ABSTRACT
Translation is best known as the fundamental mechanism by which the ribosome converts a sequence of nucleotides into a string of amino acids. Extensive research over many years has elucidated the key principles of translation, and the majority of translated regions were thought to be known. The recent discovery of wide-spread translation outside of annotated protein-coding open reading frames (ORFs) came therefore as a surprise, raising the intriguing possibility that these newly discovered translated regions might have unrecognized protein-coding or gene-regulatory functions. Here, we highlight recent findings that provide evidence that some of these newly discovered translated short ORFs (sORFs) encode functional, previously missed small proteins, while others have regulatory roles. Based on known examples we will also speculate about putative additional roles and the potentially much wider impact that these translated regions might have on cellular homeostasis and gene regulation.
KEYWORDS: Ribosome, short proteins, sORF, translation, translational regulation, uORF
Introduction
Traditionally, translation has been assumed to be largely restricted to protein-coding open reading frames (ORF). This long-standing view has been challenged by a series of studies emerging from technological advances that have enabled researchers to analyze translation genome-wide at unprecedented depth and detail. As such, it is now possible not only to predict even very short protein-coding ORFs based on homology,1,2 but also to analyze the translational state of ORFs genome-wide by ribosome profiling.3-5 Moreover, peptide products can be detected with increased sensitivity by improved mass-spectrometry.6-12 Based on these studies it is becoming increasingly clear that many regions outside of annotated protein-coding ORFs are translated. New translated regions have not only been identified in transcripts thought to be non-coding, but also upstream of a large fraction of protein-coding ORFs (so-called upstream ORFs, uORFs).4,13-21 These usually short translated ORFs were likely missed in previous mutagenesis screens due to their small size, and remained un-annotated in genome annotations due to their small size and lack of evidence for codingness.22-24 In analogy to the term ‘pervasive transcription’,25 this unforeseen prevalence of short ORF (sORF) translation has spurred the notion of ‘pervasive translation’.23,26
It should be pointed out that a certain fraction of the detected translation events will likely comprise ‘noise’, which can be of either technical or biological nature. For example, ribosome profiling enriches for 80S ribosome-protected mRNA fragments, yet other protected mRNA fragments of similar size and sedimentation behavior might be co-purified, which will generate technical noise.27 Moreover, the use of translation inhibitors as well as differences in sample preparation and downstream analyses can introduce biases in ribosome footprinting assays that might not accurately reflect the translational state in an unperturbed cell.28-30 To distinguish actual translation events from technical noise, a series of data analysis tools have been developed. These computational approaches use certain features like Ribosome Protected Fragment (RPF) abundance, length and trinucleotide periodicity, positioning of the ORF within a transcript and responsiveness to translation inhibitors to help detect “real” translation and eliminate technical noise.13,17,20,26,31,32 Apart from ‘technical noise’, there is ‘biological noise’ that originates from genuine translation, yet neither the peptide product nor the act of translation of this particular sequence might serve any specific purpose. Because there is ample evidence that at least some sORFs do have essential functions in vivo,19,33-42 we will omit further discussions of noise and focus on functional aspects of sORF translation.
For the sake of simplicity, we have divided this review into 2 main parts, namely roles of sORFs as 1) hidden sources of functional short proteins, and as 2) widespread regulatory elements conferring post-transcriptional control of gene expression. This division does however not preclude that the act of translation of regions functioning as short proteins might also have regulatory roles and vice versa. In the end we will summarize the challenges and opportunities that this newly emerging research area has.
sORFs as source of functional short proteins
Despite previous medium- to large-scale forward genetic screens in organisms ranging from yeast,43,44 plants,45,46 worms47,48 and flies49,50 to zebrafish51,52 and mice,53-55 several new, essential short proteins were discovered during the last decade in different organisms.19,35-42 The majority of these newly identified short proteins show a higher degree of amino acid sequence conservation than observed in known regulatory translation events. The modes of action of these short proteins are divers and comprise intra- as well as extracellular functions (Fig. 1). In the following part we will highlight recent examples of newly discovered short proteins regulating key processes during embryogenesis, in cell-cell communication or in cell physiology. Based on known examples, combined with the extensive body of knowledge on protein-functionalities in general, we will also speculate about other possible roles that this potentially large source of putatively bioactive short proteins might have.
Figure 1.
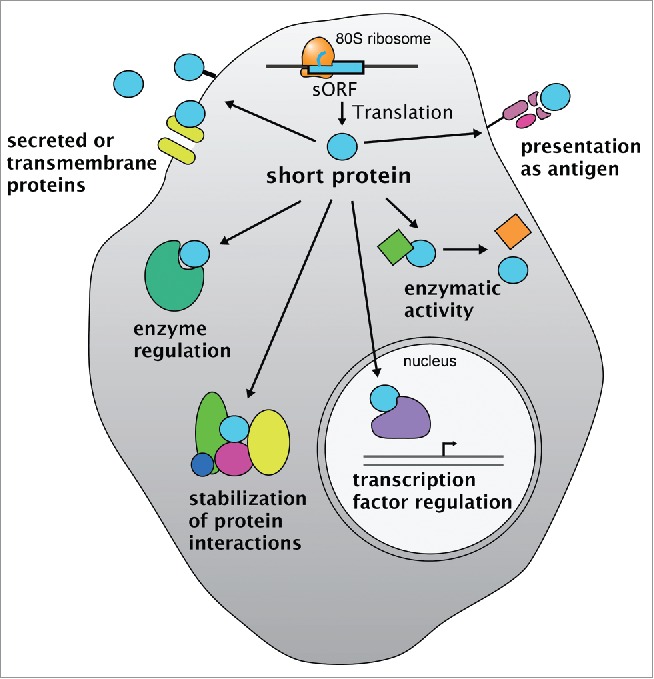
sORFs as source of functional short proteins. Translation of short open reading frames (sORFs) can generate short proteins (light blue sphere) with diverse intracellular and extracellular roles. Functions range from cytoplasmic regulation of enzymes, protein-protein interactions, enzymatic activities to regulation of transcription factors in the nucleus and extracellular roles as signals, membrane-associated proteins or antigens presented on MHC-type molecules.
Short proteins as regulators of protein-protein interaction and enzymatic activity
Due to their small size, short proteins can easily fit into the binding pockets of other proteins, which makes them candidate regulators of protein-protein interactions and enzymatic activities. For example, the binding of a peptide to an allosteric site of a protein could induce a conformational change that alters the interaction surface or the enzymatic activity of the protein. The former mode of action was proposed for the Drosophila polished-rice or tarsal-less (pri) sORF peptides, which are one of the best studied examples of short peptides with an essential function during embryogenesis.36,37,56 The pri locus is transcribed into a polycistronic mRNA encoding 4 evolutionary conserved short ORFs of only 11 to 32 amino acids (aa).36 These short peptides control the binding of the E3 ubiquitin ligase Ubr3 to the transcriptional regulator Shavenbaby (Svb) by inducing a conformational change in Ubr3 that leads to the exposure of a Svb-recognition site in Ubr3.56 Pri-induced binding of Ubr3 to Svb leads to ubiquitination of Svb, which is then N-terminally truncated by the proteasome resulting in a switch in Svb's activity from a transcriptional repressor to a transcriptional activator.37,56 By promoting the formation of Svb activator, Pri-peptides thus induce trichome formation and epidermal differentiation in Drosophila.
By modifying protein-protein interactions, short proteins are also able to alter protein localization and recruitment, as has been proposed for the Drosophila Polar granule component (Pgc). Initially thought to function as a non-coding RNA,57 the germ-cell expressed pgc transcript was subsequently shown to encode a 71 aa long protein that inhibits the phosphorylation of serine 2 residues in the C-terminal domain of RNA polymerase II by preventing the recruitment of the kinase P-TEFb (positive transcription elongation factor b) to transcription sites. As a result, RNA polymerase II-dependent transcription is repressed in germ cells, protecting them from differentiation into somatic cells.58,59
The binding of a short protein to an enzyme can also directly affect enzymatic activities. This is the case for a group of recently identified, short, α-helical transmembrane proteins (all < 50 aa) that bind to and thereby control the activity of SERCA (sacro-endoplasmic reticulum Ca2+ adenosine triphosphatase). SERCA is an ATPase located in the membrane of the sarcoplasmic reticulum (SR), where it pumps Ca2+ back into the SR after Ca2+ release upon muscle contraction. SERCA was recently shown to be inhibited by 3 short proteins, namely phospholamban (PLN/PLB), sacrolipin (SLN) and myoregulin (MLN),38,40 while the short protein DWORF (dwarf open reading frame) enhances SERCA activity by displacing those inhibitory proteins.41 Apart from allosteric regulation, it is also feasible that short proteins could negatively regulate enzyme activities by competing with enzymatic substrates for binding to the active site. While direct evidence is still missing, it is intriguing to speculate that pseudogenes, about one-third of which were recently shown to be translated into proteins of various lengths in human cells,15 might provide a source for such competitive inhibitory peptides.60
Future work might also reveal that some of the newly discovered proteins have specific enzymatic activities themselves. Examples for such small enzymes exist, such as Cytochrome C (105 aa) or the smallest known enzyme, 4-oxalocrotonate tautomerase (62 aa per monomer).61,62 Moreover, short proteins can also act as part of large protein assemblies. For example, advances in mass spectrometry have recently led to the identification of APC15 and APC16, 2 new constitutive components of the extensively studied, 1.5-MDa multi-protein complex Anaphase Promoting Complex/Cyclosome (APC/C).63-65 APC15 was subsequently shown to mediate auto-ubiquitination of Cdc20 by APC/CMCC and disassembly of the mitotic checkpoint complex (MCC).66 Another well-known large molecular machine containing a number of short proteins as structural components is the ribosome itself: Of approximately 80 eukaryotic ribosomal proteins, 21 proteins consist of only 25–100 aa.67 Based on these 2 noticeable examples, small proteins can indeed play prominent roles as subunits of well-studied large protein complexes.
Short proteins in signaling and cell-cell communication
Besides such intracellular functions, recent studies have revealed important roles for short proteins as secreted peptides and hormones. Especially in plants different classes of polypeptide hormones have been identified, several of which are involved in defense mechanisms. For example, Systemin, which is the first plant polypeptide hormone identified, only consists of 18 aa.68,69 Many other secreted short plant proteins are involved in developmental processes, growth control and stress response (for an overview see 70). This diverse group of signals includes the recently discovered ESF1 (embryo surrounding factor 1), which regulates early embryo tissue patterning,39 and the secreted phytosulfokine pentapeptides (PSK), which are encoded by 7 different precursor genes of approximately 100 aa in length and regulate plant growth and stress responses. PSK binds to the extracellular leucine-rich repeats of the PSK receptor PSKR1 and might thereby allosterically activate the Ser/Thr kinase activity of PSKR.71,72
Signaling functions for short proteins are not limited to plants and have also been discovered in vertebrates: For example, the mature, 36 aa long secreted polypeptide Apelin binds to the G protein-coupled Apelin receptor APJ and has been implicated in the regulation of various physiological processes, ranging from angiogenesis to energy metabolism, neuroendocrine stress response, cardiovascular function and fluid homeostasis (reviewed by 73). Recently, a second conserved APJ ligand named Apela/ELABELA/Toddler was identified that had been mis-annotated as a non-coding RNA.19,35 Functional analyses in zebrafish embryos revealed that Apela/ELABELA/Toddler promotes the movement of ventral and lateral mesendodermal cells during gastrulation19,35 and the migration of angioblasts during vasculogenesis.74
Apart from signaling to other cells as secreted factors, it is also feasible that membrane-bound short proteins could act as (co-)receptors or as cell adhesion molecules. While specific examples for such functionalities have to our knowledge not yet been identified, the SERCA regulators PLN, SLN, MLN and DWORF38,40,41 provide the proof-of-principle that functional, cell membrane-embedded short proteins exist.
Cell-cell communication can also occur via an entirely different route, e.g. by the presentation of peptides on the surface of a cell by Major Histocompatibility Complex class I (MHC I) molecules. Many antigens that are presented on MHC I molecules were recently shown to derive from non-conventional peptide sources, such as untranslated regions (UTRs), unannotated ORFs, introns, non-AUG start codons or from alternative translational reading frames.75-78 Short peptides presented as self-antigens on MHC-type molecules could play a role in the negative selection of T-cells during T-cell development in the thymus, or might shape the immune response during viral infection, cancer progression and autoimmune disease.26,79 Because the presentation of peptides by MHC-type molecules is largely independent of the encoded amino acid sequence, a certain fraction - if not even the entire cohort - of sORF-originating short proteins might be co-opted for such an immunological functionality.
Given the range of possible activities and the paucity of functional studies up to date, it is clear that we are only at the beginning of grasping the full impact that short proteins have.
sORFs as post-transcriptional regulators of gene expression
One hallmark of most sORFs discussed so far is that they encode evolutionarily conserved short proteins. However, this is not the case for the majority of newly identified short translated ORFs. Instead, there is increasing support for the idea that the gene-regulatory effect imposed by the act of uORF translation is conserved across vertebrates.16,20,80-83 Although translated sORFs also occur in presumably non-coding transcripts, and to a much lower extent also in 3’UTRs,8,13-15,17,21 we will focus on uORFs as most abundant and best-studied class of regulatory sORFs.
Post-transcriptional regulation plays an important role in controlling the composition of the proteome of each cell. Because it does not require transcriptional changes, this mode of regulation stands out as being fast and – if it does not trigger transcript degradation – reversible. It is well known from classical, mostly single gene studies that uORFs can repress translation of downstream ORFs by 2 means.34,84-91 uORF translation can impact the stability of the mRNA by triggering co-translational RNA decay pathways,21,92-95 and it can interfere with ribosome access to downstream ORFs91,96: in order to reach a downstream (coding) ORF, scanning ribosomes have to either read-through uORFs without initiating (‘leaky scanning’), or re-start translation after having already translated a uORF (‘reinitiation’) (Fig. 2). These mechanisms enable the cell to cope with uORFs and had been known for decades, yet the extent and possibly widespread impact of uORF-mediated regulation has only become evident over the past few years.
Figure 2.
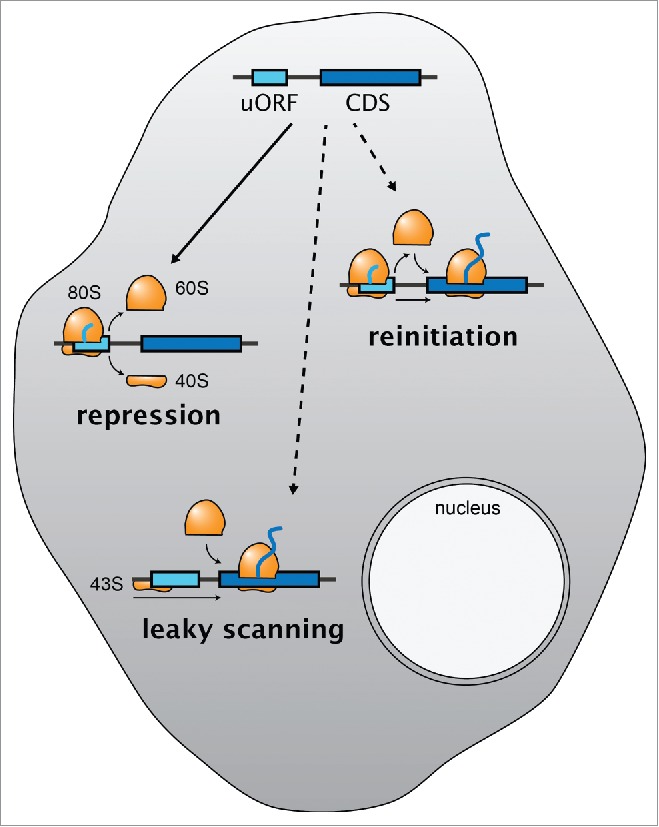
uORFs as post-transcriptional regulators of gene expression. Translation of sORFs upstream of the main coding sequence (CDS, dark blue), so-called upstream ORFs (uORFs, light blue), is generally repressive by blocking the ribosome from accessing the downstream CDS. Two strategies allow the cell to bypass the inhibitory effect of uORFs (dashed arrows): leaky scanning and reinitiation. 80S, actively translating ribosome; 60S, large ribosomal subunit; 40S, small ribosomal subunit; 43S, preinitiation complex.
uORFs as widespread repressive genetic elements
A large fraction of ribosomal footprints outside of the annotated coding sequences originates from uORFs within transcript leaders, which are conventionally yet rather misleadingly called 5’UTRs (5’ untranslated region).4,13,14,16,20,97-99 The generally repressive effect of uORFs on downstream translation has been studied at the level of individual genes,34,90,100-102 in reporter studies,16,83 and more recently also genome-wide.16,20,83
One of the best-studied examples for regulatory uORFs is the yeast transcriptional activator GCN4 (ATF4 in vertebrates).33,90 The GCN4 mRNA contains 4 short uORFs that function as translational barriers for the main coding sequence under normal conditions when GCN4 protein is not required. Upon stress, translation of the GCN4 coding sequence is induced by a mechanism that harnesses uORF1 translation to bypass the 3 strictly inhibitory uORFs 2-4. Thus, differential translation of the 4 uORFs causes differential translation of GCN4 in response to stress.
In support of uORFs being repressive genetic elements, genome-wide analyses of vertebrate ribosome profiling data revealed an inverse correlation between the number of uORFs within a transcript and the efficiency of CDS translation: CDSes of transcripts lacking uORFs are more efficiently translated than those of transcripts with uORFs; furthermore, the more uORFs a transcript has, the less the CDS is translated.16,20,103 Apart from reduced translation downstream, the presence of uORFs also correlates with reduced steady-state levels of transcripts,16 which is indicative of reduced stability of uORF-containing transcripts. Consistently, uORF-containing transcripts have been shown to be enriched in targets of nonsense-mediated mRNA decay (NMD),21,92,94,104-107 which induces rapid degradation of transcripts with premature termination codons.
A certain fraction of uORFs is likely to act constitutively. Without invoking any further regulatory mechanisms, the mere presence of a uORF amenable to translational initiation (e.g., located within a permissive sequence context) is expected to cause dampening of downstream translation and/or destabilization of the associated transcript - independent of cell type, cellular condition or environmental change. The strength of the imposed regulatory effect of constitutive uORFs is determined by cis-encoded sequence features. For example, certain scenarios like a sub-optimal sequence context around the initiation codon and close proximity to the 5’ cap interfere with recognition of initiation codons, promote leaky scanning and thus correlate with weak repressiveness of the uORF.108,109 On the other hand, long uORFs and short distances between uORF and main ORF impede reinitiation and thus correlate with increased repressiveness of the uORF.91,110,111
Regulating the regulator – how uORF translation can be controlled
Apart from such a constitutive mode of action, there is increasing evidence that uORF-mediated regulation itself can be dynamically regulated. In its simplest form of regulation, uORFs can either be selectively included or excluded during the production of the mRNA. Mechanisms generating transcript isoforms differing in the number and/or location of uORFs include alternative transcription start site (TSS) usage92,107,112,113 and alternative splicing.114-118 The impact that a different transcript leader can have on protein production is exemplified by the disease-causing splice donor mutation in the Thrombopoietin (THPO) transcript leader.119 Exon-skipping generates a THPO transcript leader lacking uORFs. Consistent with a repressive effect of the THPO uORFs, the resultant transcript variant shows increased translation of THPO and leads to an over-production of TPO protein. More interesting from a developmental point of view are regulated changes in transcript leaders that can contribute to the cell-type specific proteome. For example, differential splicing of the 5’UTR of Elk-1 removes the STOP-containing exon of the first uORF, which concomitantly places the AUG initiation codon of the second uORF in-frame within the first uORF. While the underlying molecular mechanism is not yet clear, this alternatively spliced transcript shows decreased sensitivity to mTOR inhibition by Rapamycin.118 Another example stems from differential TSS usage: Differential TSS usage during myoblasts differentiation generates a Cryab transcript isoform in myoblasts with an additional 5’-most uORF-containing exon. Lack of this 5’-most exon in differentiated myotubes could contribute to the increased Cryab protein production.120,121 Because alternative TSS usage is common during vertebrate embryogenesis122,123 and in different cell types,124-127 such a transcription-based control of uORF regulation could have broad implications in developmental regulation of gene expression.
Other mechanisms of uORF regulation require additional RNA-binding factors or translation machinery associated proteins that modulate the extent of leaky scanning or reinitiation.128 A factor that has been implicated in promoting upstream AUG start codon selection is the DExH-box helicase DHX29.129 In vitro experiments revealed that DHX29, in association with the initiation factor eIF1A and the pre-initiation complex, reduces leaky scanning, presumably by inducing a conformational change and stabilization of the pre-initiation complex. Moreover, changing the activity and concentration of eIF2 affects the general ability of ribosomes to reinitiate translation after uORFs.91,110,111 Similarly, a study carried out in Arabidopsis thaliana has identified the target-of-rapamycin (TOR) pathway in conjunction with the S6 kinase (S6K) and the plant reinitiation factor eIF3h as more general regulators of reinitiation.130 Induction of the TOR pathway by the plant hormone auxin leads to S6K-mediated phosphorylation of eIF3h, which promotes the assembly of reinitiating ribosomes.
In contrast to these globally acting trans-factors, there are several examples for RNA-binding factors that affect translation of specific uORF-containing transcripts. A good example is the trans-acting RNA binding protein Sex Lethal (SXL). SXL binds downstream of a short uORF in the male-specific lethal (msl)-2 transcript and reduces leaky scanning by promoting translation initiation at the uORF, which augments the repressive effect of the uORF on downstream translation by about 9-fold.102 Another transcript-specific modulator is the non-canonical translation initiation factor DENR-MCT (density regulated protein and multiple copies in T-cell lymphoma) complex, which promotes reinitiation at ∼100 transcripts characterized by the presence of short uORFs with strong Kozak sequences.131 DENR knockout mutants in Drosophila exhibit defects in larval growth and reduced accumulation rates of DENR-regulated proteins, especially in proliferating tissues.
Challenges and opportunities
The biggest challenge in the field of sORFs will be to tease apart those that have a specific function from those that don’t. Evolutionary conservation of either the encoded protein sequence or the regulatory effect is clearly a hint toward functionality, yet for most sORFs the most rigorous assessment of functionality, namely mutagenesis of the sORF and analyses of resultant in vivo phenotypes, remains to be done. In light of the recent finding that some uORF-, pseudogene- and dORF- (downstream ORF) encoded peptides are conserved,15 these analyses might reveal protein-coding functions for some sORFs that are currently classified as regulatory. In general, classifying sORFs into likely protein-coding versus regulatory regions is aided by the analysis of nucleotide and amino acid conservation. However, in the absence of functional data this division remains artificial and does not exclude a possible dual (regulatory and coding) role for a single sORF. Precedence for such uORF-encoded functional peptides exist from studies in plants.132,133
While not all of the conserved sORFs are expected to reveal discernable phenotypes when mutated, the chances of identifying even subtle phenotypes indicating potentially essential roles can be greatly increased by specific hypotheses that can be tested in targeted functional assays. For example, flies and mice lacking SERCA-regulating short protein-encoding sORFs are viable and do not show overt behavioral or morphological muscle phenotypes, yet detailed analyses of muscle physiology and functionality revealed altered muscle contractions and aberrant Calcium flux in mutant animals.38,40,41 Therefore, the particular challenge for identifying functions for small protein-encoding sORFs will be to narrow down the vast range of potentially affected cellular processes to formulate specific, testable hypotheses. On the other hand, big outstanding questions in the field of regulatory sORFs are regarding 1) the extent to which sORF translation really matters, 2) the extent to which sORFs are dynamically regulated, and 3) what the underlying mechanisms are. While the combination of currently available techniques like ribosome profiling, RNA-IPs and CRISPR/Cas9-based mutagenesis of potential regulators will likely address several of these questions, genome-wide technologies that can assess translational regulation at the level of individual transcripts are still missing. As such, it is currently unclear to which extent ribosome protected fragments in transcript leaders originate from the same transcript that also translates the downstream ORF, or from a different transcript at which the downstream ORF is not associated with translating ribosomes. Answers to this long-standing question will open new possibilities to globally assess the dynamics of translational regulation at unprecedented detail.
Disclosure of potential conflicts of interest.
No potential conflicts of interest were disclosed.
References
- 1. Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature 2012; 482:339–46; PMID:22337053; http://dx.doi.org/ 10.1038/nature10887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lin MF, Jungreis I, Kellis M. PhyloCSF: a comparative genomics method to distinguish protein coding and non-coding regions. Bioinformatics 2011; 27:i275-82; PMID:21685081; http://dx.doi.org/ 10.1093/bioinformatics/btr209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ingolia NT, Ghaemmaghami S, Newnam JRS, Weissman JS. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 2009; 324:218–23; PMID:19213877; http://dx.doi.org/ 10.1126/science.1168978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ingolia NT, Lareau LF, Weissman JS. Ribosome Profiling of Mouse Embryonic Stem Cells Reveals the Complexity and Dynamics of Mammalian Proteomes. Cell 2011; 147(4):1–23; PMID:22056041; http://dx.doi.org/27015305 10.1016/j.cell.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ingolia NT. Ribosome Footprint Profiling of Translation throughout the Genome. Cell 2016; 165:22–33; PMID:27015305; http://dx.doi.org/ 10.1016/j.cell.2016.02.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oyama M, Itagaki C, Hata H, Suzuki Y, Izumi T, Natsume T, Isobe T, Sugano S. Analysis of small human proteins reveals the translation of upstream open reading frames of mRNAs. Genome Res 2004; 14:2048–52; PMID:15489325; http://dx.doi.org/ 10.1101/gr.2384604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baerenfaller K, Grossmann J, Grobei MA, Hull R, Hirsch-Hoffmann M, Yalovsky S, Zimmermann P, Grossniklaus U, Gruissem W, Baginsky S. Genome-scale proteomics reveals Arabidopsis thaliana gene models and proteome dynamics. Science 2008; 320:938–41; PMID:18436743; http://dx.doi.org/ 10.1126/science.1157956 [DOI] [PubMed] [Google Scholar]
- 8. Slavoff SA, Mitchell AJ, Schwaid AG, Cabili MN, Ma J, Levin JZ, Karger AD, Budnik BA, Rinn JL, Saghatelian A. Peptidomic discovery of short open reading frame-encoded peptides in human cells. Nat Chem Biol 2012; 9:59–64; PMID:23160002; http://dx.doi.org/ 10.1038/nchembio.1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saghatelian A, Couso J-P. Discovery and characterization of smORF-encoded bioactive polypeptides. Nat Chem Biol 2015; 11:909–16; PMID:26575237; http://dx.doi.org/ 10.1038/nchembio.1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ma J, Diedrich JK, Jungreis I, Donaldson C, Vaughan J, Kellis M, Yates JR. III, Saghatelian A. Improved Identification and Analysis of Small Open Reading Frame Encoded Polypeptides. Anal Chem 2016; 88:3967–75; PMID:27010111; http://dx.doi.org/ 10.1021/acs.analchem.6b00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim M-S, Pinto SM, Getnet D, Nirujogi RS, Manda SS, Chaerkady R, Madugundu AK, Kelkar DS, Isserlin R, Jain S, et al. . A draft map of the human proteome. Nature 2014; 509:575–81; PMID:24870542; http://dx.doi.org/ 10.1038/nature13302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilhelm M, Schlegl J, Hahne H, Moghaddas Gholami A, Lieberenz M, Savitski MM, Ziegler E, Butzmann L, Gessulat S, Marx H, et al. . Mass-spectrometry-based draft of the human proteome. Nature 2014; 509:582–7; PMID:24870543; http://dx.doi.org/ 10.1038/nature13319 [DOI] [PubMed] [Google Scholar]
- 13. Bazzini AA, Johnstone TG, Christiano R, Mackowiak SD, Obermayer B, Fleming ES, Vejnar CE, Lee MT, Rajewsky N, Walther TC, et al. . Identification of small ORFs in vertebrates using ribosome footprinting and evolutionary conservation. The EMBO Journal 2014; 33:981–93; PMID:24705786; http://dx.doi.org/ 10.1002/embj.201488411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brar GA, Yassour M, Friedman N, Regev A, Ingolia NT, Weissman JS. High-Resolution View of the Yeast Meiotic Program Revealed by Ribosome Profiling. Science 2012; 335:552–7; PMID:22194413; http://dx.doi.org/ 10.1126/science.1215110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ji Z, Song R, Regev A, Struhl K. Many lncRNAs, 5’UTRs, and pseudogenes are translated and some are likely to express functional proteins. eLife 2015; 4:1–21; PMID:26687005; http://dx.doi.org/26896445 10.7554/eLife.08890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnstone TG, Bazzini AA, Giraldez AJ. Upstream ORFs are prevalent translational repressors in vertebrates. The EMBO Journal 2016; 35: 706–23; PMID:26896445; http://dx.doi.org/ 10.15252/embj.201592759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chew G-L, Pauli A, Rinn JL, Regev A, Schier AF, Valen E. Ribosome profiling reveals resemblance between long non-coding RNAs and 5' leaders of coding RNAs. Development 2013; 140:2828–34; PMID:23698349; http://dx.doi.org/ 10.1242/dev.098343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crappé J, Van Criekinge W, Trooskens G, Hayakawa E, Luyten W, Baggerman G, Menschaert G. Combining in silico prediction and ribosome profiling in a genome-wide search for novel putatively coding sORFs. BMC Genomics 2013; 14:648–12; http://dx.doi.org/ 10.1186/1471-2164-14-648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pauli A, Norris ML, Valen E, Chew G-L, Gagnon JA, Zimmerman S, Mitchell A, Ma J, Dubrulle J, Reyon D, et al. . Toddler: An Embryonic Signal That Promotes Cell Movement via Apelin Receptors. Science 2014; 343:1248636–6; PMID:24407481; http://dx.doi.org/ 10.1126/science.1248636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chew G-L, Pauli A, Schier AF. Conservation of uORF repressiveness and sequence features in mouse, human and zebrafish. Nature Communications 2016; 7:11663–10; PMID:27216465; http://dx.doi.org/ 10.1038/ncomms11663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith JE, Alvarez-Dominguez JR, Kline N, Huynh NJ, Geisler S, Hu W, Coller J, Baker KE. Translation of Small Open Reading Frames within Unannotated RNA Transcripts in Saccharomyces cerevisiae. CellReports 2014; 7:1858–66; PMID:24931603; http://dx.doi.org/16683031 10.1016/j.celrep.2014.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Frith MC, Forrest AR, Nourbakhsh E, Pang KC, Kai C, Kawai J, Carninci P, Hayashizaki Y, Bailey TL, Grimmond SM. The abundance of short proteins in the mammalian proteome. PLoS Genet 2006; 2:e52-14; PMID:16683031; http://dx.doi.org/ 10.1371/journal.pgen.0020052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pauli A, Valen E, Schier AF. Identifying (non-)coding RNAs and small peptides: challenges and opportunities. BioEssays 2015; 37:103–12; PMID:25345765; http://dx.doi.org/ 10.1002/bies.201400103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cohen SM. Everything old is new again: (linc)RNAs make proteins! The EMBO Journal 2014; 33:937–8; PMID:24719208; http://dx.doi.org/ 10.1002/embj.201488303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. ENCODE Project Consortium , Birney E, Stamatoyannopoulos JA, Dutta A, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, Thurman RE, Kuehn MS, et al. . Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 2007; 447:799–816; PMID:17571346; http://dx.doi.org/ 10.1038/nature05874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ingolia NT, Brar GA, Stern-Ginossar N, Harris MS, Talhouarne GJS, Jackson SE, Wills MR, Weissman JS. Ribosome Profiling Reveals Pervasive Translation Outside of Annotated Protein-Coding Genes. CellReports 2014; 8:1365–79; PMID:25159147; http://dx.doi.org/23810193 10.1016/j.celrep.2014.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guttman M, Russell P, Ingolia NT, Weissman JS, Lander ES. Ribosome Profiling Provides Evidence that Large Noncoding RNAs Do Not Encode Proteins. Cell 2013; 154:240–51; PMID:23810193; http://dx.doi.org/ 10.1016/j.cell.2013.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jackson RJ, Standart N. The awesome power of ribosome profiling. RNA 2015; 21:652–4; PMID:25780177; http://dx.doi.org/ 10.1261/rna.049908.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bartholomäus A, Del Campo C, Ignatova Z. Mapping the non-standardized biases of ribosome profiling. Biological Chemistry 2015; 397:1–13; PMID:26351919; http://dx.doi.org/25056308 10.1515/hsz-2015-0197 [DOI] [PubMed] [Google Scholar]
- 30. Gerashchenko MV, Gladyshev VN. Translation inhibitors cause abnormalities in ribosome profiling experiments. Nucleic Acids Res 2014; 42:e134-4; PMID:25056308; http://dx.doi.org/ 10.1093/nar/gku671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Calviello L, Mukherjee N, Wyler E, Zauber H, Hirsekorn A, Selbach M, Landthaler M, Obermayer B, Ohler U. Detecting actively translated open reading frames in ribosome profiling data. Nature Methods 2015; 13:165–70; PMID:26657557; http://dx.doi.org/ 10.1038/nmeth.3688 [DOI] [PubMed] [Google Scholar]
- 32. Raj A, Wang SH, Shim H, Harpak A, Li YI, Engelmann B, Stephens M, Gilad Y, Pritchard JK. Thousands of novel translated open reading frames in humans inferred by ribosome footprint profiling. eLife 2016; 5:1–24; PMID:27232982; http://dx.doi.org/3516411 10.7554/eLife.13328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mueller PP, Hinnebusch AG. Multiple upstream AUG codons mediate translational control of GCN4. Cell 1986; 45:201–7; PMID:3516411; http://dx.doi.org/ 10.1016/0092-8674(86)90384-3 [DOI] [PubMed] [Google Scholar]
- 34. Wethmar K, Schulz J, Muro EM, Talyan S, Andrade-Navarro MA, Leutz A. Comprehensive translational control of tyrosine kinase expression by upstream open reading frames. Oncogene 2015; 35(13):1736-42; PMID:26096937; http://dx.doi.org/24316148 10.1038/onc.2015.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chng SC, Ho L, Tian J, Reversade B. ELABELA: a hormone essential for heart development signals via the apelin receptor. Developmental Cell 2013; 27:672–80; PMID:24316148; http://dx.doi.org/ 10.1016/j.devcel.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 36. Kondo T, Hashimoto Y, Kato K, Inagaki S, Hayashi S, Kageyama Y. Small peptide regulators of actin-based cell morphogenesis encoded by a polycistronic mRNA. Nat Cell Biol 2007; 9:660–5; PMID:17486114; http://dx.doi.org/ 10.1038/ncb1595 [DOI] [PubMed] [Google Scholar]
- 37. Kondo T, Plaza S, Zanet J, Benrabah E, Valenti P, Hashimoto Y, Kobayashi S, Payre F, Kageyama Y. Small Peptides Switch the Transcriptional Activity of Shavenbaby During Drosophila Embryogenesis. Science 2010; 329:336–9; PMID:20647469; http://dx.doi.org/ 10.1126/science.1188158 [DOI] [PubMed] [Google Scholar]
- 38. Magny EG, Pueyo JI, Pearl FMG, Cespedes MA, Niven JE, Bishop SA, Couso J-P. Conserved regulation of cardiac calcium uptake by peptides encoded in small open reading frames. Science 2013; 341:1116–20; PMID:23970561; http://dx.doi.org/ 10.1126/science.1238802 [DOI] [PubMed] [Google Scholar]
- 39. Costa LM, Marshall E, Tesfaye M, Silverstein KAT, Mori M, Umetsu Y, Otterbach SL, Papareddy R, Dickinson HG, Boutiller K, et al. . Central Cell-Derived Peptides Regulate Early Embryo Patterning in Flowering Plants. Science 2014; 344:168–72; PMID:24723605; http://dx.doi.org/ 10.1126/science.1243005 [DOI] [PubMed] [Google Scholar]
- 40. Anderson DM, Anderson KM, Chang C-L, Makarewich CA, Nelson BR, McAnally JR, Kasaragod P, Shelton JM, Liou J, Bassel-Duby R, et al. . A Micropeptide Encoded by a Putative Long Noncoding RNA Regulates Muscle Performance. Cell 2015; 160:595–606; PMID:25640239; http://dx.doi.org/ 10.1016/j.cell.2015.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nelson BR, Makarewich CA, Anderson DM, Winders BR, Troupes CD, Wu F, Reese AL, McAnally JR, Chen X, Kavalali ET, et al. . A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science 2016; 351:271–5; PMID:26816378; http://dx.doi.org/ 10.1126/science.aad4076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Andrews SJ, Rothnagel JA. Emerging evidence for functional peptides encoded by short open reading frames. Nat Rev Genet 2014; 15:193–204; PMID:24514441; http://dx.doi.org/ 10.1038/nrg3520 [DOI] [PubMed] [Google Scholar]
- 43. Oliver SG. From gene to screen with yeast. Current Opinion in Genetics & Development 1997; 7:405–9; PMID:9229118; http://dx.doi.org/ 10.1016/S0959-437X(97)80156-6 [DOI] [PubMed] [Google Scholar]
- 44. Kumar A, Snyder M. Emerging technologies in yeast genomics. Nat Rev Genet 2001; 2:302–12; PMID:11283702; http://dx.doi.org/ 10.1038/35066084 [DOI] [PubMed] [Google Scholar]
- 45. Mayer U, Ruiz RAT, Berleth T, Miseéra S, Juürgens G. Mutations affecting body organization in the Arabidopsis embryo. Nature 1991; 353:402–7; http://dx.doi.org/ 10.1038/353402a0 [DOI] [Google Scholar]
- 46. Browse J, McCourt P, Somerville CR. A mutant of Arabidopsis lacking a chloroplast-specific lipid. Science 1985; 227:763–5; PMID:17796728; http://dx.doi.org/ 10.1126/science.227.4688.763 [DOI] [PubMed] [Google Scholar]
- 47. Brenner S. The genetics of Caenorhabditis elegans. Genetics 1974; 77:71–94; PMID:4366476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ferguson EL, Horvitz HR. Identification and characterization of 22 genes that affect the vulval cell lineages of the nematode Caenorhabditis elegans. Genetics 1985; 110:17–72; PMID:3996896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature 1980; 287:795–801; PMID:6776413; http://dx.doi.org/ 10.1038/287795a0 [DOI] [PubMed] [Google Scholar]
- 50. St Johnston D. The art and design of genetic screens: Drosophila melanogaster. Nat Rev Genet 2002; 3:176–88; PMID:11972155; http://dx.doi.org/ 10.1038/nrg751 [DOI] [PubMed] [Google Scholar]
- 51. Driever W, Solnica-Krezel L, Schier AF, Neuhauss SC, Malicki J, Stemple DL, Stainier DY, Zwartkruis F, Abdelilah S, Rangini Z, et al. . A genetic screen for mutations affecting embryogenesis in zebrafish. Development 1996; 123:37–46; PMID:9007227 [DOI] [PubMed] [Google Scholar]
- 52. Haffter P, Granato M, Brand M, Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, van Eeden FJ, Jiang YJ, Heisenberg CP, et al. . The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 1996; 123:1–36; PMID:9007226 [DOI] [PubMed] [Google Scholar]
- 53. Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 1994; 264:719–25; PMID:8171325; http://dx.doi.org/ 10.1126/science.8171325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nolan PM, Peters J, Strivens M, Rogers D, Hagan J, Spurr N, Gray IC, Vizor L, Brooker D, Whitehill E, et al. . A systematic, genome-wide, phenotype-driven mutagenesis programme for gene function studies in the mouse. Nature Genetics 2000; 25:440–3; PMID:10932191; http://dx.doi.org/ 10.1038/78140 [DOI] [PubMed] [Google Scholar]
- 55. Hrabé de Angelis MH, Flaswinkel H, Fuchs H, Rathkolb B, Soewarto D, Marschall S, Heffner S, Pargent W, Wuensch K, Jung M, et al. . Genome-wide, large-scale production of mutant mice by ENU mutagenesis. Nature Genetics 2000; 25:444–7; PMID:10932192; http://dx.doi.org/ 10.1038/78146 [DOI] [PubMed] [Google Scholar]
- 56. Zanet J, Benrabah E, Li T, Pelissier-Monier A, Chanut-Delalande H, Ronsin B, Bellen HJ, Payre F, Plaza S. Pri sORF peptides induce selective proteasome-mediated protein processing. Science 2015; 349:1356–8; PMID:26383956; http://dx.doi.org/ 10.1126/science.aac5677 [DOI] [PubMed] [Google Scholar]
- 57. Nakamura A, Amikura R, Mukai M, Kobayashi S, Lasko PF. Requirement for a Noncoding RNA in Drosophila Polar Granules for Germ Cell Establishment. Science 1996; 274:2075–9; PMID:8953037; http://dx.doi.org/ 10.1126/science.274.5295.2075 [DOI] [PubMed] [Google Scholar]
- 58. Hanyu-Nakamura K, Sonobe-Nojima H, Tanigawa A, Lasko P, Nakamura A. Drosophila Pgc protein inhibits P-TEFb recruitment to chromatin in primordial germ cells. Nature 2008; 451:730–3; PMID:18200011; http://dx.doi.org/ 10.1038/nature06498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Timinszky G, Bortfeld M, Ladurner AG. Repression of RNA Polymerase II Transcription by a Drosophila Oligopeptide. PLoS ONE 2008; 3:e2506-7; PMID:18575576; http://dx.doi.org/ 10.1371/journal.pone.0002506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McCarrey JR, Riggs AD. Determinator-inhibitor pairs as a mechanism for threshold setting in development: a possible function for pseudogenes. Proc Natl Acad Sci USA 1986; 83:679–83; PMID:2418440; http://dx.doi.org/ 10.1073/pnas.83.3.679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen LH, Kenyon GL, Curtin F, Harayama S, Bembenek ME, Hajipour G, Whitman CP. 4-Oxalocrotonate tautomerase, an enzyme composed of 62 amino acid residues per monomer. J Biol Chem 1992; 267:17716–21; PMID:1339435 [PubMed] [Google Scholar]
- 62. Tiessen A, Pérez-Rodríguez P, Delaye-Arredondo LJ. Mathematical modeling and comparison of protein size distribution in different plant, animal, fungal and microbial species reveals a negative correlation between protein size and protein number, thus providing insight into the evolution of proteomes. BMC Res Notes 2012; 5:85–23; PMID:22296664; http://dx.doi.org/ 10.1186/1756-0500-5-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hubner NC, Bird AW, Cox J, Splettstoesser B, Bandilla P, Poser I, Hyman A, Mann M. Quantitative proteomics combined with BAC TransgeneOmics reveals in vivo protein interactions. J Cell Biol 2010; 189:739–54; PMID:20479470; http://dx.doi.org/ 10.1083/jcb.200911091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mansfeld J, Collin P, Collins MO, Choudhary JS, Pines J. APC15 drives the turnover of MCC-CDC20 to make the spindle assembly checkpoint responsive to kinetochore attachment. Nat Cell Biol 2011; 13:1234–43; PMID:21926987; http://dx.doi.org/ 10.1038/ncb2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hutchins JRA, Toyoda Y, Hegemann B, Poser I, Hériché J-K, Sykora MM, Augsburg M, Hudecz O, Buschhorn BA, Bulkescher J, et al. . Systematic analysis of human protein complexes identifies chromosome segregation proteins. Science 2010; 328:593–9; PMID:20360068; http://dx.doi.org/ 10.1126/science.1181348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Uzunova K, Dye BT, Schutz H, Ladurner R, Petzold G, Toyoda Y, Jarvis MA, Brown NG, Poser I, Novatchkova M, et al. . APC15 mediates CDC20 autoubiquitylation by APC/C(MCC) and disassembly of the mitotic checkpoint complex. Nat Struct Mol Biol 2012; 19:1116–23; PMID:23007861; http://dx.doi.org/ 10.1038/nsmb.2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Planta RJ, Mager WH. The list of cytoplasmic ribosomal proteins of Saccharomyces cerevisiae. Yeast 1998; 14:471–7; PMID:9559554; http://dx.doi.org/ 10.1002/(SICI)1097-0061(19980330)14:5%3c471::AID-YEA241%3e3.0.CO;2-U [DOI] [PubMed] [Google Scholar]
- 68. Pearce G, Strydom D, Johnson S, Ryan CA. A Polypeptide from Tomato Leaves Induces Wound-Inducible Proteinase Inhibitor Proteins. Science 1991; 253:895–7; PMID:17751827; http://dx.doi.org/ 10.1126/science.253.5022.895 [DOI] [PubMed] [Google Scholar]
- 69. McGurl B, Pearce G, Orozco-Cardenas M, Ryan C. Structure, expression, and antisense inhibition of the systemin precursor gene. Science 1992; 255:1570–3; PMID:1549783; http://dx.doi.org/ 10.1126/science.1549783 [DOI] [PubMed] [Google Scholar]
- 70. Motomitsu A, Sawa S, Ishida T. Plant peptide hormone signalling. Essays Biochem 2015; 58:115–31; PMID:26374891; http://dx.doi.org/ 10.1042/bse0580115 [DOI] [PubMed] [Google Scholar]
- 71. Sauter M. Phytosulfokine peptide signalling. J Exp Botany 2015; 66:5161–9; PMID:25754406; http://dx.doi.org/ 10.1093/jxb/erv071 [DOI] [PubMed] [Google Scholar]
- 72. Wang J, Li H, Han Z, Zhang H, Wang T, Lin G, Chang J, Yang W, Chai J. Allosteric receptor activation by the plant peptide hormone phytosulfokine. Nature 2015; 525:265–8; PMID:26308901; http://dx.doi.org/ 10.1038/nature14858 [DOI] [PubMed] [Google Scholar]
- 73. O'Carroll AM, Lolait SJ, Harris LE, Pope GR. The apelin receptor APJ: journey from an orphan to a multifaceted regulator of homeostasis. J Endocrinol 2013; 219:R13–R35; PMID:23943882; http://dx.doi.org/ 10.1530/JOE-13-0227 [DOI] [PubMed] [Google Scholar]
- 74. Helker CSM, Schuermann A, Pollmann C, Chng SC, Kiefer F, Reversade B, Herzog W. The hormonal peptide Elabela guides angioblasts to the midline during vasculogenesis. eLife 2015; 4:1–13; PMID: 26017639; http://dx.doi.org/21390547 10.7554/eLife.06726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Starck SR, Shastri N. Non-conventional sources of peptides presented by MHC class I. Cell Mol Life Sci 2011; 68:1471–9; PMID:21390547; http://dx.doi.org/ 10.1007/s00018-011-0655-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shastri N, Schwab S, Serwold T. Producing nature's gene-chips: the generation of peptides for display by MHC class I molecules. Annu Rev Immunol 2002; 20:463–93; PMID:11861610; http://dx.doi.org/ 10.1146/annurev.immunol.20.100301.064819 [DOI] [PubMed] [Google Scholar]
- 77. Starck SR, Jiang V, Pavon-Eternod M, Prasad S, McCarthy B, Pan T, Shastri N. Leucine-tRNA initiates at CUG start codons for protein synthesis and presentation by MHC class I. Science 2012; 336:1719–23; PMID:22745432; http://dx.doi.org/ 10.1126/science.1220270 [DOI] [PubMed] [Google Scholar]
- 78. Starck SR, Tsai JC, Chen K, Shodiya M, Wang L, Yahiro K, Martins-Green M, Shastri N, Walter P. Translation from the 5' untranslated region shapes the integrated stress response. Science 2016; 351:aad3867-7; PMID:26823435; http://dx.doi.org/ 10.1126/science.aad3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Engelhard VH. The contributions of mass spectrometry to understanding of immune recognition by T lymphocytes. Int J Mass Spectrom 2007; 259:32–9; PMID:18167512; http://dx.doi.org/ 10.1016/j.ijms.2006.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Churbanov A. Evolutionary conservation suggests a regulatory function of AUG triplets in 5'-UTRs of eukaryotic genes. Nucleic Acids Res 2005; 33:5512–20; PMID:16186132; http://dx.doi.org/ 10.1093/nar/gki847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Iacono M, Mignone F, Pesole G. uAUG and uORFs in human and rodent 5' untranslated mRNAs. Gene 2005; 349:97–105; PMID:15777708; http://dx.doi.org/ 10.1016/j.gene.2004.11.041 [DOI] [PubMed] [Google Scholar]
- 82. Crowe ML, Wang X-Q, Rothnagel JA. Evidence for conservation and selection of upstream open reading frames suggests probable encoding of bioactive peptides. BMC Genomics 2006; 7:16–10; PMID:16438715; http://dx.doi.org/ 10.1186/1471-2164-7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Calvo SE, Pagliarini DJ, Mootha VK. Upstream open reading frames cause widespread reduction of protein expression and are polymorphic among humans. Proc Natl Acad Sci USA 2009; 106:7507–12; PMID:19372376; http://dx.doi.org/ 10.1073/pnas.0810916106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ruan H, Hill JR, Fatemie-Nainie S, Morris DR. Cell-specific translational regulation of S-adenosylmethionine decarboxylase mRNA. Influence of the structure of the 5' transcript leader on regulation by the upstream open reading frame. J Biol Chem 1994; 269:17905–10; PMID:8027046 [PubMed] [Google Scholar]
- 85. Warnakulasuriyarachchi D, Ungureanu NH, Holcík M. The translation of an antiapoptotic protein HIAP2 is regulated by an upstream open reading frame. Cell Death Differ 2003; 10:899–904; PMID:12867997; http://dx.doi.org/ 10.1038/sj.cdd.4401256 [DOI] [PubMed] [Google Scholar]
- 86. Meijer HA. Ribosomes stalling on uORF1 in the Xenopus Cx41 5' UTR inhibit downstream translation initiation. Nucleic Acids Res 2003; 31:3174–84; PMID:12799445; http://dx.doi.org/ 10.1093/nar/gkg429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mehta A. Derepression of the Her-2 uORF is mediated by a novel post-transcriptional control mechanism in cancer cells. Genes & Development 2006; 20:939–53; PMID:16598037; http://dx.doi.org/ 10.1101/gad.1388706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gunišová S, Beznosková P, Mohammad MP, Vlčková V, Valášek LS. In-depth analysis of cis-determinants that either promote or inhibit reinitiation on GCN4 mRNA after translation of its four short uORFs. RNA 2016; 23(4):542-58; PMID:26822200; http://dx.doi.org/23950723 10.1261/rna.055046.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Barbosa C, Peixeiro I, Romão L. Gene Expression Regulation by Upstream Open Reading Frames and Human Disease. PLoS Genet 2013; 9:e1003529-12; PMID:23950723; http://dx.doi.org/ 10.1371/journal.pgen.1003529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol 2005; 59:407–50; PMID:16153175; http://dx.doi.org/ 10.1146/annurev.micro.59.031805.133833 [DOI] [PubMed] [Google Scholar]
- 91. Kozak M. Pushing the limits of the scanning mechanism for initiation of translation. Gene 2002; 299:1–34; PMID:12459250; http://dx.doi.org/ 10.1016/S0378-1119(02)01056-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Arribere JA, Gilbert WV. Roles for transcript leaders in translation and mRNA decay revealed by transcript leader sequencing. Genome Res 2013; 23:977–87; PMID:23580730; http://dx.doi.org/ 10.1101/gr.150342.112; PMID:24529707; http://dx.doi.org/16285926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Decourty L, Doyen A, Malabat C, Frachon E, Rispal D, Séraphin B, Feuerbach F, Jacquier A, Saveanu C. Long Open Reading Frame Transcripts Escape Nonsense-Mediated mRNA Decay in Yeast. CellReports 2014; 6:593–8; PMID:24529707; http://dx.doi.org/16285926 10.1016/j.celrep.2014.01.025 [DOI] [PubMed] [Google Scholar]
- 94. Gaba A, Jacobson A, Sachs MS. Ribosome Occupancy of the Yeast CPA1 Upstream Open Reading Frame Termination Codon Modulates Nonsense-Mediated mRNA Decay. Molecular Cell 2005; 20:449–60; PMID:16285926; http://dx.doi.org/ 10.1016/j.molcel.2005.09.019 [DOI] [PubMed] [Google Scholar]
- 95. Rebbapragada I, Lykke-Andersen J. Execution of nonsense-mediated mRNA decay: what defines a substrate? Curr Opin Cell Biol 2009; 21:394–402; PMID:19359157; http://dx.doi.org/ 10.1016/j.ceb.2009.02.007 [DOI] [PubMed] [Google Scholar]
- 96. Morris DR, Geballe AP. Upstream open reading frames as regulators of mRNA translation. Mol Cell Biol 2000; 20:8635–42; PMID:11073965; http://dx.doi.org/ 10.1128/MCB.20.23.8635-8642.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Janich P, Arpat AB, Castelo-Szekely V, Lopes M, Gatfield D. Ribosome profiling reveals the rhythmic liver translatome and circadian clock regulation by upstream open reading frames. Genome Res 2015; 25:1848–59; PMID:26486724; http://dx.doi.org/ 10.1101/gr.195404.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lee S, Liu B, Lee S, Huang S-X, Shen B, Qian S-B. Global mapping of translation initiation sites in mammalian cells at single-nucleotide resolution. Proc Natl Acad Sci USA 2012; 109:E2424–32; PMID:22927429; http://dx.doi.org/ 10.1073/pnas.1207846109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Juntawong P, Girke T, Bazin J, Bailey-Serres J. Translational dynamics revealed by genome-wide profiling of ribosome footprints in Arabidopsis. Proc Natl Acad Sci USA 2014; 111:E203–12; PMID:24367078; http://dx.doi.org/ 10.1073/pnas.1317811111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hernández-Sánchez C, Mansilla A, la Rosa EJ, Pollerberg GE, Martínez-Salas E, de Pablo F. Upstream AUGs in embryonic proinsulin mRNA control its low translation level. EMBO J 2003; 22:5582–92; http://dx.doi.org/ 10.1093/emboj/cdg515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wethmar K. The regulatory potential of upstream open reading frames in eukaryotic gene expression. WIREs RNA 2014; 5:765–8; PMID:24995549; http://dx.doi.org/ 10.1002/wrna.1245 [DOI] [PubMed] [Google Scholar]
- 102. Medenbach J, Seiler M, Hentze MW. Translational Control via Protein-Regulated Upstream Open Reading Frames. Cell 2011; 145:902–13; PMID:21663794; http://dx.doi.org/ 10.1016/j.cell.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 103. Pelechano V, Wei W, Steinmetz LM. Extensive transcriptional heterogeneity revealed by isoform profiling. Nature 2013; 497:127–31; PMID:23615609; http://dx.doi.org/ 10.1038/nature12121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. He F, Li X, Spatrick P, Casillo R, Dong S, Jacobson A. Genome-Wide Analysis of mRNAs Regulated by the Nonsense-Mediated and 5' to 3' mRNA Decay Pathways in Yeast. Mol Cell 2003; 12:1439–52; PMID:14690598; http://dx.doi.org/ 10.1016/S1097-2765(03)00446-5 [DOI] [PubMed] [Google Scholar]
- 105. Mendell JT, Sharifi NA, Meyers JL, Martinez-Murillo F, Dietz HC. Nonsense surveillance regulates expression of diverse classes of mammalian transcripts and mutes genomic noise. Nat Genet 2004; 36:1073–8; PMID:15448691; http://dx.doi.org/ 10.1038/ng1429 [DOI] [PubMed] [Google Scholar]
- 106. Hurt JA, Robertson AD, Burge CB. Global analyses of UPF1 binding and function reveal expanded scope of nonsense-mediated mRNA decay. Genome Res 2013; 23:1636–50; PMID:23766421; http://dx.doi.org/ 10.1101/gr.157354.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Malabat C, Feuerbach F, Ma L, Saveanu C, Jacquier A. Quality control of transcription start site selection by nonsense-mediated-mRNA decay. eLife 2015; 4:1–24; PMID:25905671; http://dx.doi.org/1939050 10.7554/eLife.06722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem 1991; 266:19867–70; PMID:1939050 [PubMed] [Google Scholar]
- 109. Pisarev AV. Specific functional interactions of nucleotides at key -3 and +4 positions flanking the initiation codon with components of the mammalian 48S translation initiation complex. Gen Dev 2006; 20:624–36; PMID:16510876; http://dx.doi.org/ 10.1101/gad.1397906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Andreev DE, O'Connor PBF, Fahey C, Kenny EM, Terenin IM, Dmitriev SE, Cormican P, Morris DW, Shatsky IN, Baranov PV. Translation of 5' leaders is pervasive in genes resistant to eIF2 repression. eLife 2015; 4:e03971-21; PMID:25621764; http://dx.doi.org/ 10.7554/eLife.03971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Terenin IM, Akulich KA, Andreev DE, Polyanskaya SA, Shatsky IN, Dmitriev SE. Sliding of a 43S ribosomal complex from the recognized AUG codon triggered by a delay in eIF2-bound GTP hydrolysis. Nucleic Acids Res 2015; 44(4):1882-93; PMID:26717981; http://dx.doi.org/23105001 10.1093/nar/gkv1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Floor SN, Doudna JA. Tunable protein synthesis by transcript isoforms in human cells. eLife 2016; 5:1–25; http://dx.doi.org/ 10.7554/eLife.10921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rojas-Duran MF, Gilbert WV. Alternative transcription start site selection leads to large differences in translation activity in yeast. RNA 2012; 18:2299–305; PMID:23105001; http://dx.doi.org/ 10.1261/rna.035865.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Capell A, Fellerer K, Haass C. Progranulin transcripts with short and long 5' untranslated regions (UTRs) are differentially expressed via posttranscriptional and translational repression. J Biol Chem 2014; 289:25879–89; PMID:25056957; http://dx.doi.org/ 10.1074/jbc.M114.560128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kalyna M, Simpson CG, Syed NH, Lewandowska D, Marquez Y, Kusenda B, Marshall J, Fuller J, Cardle L, McNicol J, et al. . Alternative splicing and nonsense-mediated decay modulate expression of important regulatory genes in Arabidopsis. Nucleic Acids Res 2012; 40:2454–69; PMID:22127866; http://dx.doi.org/ 10.1093/nar/gkr932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Rahim G, Araud T, Jaquier-Gubler P, Curran J. Alternative Splicing within the elk-1 5' Untranslated Region Serves To Modulate Initiation Events Downstream of the Highly Conserved Upstream Open Reading Frame 2. Mol Cell Biol 2012; 32:1745–56; PMID:22354998; http://dx.doi.org/ 10.1128/MCB.06751-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Resch AM, Ogurtsov AY, Rogozin IB, Shabalina SA, Koonin EV. Evolution of alternative and constitutive regions of mammalian 5'UTRs. BMC Genomics 2009; 10:162–14; PMID:19371439; http://dx.doi.org/ 10.1186/1471-2164-10-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Araud T, Genolet R, Jaquier-Gubler P, Curran J. Alternatively spliced isoforms of the human elk-1 mRNA within the 5' UTR: implications for ELK-1 expression. Nucleic Acids Res 2007; 35:4649–63; PMID:17591614; http://dx.doi.org/ 10.1093/nar/gkm482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wiestner A, Schlemper RJ, van der Maas AP, Skoda RC. An activating splice donor mutation in the thrombopoietin gene causes hereditary thrombocythaemia. Nat Genet 1998; 18:49–52; PMID:9425899; http://dx.doi.org/ 10.1038/ng0198-49 [DOI] [PubMed] [Google Scholar]
- 120. de Klerk E, Fokkema IFAC, Thiadens KAMH, Goeman JJ, Palmblad M, Dunnen den JT, Lindern von M, 't Hoen PAC. Assessing the translational landscape of myogenic differentiation by ribosome profiling. Nucleic Acids Res 2015; 43:4408–28; PMID:25873627; http://dx.doi.org/ 10.1093/nar/gkv281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Singh BN, Rao KS, Rao CM. Ubiquitin–proteasome-mediated degradation and synthesis of MyoD is modulated by αB-crystallin, a small heat shock protein, during muscle differentiation. BBA - Molecular Cell Research 2010; 1803:288–99; PMID:20005263; http://dx.doi.org/ 10.1016/j.bbamcr.2009.11.009 [DOI] [PubMed] [Google Scholar]
- 122. Nepal C, Hadzhiev Y, Previti C, Haberle V, Li N, Takahashi H, Suzuki AMM, Sheng Y, Abdelhamid RF, Anand S, et al. . Dynamic regulation of the transcription initiation landscape at single nucleotide resolution during vertebrate embryogenesis. Genome Res 2013; 23:1938–50; PMID:24002785; http://dx.doi.org/ 10.1101/gr.153692.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Haberle V, Li N, Hadzhiev Y, Plessy C, Previti C, Nepal C, Gehrig J, Dong X, Akalin A, Suzuki AM, et al. . Two independent transcription initiation codes overlap on vertebrate core promoters. Nature 2014; 507:381–5; PMID:24531765; http://dx.doi.org/ 10.1038/nature12974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Davuluri RV, Suzuki Y, Sugano S, Plass C, Huang THM. The functional consequences of alternative promoter use in mammalian genomes. Trends in Genetics 2008; 24:167–77; PMID:18329129; http://dx.doi.org/ 10.1016/j.tig.2008.01.008 [DOI] [PubMed] [Google Scholar]
- 125. Dieudonné F-X, O'Connor PBF, Gubler-Jaquier P, Yasrebi H, Conne B, Nikolaev S, Antonarakis S, Baranov PV, Curran J. The effect of heterogeneous Transcription Start Sites (TSS) on the translatome: implications for the mammalian cellular phenotype. BMC Genomics 2015; 1–15; PMID:26589636; http://dx.doi.org/18978772 10.1186/s12864-015-2179-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB. Alternative isoform regulation in human tissue transcriptomes. Nature 2008; 456:470–6; PMID:18978772; http://dx.doi.org/ 10.1038/nature07509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. White NL, Higgins CF, Trezise AE. Tissue-specific in vivo transcription start sites of the human and murine cystic fibrosis genes. Hum Mol Genet 1998; 7:363–9; PMID:9466991; http://dx.doi.org/ 10.1093/hmg/7.3.363 [DOI] [PubMed] [Google Scholar]
- 128. Sonenberg N, Hinnebusch AG. Regulation of Translation Initiation in Eukaryotes: Mechanisms and Biological Targets. Cell 2009; 136:731–45; PMID:19239892; http://dx.doi.org/ 10.1016/j.cell.2009.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Pisareva VP, Pisarev AV. DHX29 reduces leaky scanning through an upstream AUG codon regardless of its nucleotide context. Nucleic Acids Res 2016; 44(9):4252-65; PMID:27067542; http://dx.doi.org/23524850 10.1093/nar/gkw240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Schepetilnikov M, Dimitrova M, nez EM-MI, le Geldreich AE, Keller M, Ryabova LA. TOR and S6K1 promote translation reinitiation of uORF-containing mRNAs via phosphorylation of eIF3h. EMBO J 2013; 32:1087–102; PMID:23524850; http://dx.doi.org/ 10.1038/emboj.2013.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Schleich S, Strassburger K, Janiesch PC, Koledachkina T, Miller KK, Haneke K, Cheng Y-S, Küchler K, Stoecklin G, Duncan KE, et al. . DENR-MCT-1 promotes translation re-initiation downstream of uORFs to control tissue growth. Nature 2014; 512:208–12; PMID:25043021; http://dx.doi.org/ 10.1038/nature13401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Rahmani F, Hummel M, Schuurmans J, Wiese-Klinkenberg A, Smeekens S, Hanson J. Sucrose Control of Translation Mediated by an Upstream Open Reading Frame-Encoded Peptide. PLANT PHYSIOLOGY 2009; 150:1356–67; PMID:19403731; http://dx.doi.org/ 10.1104/pp.109.136036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ebina I, Takemoto-Tsutsumi M, Watanabe S, Koyama H, Endo Y, Kimata K, Igarashi T, Murakami K, Kudo R, Ohsumi A, et al. . Identification of novel Arabidopsis thaliana upstream open reading frames that control expression of the main coding sequences in a peptide sequence-dependent manner. Nucleic Acids Res 2015; 43:1562–76; PMID:25618853; http://dx.doi.org/ 10.1093/nar/gkv018 [DOI] [PMC free article] [PubMed] [Google Scholar]


