 3.
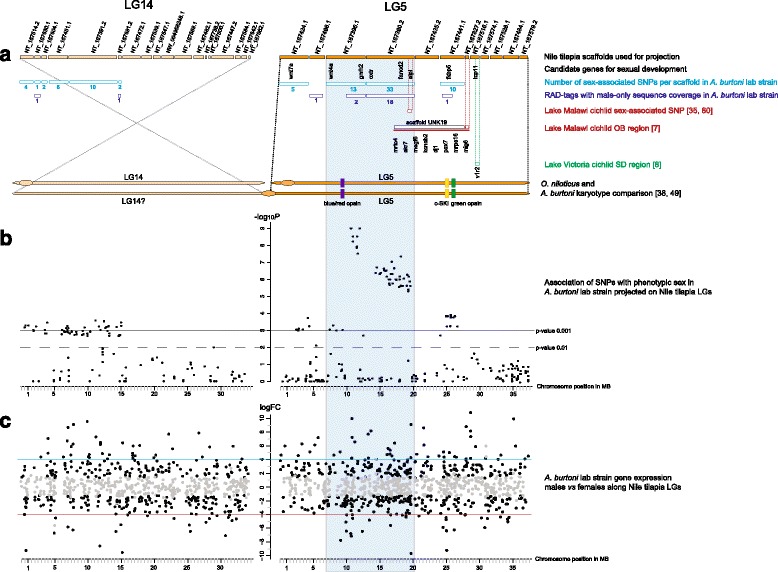
Comparative genomics of LG5 in haplochromine cichlids of Lakes Tanganyika, Malawi and Victoria. In A. burtoni, LG5 has a centric fusion to another chromosome upstream of the blue/red opsin genes [38], probably LG14, here shown on the left site. a LG5 and LG14 show sex association in A. burtoni lab strain. SNP inheritance patterns on LG5 support an XX-XY system on this chromosome in A. burtoni lab strain. LG5 carries a ZZ-ZW SD locus in Lake Malawi cichlids as well as a SD locus in Lake Victoria cichlids. Light blue numbers and boxes correspond to sex-associated SNPs in this study. Gene candidates for sex determination on LG5 are depicted below the corresponding scaffolds of the Nile tilapia. Dark blue boxes and numbers indicate location of male-specific loci. Sex-determining regions of other cichlids are indicated for Lake Malawi in red and Lake Victoria in green. b The plots show the –log10 p-values of genotypes associated with sex plotted against the Nile tilapia chromosomes LG5 and LG14 as higher resolution of Fig. 1. c Gene expression does not show a particular accumulation of sex-biased genes on LG14 or LG5 in A. burtoni lab strain (black dots correspond to significantly differentially expressed genes, grey dots correspond to not differentially expressed genes; values above 0 correspond to overexpression in males, values below 0 to overexpression in females, see Additional file 8: Table S6 for details). The blue box highlights the region with the highest density of Y-linked loci and sex-associated SNPs with the most significant p-values, referred to as SD region
An examination of the parental origin and scaffold location of sex-associated SNPs in the lab strain fish showed that the majority of SNPs (48 out of 52) that show clear transmission of paternal alleles to only sons, are on LG5, while the remaining 5 SNPs mapped to unplaced scaffolds (Additional file 3: Table S2). These loci could either be minor sex modifiers not placed on the sex chromosomes, be wrongly assembled in the Nile tilapia genome or be rearranged in the A. burtoni genome.
In the Chipwa fish, 12 out of the 19 SNPs that show inheritance patterns supportive of an XX-XY system mapped to LG18 spanning ~16 MB, the remaining ones mapped to LGs 1, 20, 12, 8-24 and to unplaced scaffolds (Additional file 4: Table S3).
Confirmation of the sex chromosome in A. burtoni by sequence coverage analysis
Despite the fact that sex chromosomes usually have high repeat content, some RAD-tags might be predicted to be only on the Y-chromosome and some only on the X-chromosome, being located in the non-recombining part of the sex chromosome. For such loci, differences in sequence coverage in males and females enabled us to perform a second – and independent – approach to identify ancestral LGs in the A. burtoni sex chromosome. In an XX-XY sex-determining system, sequences present on the Y but absent from the X chromosome should have sequence coverage only in males, whereas sequences in an X-limited region should be twice as frequent in XX females compared to XY males. In contrast, sequences in pseudo-autosomal regions (PARs), should have the same coverage in males and females like autosomal region. This copy number variation strategy has previously been used to identify non-recombining regions on fish sex chromosomes [39, 40]. To identify male-limited loci, we built a RAD locus catalog from the father as a reference (instead of using the female reference genome, which would lack Y-limited sites) and mapped reads of each son and each daughter to this reference. We then calculated mean coverage for all sites in these loci (Fig. 2a). We detected 41 loci with male-only coverage (orange dots on Fig. 2), with an over representation of such loci on LG5 in the Nile tilapia genome (p-value < 2.2e-16; for further RADtag distribution see Additional file 2: Table S1). We inspected all male-only coverage loci in the initial Stacks catalog and constructed PCR-primer pairs for the 19 loci present in at least 10 sons to test their presence/absence by PCR on male and female DNA. Two loci indeed amplified only from DNA from sons of the cross. However, when extended to more individuals of our lab strain, the two markers occasionally amplified bands of the expected size also in females. This result could indicate either sex reversed individuals or recombination events between the sex-linked markers and the sex-determining locus.
Fig. 2.
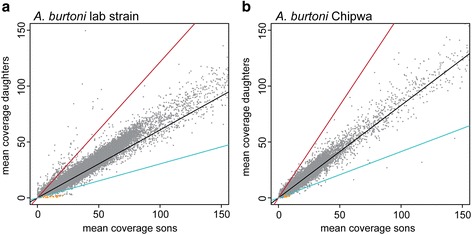
Sequence coverage of paternal RAD loci in sons and daughters of a lab strain and b Chipwa wild-caught fish. Autosomal loci should show equal coverage in both, males and females (along black line), whereas X-linked loci should have twice the coverage in females compared to males (red line, the expectation for W-linked loci in a ZZ-ZW system is shown as blue line, corresponding to double coverage in males; lines are adjusted for differences in sample size and mean coverage). Most loci show similar coverage in sons and daughters. Note that some loci have coverage only in sons, especially in the lab strain, and are depicted in orange
Concerning loci with higher coverage in daughters than sons (ratio daughter/son >1.5 with an initial minimum coverage >2 in females to exclude false positives caused by too low sequencing depth), we also found an over representation on LG5 (p-value = 7.894e-06; 201 total loci, 128 placed on LGs, 22 on LG5) but also on LG1 (p-value = 5.3e-11, 27 loci).
In the Chipwa cross, again, we detected fewer loci both with male-only and daughter/son >1.5 coverage than in the lab strain cross (Fig. 2b and Additional file 5: Table S4). Nevertheless, LG18 has an over representation of these loci (29 loci with male-only coverage, 27 loci placed on LGs, 6 on LG18, p-value = 0.0002; 102 loci with daughter/son >1.5, 78 placed on LGs, 14 on LG1, p-value = 2.361e-07).
Candidate genes on A. burtoni sex chromosomes
Some genes have repeatedly evolved as master sex determiners in tetrapods, including Sox and DM domain factors and, especially in fish, also members of the TGF-beta signaling cascade (reviewed in [5]). These “usual suspects” [41] derived by duplication or allelic diversification from genes with a known function in sex differentiation or gonad development. However, sex-determining genes can also be “newcomers” [41] as the sdY gene in the rainbow trout, which shows no similarity to any described gene with a function in sexual development [42].
In the Chipwa fish, the region with SNPs associated with sex on LG18 spans ~16 MB reaching from position 3.9 MB to 19 MB and containing 731 genes. None of these genes has been described as a sex-determining gene before.
In the lab strain, the sex-associated region on LG5 spans the first 28 MB of this 37 MB-long chromosome. This region has 966 annotated genes in Nile tilapia. The region on LG14 spans the first 15 MB and includes 397 annotated genes.
LG5 is also sex-associated in other cichlids. In addition, it contains several genes that have already been implicated in sexual development and reproduction, including two secreted signaling proteins wnt7a and wnt4a, which are known to control cell fate choices [43]. Wnt4 is an especially likely candidate in sex determination and plays an important role in gonad development in mammals [44–46]. It is located at the beginning of the SD region of A. burtoni lab strain (Fig. 3a). Two other genes on LG5 play a role in hormonal regulation of reproduction, including gnrh2 (gonadotropin-releasing hormone 2), and oxtr (the oxytocin receptor). The final candidate gene is tcp11 (T-complex protein 11 homolog), which might play a role in sperm function and fertility ([47] and references therein).
To characterize further candidate genes in chromosomal regions with sex-associated SNPs, we examined genes placed in the three regions on LG5, 14 and 18 for their gene ontology (GO) terms searching for categories related to sexual development and reproduction (“sex determination GO:0007530, sex differentiation GO:0007548, developmental process involved in reproduction GO:0003006, reproductive process GO:0022414 and reproduction GO:0000003), allowing a hunt for the “usual suspects” (Additional file 6: Table S5). We only identified two genes in this way, alpl (alkaline phosphatase) with a GO annotation GO:0003006, and fancd2 (fanconi anemia complementation group d2) with GO:0000003, both located on LG5 (see Fig. 3 for location of all candidate genes). The FANC gene family has especially been investigated for its role in zebrafish sex determination [48].
Male and female gene expression in A. burtoni lab strain
Sex chromosomes often show a sex-specific gene content, which can also be reflected in gene expression. For the A. burtoni lab strain, male and female expression data are available from [22], unfortunately no expression data are currently available for the Chipwa population.
We here investigated gene expression along LG14 and LG5 using adult male and female gonad RNA sequencing reads from [22]. Sex-biased genes were not accumulated on LG5 or LG14 and gene expression did not show a particular pattern on these or any other chromosomes (Fig. 3c and Additional file 7: Figure S2 for all other chromosomes). We inspected the genes that showed the strongest sex-bias on these two chromosomes (logFC >4, genes above blue line in Fig. 3c are highly male-biased, genes below the red line highly female biased, Additional file 8: Table S6), and investigated gene expression of the above mentioned candidate genes (Additional file 8: Table S6). From the candidates, wnt4a is the only one that shows over expression in male gonads, while wnt7a, fkbp5 and tcp11 are overexpressed in females. Gnrh2, otxr, fancd2, and alpl are not differentially expressed. Note however, that downstream of wnt4a several genes show strong sex biased expression (Fig. 3. blue box, Additional file 8: Table S6).
The genes that are most differentially expressed and show the highest expression in males on LG5 include another member of the wnt pathway (calcoco1), several transcription factors, and genes involved in cell migration/motility. The gene with the strongest male-bias is sycp1, synaptonemal complex protein 1 (at ~29 MB of LG5 in the Nile tilapia, Fig. 3c), reflecting the many meioses occurring in testes. Genes with a strong female bias on LG5 also include several transcription factors. The gene with the strongest female-bias is histone H100-like (at ~20 MB of LG5 in the Nile tilapia, Fig. 3c).
Discussion
The sex chromosome in A. burtoni lab strain is a fused chromosome
The SD region that we identified in A. burtoni lab strain is located between position 6.5 MB and 20 MB on LG5 of the Nile tilapia. In Nile tilapia, the telocentric centromere is placed close to this region [38, 49]. The homologous chromosome to LG5 in A. burtoni is a large, metacentric chromosome, likely the result of a centric fusion of two subtelocentric/acrocentric chromosomes [38]. So far only LG5 was known to be involved in this fusion [38]. In the lab strain fish, we detected sex-association with a second Nile tilapia chromosome, LG14 (smaller peak in Fig. 1a), and hence suggest that this chromosome is the one fused to LG5 in A. burtoni. Our association pattern with sex on both Nile tilapia chromosomes LG5 and LG14 in vicinity to the telocentric centromers, supports a centric fusion as previously proposed for this chromosome in A. burtoni (Fig. 3a, [38]). Numerous neo-sex chromosomes have been characterized in fish, most often generated by Y-autosome fusion [50, 51]. Such fusions are easily recognizable because they cause an odd number of chromosomes in a diploid karyotype of one sex and might be implicated with speciation (e.g. [50, 52, 53]). In A. burtoni, males and females have the same karyotype of 2n = 40 ([12], personal communication with Cesar Martins) indicating that chromosome fusions were not causing the formation of neo-sex chromosomes. Most other African cichlids show a 2n = 44 karyotype [12]. However, data are not sufficient to date the two fusions in A. burtoni nor to correlate them with the emergence of SD loci on LG5.
Varying sex determination systems in two populations of A. burtoni
Usage of different sex chromosomes in closely related sister species has previously been described for the fish genus Oryzias (reviewed in [54]) as well as in populations of sticklebacks [55]. Our laboratory population of A. burtoni provided strong genetic evidence for an XX-XY sex-determining system located on the fused chromosome LG5/LG14 ([10] and this study). In addition, Roberts et al (DOI 10.1186/s12864-016-3177-1) found a sex-asociated locus on LG13 in some families of their laboratory population that we did not detect. In offspring of Chipwa fish, we have support for yet another XX-XY locus on LG18 although the genetic signal is somewhat weaker than the one detected in the lab strain (Fig. 1). The wild-caught fish studied here are offspring from a couple caught at the Chipwa village at the estuary of the Kalambo river, which flows into LT (Additional file 1: Figure S1). The lab strain was originally derived from another population, which is closely related to current populations from the northern part of the lake, about 600 km away from Chipwa (Pauquet et al. in preparation). Note that the A. burtoni genome was also derived from a female of a laboratory strain, the one of the Hofmann lab (inbred line for ~60 generations [20]), which belongs to the same clade as the lab strain investigated here. Hence, the two studied crosses belong to geographically as well as genetically distantly related populations (Pauquet et al. in preparation), which could account for differences in their sex chromosomal systems.
In zebrafish, domesticated strains have lost the natural sex-determining system probably as a by-product of selection under laboratory conditions [31]. Under laboratory rearing, fish are often selected for early maturation and breeding as well as fast growth and probably also balanced sex ratios. A second possible explanation for differences in the sex-determining system and also their degree of differentiation in the two investigated A. burtoni populations is that the sex-determining system in our A. burtoni lab strain has been co-selected in such a process.
The sex-determining regions of A. burtoni
The SD regions of A. burtoni span ~14 MB in the lab strain (blue box in Fig. 3) and ~16 MB in the wild-caught fish. In the lab strain, sex-associated SNPs can even be seen along a region of much longer size (~28 MB on LG5 and ~14 MB on LG14). The association signal of sex determination in the wild-caught Chipwa population is weaker than the one in the lab strain, which might be explained by a generally higher level of heterozygosity in wild compared to inbred fish. It could also be that the sex chromosomes of the Chipwa population are even younger or less differentiated than the one of the lab strain/northern populations or that sex determination is polygenic.
The same RAD-sequencing method detected comparable regions of sex-association of 18.2 MB around the SD locus in the medaka [31] in which the Y-chromosome specific part is only 250 kb long and the SD system is about 5–10 million years old. ([56] and references therein). In the three-spined stickleback with ~10my old sex chromosomes, sequence differentiation between X and Y as reflected by elevated nucleotide divergence, accumulation of Y-specific alleles as well as rearrangements covers a region of 16.4 Mb (over 80 % of the X-chromosome length), however being much larger than the actual SD locus [57, 58]. We hence expect that the effective locus responsible for SD in A. burtoni will be smaller than the 14 and 16 MB, respectively. Regions around loci under sexually-antagonistic selection (as the color and vision genes on LG5, Fig. 3) can show elevated divergence between X and Y and linkage to the SD locus can make the divergence peak much broader than without this linkage (e.g., on an autosome) [59]. The rather large regions of sex-association that we detect could reflect this pattern. A possible explanation for increased heteromorphism on (young) sex chromosomes along a rather large region are structural rearrangements such as deletions or inversions accompanied by a reduction in recombination [57]. The causality or order of these events is however debated ([60] and references therein).
Lack of data from more closely related species as well as the sequencing resolution makes dating of the sex chromosomal system as well as defining the SD locus in A. burtoni difficult. Comparing wild-caught fish and lab fish, artificial selection under laboratory conditions could have slightly speeded up the process of sex chromosome differentiation in the lab strain as well as reduced the level of heterozygosity accounting for the broader distribution of sex-associated SNPs as well as the higher significance levels of association (visible when comparing the Chipwa fish and lab fish, Fig. 1a and b).
Sequence coverage comparison of males and females confirms sex-association with LG5 in the lab strain and LG18 in the wild-caught fish. However, it does not indicate sequence differentiation along those chromosomes as, for example, it did in sticklebacks [39]. PCR of potential Y-markers identified in the coverage analysis, did not detect complete sex linkage in individuals of the lab strain that were not members of the investigated cross. Also, these markers are present in the A. burtoni reference genome, which is derived from a female individual. This finding suggests that the regions we identified are still quite similar between X and Y and recombine over most their length, which are usually thought to be properties of rather young sex chromosomes.
Other explanations are that sex reversal [23] or intersex individuals can occur in our lab strain (we occasionally observed individuals that had a male phenotype but lay eggs as well as one intersex individual with a mixed gonad, A. Böhne and B. Egger, personal observations). Sex reversal can counteract Muller’s ratchet by purging deleterious mutations through occasional recombination in XY females, which results in rejuvenating sex chromosomes, preventing them from strong differentiation and decay and thus accounting for homomorphic sex chromosomes [60, 61]. It could also be that genetic sex determination is incomplete in A. burtoni and can be impacted by environmental factors.
Differentiated (heteromorphic) sex chromosomes often show a non-random distribution of sex-biased genes [62–65]. We did not, however, see any sex-bias in gene expression along LGs 5 and 14, a result that also indicates not yet specialized, young chromosomes. We previously showed that the ancient LG1 sex chromosome contains an over-representation of genes commonly expressed in a female-biased manner in several LT cichlids [22].
Overall, our method identified the sex-determining regions of two A. burtoni populations but it has not yet identified the non-recombining Y-limited regions and suggests that sex chromosomes in both populations are rather young and undifferentiated. Because our RAD marker density is estimated to average about one cutting site every ~30 kb, it could miss a small Y-limited region. This situation was indeed the case in the medaka [31], where the major SD gene is on an unassembled scaffold with no SbfI restriction site.
“Limited options” – wnt4 is a promising candidate for sex determination in A. burtoni
Except for birds or mammals, sex-determining genes do not tend to be strongly conserved within vertebrates. Even novel genes not related to any known sex-differentiating gene can become a master SD locus [42]. Still, it seems, that a pool of genes referred to as “usual suspects” [41] or “limited options” [66] has repeatedly evolved as master SD genes, especially in fish.
Besides sdY, the novel gene in rainbow trout [42], the described teleost master SD genes either belong to the DM domain transcription factor family (dmY/dmrt1bY in the medaka Oryzias latipes [67, 68], and Oryzias curvinotus [69], dmrt1 in Chinese tongue sole [40]), the Sox family (sox3Y in Oryzias dancena [70]) or the TGFbeta signaling pathway (gsdfY in Oryzias luzonensis [71] and sablefish [72], amhY in Patagonian pejerrey [73] and amhr2Y in Fugu [74]).
Also belonging to this pool of usual suspects is wnt4, a key gene of gonad development and sex determination in mammals [44], which is located at the beginning of the SD region of the A. burtoni lab strain (Fig. 3a) and presents our prime candidate for sex determination. Cichlids, like other teleosts, possess two copies of wnt4 as they do for most of their genes as the result of a whole genome duplication at the origin of the teleostean lineage [75–77]. In a previous study, we showed that the expression profile of wnt4b (located on LG11) strongly resembles that of a male SD gene with an early expression peak in developing male gonads [10]. However, we did not detect any sequence difference between male and female individuals. In that study, we also profiled the expression of wnt4a, the gene copy located on cichlid LG5 (figure 3 in [10]). Within the time window studied, wnt4a did not show a peak in gonadal expression as sharp as its ohnolog wnt4b. Still, wnt4a expression showed a reduction in expression level throughout the experiment and a maximal expression level at the same time point as wnt4b. In addition, we detected here over-expression of wnt4a in adult testes. In olive flounder (Paralichthys olivaceus), overexpression of wnt4a in the developing male gonad has also been described [78]. Taken together, these findings support a possible function in SD or gonad development for wnt4a in A. burtoni.
Comparative genomics of LG5 in cichlids
The concept of ‘limited options’ appears to apply not only to genes, but also to entire chromosomes [66, 79]. LG5 seems to be one such chromosome in teleosts. Different studies have identified LG5 as a sex chromosome in cichlids, including species from LM and LV, all belonging to the haplochromine lineage, which also contains A. burtoni.
A sex-associated SNP has been identified in LM cichlids with a ZZ-ZW sex-determining system in the same region on LG5 (at ~19.5 MB, [35, 80]). This ZZ-ZW system is coupled to the orange blotch (OB) genetic determinant in LM cichlids, located on LG5, with pax7 as the responsible gene [7]. Of the OB region [7], only mig6 is located on LG5, at 28 MB of the Nile tilapia, further downstream of the sex-SNP identified by [35]. The rest of the OB region is on the unassembled scaffold UNK19 of the Nile tilapia, which itself shows conserved chromosomal linkage in several fish genomes, including zebrafish, medaka, stickleback and the two pufferfish Fugu and Tetraodon, suggesting its placement on cichlid LG5 [7]. Note that in the A. burtoni lab strain, one of the significantly sex-associated SNPs supporting an XX-XY system is located on UNK19 (Additional file 3: Table S2), in agreement with linkage to LG5.
The third SD region on LG5 was identified in LV cichlids, located close to the pheromone receptor v1r2 (figure 4 in [8]), at position ~30 MB on LG5, not overlapping with the SD regions in LM or LT. It is not clear whether this sex-determining system is chromosomally XX-XY or ZZ-ZW.
Some chromosomes are more often and repeatedly used as sex chromosomes than others [66, 79]. The preferential co-option and re-usage of a specific chromosome as sex chromosome could be associated with particular gene functions or sexual antagonistic effects of genes those chromosomes carry [81]. LG5 of cichlids seems to be one such chromosome. It has (most likely) independently been used as a sex chromosome in cichlids of the three Great Lakes possessing different heterogametic statuses. The SD region in LV cichlids does not overlap with the one in LM or LT cichlids. The XX-XY system on LG7 in LM is ancestral in this cichlid lineage [13] dating the emergence of the ZZ-ZW locus on LG5 after the split from LT cichlids, arguing also for independent evolution of LG5 as sex chromosome in cichlids of all lakes. However, the repeated co-option of LG5 as sex chromosome could still represent the reuse of a shared ancestral polymorphism responsible for SD in cichlids from the different lakes.
The evolution of this chromosome as sex chromosome is not confined to cichlids but chromosomes homologous to cichlid LG5 repeatedly evolved as sex chromosomes in other teleosts. Cichlid LG5 corresponds to TEL3 [49], the reconstructed ancestral teleost chromosome 3 [82], which is ancestral to the sex chromosomes LG12 in Ninespine stickleback, LG19 in Tiger pufferfish and LG5 in Oryzias hubbsi (reviewed in [66]). This repeated usage of descendants of TEL3 as a sex chromosome could be linked to the presence of sexually antagonistic genes on TEL3. On fish sex chromosomes, sexually antagonistic genes are often involved in pigmentation (table 2 in [83]). The homologs of TEL3 carry several pigmentation genes as well as some of the color vision opsin genes ([38], table 2 in [66]), making this chromosome one of the “limited options” that could evolutionarily be favored as sex chromosome due to location of sexually antagonistic loci on it and their linkage to SD genes.
Conclusion
Although sex chromosomes are not conserved among fish species, particular chromosomes seem to be more likely to evolve as sex chromosomes than others. Cichlids, and especially the haplochromine lineage, are a perfect system to investigate sex chromosomes of different evolutionary ages or stages. Furthermore, they offer the possibility to study transitions between different sex chromosomal loci as well as their linkage to sexually antagonistic and novel key traits and eventually their contribution to speciation. Whole genome sequencing of different A. burtoni strains and populations might facilitate the identification of Y-chromosomal regions and shed light on the origin and potential turnover of sex chromosomes in haplochromines as well as help in the identification of novel genes located on the sex chromosomes although assembly of repeat-rich sex chromosome regions remains a huge challenge. Comparisons with other cichlids from LT will answer the question if LG5 has indeed more often and repeatedly evolved as a sex chromosome in cichlids and if the SD regions represent the co-option of an ancestral polymorphism. Extension to further species from LT will answer the question if this pattern is confined to the haplochromine lineage.
Acknowledgements
We thank the support team of sciCORE (center for scientific computing, University of Basel, http://scicore.unibas.ch/) for providing access to computational resources and advice and especially Pablo Escobar Lopez for help with the Stacks analyses pipeline. We further thank Attila Rüegg and Adrian Indermaur for fish keeping and Fabrizia Ronco, Marius Rösti and Steffen Fehrmann for helpful discussion on analyses.
Funding
This project was funded by the Volkswagen Science Foundation (program Evolutionary biology, project 86031) and the Swiss National Science Foundation (SNSF Grant international short visit IZK0Z3_154111) to AB and the European research council (ERC grant CICHLID ~ X) and the Swiss National Science Foundation to WS, and National Institutes of Health (NIH grant R01GM085318) to JHP.
Availability of data and materials
RAD sequencing raw reads have been deposited in the NCBI SRA Sequence Read Archive https://www.ncbi.nlm.nih.gov/sra under the accession number SRR4408339, scripts are available upon request to the corresponding author; RNA sequencing information was retrieved from [22]. All other datasets supporting the conclusions of this article are included within the article and its Additional files 1, 2, 3, 4, 5, 6, 7 and 8.
Authors’ contributions
AB designed the study and drafted the manuscript. All authors contributed to manuscript finalization and read and approved the final manuscript. AB and CAW constructed RAD-sequencing libraries, AB analyzed data.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Research involving animals was performed with approval of the Swiss authorities under the veterinary permits no. 2317 and 2620 issued by the Kanton Basel City and for fish caught at Lake Tanganyika under a research permit issued by the Lake Tanganyika Research Unit, Department of Fisheries, Mpulungu, Zambia.
Abbreviations
- ESD
Environmental sex determination
- GO
Gene ontology
- GSD
Genetic sex determination
- kb
Kilo bases
- km
Kilo meters
- LG
Linkage group
- LM
Lake Malawi
- LT
Lake Tanganyika
- LV
Lake Victoria
- MB
Mega bases
- my
Million years
- OB
Orange blotch
- RAD sequencing
Restriction site associated DNA sequencing
- SD
Sex determination
- SNP
Single nucleotide polymorphism
Additional files
Sampling location of the “Chipwa wild-caught” fish parents used in this study. (PNG 2140 kb)
Loci with male-specific coverage and higher coverage in daughters of the A. burtoni lab strain cross with corresponding location in BLAST on O. niloticus genome and PCR conditions and results for male specific loci. (XLSX 1227 kb)
RAD SNPs differentiating males and females of the A. burtoni lab strain with corresponding BLAST results on the O. niloticus and A. burtoni genome. SNPs in bold show inheritance of alleles from father to sons only, supporting an XX-XY system. (XLS 56 kb)
RAD loci differentiating males and females of the A. burtoni Chipwa wild-caught fish with corresponding BLAST results on the O. niloticus genome. SNPs in bold show inheritance of alleles from father to sons only, supporting an XX-XY system. (XLS 807 kb)
Loci with male-specific coverage and higher coverage in daughters of the A. burtoni Chipwa wild-caught cross with corresponding location in BLAST on O. niloticus genome. (XLSX 83 kb)
GO annotations of O. niloticus genes located in the potential sex-determining regions on LG5, LG14 and LG18. (XLSX 64 kb)
Gene expression of A. burtoni lab strain along the reference chromosomes of the Nile tilapia. Gene expression in male and female gonads of the A. burtoni lab strain along the reference chromosomes of the Nile tilapia as in Fig. 3c. (ZIP 329 kb)
Gene expression details of genes with strongest male and female bias on LGs 5 and 14 as well as gene candidates on LG5. (XLSX 30 kb)
Contributor Information
Astrid Böhne, Email: astrid.boehne@unibas.ch.
Catherine A. Wilson, Email: cwilson@uoneuro.uoregon.edu
John H. Postlethwait, Email: jpostle@uoneuro.uoregon.edu
Walter Salzburger, Email: walter.salzburger@unibas.ch.
References
- 1.Goodenough U, Heitman J. Origins of eukaryotic sexual reproduction. Cold Spring Harb Perspect Biol. 2014: 6(3). [DOI] [PMC free article] [PubMed]
- 2.Charlesworth D, Charlesworth B, Marais G. Steps in the evolution of heteromorphic sex chromosomes. Heredity. 2005;95(2):118–128. doi: 10.1038/sj.hdy.6800697. [DOI] [PubMed] [Google Scholar]
- 3.Bachtrog D. A dynamic view of sex chromosome evolution. Curr Opin Genet Dev. 2006;16(6):578–585. doi: 10.1016/j.gde.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Charlesworth B, Charlesworth D. The degeneration of Y chromosomes. Philos Trans R Soc Lond B Biol Sci. 2000;355(1403):1563–1572. doi: 10.1098/rstb.2000.0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heule C, Salzburger W, Böhne A. Genetics of sexual development – an evolutionary playground for fish. Genetics. 2014;196(3):579–591. doi: 10.1534/genetics.114.161158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salzburger W, Van Bocxlaer B, Cohen AS. The ecology and and evolution of the African Great Lakes and their faunas. Annu Rev Ecol Evol Syst. 2014;5:19–45. [Google Scholar]
- 7.Roberts RB, Ser JR, Kocher TD. Sexual conflict resolved by invasion of a novel sex determiner in Lake Malawi cichlid fishes. Science. 2009;326(5955):998–1001. doi: 10.1126/science.1174705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kudo Y, Nikaido M, Kondo A, Suzuki H, Yoshida K, Kikuchi K, Okada N. A microsatellite-based genetic linkage map and putative sex-determining genomic regions in Lake Victoria cichlids. Gene. 2015;560(2):156–164. doi: 10.1016/j.gene.2015.01.057. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida K, Terai Y, Mizoiri S, Aibara M, Nishihara H, Watanabe M, Kuroiwa A, Hirai H, Hirai Y, Matsuda Y, et al. B chromosomes have a functional effect on female sex determination in Lake Victoria cichlid fishes. PLoS Genet. 2011;7(8):e1002203. doi: 10.1371/journal.pgen.1002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heule C, Göppert C, Salzburger W, Böhne A. Genetics and timing of sex determination in the East African cichlid fish Astatotilapia burtoni. BMC Genet. 2014;15(1):140. doi: 10.1186/s12863-014-0140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wirtz Ocana S, Meidl P, Bonfils D, Taborsky M. Y-linked Mendelian inheritance of giant and dwarf male morphs in shell-brooding cichlids. P Roy Soc B-Biol Sci. 2014;281(1794):20140253. doi: 10.1098/rspb.2014.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poletto A, Ferreira I, Cabral-de-Mello D, Nakajima R, Mazzuchelli J, Ribeiro H, Venere P, Nirchio M, Kocher T, Martins C. Chromosome differentiation patterns during cichlid fish evolution. BMC Genet. 2010;11(1):50. doi: 10.1186/1471-2156-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ser JR, Roberts RB, Kocher TD. Multiple interacting loci control sex determination in lake Malawi cichlid fish. Evolution. 2010;64(2):486–501. doi: 10.1111/j.1558-5646.2009.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cnaani A. The tilapias’ chromosomes influencing sex determination. Cytogenet Genome Res. 2013;141(2-3):195–205. doi: 10.1159/000355304. [DOI] [PubMed] [Google Scholar]
- 15.Baroiller JF, D’Cotta H, Bezault E, Wessels S, Hoerstgen-Schwark G. Tilapia sex determination: Where temperature and genetics meet. Comp Biochem Physiol A Mol Integr Physiol. 2009;153(1):30–38. doi: 10.1016/j.cbpa.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Huffman LS, O’Connell LA, Hofmann HA. Aromatase regulates aggression in the African cichlid fish Astatotilapia burtoni. Physiol Behav. 2013;112–113:77–83. doi: 10.1016/j.physbeh.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Kidd MR, O’Connell LA, Kidd CE, Chen CW, Fontenot MR, Williams SJ, Hofmann HA. Female preference for males depends on reproductive physiology in the African cichlid fish Astatotilapia burtoni. Gen Comp Endocrinol. 2013;180:56–63. doi: 10.1016/j.ygcen.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Theis A, Salzburger W, Egger B. The function of anal fin egg-spots in the cichlid fish Astatotilapia burtoni. PLoS One. 2012;7(1):e29878. doi: 10.1371/journal.pone.0029878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theis A, Ronco F, Indermaur A, Salzburger W, Egger B. Adaptive divergence between lake and stream populations of an East African cichlid fish. Mol Ecol. 2014;23(21):5304–5322. doi: 10.1111/mec.12939. [DOI] [PubMed] [Google Scholar]
- 20.Brawand D, Wagner CE, Li YI, Malinsky M, Keller I, Fan S, Simakov O, Ng AY, Lim ZW, Bezault E, et al. The genomic substrate for adaptive radiation in African cichlid fish. Nature. 2014;513(7518):375–381. doi: 10.1038/nature13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Böhne A, Heule C, Boileau N, Salzburger W. Expression and sequence evolution of aromatase cyp19a1 and other sexual development genes in East African cichlid fishes. Mol Biol Evol. 2013;30(10):2268–2285. doi: 10.1093/molbev/mst124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Böhne A, Sengstag T, Salzburger W. Comparative transcriptomics in East African cichlids reveals sex- and species-specific expression and new candidates for sex differentiation in fishes. Genome Biol Evol. 2014;6(9):2567–2585. doi: 10.1093/gbe/evu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Göppert C, Harris RM, Theis A, Boila A, Hohl S, Rüegg A, Hofmann HA, Salzburger W, Böhne A. Aromatase inhibition in a cichlid fish induces partial sex change indicating distinct functions for sex steroids in brains and gonads. Sex Dev. 2016;10(2):97–110. doi: 10.1159/000445463. [DOI] [PubMed] [Google Scholar]
- 24.Salzburger W, Renn S, Steinke D, Braasch I, Hofmann H, Meyer A. Annotation of expressed sequence tags for the East African cichlid fish Astatotilapia burtoni and evolutionary analyses of cichlid ORFs. BMC Genomics. 2008;9(1):96. doi: 10.1186/1471-2164-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salzburger W, Mack T, Verheyen E, Meyer A. Out of Tanganyika: Genesis, explosive speciation, key-innovations and phylogeography of the haplochromine cichlid fishes. BMC Evol Biol. 2005;5(1):17. doi: 10.1186/1471-2148-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gamble T, Coryell J, Ezaz T, Lynch J, Scantlebury DP, Zarkower D. Restriction site-associated DNA sequencing (RAD-seq) reveals an extraordinary number of transitions among gecko sex-determining systems. Mol Biol Evol. 2015;32(5):1296–1309. doi: 10.1093/molbev/msv023. [DOI] [PubMed] [Google Scholar]
- 27.Anderson JL, Rodríguez Marí A, Braasch I, Amores A, Hohenlohe P, Batzel P, Postlethwait JH. Multiple sex-associated regions and a putative sex chromosome in zebrafish revealed by RAD mapping and population genomics. PLoS One. 2012;7(7):e40701. doi: 10.1371/journal.pone.0040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amores A, Catchen J, Ferrara A, Fontenot Q, Postlethwait JH. Genome evolution and meiotic maps by massively parallel DNA sequencing: Spotted gar, an outgroup for the teleost genome duplication. Genetics. 2011;188(4):799–808. doi: 10.1534/genetics.111.127324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Catchen J, Hohenlohe PA, Bassham S, Amores A, Cresko WA. Stacks: an analysis tool set for population genomics. Mol Ecol. 2013;22(11):3124–3140. doi: 10.1111/mec.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Catchen JM, Amores A, Hohenlohe P, Cresko W, Postlethwait JH. Stacks: Building and genotyping loci de novo from short-read sequences. G3. 2011;1(3):171–182. doi: 10.1534/g3.111.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson CA, High SK, McCluskey BM, Amores A, Yan Y-l, Titus TA, Anderson JL, Batzel P, Carvan MJ, Schartl M, et al. Wild sex in zebrafish: Loss of the natural sex determinant in domesticated strains. Genetics. 2014;198(3):1291–1308. doi: 10.1534/genetics.114.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Meth. 2012;9(4):357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, Subgroup GPDP. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26(6):841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parnell NF, Streelman JT. Genetic interactions controlling sex and color establish the potential for sexual conflict in Lake Malawi cichlid fishes. Heredity. 2013;110(3):239–246. doi: 10.1038/hdy.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braasch I, Peterson SM, Desvignes T, McCluskey BM, Batzel P, Postlethwait JH. A new model army: Emerging fish models to study the genomics of vertebrate Evo-Devo. J Exp Zool B Mol Dev Evol. 2015;324(4):316–341. doi: 10.1002/jez.b.22589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazzuchelli J, Kocher T, Yang F, Martins C. Integrating cytogenetics and genomics in comparative evolutionary studies of cichlid fish. BMC Genomics. 2012;13(1):463. doi: 10.1186/1471-2164-13-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roesti M, Moser D, Berner D. Recombination in the threespine stickleback genome—patterns and consequences. Mol Ecol. 2013;22(11):3014–3027. doi: 10.1111/mec.12322. [DOI] [PubMed] [Google Scholar]
- 40.Chen S, Zhang G, Shao C, Huang Q, Liu G, Zhang P, Song W, An N, Chalopin D, Volff J-N, et al. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nat Genet. 2014;46(3):253–260. doi: 10.1038/ng.2890. [DOI] [PubMed] [Google Scholar]
- 41.Herpin A, Schartl M. Plasticity of gene‐regulatory networks controlling sex determination: of masters, slaves, usual suspects, newcomers, and usurpators. EMBO Rep. 2015;16(10):1260–1274. doi: 10.15252/embr.201540667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yano A, Guyomard R, Nicol B, Jouanno E, Quillet E, Klopp C, Cabau C, Bouchez O, Fostier A, Guiguen Y. An immune-related gene evolved into the master sex-determining gene in rainbow trout, Oncorhynchus mykiss. Curr Biol. 2012;22(15):1423–1428. doi: 10.1016/j.cub.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 43.Bejsovec A. Signal transduction: Wnt signalling shows its versatility. Curr Biol. 1999;9(18):R684–R687. doi: 10.1016/S0960-9822(99)80439-4. [DOI] [PubMed] [Google Scholar]
- 44.Bernard P, Harley V. Wnt4 action in gonadal development and sex determination. Int J Biochem Cell Biol. 2007;39(1):31–43. doi: 10.1016/j.biocel.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Jeays-Ward K, Dandonneau M, Swain A. Wnt4 is required for proper male as well as female sexual development. Dev Biol. 2004;276(2):431–440. doi: 10.1016/j.ydbio.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 46.Kim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, Chaboissier M-C, Poulat F, Behringer RR, Lovell-Badge R, Capel B. Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol. 2006;4(6):e187. doi: 10.1371/journal.pbio.0040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma Y, Zhang S, Xia Q, Zhang G, Huang X, Huang M, Xiao C, Pan A, Sun Y, Lebo R, et al. Molecular characterization of the TCP11 gene which is the human homologue of the mouse gene encoding the receptor of fertilization promoting peptide. Mol Hum Reprod. 2002;8(1):24–31. doi: 10.1093/molehr/8.1.24. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez-Mari A, Postlethwait JH. The role of Fanconi anemia/BRCA genes in zebrafish sex determination. Methods Cell Biol. 2011;105:461–490. doi: 10.1016/B978-0-12-381320-6.00020-5. [DOI] [PubMed] [Google Scholar]
- 49.Guyon R, Rakotomanga M, Azzouzi N, Coutanceau JP, Bonillo C, D’Cotta H, Pepey E, Soler L, Rodier-Goud M, D’Hont A, et al. A high-resolution map of the Nile tilapia genome: a resource for studying cichlids and other percomorphs. BMC Genomics. 2012;13(1):1–17. doi: 10.1186/1471-2164-13-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitano J, Peichel C. Turnover of sex chromosomes and speciation in fishes. Environ Biol Fishes. 2012;94(3):549–558. doi: 10.1007/s10641-011-9853-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pennell MW, Kirkpatrick M, Otto SP, Vamosi JC, Peichel CL, Valenzuela N, Kitano J. Y fuse? Sex chromosome fusions in fishes and reptiles. PLoS Genet. 2015;11(5):e1005237. doi: 10.1371/journal.pgen.1005237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith DA, Gordon IJ, Traut W, Herren J, Collins S, Martins DJ, Saitoti K, Ireri P, Ffrench-Constant R. A neo-W chromosome in a tropical butterfly links colour pattern, male-killing, and speciation. Proc Biol Sci. 2016: 283(1835). [DOI] [PMC free article] [PubMed]
- 53.Graves JA. Did sex chromosome turnover promote divergence of the major mammal groups?: De novo sex chromosomes and drastic rearrangements may have posed reproductive barriers between monotremes, marsupials and placental mammals. Bioessays. 2016;38(8):734–743. doi: 10.1002/bies.201600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsuda M, Sakaizumi M. Evolution of the sex-determining gene in the teleostean genus Oryzias. Gen Comp Endocrinol. 2015. in press. [DOI] [PubMed]
- 55.Kitano J, Ross JA, Mori S, Kume M, Jones FC, Chan YF, Absher DM, Grimwood J, Schmutz J, Myers RM, et al. A role for a neo-sex chromosome in stickleback speciation. Nature. 2009;461(7267):1079–1083. doi: 10.1038/nature08441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herpin A, Schartl M. Molecular mechanisms of sex determination and evolution of the Y-chromosome: insights from the medaka fish (Oryzias latipes) Mol Cell Endocrinol. 2009;306(1-2):51–58. doi: 10.1016/j.mce.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 57.Peichel CL, Ross JA, Matson CK, Dickson M, Grimwood J, Schmutz J, Myers RM, Mori S, Schluter D, Kingsley DM. The master sex-determination locus in threespine sticklebacks is on a nascent Y chromosome. Curr Biol. 2004;14(16):1416–1424. doi: 10.1016/j.cub.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 58.Shikano T, Natri HM, Shimada Y, Merilä J. High degree of sex chromosome differentiation in stickleback fishes. BMC Genomics. 2011;12(1):1–10. doi: 10.1186/1471-2164-12-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kirkpatrick M, Guerrero RF. Signatures of sex-antagonistic selection on recombining sex chromosomes. Genetics. 2014;197(2):531–541. doi: 10.1534/genetics.113.156026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perrin N. Sex reversal: A fountain of youth for sex chromosomes? Evolution. 2009;63(12):3043–3049. doi: 10.1111/j.1558-5646.2009.00837.x. [DOI] [PubMed] [Google Scholar]
- 61.Grossen C, Neuenschwander S, Perrin N. The evolution of XY recombination: sexually antagonistic selection versus deleterious mutation load. Evolution. 2012;66(10):3155–3166. doi: 10.1111/j.1558-5646.2012.01661.x. [DOI] [PubMed] [Google Scholar]
- 62.Wang PJ, McCarrey JR, Yang F, Page DC. An abundance of X-linked genes expressed in spermatogonia. Nat Genet. 2001;27(4):422–426. doi: 10.1038/86927. [DOI] [PubMed] [Google Scholar]
- 63.Parisi M, Nuttall R, Naiman D, Bouffard G, Malley J, Andrews J, Eastman S, Oliver B. Paucity of genes on the Drosophila X chromosome showing male-biased expression. Science. 2003;299(5607):697–700. doi: 10.1126/science.1079190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lercher MJ, Urrutia AO, Hurst LD. Evidence that the human X chromosome is enriched for male-specific but not female-specific genes. Mol Biol Evol. 2003;20(7):1113–1116. doi: 10.1093/molbev/msg131. [DOI] [PubMed] [Google Scholar]
- 65.Kaiser VB, Ellegren H, Noor M. Nonrandom distribution of genes with sex-biased expression in the chicken genome. Evolution. 2006;60(9):1945–1951. doi: 10.1111/j.0014-3820.2006.tb00537.x. [DOI] [PubMed] [Google Scholar]
- 66.Marshall Graves JA, Peichel CL. Are homologies in vertebrate sex determination due to shared ancestry or to limited options? Genome Biol. 2010;11:205. doi: 10.1186/gb-2010-11-4-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nanda I, Kondo M, Hornung U, Asakawa S, Winkler C, Shimizu A, Shan Z, Haaf T, Shimizu N, Shima A, et al. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proc Natl Acad Sci U S A. 2002;99(18):11778–11783. doi: 10.1073/pnas.182314699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matsuda M, Nagahama Y, Shinomiya A, Sato T, Matsuda C, Kobayashi T, Morrey CE, Shibata N, Asakawa S, Shimizu N, et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature. 2002;417:559–563. doi: 10.1038/nature751. [DOI] [PubMed] [Google Scholar]
- 69.Matsuda M, Sato T, Toyazaki Y, Nagahama Y, Hamaguchi S, Sakaizumi M. Oryzias curvinotus has DMY, a gene that is required for male development in the medaka, O. latipes. Zoolog Sci. 2003;20(2):159–161. doi: 10.2108/zsj.20.159. [DOI] [PubMed] [Google Scholar]
- 70.Takehana Y, Matsuda M, Myosho T, Suster ML, Kawakami K, Shin-I T, Kohara Y, Kuroki Y, Toyoda A, Fujiyama A, et al. Co-option of Sox3 as the male-determining factor on the Y chromosome in the fish Oryzias dancena. Nat Commun. 2014;5:4157. doi: 10.1038/ncomms5157. [DOI] [PubMed] [Google Scholar]
- 71.Myosho T, Otake H, Masuyama H, Matsuda M, Kuroki Y, Fujiyama A, Naruse K, Hamaguchi S, Sakaizumi M. Tracing the emergence of a novel sex-determining gene in medaka, Oryzias luzonensis. Genetics. 2012;191(1):163–170. doi: 10.1534/genetics.111.137497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rondeau E, Messmer A, Sanderson D, Jantzen S, von Schalburg K, Minkley D, Leong J, Macdonald G, Davidsen A, Parker W, et al. Genomics of sablefish (Anoplopoma fimbria): expressed genes, mitochondrial phylogeny, linkage map and identification of a putative sex gene. BMC Genomics. 2013;14(1):452. doi: 10.1186/1471-2164-14-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hattori RS, Murai Y, Oura M, Masuda S, Majhi SK, Sakamoto T, Fernandino JI, Somoza GM, Yokota M, Strüssmann CA. A Y-linked anti-Müllerian hormone duplication takes over a critical role in sex determination. Proc Natl Acad Sci U S A. 2012;109(8):2955–2959. doi: 10.1073/pnas.1018392109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kamiya T, Kai W, Tasumi S, Oka A, Matsunaga T, Mizuno N, Fujita M, Suetake H, Suzuki S, Hosoya S, et al. A trans-species missense SNP in amhr2 is associated with sex determination in the tiger pufferfish, Takifugu rubripes (Fugu) PLoS Genet. 2012;8(7):e1002798. doi: 10.1371/journal.pgen.1002798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meyer A, Van de Peer Y. From 2R to 3R: evidence for a fish-specific genome duplication (FSGD) Bioessays. 2005;27(9):937–945. doi: 10.1002/bies.20293. [DOI] [PubMed] [Google Scholar]
- 76.Amores A, Force A, Yan Y-L, Joly L, Amemiya C, Fritz A, Ho RK, Langeland J, Prince V, Wang Y-L, et al. Zebrafish hox clusters and vertebrate genome evolution. Science. 1998;282(5394):1711–1714. doi: 10.1126/science.282.5394.1711. [DOI] [PubMed] [Google Scholar]
- 77.Taylor JS, Braasch I, Frickey T, Meyer A, Van de Peer Y. Genome duplication, a trait shared by 22,000 species of ray-finned fish. Genome Res. 2003;13(3):382–390. doi: 10.1101/gr.640303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weng S, You F, Fan Z, Wang L, Wu Z, Zou Y. Molecular cloning and sexually dimorphic expression of wnt4 in olive flounder (Paralichthys olivaceus). Fish Physiol Biochem. 2016;42(4):1167-76. [DOI] [PubMed]
- 79.Brelsford A, Stöck M, Betto-Colliard C, Dubey S, Dufresnes C, Jourdan-Pineau H, Rodrigues N, Savary R, Sermier R, Perrin N. Homologous sex chromosomes in three deeply divergent anuran species. Evolution. 2013;67(8):2434–2440. doi: 10.1111/evo.12151. [DOI] [PubMed] [Google Scholar]
- 80.Parnell NF, Hulsey CD, Streelman JT. The genetic basis of a complex functional system. Evolution. 2012;66(11):3352–3366. doi: 10.1111/j.1558-5646.2012.01688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blaser O, Neuenschwander S, Perrin N. Sex-chromosome turnovers: The hot-potato model. Am Nat. 2014;183(1):140–146. doi: 10.1086/674026. [DOI] [PubMed] [Google Scholar]
- 82.Kohn M, Högel J, Vogel W, Minich P, Kehrer-Sawatzki H, Graves JAM, Hameister H. Reconstruction of a 450-My-old ancestral vertebrate protokaryotype. Trends Genet. 2006;22(4):203–210. doi: 10.1016/j.tig.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 83.Lindholm A, Breden F. Sex chromosomes and sexual selection in poeciliid fishes. Am Nat. 2002;160(Suppl 6):S214–224. doi: 10.1086/342898. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
RAD sequencing raw reads have been deposited in the NCBI SRA Sequence Read Archive https://www.ncbi.nlm.nih.gov/sra under the accession number SRR4408339, scripts are available upon request to the corresponding author; RNA sequencing information was retrieved from [22]. All other datasets supporting the conclusions of this article are included within the article and its Additional files 1, 2, 3, 4, 5, 6, 7 and 8.


