Abstract
Objectives
To evaluate growth patterns of ambulatory males with Duchenne muscular dystrophy (DMD) treated with corticosteroids compared with ambulatory, steroid-naïlve males with DMD and age-matched unaffected general-population males and to test associations between growth and steroid treatment patterns among treated males.
Study design
Using data from the Muscular Dystrophy Surveillance, Tracking, and Research Network, we identified a total of 1768 height, 2246 weight, and 1755 body mass index (BMI) measurements between age 2 and 12 years for 324 ambulatory males who were treated with corticosteroids for at least 6 months. Growth curve comparisons and linear mixed-effects modeling, adjusted for race/ethnicity and birth year, were used to evaluate growth and steroid treatment patterns (age at initiation, dosing interval, duration, cumulative dose).
Results
Growth curves for ambulatory males treated with corticosteroids showed significantly shorter stature, heavier weight, and greater BMI compared with ambulatory, steroid-naïlve males with DMD and general-population US males. Adjusted linear mixed-effects models for ambulatory males treated with corticosteroids showed that earlier initiation, daily dosing, longer duration, and greater dosages predicted shorter stature with prednisone. Longer duration and greater dosages predicted shorter stature for deflazacort. Daily prednisone dosing predicted lighter weight, but longer duration, and greater dosages predicted heavier weight. Early initiation, less than daily dosing, longer duration, and greater doses predicted greater BMIs. Deflazacort predicted shorter stature, but lighter weight, compared with prednisone.
Conclusion
Prolonged steroid use is significantly associated with short stature and heavier weight. Growth alterations associated with steroid treatment should be considered when making treatment decisions for males with DMD.
Corticosteroid (“steroid”) treatment in patients with Duchenne muscular dystrophy (DMD) has been shown to preserve1–4 or improve5–8 muscle strength and motor function, prolong independent ambulation,9–13 reduce or delay the onset of scoliosis,1,10,11,13–15 preserve respiratory function,1,4,16 and delay the onset of cardiomyopathy.10,16–18 The American Academy of Neurology has recommended treatment with steroids for males with DMD, and this recommendation was reiterated in the Centers for Disease Control and Prevention (CDC) in its supportive care guidelines for DMD.19
Excess weight gain is the most frequently reported side effect of steroid treatment1,7,10,20,21 and is one of the most common reasons for discontinuation.22 Long-term daily use of corticosteroids also has been shown to slow linear growth10,16,20 and may exacerbate the short stature associated with DMD.3,23–25 Although many studies have summarized their observations of these growth-related side effects, none have reported the effects of steroid treatment on growth by steroid type, dosing frequency, cumulative dose, duration of steroid treatment, and age at initiation of steroid treatment in the same cohort of individuals. In short, even though the side effects of corticosteroids on growth are well known, the effect of corticosteroids on growth has not been quantified in sufficient detail to enable males with DMD and their families to make informed treatment decisions.
The objective of this analysis was to estimate the associations between steroid use and measures of height, weight, and body mass index (BMI) by the use of 2 separate analysis strategies: (1) compare growth curves for height, weight, and BMI for ambulatory males with DMD treated with corticosteroids with previously published height, weight, and BMI curves for ambulatory, steroid-naïlve males with DMD25 identified by the Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet) cohort, as well as to those for age-matched males from the general US male population26; and (2) estimate the associations between corticosteroids and each growth measure by steroid type (prednisone vs deflazacort), age at initiation of steroid treatment (years), dosing interval (at least daily vs less than daily), duration of steroid treatment (years), and cumulative dose (mg: dose × frequency × duration) among ambulatory males with DMD treated with corticosteroids.
Methods
The MD STARnet is a population-based surveillance system that aims to identify all individuals with childhood-onset DMD or Becker muscular dystrophy born between 1982 and 2011 and who have ever resided near an MD STARnet site. Details of the surveillance methodology and case classification have been published previously.27,28 To summarize in brief, we retrospectively identified cases, starting in 2004, in Arizona, Colorado, Iowa, and Western New York. Georgia was added in 2005, and Hawaii was added in 2008. Annual medical record abstraction was completed through December 31, 2011, for those cases identified before 2011 and December 31, 2012, for those cases identified in 2011. Potential cases had to show symptoms before they turned 21 years of age. The case-finding methodology used by MD STARnet was based on active review of source records in neuromuscular clinics, hospital discharge databases, private physician practices, service sites for children with special health care needs, and birth defect surveillance programs.27 Each participating site obtained permission for case finding and medical record abstraction either through institutional review board approval or by state-mandated public health reporting.
Critical diagnostic elements of each abstracted case record were reviewed independently by clinicians from each site and assigned by consensus a case definition category of “possible,” “probable,” “definite,” “asymptomatic,” or “affected female.”28 All possible cases had recorded clinical symptoms related to a dystrophinopathy and elevated creatine kinase. Probable cases also had an X-linked pedigree consistent with a dystrophinopathy. Definite cases also had a confirmed DMD mutation, a muscle biopsy showing absent dystrophin, or an X-linked pedigree and an affected family member with a DMD mutation or diagnostic muscle biopsy. Cases who met the criteria for definite, but did not show any clinical symptoms, were defined as asymptomatic. Affected females who were diagnosed with a dystrophinopathy before age 21 years and had a DMD mutation or diagnostic muscle biopsy also were ascertained.
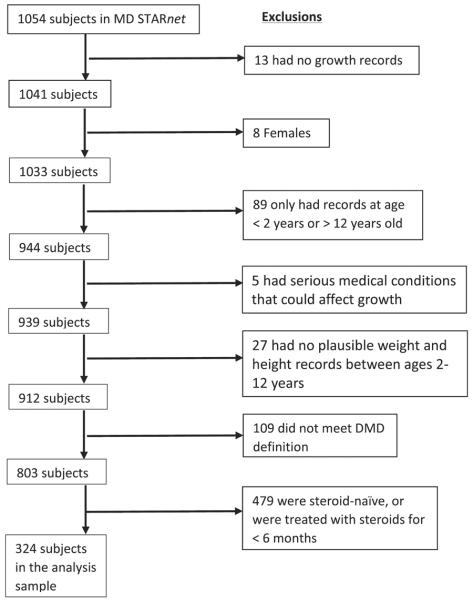
To ensure comparability, the inclusion criteria for the analysis cohort represents that used by West et al25 and were limited to males with at least 1 plausible growth record between ages 2 and 12 years, the absence of other serious medical conditions that could affect growth (eg, cancer, heart condition), clinical symptoms consistent with DMD, and treatment with corticosteroids for at least 6 months before loss of ambulation (Figure 1; available at www.jpeds.com). A total of 324 males, from whom 13 sibling pairs and 3 sibling triads were identified, comprised the analysis sample.
Figure 1.
Flowchart of exclusions to produce the analysis sample: the MD STARnet 2004-2012.
DMD
The following criteria were used to classify DMD in males treated with corticosteroids: (1) loss of ambulation before age of 16 years when treated with corticosteroids for at least 6 months before the loss of ambulation; or (2) younger than 16 years of age at their last clinic visit who were still ambulating, with onset of earliest signs or symptoms before the sixth birthday. Clinical signs or symptoms included a positive Gower sign, abnormal gait, difficulty running or jumping, frequent falling, inability to keep up with peers, and gross motor delay or muscle weakness.
Growth Data
Weight and height data were obtained retrospectively from annual medical record abstraction. We excluded growth measurements collected before 6 months of cumulative steroid treatment (daily or intermittent regimens). We also excluded growth measurements collected after initiation of growth hormone therapy in the 18 males who received growth hormone therapy. We included growth measurements collected up to 12 years of age if still ambulating, or age at loss of ambulation, whichever came first, to align this analysis with the previously published analysis of growth in steroid-naïlve males with DMD in the MD STARnet.25 A total of 1768 height, 2246 weight, and 1755 BMI measurements were available for analyses.
Steroid Treatment
Steroid treatment was categorized according to drug type (deflazacort, prednisone), age at initiation, and 3 steroid treatment variables (dose interval, cumulative duration, and cumulative dosage). Steroid dose interval was dichotomized from recorded dosing intervals to “at least daily” (1) or “less than daily” (0). Cumulative duration of steroid treatment was calculated as a count of days during which the child was determined to have been treated with corticosteroids, on the basis of recorded steroid start and stop dates. The number of days was converted into number of years for analysis.
Cumulative dosage of corticosteroids was calculated as the frequency of steroid treatment multiplied by the dose of steroid multiplied by the duration of treatment and was summed for each reported change in steroid dose (reported as mg/kg). Each steroid treatment variable was calculated for prednisone and deflazacort separately.
Statistical Analyses
Two separate analysis strategies were conducted for growth curve comparisons and linear mixed-effects modeling. We used the same analysis cohort for both strategies. SAS statistical package 9.4 (SAS Institute Inc, Cary, North Carolina) was used for all analyses.
The first analysis strategy compared the growth curves for ambulatory males with DMD treated with corticosteroids with previously published growth curves for ambulatory, steroid-naïve males with DMD identified by the MD STARnet,25 as well as with growth curves for age-matched general-population US males.26 To construct the growth curves for height, weight, and BMI for the ambulatory males with DMD treated with corticosteroids, we calculated the 10th, 25th, 50th, 75th, and 90th percentiles for height, weight, and BMI in 6-month increments between the ages of 3.5 and 12 years (the small number of records collected between 2 and 3.5 years of age made growth curve generation unstable). If more than 1 growth measurement was available in a given 6-month increment, then those measurements were averaged, and the average value was used to create the growth charts. To produce percentile curves that could be directly compared with the previously published steroid-naïve growth charts for boys with DMD,25 as well as the CDC general-population growth charts,26 linear smoothing procedures similar to the smoothing procedures used by the 2000 CDC Growth Charts for the US were applied in 2 stages to the irregular plots of the empirical percentile values.26
Graphical comparisons with the growth charts for steroid-naïve males with DMD,25 as well as for the age-matched general US population males, were made to examine differences and similarities between the curves. Further comparison between the DMD-specific growth curves of males with DMD treated with corticosteroids and both the steroid-naïve males with DMD and the general-population growth curves was accomplished by standardizing our data using the CDC age-specific means and SDs and then calculating a weighted average of the standardized height, weight, and BMI scores across the age range.26 The resulting standardized variables were compared separately using t tests.
The second analysis strategy analyzed associations between growth and measures of steroid treatment using only those ambulatory males with DMD treated with corticosteroids. We used a linear mixed-effects modeling approach, which allows for intrasubject correlation of repeated measures of growth measurements on each child and the age at which the growth measurements were collected. Separate linear mixed-effects models were run for each growth outcome z-score (dependent variables) and each steroid treatment variable of interest (independent variables). We adjusted for birth year and ethnicity, because these factors showed association with both the independent variables of interest and the outcomes, and thus may be potential confounders.
Results
Table I shows the demographic characteristics of the analysis cohort. Sixty-four percent of males with at least 6 months of documented steroid treatment used prednisone, 24% used deflazacort, and 11% had used both drugs (not concurrently) at some time between ages 2 and 12 years. Steroid dose intervals ranged from 3 times a day to once a week. This diversity in dosing schedules likely reflects the treatment experience of boys with DMD in the US. Steroid treatment was started and stopped up to 3 times among MD STARnet males in the age range of interest (2-12 years of age). Eighty-five percent of males in the analysis cohort were still being treated with corticosteroids at age at last ambulation (or at 12 years old, if still ambulating). Average age at steroid initiation was 6.6 years, and average duration of steroid treatment before loss of ambulation or age 12 years, whichever occurred first, was 3.2 years (Table I).
Table I.
Demographic and steroid treatment characteristics of ambulatory treatment using corticosteroids in males with DMD ages 2–12 years (n = 324): The MD STARnet 2004–2012
| N (%) or mean (SD) | |
|---|---|
| Race/ethnicity | |
| Non-Hispanic white | 227 (70%) |
| Hispanic | 42 (13%) |
| Black | 10 (3%) |
| Other | 9 (3%) |
| Unknown | 36 (11%) |
| Current corticosteroid treatment at final observation (age at last ambulation or age 12 y if still ambulatory) | 276 (85%) |
| Type of steroid used | |
| Prednisone | 208 (64%) |
| Treatment (N = 136*): at least daily | 102 (80%) |
| Average dosage >0.3 mg/kg/d | 28 (19%) |
| Deflazacort | 78 (24%) |
| Treatment (N = 56*): at least daily | 45 (75%) |
| Average dosage >0.5 mg/kg/d | 15 (22%) |
| Both (not concurrently) | 38 (11%) |
| Age at steroid initiation, y | 6.6 (1.8) |
| Treated with prednisone only | 6.6 (1.8) |
| Treated with deflazacort only | 6.5 (1.6) |
| Age at most recent visit, y | 10.1 (1.7) |
| Treated with prednisone only | 10.0 (1.8) |
| Treated with deflazacort only | 10.1 (1.7) |
| Duration of corticosteroid use prior to ambulation loss or at age 12 if still ambulatory, y | 3.2 (1.9) |
| Treated with prednisone only | 3.1 (1.9) |
| Treated with deflazacort only | 3.4 (1.8) |
| Weekly dose at final measurement (in those treated less than daily) | |
| Treated with prednisone only, mg/kg/wk | 3.1 (2.0) |
| Treated with deflazacort only, mg/kg/wk | 2.9 (1.7) |
Number with dosing frequency data available.
Growth Curve Analysis
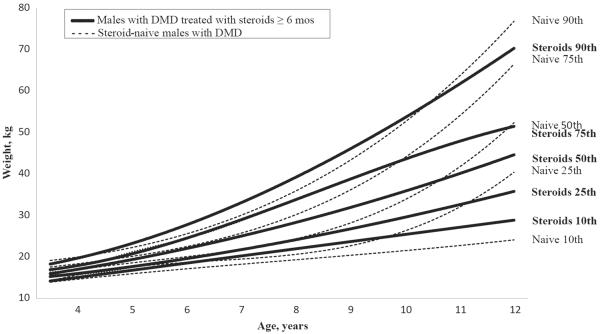
The 50th percentile of the growth curves for ambulatory males with DMD who were treated with corticosteroids showed significantly shorter stature (P < .0001), heavier weight (P < .0001), and greater BMI (P < .0001), compared with ambulatory, steroid-naïve males with DMD (Figures 2–4; Figure 4 available at www.jpeds.com). Although ambulatory males with DMD treated with corticosteroids have significantly greater weight on average, visual inspection of the growth curve (Figure 3) suggests that they also exhibited less extreme (very high or very low) weights than the ambulatory, steroid-naïve males with DMD. The 50th percentile of the growth curves for ambulatory males with DMD treated with corticosteroids also were significantly different from the CDC growth charts for general-population US males, with males with DMD treated with corticosteroids having shorter height, heavier weight, and greater BMI (all P values <.0001). The observed means, SDs, and selected percentiles for height, weight, and BMI among males with DMD treated with corticosteroids, age 3.5–12 years can be found in Figures 2–4.
Figure 2.
Height in males with DMD who were treated with corticosteroids for ≥6 months compared with height in steroid-naïve males with DMD ages 2-12 years: the MD STARnet 2004-2012.
Figure 4.
BMI in males with DMD who were treated with corticosteroids for ≥6 months compared with BMI in steroid-naïve males with DMD ages 2-12 years: the MD STARnet 2004-2012.
Figure 3.
Weight in males with DMD who were treated with corticosteroids for ≥6 months compared with weight in steroid-naïve males with DMD ages 2-12 years: the MD STARnet 2004-2012.
Linear Mixed-Effects Modeling
Drug Type
Adjusted analyses comparing prednisone and deflazacort showed that treatment with deflazacort was significantly associated with shorter stature (P < .0001) but lighter weight (P = .005) compared with prednisone treatment. As a result, BMI was equivalent among users of prednisone and deflazacort (P = .53; Table II).
Table II.
Linear mixed-effects analyses* of corticosteroid treatment and growth among ambulatory males with DMD age 2–12 years (n = 324): The MD STARnet 2004–12
| Height z score |
Weight z score |
BMI z score |
||||
|---|---|---|---|---|---|---|
| Estimate (SE) | P value | Estimate (SE) | P value | Estimate (SE) | P value | |
| Prednisone treatment | ||||||
| Age at initiation, y | 0.11 (0.02) | <.0001 | 0.02 (0.03) | .35 | −0.08 (0.03) | .005 |
| At least daily dosing (vs < daily) | −0.31 (0.09) | .0007 | −0.25 (0.08) | .003 | −0.19 (0.09) | .04 |
| Duration of use, y | −0.18 (0.02) | <.0001 | 0.06 (0.02) | .013 | 0.09 (0.03) | .0005 |
| Cumulative dose, mg | −0.00001 (3.6E-6) | .0008 | 0.00003 (3.7E-6) | <.00001 | 0.00002 (4.0E-6) | <.0001 |
| Deflazacort treatment | ||||||
| Age at initiation, y | 0.04 (0.04) | .23 | −0.04 (0.04) | .39 | −0.11 (0.03) | .002 |
| At least daily dosing (vs < daily) | −0.10 (0.13) | .47 | −0.64 (0.15) | <.0001 | −0.41 (0.13) | .003 |
| Duration of use, y | −0.23 (0.03) | <.0001 | −0.03 (0.04) | .35 | 0.09 (0.03) | .004 |
| Cumulative dose, mg | −0.00002 (3.3E-6) | <.0001 | 7.7E-7 (4.1E-6) | .85 | 0.00001 (3.3E-6) | .0008 |
| Drug comparison | ||||||
| Deflazacort vs prednisone (ref) | −0.41 (0.06) | <.0001 | −0.20 (0.07) | .005 | 0.04 (0.07) | .53 |
Adjusted for year of birth and race/ethnicity.
Prednisone
The adjusted linear mixed-effects model analyses of each treatment indicator for prednisone (age at initiation, dosing interval, duration, and cumulative dose) demonstrated that, for height, later age at steroid initiation was associated with taller height (P < .001) whereas at-least-daily dosing (P < .001), longer duration (P < .001), and greater cumulative dosage (P < .001) were associated with shorter height (Table II). For weight, results showed at-least-daily dosing interval of prednisone was associated with lower weight (P < .01) but longer treatment duration (P < .01) and greater cumulative dosage (P < .001) were associated with greater weight. The treatment model results for BMI showed lower BMI when prednisone was initiated later (P < .01) and at-least-daily dosing was prescribed (P < .05) but greater BMI with longer duration (P < .05) and greater cumulative dose (P < .001). There were no significant differences in cumulative dosage, duration of dosing, or age at last record of treatment between children that were on an “at-least-daily” vs a “less-than-daily” dosing schedule.
As an example of how to translate these results into clinically useful numbers, an estimate of 0.11 (Table II) means that for every year later a child begins prednisone treatment, the height increases by approximately 0.11 SDs per year. By referring to the CDC growth z-score charts (http://www.cdc.gov/growthcharts/zscore.htm) and using the height information for 8-year-old boys, we see that this is roughly the equivalent of gaining 0.6 cm more height per year throughout childhood if steroid initiation is delayed 1 year. Likewise, shorter stature results from being treated with prednisone at least daily (roughly 1.8 cm shorter on average for every year of daily treatment), longer duration of use (roughly 1.05 cm for every year of prednisone treatment), and greater cumulative dosage (0.06 cm per g of prednisone). We caution, however, against attempting to make such direct calculations to predict the growth of any individual child, because a boy's past and current drug, dose, frequency, and duration all affect the growth pattern simultaneously.
Deflazacort
The adjusted linear mixed-effects analyses of each deflazacort treatment variable showed longer duration (P < .001) and greater cumulative dosage (P < .001) were associated with shorter height (Table II). Approximately 80% of boys with DMD being treated with deflazacort in our analysis were on an “at-least-daily” dosing schedule. For weight, at least daily dosing (P < .001) was associated with lower weight. For BMI, later age at deflazacort initiation (P < .01) and at-least-daily dosing (P < .01) were associated with lower BMI; longer duration (P = .03) and greater cumulative dose (P < .001) were associated with greater BMI (Table II).
Discussion
Using data from the population-based MD STARnet, we showed significant associations between steroid treatment and growth among ambulatory males with DMD who were treated with corticosteroids for at least 6 months. These males were shorter than ambulatory, steroid-naïve males with DMD, who are already shorter than males without DMD.25 Furthermore, visual inspection of the growth curves for males with DMD treated with corticosteroids suggests that the average height of males with DMD becomes increasingly arrested as steroid treatment continues. Although short stature may be a socially undesirable side effect in a population that already experiences short height, it has been proposed that short stature may potentially confer a mechanical advantage by reducing muscle stress and damage29,30 and may be one of the mechanisms by which corticosteroids prolong ambulation in males with DMD.31
Our findings were consistent with previous research1,7,10,20,21 showing that treatment with steroids may exacerbate weight gain in a population already susceptible to obesity.1,7,10,20,21,25,32 Excess weight gain may contribute to a variety of undesirable health issues, such as decreased time of independent ambulation, respiratory impairment, insulin resistance, complicated surgeries, aggravated bowel problems, and risk of cardiomyopathy.33 Interestingly, males with DMD treated with corticosteroids have significantly increased weight on average but less extreme (both very high and very low) weights than the steroid-naïve males with DMD, possibly because of more intense weight management of males treated with corticosteroids. Thus, the decision to treat with corticosteroids is complex, because the long-term health effects of growth alterations caused by steroid treatment must be considered.
Much research has been conducted to determine the right balance of benefits and harms of treatment with steroids. As expected, our results suggest a dose–response relationship for both prednisone and deflazacort, in that greater cumulative dosage of corticosteroids, as well as longer duration of use, were each associated with shorter stature, greater weight (for prednisone only), and greater BMI. Although families and medical providers may be tempted to lower the dose of corticosteroids to reduce side effects, a randomized controlled trial has shown a daily dose of 0.75 mg/kg of prednisone (an equivalent deflazacort dose would be 0.9 mg/kg) to be a more effective regimen than 0.3 mg/kg per day for improving muscle strength, and weight gain was observed at both doses.34 In addition, boys with DMD who have shorter stature appear to maintain independent ambulation longer,30 and a biologic mechanism has been proposed.29 Future studies of larger cohorts are needed to describe this potential relationship, so that families and clinicians can appropriately evaluate the risks and benefits of steroid treatment.
In an effort to maximize the benefits and minimize the harms of treatment with steroids, clinicians and researchers have also tested a variety of different dosing intervals.2,35–39 In MD STARnet, dosing intervals as frequent as 3 times a day and as infrequent as once a week were reported. Although our sample size was too small to test each dosing regimen separately, our results show that a dosing interval of at-least-daily dosing (prednisone only) reduces linear growth but may minimize weight gain and excess BMI. A National Institutes of Health–sponsored clinical trial is currently underway to determine the best steroid regimen.40 Approximately 64% of males with DMD who were treated with corticosteroids used prednisone only, 24% used deflazacort only, and 11% tried both. Although the side effects of the 2 drugs are similar, we did observe that deflazacort was associated with significantly less weight gain compared with prednisone and was associated with shorter stature. Other studies have supported this finding that deflazacort is associated with less weight gain than prednisone.14,41 There is still a lack of consensus on the most effective steroid type (prednisone or deflazacort), optimal dose, and dosing regimen (eg, daily vs intermittent administration) to maintain efficacy but minimize adverse effects.42,43
Long-term treatment with steroids is effective at prolonging a variety of functions in males with DMD,44 and clinicians are initiating steroid treatment in males with DMD at younger ages in an effort to maximize the potential benefits. Our results suggest that earlier age at steroid initiation is associated with greater BMI, regardless of steroid type. Earlier age at initiation of prednisone also is associated with shorter stature, in agreement with a study on the early initiation of corticosteroids in males with DMD.45 Thus, families and clinicians considering steroid initiation at a young age should discuss the potential difficulties associated with shorter stature and greater BMI.
Strengths of this study include the classification of DMD using a standard protocol by a review committee consisting of neuromuscular clinicians from each site. These data are derived from a population-based cohort and are thus likely to be an accurate representation of growth attained at specific ages by ambulatory males with DMD. Furthermore, the MD STARnet surveillance methodology collects data longitudinally, which permits evaluation of growth throughout childhood. Adjustment for birth year enabled us to reduce cohort effects that may have been present because of the fact that clinical practice in prescribing corticosteroids to males with DMD has changed greatly over the past few decades, and children's growth in general has accelerated.46 A limitation of these data is the use of clinical measurements extracted retrospectively from medical records. We also relied on the medical record for information about steroid treatment, which may be incomplete and may have resulted in misclassification. We did not have sufficient detail on measures of motor status other than ambulation loss to be able to explore associations between steroid treatment and other motor function outcomes. Finally, the number of growth measurements was small at the younger ages because of varying age at steroid initiation, which limited the precision of some of the agespecific estimates.
In conclusion, our results suggest that prolonged steroid treatment may significantly alter the growth of males with DMD. The increased weight may cause a number of other undesirable side effects, and although steroid treatment often prolongs ambulation time, this effect is not consistent.47 The growth charts for steroid treated boys with DMD that we have provided should be very useful to both clinicians and families in ongoing monitoring of steroid treatment, as well as decisions regarding initiation of treatment. Physicians and families should consider the growth alterations associated with steroid treatment, and their potential long-term health effects, when making treatment decisions for males with DMD.
Acknowledgments
Supported by the Centers for Disease Control and Prevention (DD000187, DD000189, DD000190, and DD000191). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
Glossary
- BMI
Body mass index
- CDC
Centers for Disease Control and Prevention
- DMD
Duchenne muscular dystrophy
- MD STARnet
Muscular Dystrophy Surveillance, Tracking, and Research Network
Appendix
Additional members of MD STARnet include:
University of Arizona, Tucson, AZ: Chris Cunniff, MD (Epi PI), John Meaney, MD (Epi PI), Jennifer Andrews, MBA (Project manager), Kathleen Pettit (data manager), Sydney Pettygrove, PhD (Interview coordinator); University of Colorado, Aurora, CO: Lisa Miller, MD (PI), Dennis Matthews, MD (clinical PI), April Montgomery, MHA (Program Coordinator), Jennifer Donnelly (Data Manager); Centers for Disease Control and Prevention, Atlanta, GA: Julie Bolen, PhD (Epi PI), Natalie Street, MS (Program Coordinator), Bobby Lyles (Data manager); Hawaii Department of Health, Honolulu, HI: Sylvia Mann, MS (Epi PI); University of Iowa, Iowa City, IA: Paul Romitti, PhD (Epi PI), Katherine Mathews, MD (Clinical PI), Kristin Caspers Conway, PhD (Co-Investigator), Soman Puzhankara, MS (Data Coordinator), Florence Foo, MA (Data Manager); University of Rochester, Rochester, NY: Shree Pandya, PT, MS (Clinical PI), Christina Westfield, RN (Abstractor, Data QA/QC Coordinator); New York State Department of Health, Albany, NY: Charlotte Druschel, MD, MPH (Epi PI), Kim Campbell, PhD (Data manager), Deborah Fox, MS (Study coordinator).
Footnotes
The authors declare no conflicts of interest.
References
- 1.Balaban B, Matthews DJ, Clayton GH, Carry T. Corticosteroid treatment and functional improvement in Duchenne muscular dystrophy: long-term effect. Am J Phys Med Rehabil. 2005;84:843–50. doi: 10.1097/01.phm.0000184156.98671.d0. [DOI] [PubMed] [Google Scholar]
- 2.Beenakker EA, Fock JM, Van Tol MJ, Maurits NM, Koopman HM, Brouwer OF, et al. Intermittent prednisone therapy in Duchenne muscular dystrophy: a randomized controlled trial. Arch Neurol. 2005;62:128–32. doi: 10.1001/archneur.62.1.128. [DOI] [PubMed] [Google Scholar]
- 3.Biggar WD, Harris VA, Eliasoph L, Alman B. Long-term benefits of deflazacort treatment for boys with Duchenne muscular dystrophy in their second decade. Neuromuscul Disord. 2006;16:249–55. doi: 10.1016/j.nmd.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Henricson EK, Abresch RT, Cnaan A, Hu F, Duong T, Arrieta A, et al. The cooperative international neuromuscular research group Duchenne natural history study: glucocorticoid treatment preserves clinically meaningful functional milestones and reduces rate of disease progression as measured by manual muscle testing and other commonly used clinical trial outcome measures. Muscle Nerve. 2013;48:55–67. doi: 10.1002/mus.23808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angelini C, Pegoraro E, Turella E, Intino MT, Pini A, Costa C. Deflazacort in Duchenne dystrophy: study of long-term effect. Muscle Nerve. 1994;17:386–91. doi: 10.1002/mus.880170405. [DOI] [PubMed] [Google Scholar]
- 6.Fenichel GM, Florence JM, Pestronk A, Mendell JR, Moxley RT, III, Griggs RC, et al. Long-term benefit from prednisone therapy in Duchenne muscular dystrophy. Neurology. 1991;41:1874–7. doi: 10.1212/wnl.41.12.1874. [DOI] [PubMed] [Google Scholar]
- 7.Mendell JR, Moxley RT, Griggs RC, Brooke MH, Fenichel GM, Miller JP, et al. Randomized, double-blind six-month trial of prednisone in Duchenne's muscular dystrophy. N Engl J Med. 1989;320:1592–7. doi: 10.1056/NEJM198906153202405. [DOI] [PubMed] [Google Scholar]
- 8.Rahman MM, Hannan MA, Mondol BA, Bhoumick NB, Haque A. Prednisolone in Duchenne muscular dystrophy. Bangladesh Med Res Counc Bull. 2001;27:38–42. [PubMed] [Google Scholar]
- 9.DeSilva S, Drachman DB, Mellits D, Kuncl RW. Prednisone treatment in Duchenne muscular dystrophy. Long-term benefit. Arch Neurol. 1987;44:818–22. doi: 10.1001/archneur.1987.00520200022012. [DOI] [PubMed] [Google Scholar]
- 10.Houde S, Filiatrault M, Fournier A, Dube J, D'Arcy S, Berube D, et al. Deflazacort use in Duchenne muscular dystrophy: an 8-year follow-up. Pediatr Neurol. 2008;38:200–6. doi: 10.1016/j.pediatrneurol.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 11.King WM, Ruttencutter R, Nagaraja HN, Matkovic V, Landoll J, Hoyle C, et al. Orthopedic outcomes of long-term daily corticosteroid treatment in Duchenne muscular dystrophy. Neurology. 2007;68:1607–13. doi: 10.1212/01.wnl.0000260974.41514.83. [DOI] [PubMed] [Google Scholar]
- 12.Pradhan S, Ghosh D, Srivastava NK, Kumar A, Mittal B, Pandey CM, et al. Prednisolone in Duchenne muscular dystrophy with imminent loss of ambulation. J Neurol. 2006;253:1309–16. doi: 10.1007/s00415-006-0212-1. [DOI] [PubMed] [Google Scholar]
- 13.Yilmaz O, Karaduman A, Topaloglu H. Prednisolone therapy in Duchenne muscular dystrophy prolongs ambulation and prevents scoliosis. Eur J Neurol. 2004;11:541–4. doi: 10.1111/j.1468-1331.2004.00866.x. [DOI] [PubMed] [Google Scholar]
- 14.Alman BA, Raza SN, Biggar WD. Steroid treatment and the development of scoliosis in males with Duchenne muscular dystrophy. J Bone Joint Surg Am. 2004;86-A:519–24. doi: 10.2106/00004623-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Lebel DE, Corston JA, McAdam LC, Biggar WD, Alman BA. Glucocorticoid treatment for the prevention of scoliosis in children with Duchenne muscular dystrophy: long-term follow-up. J Bone Joint Surg Am. 2013;95:1057–61. doi: 10.2106/JBJS.L.01577. [DOI] [PubMed] [Google Scholar]
- 16.Silversides CK, Webb GD, Harris VA, Biggar DW. Effects of deflazacort on left ventricular function in patients with Duchenne muscular dystrophy. Am J Cardiol. 2003;91:769–72. doi: 10.1016/s0002-9149(02)03429-x. [DOI] [PubMed] [Google Scholar]
- 17.Schram G, Fournier A, Leduc H, Dahdah N, Therien J, Vanasse M, et al. All-cause mortality and cardiovascular outcomes with prophylactic steroid therapy in Duchenne muscular dystrophy. J Am Coll Cardiol. 2013;61:948–54. doi: 10.1016/j.jacc.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Markham LW, Kinnett K, Wong BL, Woodrow Benson D, Cripe LH. Corticosteroid treatment retards development of ventricular dysfunction in Duchenne muscular dystrophy. Neuromuscul Disord. 2008;18:365–70. doi: 10.1016/j.nmd.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9:77–93. doi: 10.1016/S1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- 20.Griggs RC, Moxley RT, III, Mendell JR, Fenichel GM, Brooke MH, Pestronk A, et al. Duchenne dystrophy: randomized, controlled trial of prednisone (18 months) and azathioprine (12 months) Neurology. 1993;43:520–7. doi: 10.1212/wnl.43.3_part_1.520. [DOI] [PubMed] [Google Scholar]
- 21.Moxley RT, III, Ashwal S, Pandya S, Connolly A, Florence J, Mathews K, et al. Practice parameter: corticosteroid treatment of Duchenne dystrophy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2005;64:13–20. doi: 10.1212/01.WNL.0000148485.00049.B7. [DOI] [PubMed] [Google Scholar]
- 22.Matthews DJ, James KA, Miller LA, Pandya S, Campbell KA, Ciafaloni E, et al. Use of corticocorticosteroids in a population-based cohort of boys with Duchenne and Becker muscular dystrophy. J Child Neurol. 2010;25:1319–24. doi: 10.1177/0883073810362762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAdam LC, Mayo AL, Alman BA, Biggar WD. The Canadian experience with long-term deflazacort treatment in Duchenne muscular dystrophy. Acta Myol. 2012;31:16–20. [PMC free article] [PubMed] [Google Scholar]
- 24.Nagel BH, Mortier W, Elmlinger M, Wollmann HA, Schmitt K, Ranke MB. Short stature in Duchenne muscular dystrophy: a study of 34 patients. Acta Paediatr. 1999;88:62–5. [PubMed] [Google Scholar]
- 25.West NA, Yang ML, Weitzenkamp DA, Andrews J, Meaney FJ, Oleszek J, et al. Patterns of growth in ambulatory males with Duchenne muscular dystrophy. J Pediatr. 2013;163:1759–63.e1. doi: 10.1016/j.jpeds.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002;246:1–190. [PubMed] [Google Scholar]
- 27.Miller LA, Romitti PA, Cunniff C, Druschel C, Mathews KD, Meaney FJ, et al. The muscular Dystrophy Surveillance Tracking and Research Network (MD STARnet): surveillance methodology. Birth Defects Res Part A Clin Mol Teratol. 2006;76:793–7. doi: 10.1002/bdra.20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathews KD, Cunniff C, Kantamneni JR, Ciafaloni E, Miller T, Matthews D, et al. Muscular Dystrophy Surveillance Tracking and Research Network (MD STARnet): case definition in surveillance for childhood-onset Duchenne/Becker muscular dystrophy. J Child Neurol. 2010;25:1098–102. doi: 10.1177/0883073810371001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bodor M, McDonald CM. Why short stature is beneficial in Duchenne muscular dystrophy. Muscle Nerve. 2013;48:336–42. doi: 10.1002/mus.23793. [DOI] [PubMed] [Google Scholar]
- 30.Zatz M, Rapaport D, Vainzof M, Rocha JM, Pavanello Rde C, Colletto GM, et al. Relation between height and clinical course in Duchenne muscular dystrophy. Am J Med Genet. 1988;29:405–10. doi: 10.1002/ajmg.1320290223. [DOI] [PubMed] [Google Scholar]
- 31.Biggar WD, Gingras M, Fehlings DL, Harris VA, Steele CA. Deflazacort treatment of Duchenne muscular dystrophy. J Pediatr. 2001;138:45–50. doi: 10.1067/mpd.2001.109601. [DOI] [PubMed] [Google Scholar]
- 32.Davidson ZE, Ryan MM, Kornberg AJ, Sinclair K, Cairns A, Walker KZ, et al. Observations of body mass index in Duchenne muscular dystrophy: a longitudinal study. Eur J Clin Nutr. 2014;68:892–7. doi: 10.1038/ejcn.2014.93. [DOI] [PubMed] [Google Scholar]
- 33.Goemans N, Buyse G. Current treatment and management of dystrophinopathies. Curr Treat Options Neurol. 2014;16:287. doi: 10.1007/s11940-014-0287-4. [DOI] [PubMed] [Google Scholar]
- 34.Griggs RC, Moxley RT, III, Mendell JR, Fenichel GM, Brooke MH, Pestronk A, et al. Prednisone in Duchenne dystrophy. A randomized, controlled trial defining the time course and dose response. Clinical Investigation of Duchenne Dystrophy Group. Arch Neurol. 1991;48:383–8. doi: 10.1001/archneur.1991.00530160047012. [DOI] [PubMed] [Google Scholar]
- 35.Escolar DM, Hache LP, Clemens PR, Cnaan A, McDonald CM, Viswanathan V, et al. Randomized, blinded trial of weekend vs daily prednisone in Duchenne muscular dystrophy. Neurology. 2011;77:444–52. doi: 10.1212/WNL.0b013e318227b164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fenichel GM, Mendell JR, Moxley RT, III, Griggs RC, Brooke MH, Miller JP, et al. A comparison of daily and alternate-day prednisone therapy in the treatment of Duchenne muscular dystrophy. Arch Neurol. 1991;48:575–9. doi: 10.1001/archneur.1991.00530180027012. [DOI] [PubMed] [Google Scholar]
- 37.Griggs RC, Herr BE, Reha A, Elfring G, Atkinson L, Cwik V, et al. Corticocorticosteroids in Duchenne muscular dystrophy: major variations in practice. Muscle Nerve. 2013;48:27–31. doi: 10.1002/mus.23831. [DOI] [PubMed] [Google Scholar]
- 38.Ricotti V, Ridout DA, Scott E, Quinlivan R, Robb SA, Manzur AY, et al. Long-term benefits and adverse effects of intermittent versus daily glucocorticoids in boys with Duchenne muscular dystrophy. J Neurol Neurosurg Psychiatry. 2013;84:698–705. doi: 10.1136/jnnp-2012-303902. [DOI] [PubMed] [Google Scholar]
- 39.Straathof CS, Overweg-Plandsoen WC, van den Burg GJ, van der Kooi AJ, Verschuuren JJ, de Groot IJ. Prednisone 10 days on/10 days off in patients with Duchenne muscular dystrophy. J Neurol. 2009;256:768–73. doi: 10.1007/s00415-009-5012-y. [DOI] [PubMed] [Google Scholar]
- 40. [Accessed July 23, 2015];Finding the Optimum Regimen for Duchenne Muscular Dystrophy (FOR-DMD) http://www.ninds.nih.gov/disorders/clinical_trials/NCT01603407.htm.
- 41.Bonifati MD, Ruzza G, Bonometto P, Berardinelli A, Gorni K, Orcesi S, et al. A multicenter, double-blind, randomized trial of deflazacort versus prednisone in Duchenne muscular dystrophy. Muscle Nerve. 2000;23:1344–7. doi: 10.1002/1097-4598(200009)23:9<1344::aid-mus4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 42.Connolly AM, Schierbecker J, Renna R, Florence J. High dose weekly oral prednisone improves strength in boys with Duchenne muscular dystrophy. Neuromuscul Disord. 2002;12:917–25. doi: 10.1016/s0960-8966(02)00180-3. [DOI] [PubMed] [Google Scholar]
- 43.Dubowitz V, Kinali M, Main M, Mercuri E, Muntoni F. Remission of clinical signs in early Duchenne muscular dystrophy on intermittent low-dosage prednisolone therapy. Eur J Paediatr Neurol. 2002;6:153–9. doi: 10.1053/ejpn.2002.0583. [DOI] [PubMed] [Google Scholar]
- 44.Moxley RT, III, Pandya S, Ciafaloni E, Fox DJ, Campbell K. Change in natural history of Duchenne muscular dystrophy with long-term corticosteroid treatment: implications for management. J Child Neurol. 2010;25:1116–29. doi: 10.1177/0883073810371004. [DOI] [PubMed] [Google Scholar]
- 45.Merlini L, Cicognani A, Malaspina E, Gennari M, Gnudi S, Talim B, et al. Early prednisone treatment in Duchenne muscular dystrophy. Muscle Nerve. 2003;27:222–7. doi: 10.1002/mus.10319. [DOI] [PubMed] [Google Scholar]
- 46.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. J Am Med Assoc. 2008;299:2401–5. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 47.Kim S, Campbell KA, Fox DJ, Matthews DJ, Valdez R. Corticosteroid treatments in males with Duchenne muscular dystrophy: treatment duration and time to loss of ambulation. J Child Neurol. 2015;30:1275–80. doi: 10.1177/0883073814558120. [DOI] [PMC free article] [PubMed] [Google Scholar]