Abstract
Background: In vitro and observational epidemiological studies suggest that vitamin D may play a role in cancer prevention. However, the relationship between vitamin D and ovarian cancer is uncertain, with observational studies generating conflicting findings. A potential limitation of observational studies is inadequate control of confounding. To overcome this problem, we used Mendelian randomization (MR) to evaluate the association between single nucleotide polymorphisms (SNPs) associated with circulating 25-hydroxyvitamin D [25(OH)D] concentration and risk of ovarian cancer.
Methods: We employed SNPs with well-established associations with 25(OH)D concentration as instrumental variables for MR: rs7944926 (DHCR7), rs12794714 (CYP2R1) and rs2282679 (GC). We included 31 719 women of European ancestry (10 065 cases, 21 654 controls) from the Ovarian Cancer Association Consortium, who were genotyped using customized Illumina Infinium iSelect (iCOGS) arrays. A two-sample (summary data) MR approach was used and analyses were performed separately for all ovarian cancer (10 065 cases) and for high-grade serous ovarian cancer (4121 cases).
Results: The odds ratio for epithelial ovarian cancer risk (10 065 cases) estimated by combining the individual SNP associations using inverse variance weighting was 1.27 (95% confidence interval: 1.06 to 1.51) per 20 nmol/L decrease in 25(OH)D concentration. The estimated odds ratio for high-grade serous epithelial ovarian cancer (4121 cases) was 1.54 (1.19, 2.01).
Conclusions: Genetically lowered 25-hydroxyvitamin D concentrations were associated with higher ovarian cancer susceptibility in Europeans. These findings suggest that increasing plasma vitamin D levels may reduce risk of ovarian cancer.
Key Messages
Previous observational studies have reported conflicting findings on the association between serum 25(OH)D concentration and ovarian cancer.
Results from this study suggest that lower 25(OH)D concentration associates with higher susceptibility to ovarian cancer.
Among different ovarian cancer subtypes, the magnitude of association was the highest for high-grade serous ovarian cancer.
Introduction
Ovarian cancer is one of the most fatal cancers among women.1 Survival following diagnosis is poor (less than 50% at 5 years post-diagnosis) with a mortality rate of 152 000 per year worldwide.2,3 The most common histological subtype is serous carcinoma (further classified into high-grade serous and low-grade serous); other subtypes include mucinous, clear cell and endometrioid carcinomas.4 Higher parity and oral contraceptive use reduce risk whereas established risk factors include a history of endometriosis, obesity and family history of ovarian or breast cancer.5 Several recent studies have examined whether or not serum 25-hydroxyvitamin D [25(OH)D] concentrations are associated with ovarian cancer risk or mortality.6–12
Vitamin D is produced in the skin when 7-dehydrocholesterol is exposed to Ultraviolet B. It is transported to the liver, where it is hydroxylated to become 25(OH)D. It then undergoes a second hydroxylation step, primarily in the liver, to become the active form, 1,25-dihydroxyvitamin D (calcitriol). Whereas 25(OH)D is relatively inactive, it has a long half-life and its production is loosely regulated, making it a useful indicator of vitamin D status. In vitro and animal studies suggest that calcitriol has a variety of anti-cancer effects, including the prevention of cell disjunction,13–16 preventing overgrowth and exerting multiple anti-proliferative and anti-inflammatory effects.17
The association between vitamin D and ovarian cancer is controversial. Most recent observational studies found no strong evidence for an association between circulating 25(OH)D and risk for this cancer.7,8,10,18–20 One limitation of these studies is that their findings may only be generalized for specific populations because of the latitudes in which they were conducted. Furthermore, the variety of different 25(OH)D measurement techniques as well as the different subtype distribution of ovarian cancers used in the various studies might have also affected the results.8 More fundamentally, a limitation of observational studies is that confounding and reverse causation can make it difficult to interpret the results. For example, affected individuals may have altered vitamin D levels due to their disease status. Randomized clinical trials (RCT) are an attractive alternative to observational studies, as these remove biases from confounding and reverse causation. However, RCTs are costly and logistically cumbersome, and there are no published RCTs assessing the relationship between 25(OH)D levels and risk of epithelial ovarian cancer.
Mendelian randomization (MR) is an approach for evaluating associations of an exposure with a disease.21,22 This technique utilizes the fact that allelic variants are assigned at random during meiosis, making them potentially robust and unbiased (free from confounding effects) instruments to gauge the effect of an exposure (e.g. low vitamin D) on a trait (e.g. cancer).22 An instrumental variable (SNP) used in a MR study also has to satisfy the following assumptions21,22: (i) the instrumental variable is associated with the exposure of interest, (ii) the instrumental variable is independent of confounding factors that might confound the association of the exposure with the outcome and (iii) the instrumental variable is only associated with the outcome through the exposure (Figure 1). Two key determinants of the power of an MR study are the variance in the modifiable exposure explained by the genetic variants (SNPs) and the sample size of the study associating the relevant SNPs with the trait of interest. To date, SNPs associated with vitamin D level explain only a very small proportion (approximately 1–4%) of the trait variance. Therefore, for MR to be informative for vitamin D concentrations, large sample sizes are needed. Here we use large-scale data from the Ovarian Cancer Association Consortium (OCAC) in an MR framework to assess whether or not SNPs associated with 25(OH)D concentration are related to risk of ovarian cancer.
Figure 1.
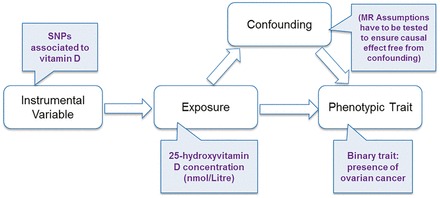
Schematic of the Mendelian randomization framework in our study using vitamin D SNPs as instrumental variables.
Methods
Data sources
Individual-level genetic data from the OCAC were used in this study. Participants from 43 studies from around the world were genotyped using the Illumina Infinium iSelect (iCOGS) array.23 Quality control was as per previous work, with related individuals and ancestry outliers removed.4 We excluded 13 studies of individuals of non-European ancestry4; the remaining studies that contributed to our analysis are listed in Supplementary Table 4 (available as Supplementary data at IJE online). For examination of all histotypes of ovarian cancer combined, we had 10 065 cases and 21 654 controls for analysis. The distribution of histological subtypes is shown in Table 1. For high-grade serous ovarian cancer, 4121 cases were available. We also performed MR analysis on the other subtypes individually, although sample sizes were much smaller than for high-grade serous cancer.
Table 1.
Distribution of cases based on epithelial ovarian carcinoma subtypes
| EOC subtypes | Number of cases |
|---|---|
| High-grade serous | 4121 |
| Low-grade serous | 363 |
| Serous of unknown grade | 1344 |
| Mucinous | 662 |
| Clear cell | 621 |
| Endometroid | 1350 |
| Others | 1604 |
SNP selection criteria
Several SNPs have been observed in association with 25(OH)D concentrations: rs6013897 in the Cytochrome P450, family 24, subfamily A, polypeptide 1 (CYP24A1) gene; rs2282679 and rs7041 in the Group-Specific Component (GC) gene; rs12800438 and rs7944926 near the 7-Dehydrocholesterol Reductase (DHCR7) gene; and rs10741657 and rs12794714 in the Cytochrome P450, family 2, subfamily R, polypeptide 1 (CYP2R1) gene.24–30 The iCOGs array directly genotyped rs12794714 and rs2282679; rs7944926 was the best imputed DHCR7 SNPs (imputation quality score of 0.92) described by previous study.31 We were unable to include rs6013897 in CYP24A1, as there were no SNPs in adequate linkage disequilibrium (>0.3) genotyped on our arrays. These SNPs are potential instrumental variables with respect to 25(OH)D concentrations. To ensure that these SNPs instruments can be applied to the MR via summary statistics approach, we first required accurate 25(OH)D association estimates for each of the SNP—the most accurate estimates available were those from Afzal et al.31 for the SNPs within/near DHCR7 and CYP2R1, whereas the estimates for the GC SNP is only available in Mokry et al.26 [the effect of the GC SNP on 25(OH)D was only estimated based on 2347 individuals26 whereas the estimates for DHCR7 and CYP2R1 were derived based on 30 792 individuals31]. We then examined their associations with various potential confounders using publicly available GWAS datasets (the complete list of potential confounders that were investigated is available in Supplementary Table 1, available as Supplementary data at IJE online).
Statistical analyses
MR operates by comparing the estimated magnitude of the association of the SNPs on the modifiable risk factor [25(OH)D concentration] with the magnitude of the association of the SNP on the outcome of interest (ovarian cancer). Estimates of the association of the relevant SNPs with ovarian cancer status were derived using logistic regressions using SNPTEST.32 We adjusted for intra-ethnic (i.e. within Europeans) population differences by incorporating the first six principal components and indicators for study number as covariates in the SNP-outcome regressions. To check for evidence of residual population stratification, we computed the genomic control lambda value from 195 183 directly genotyped autosomal SNPs genome-wide. Additional confounding variables such as time spent outdoors, socio-economic status and body mass index (BMI) were not adjusted in our model, as this information was not available on all individuals in our dataset. Instead, samples with available confounder data (n < 26 000) were retained for subsequent sensitivity analysis (see the ‘Discussion’ section).
In the absence of information on 25(OH)D concentration levels in the OCAC dataset, we applied a two-sample approach that uses only summary data to assess indirect associations33 where estimates for the SNP-outcome associations are from a different sample than the SNP-exposure associations. Here, we obtain 25(OH)D association estimates from GWAS summary statistics for SNP instruments that passed the selection criteria mentioned above. Combining these magnitudes of association, the association of 25(OH)D concentration levels on ovarian cancer, the weighted estimate can be computed using the Wald-type ratio estimator.21 The weighted model that was used to obtain the instrumental variable estimates are shown in the Supplementary section (available as Supplementary data at IJE online). Analyses were performed for all epithelial ovarian cancers irrespective of histological subtype and separately for high-grade serous epithelial ovarian cancer. To be compatible with previous studies,31,34 estimates were scaled to a 20-nmol/L change in 25(OH)D level; 20 nmol/L is approximately the inter-tertile range (66th percentile to 33rd percentile) observed in a large European study.31
Results
Validation of instrument strength
We examined each of the MR assumptions in turn. To satisfy the first MR assumption, our SNPs must be clearly associated with 25(OH)D concentrations; typically, an F-statistic > 10 is a commonly used threshold for a strong instrument. We specifically chose SNPs from DHCR7, CYP2R1 and GC, which have been clearly shown to be associated with 25(OH)D concentrations. In Afzal et al.,31 the SNPs we use are very strongly associated where the F-statistics for each SNP is >90. For the GC SNP, the association of this variant with log-transformed 25(OH)D were adequate with an F-statistic of 13.38. The SNPs combined explain about 1.3% of the variance in 25(OH)D concentration. It is important to note that these studies were among few of the many studies linking these SNPs to 25(OH)D concentrations.24,26,28,29,34 This evidence combined suggests that the SNPs we used are valid instruments (i.e. weak instrument bias is not a problem in our study).
Assessment for pleiotropy
Next we assessed possible pleiotropy. Of the known ovarian cancer risk factors, some have an established genetic component, with large GWASs conducted. Examining these GWAS findings, we found no evidence for association between the SNPs in DHCR7 and CYP2R1 and potential confounders such as smoking behaviour (Supplementary Table 1), hence satisfying the second MR assumption. We found that neither the lead SNPs nor any SNPs correlated with them were associated with the possible confounders after Bonferroni corrections. For the other ovarian cancer risk factors (OC use, parity), large-scale GWASs have not been conducted because inherited genetic factors are unlikely to play a major role. The third MR assumption can be difficult to test directly although the vitamin D metabolism pathway is well understood and there is substantial evidence that DHCR7 and CYP2R1 play roles in determining or modulating 25(OH)D concentration.24,25,34
Population stratification
MR analyses are unbiased when they reflect the true relationship between genotype and phenotype (rather than e.g. artefactual associations from unmodelled population structure). Our estimated genomic control lambda value (rescaled to 1000 cases and controls) was λ1000 = 1.005, implying no major effects of population structure. Principal component analysis showed that the OCAC cases and controls were well matched for ancestry (Supplementary Figures 2 and 3, available as Supplementary data at IJE online).
Association of SNPs to 25(OH)D concentration
To estimate the association of the chosen SNPs on 25(OH)D concentrations, we used SNP-25(OH)D association estimates from both published studies26,31 that were corrected for seasonal variation. It was shown that the variant rs7944926 near DHCR7 reduced 25(OH)D concentration levels by 2.0 nmol/L per risk allele (A) and the variant rs12794714 in CYP2R1 reduced 25(OH)D concentration levels by 3.0 nmol/L per risk allele (A). Upon performing conversion of the 25(OH)D estimates from the natural logarithm scale,26 the variant rs2282679 near GC was shown to reduce 25(OH)D levels by approximately 2.5 nmol/L per 25(OH)D decreasing allele (C).
MR analysis for all ovarian cancer subtypes
We determined the associations between the 25(OH)D associated SNPs (rs7944926 and rs12794714) and risk of ovarian cancer in Table 2. rs12794714 and rs2282679 was directly genotyped in our dataset, whereas rs7944926 was well imputed (imputation quality score 0.92). For all epithelial ovarian cancer subtypes combined, the estimated magnitude of association for a 1.0-nmol/L change in 25(OH)D level was −0.0076 [standard error (SE) = 0.0109] for the MR analysis performed via rs7944926 in DHCR7. This translates into an odds ratio (OR) of 1.17 (0.76–1.78) per 20-nmol/L decrease in 25(OH)D levels. Similarly, the magnitude of association was −0.0137, SE = 0.0063 for rs12794714 in CYP2R1, with corresponding OR of 1.31 (1.03–1.69) per 20-nmol/L decrease in 25(OH)D and the magnitude of association is –0.0110, SE = 0.0082 with OR of 1.25(0.90–1.71) for rs2282679 in GC. Since all these SNPs are independent, a more accurate estimate will be obtained from the combined associations of the three SNPs. The combined weighted magnitude of association is −0.0118, with a SE of 0.0045. The resultant OR per 20-nmol/L change in 25(OH)D on all epithelial ovarian cancer subtypes combined is 1.27 (1.06–1.51).
Table 2.
Mendelian randomization results: 25(OH)D concentration and ovarian cancer
| SNPs | EA/NEA | 25(OH)D per 25(OH)D decreasing allele (nmol/L) |
All epithelial ovarian subtype (n = 10 065 cases) |
Only high-grade serous epithelial ovarian subtype (n = 4 121 cases) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| βzx | σzx | R2 | βzy | σzy | βIVW | σIVW | βzy | σzy | βIVW | σIVW | ||
| rs7944926 | A/G | –2 | 0.19 | 0.40% | 0.0153 | 0.0217 | –0.0076 | 0.0109 | 0.0418 | 0.0309 | –0.0209 | 0.0154 |
| rs12794714 | A/G | –3 | 0.22 | 0.60% | 0.0412 | 0.0189 | –0.0137 | 0.0063 | 0.0772 | 0.0270 | –0.0257 | 0.0091 |
| rs2282679 | C/A | –2.5 | 0.70 | 0.30% | 0.0276 | 0.0205 | –0.0110 | 0.0082 | 0.0432 | 0.0292 | –0.0173 | 0.0117 |
| Combined | – | – | – | 1.30% | – | – | –0.0118 | 0.0045 | – | – | –0.0218 | 0.0067 |
EA/NEA refers to the Effect Allele and Non-Effect Allele. βzy denotes the magnitude of association of the SNP-outcome estimate. σzx is the standard error of the SNP-exposure estimate. βzx denotes the magnitude of association of Z, the SNP instrument on X, the modifiable exposure level (25(OH)D). σzy is the standard error of βzy.R2 is the proportion of variance in 25(OH)D explained by the SNP(s). βIVW is the estimate and σIVW its standard deviation. βzy is presented on the log(OR) scale. βIVW is presented on the log(OR) scale for a single unit (1-nmol/L) change in 25(OH)D—see text for OR scale changes for a 20-unit (nmol/L) change in 25(OH)D. Note: the βzx estimate for rs2282679 is obtained from Mokry et al. and transformed to natural scale (from natural logarithm) using an intercept at e4 (∼54.59) nmol/L of 25(OH)D. Standard errors for these estimates were calculated from F-statistics. The variance explained (R2) for rs12794714 and rs7944926 were obtained directly from Afzal et al., whereas the R2 for rs2822679 was computed from Mokry et al.
MR analysis for high-grade serous ovarian cancer
Similar associations were observed between SNPs for 25(OH)D concentration and high-grade serous epithelial ovarian cancer. We obtained a magnitude of association estimate of −0.0209 (SE = 0.0154) and −0.0257 (SE = 0.0091) and −0.0173 (SE = 0.0117) for rs7944926, rs12794714 and rs2282679, respectively. This resulted in an OR of 1.51 (0.83–2.78) using rs7944926, 1.67 (1.18–2.38) using rs12794714 and 1.41 (0.89–2.23) per 20-nmol/L decrease in 25(OH)D. Weighting across all SNP instruments yielded an estimated magnitude of −0.0218 (SE = 0.0067). Hence, a 20-nmol/L decrease in 25(OH)D corresponds to an OR of 1.54 (1.19–2.01) for high-grade serous ovarian cancer (see Figures 2 and 3).
Figure 2.
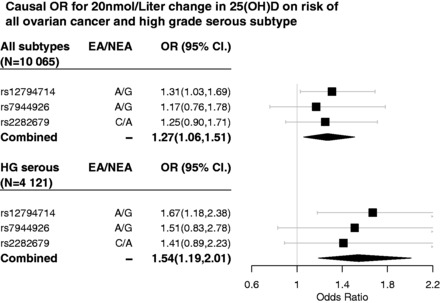
Causal OR of 25(OH)D on all ovarian cancer and high grade serous ovarian cancer.
Figure 3.
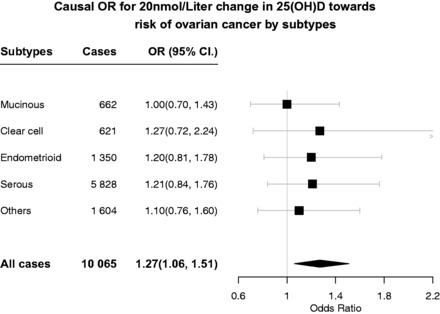
Causal OR of 25(OH)D on individual ovarian cancer subtypes.
Discussion
Even though the SNPs chosen in our study only explain a small fraction (∼1.3%) of the variance of 25(OH)D concentration, because our case–control sample was so large, we were able to demonstrate associations with ovarian cancer risk. A genetically scored decrease of 20 nmol/L of serum 25(OH)D concentration levels increased the risk of epithelial ovarian cancer by about 30% in European-ancestry women, with a larger association seen in high-grade serous disease.
Comparison with previous findings
A recent Danish study31 used MR to show that low circulating 25(OH)D concentrations were associated with cancer mortality among Europeans. That study did not separate the associations of risk and mortality and was underpowered to draw conclusions on any specific cancer type. Here, for the first time, we demonstrate that, for epithelial ovarian cancer, there is a causal effect of low 25(OH)D concentrations on risk.
Our results are inconsistent with some previous studies that have reported no associations between 25(OH)D and ovarian cancer status. The recent meta-analysis8 of 10 individual cohort studies (884 cases and 1605 controls) found no association between 25(OH)D concentration and development of ovarian cancer. Findings from epidemiologic studies may differ from our MR-based results because observational studies can be affected by confounding and reverse causation, though cohort studies such as Yin et al.8 would be expected to be less affected.
Strength and limitations
A strength of our study is that the mechanism through which our chosen SNPs influence 25(OH)D levels is well understood. DHCR7 encodes the enzyme 7-dehydrocholestrol reductase, which is responsible for the conversion of 7-dehydrocholestrol to cholesterol. Reduced activities of 7-dehydrocholestrol reductase, leading to low cholesterol and accumulation of 7-dehydrocholestrol, are partially attributable to DHCR7 variants.24,25,29 Although rs7944926 lies outside DHCR7, this variant modulates expression of DHCR7.35CYP2R1 is an enzyme which converts vitamin D3 to 25(OH)D in the liver,36 with rs12794714 unambiguously associated with 25(OH)D concentrations via GWAS.29 The GC gene has a primary role in vitamin D transport. Previous studies shown that the rs2282679 variant in particular were also strongly associated (P = 4.0 × 1042) with serum vitamin D binding protein (DBP) based on the study performed on 1674 individuals in the Twins UK cohort.29 The GC variants were also hypothesized to affect bioavailability of vitamin D through variation in circulating DBP. In view of evidence for its association towards vitamin D, the rs2282679 SNP is among one of the most associated variants with 25(OH)D (P = 1.9 × 10–109) in the SUNLIGHT GWAS.29 These variants (rs7944926, rs12794714 and rs2282679) thus affect 25(OH)D levels through varying vitamin D metabolism, bioavailability or transport, rendering them appropriate instrumental variables for use in MR.26,27,31,34
One limitation is that our two-sample MR analysis assumes that the standard error of the exposure [SNP to 25(OH)D] estimates is negligibly small.33,37—given the large sample size in the Danish study,31 this is a reasonable assumption. In addition, the MR framework assumes a linear relationship in the association of the SNP instruments on the underlying exposure. Although our MR estimates indicate that a decrease of 20 nmol/L in 25(OH)D concentration is associated with a 30% increased risk of epithelial ovarian cancer, this estimated effect size is derived from a larger sample size of women with a range of 25(OH)D concentrations. Previous studies using MR to examine 25(OH)D concentrations with different outcomes have dealt with this in various ways. For example, the published study that we used31 assumed linearity of change across raw 25(OH)D values. In contrast, the study by Mokry et al.26 on vitamin D and multiple sclerosis (MS) considered the association to be linear on log-transformed 25(OH)D.
We examined the implications of these approaches by re-computing our findings based on exposure estimates on the original scale (from the Danish study31) and on the log scale (from MR study on MS26) (see Supplementary Table 2, available as Supplementary data at IJE online). We note that, in addition to the scale differences, the estimates of the magnitude of association of each SNP on 25(OH)D differed due to random sampling error (with estimates from the Danish study31 derived from a much larger sample size than those in the MS study26). We hence repeated our analysis by adopting SNP-exposure estimates used by the MS study26 for the SNP rs12785878 (LD to rs7944926 with r2 = 1.0) in the DHCR7 gene. Although our result was robust to differences in scaling [log-transformed or non-transformed 25(OH)D concentrations, see Supplementary Table 2, available as Supplementary data at IJE online], in practice, a 20-nmol/L increase is more likely to make an impact on women with low 25(OH)D concentrations than those whose concentration is already high.
In our main analysis, there were concerns that the effect of the GC SNP on 25(OH)D was not estimated with high accuracy (GC SNP estimates were based on 2347 individuals26 whereas the estimates for DHCR7 and CYP2R1 were derived based on 30 792 individuals31), as well as concerns that the GC SNP may not influence 25-hydroxyvitamin D’s biological activity in a predictable way.31,38,39 Nonetheless, we conducted a sensitivity analyses to examine the effect of excluding this SNP. When the GC SNP was excluded, our results were unchanged (the association with ovarian cancer of the combined effect of the three SNPs was very similar to that obtained using just two SNPs; see Supplementary Table 5, available as Supplementary data at IJE online).
Another potential limitation of our analysis is residual pleiotropy. We found no evidence for SNP–confounder association based on the subset of participants with available confounder information (Supplementary Table 6, available as Supplementary data at IJE online), although we cannot rule out associations with unmeasured confounders. An approach such as Egger regression40 can potentially be applied to further test the MR assumptions but these require more SNPs than the three employed here.
Interpretation of findings
Observation of a larger magnitude of association (OR = 1.54) with high-grade serous cancer for lower 25(OH)D concentration suggests that the association of circulating 25(OH)D with risk of ovarian cancer may be confined to the high-grade serous type, although the confidence limits of the two ORs are overlapping and high-grade serous cancer is contained within all ovarian cancer. The results for histological subtypes other than high-grade serous carcinoma are shown in Figure 3 (for association of each individual SNP, see Supplementary Table 3, available as Supplementary data at IJE online) and there is no evidence for association for non-serous disease. For all non-high-grade serous cancers combined, the OR was 1.12 (0.89–1.41).
The association of lower circulating vitamin D [25(OH)D] levels to risk of epithelial ovarian cancer appear to be consistent with a recent MR study31 looking at all-cancer mortality. Vitamin D activating enzymes and vitamin D receptors are present in many tissues, with the regulation of 1–3% of gene expression in these tissues attributable to vitamin D.35 Studies have also shown that vitamin D is involved in the regulation of cell processes (proliferation, differentiation and apoptosis) in several cell types that are central to the development of cancer.14,41–43 Thus, our findings warrant further investigations on the biological role of vitamin D [specifically 25(OH)D] in mortality as well as risk of ovarian cancer.
In conclusion, we demonstrate an association between low 25(OH)D concentration and risk of ovarian cancer in women of European ancestry, with our MR approach providing estimates which are unaffected by the confounding or biases present in observational studies. Whilst our results cannot guarantee causality, placed in the context of other epidemiological studies, they provide additional evidence supportive of a causal link between vitamin D and risk of ovarian cancer.
Funding
This work was supported by the COGS project, which is funded through a European Commission’s Seventh Framework Programme Grant (agreement number 223175 HEALTH F2 2009–223175); the Genetic Associations and Mechanisms in Oncology (GAME‐ ON): a NCI Cancer Post-GWAS Initiative (U19-CA148112); the Ovarian Cancer Association Consortium is supported by a grant from the Ovarian Cancer Research Fund thanks to donations by the family and friends of Kathryn Sladek Smith (PPD/RPCI.07). Funding of the constituent studies was provided by the California Cancer Research Program (00–01389V-20170, N01-CN25403, 2II0200); the Canadian Institutes of Health Research (MOP-86727); Cancer Australia; Cancer Council Victoria; Cancer Council Queensland; Cancer Council New South Wales; Cancer Council South Australia; Cancer Council Tasmania; Cancer Foundation of Western Australia; the Cancer Institute of New Jersey; Cancer Research UK (C490/A6187, C490/A10119, C490/A10124); the Danish Cancer Society (94–222–52); the ELAN Program of the University of Erlangen-Nuremberg; the Eve Appeal; the Helsinki University Central Hospital Research Fund; Helse Vest; the Norwegian Cancer Society; the Norwegian Research Council; the Ovarian Cancer Research Fund; Nationaal Kankerplan of Belgium; the L & S Milken Foundation; the Polish Ministry of Science and Higher Education (4 PO5C 028 14, 2 PO5A 068 27); the Roswell Park Cancer Institute Alliance Foundation; the US National Cancer Institute (K07-CA095666, K07-CA80668, K07-CA143047, K22-CA138563, N01-CN55424, N01-PC67001, N01-PC067010, N01-PC035137, P01-CA017054, P01-CA087696, P30-CA072720, P30-CA15083, P30-CA008748, P50-CA159981, P50-CA105009, P50-CA136393, R01-CA149429, R01-CA014089, R01-CA016056, R01-CA017054, R01-CA049449, R01-CA050385, R01-CA054419, R01-CA058598, R01-CA058860, R01-CA061107, R01-CA061132, R01-CA063678, R01-CA063682, R01-CA067262, R01-CA071766, R01-CA074850, R01-CA080978, R01-CA083918, R01-CA087538, R01-CA092044, R01-CA095023, R01-CA122443, R01-CA112523, R01-CA114343, R01-CA126841, R01-CA136924, R03-CA113148, R03-CA115195, U01-CA069417, U01-CA071966, UM1-CA186107, UM1-CA176726 and Intramural research funds); the NIH/National Center for Research Resources/General Clinical Research Center (MO1-RR000056); the US Army Medical Research and Material Command (DAMD17‐01–1–0729, DAMD17‐02–1–0666, DAMD17‐02–1–0669, W81XWH-07–0449, W81XWH-10–1–02802); the US Public Health Service (PSA-042205); the National Health and Medical Research Council of Australia (199600 and 400281); the German Federal Ministry of Education and Research of Germany Programme of Clinical Biomedical Research (01GB 9401); the State of Baden-Wurttemberg through Medical Faculty of the University of Ulm (P.685); the German Cancer Research Center; the Minnesota Ovarian Cancer Alliance; the Mayo Foundation; the Fred C. and Katherine B. Andersen Foundation; the Lon V. Smith Foundation (LVS-39420); the Oak Foundation; Eve Appeal; the OHSU Foundation; the Mermaid I project; the Rudolf-Bartling Foundation; the UK National Institute for Health Research Biomedical Research Centres at the University of Cambridge, Imperial College London, University College Hospital ‘Womens Health Theme’ and the Royal Marsden Hospital; and Work Safe BC 14. Investigator-specific funding: JS and GC thank the University of Queensland and QIMR Berghofer Medical Research Institute for scholarship support. YL was supported by the NHMRC Early Career Fellowship. GCT, REN and PMW are supported by the National Health and Medical Research Council. SM acknowledges funding support from an Australian Research Council Future Fellowship.
Supplementary Material
Acknowledgements
We acknowledge with appreciation all the individuals who participated in the OCAC studies, the many hospital directors and staff, gynaecologists, general practitioners and pathology services who provided assistance with confirmation of diagnoses, and the many research assistants and interviewers for assistance with the studies. For the OCAC studies, we thank: D Bowtell, A deFazio, D Gertig, A Green, P Parsons, N Hayward, P Webb and D Whiteman (AUS); G Peuteman, T Van Brussel and D Smeets (BEL); L Gacucova (HMO); P Schurmann, F Kramer, W Zheng, TW Park-Simon, K Beer-Grondke and D Schmidt (HJO); S Windebank, C Hilker and J Vollenweider (MAY); the state cancer registries of AL, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA and WYL (NHS); L Paddock, M King, L Rodriguez-Rodriguez, A Samoila and Y Bensman (NJO); M Sherman, A Hutchinson, N Szeszenia-Dabrowska, B Peplonska, W Zatonski, A Soni, P Chao and M Stagner (POL); C Luccarini, P Harrington the SEARCH team and ECRIC (SEA); I Jacobs, M Widschwendter, E Wozniak, N Balogun, A Ryan, C Karpinskyj and J Ford (UKO); Carole Pye (UKR); A Amin Al Olama, J Dennis, E Dicks, K Michilaidou and K Kuchenbaker (COGS).
Conflict of interest: Mark T Goodman is a consultant for Johnson and Johnson Ltd. Usha Menon has stock ownership and research funding from Abcodia Ltd, a UCL spin-out company with interest in biomarkers and ovarian cancer screening. Peter Fasching conducts research with grants from Amgen and Novartis, and received honoraria from Amgen, Novartis, Roche, Celgene, Nanostring, Genomic Health and TEVA. All remaining authors declare no competing interest.
References
- 1. Sung PL, Chang YH, Chao KC, Chuang CM, Task Force on Systematic R, Meta-analysis of Ovarian C. Global distribution pattern of histological subtypes of epithelial ovarian cancer: a database analysis and systematic review. Gynecol Oncol 2014;133(2):147–54. [DOI] [PubMed] [Google Scholar]
- 2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61(2):69–90. [DOI] [PubMed] [Google Scholar]
- 3. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136(5):E359–86. [DOI] [PubMed] [Google Scholar]
- 4. Lu Y, Cuellar-Partida G, Painter JN, et al. Shared genetics underlying epidemiological association between endometriosis and ovarian cancer. Hum Mol Genet 2015;24(20):5955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salehi F, Dunfield L, Phillips KP, Krewski D, Vanderhyden BC. Risk factors for ovarian cancer: an overview with emphasis on hormonal factors. J Toxicol Environ Health B Crit Rev 2008;11(3–4):301–21. [DOI] [PubMed] [Google Scholar]
- 6. Prescott J, Bertrand KA, Poole EM, Rosner BA, Tworoger SS. Surrogates of long-term vitamin d exposure and ovarian cancer risk in two prospective cohort studies. Cancers 2013;5(4):1577–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cook LS, Neilson HK, Lorenzetti DL, Lee RC. A systematic literature review of vitamin D and ovarian cancer. Am J Obstet Gynecol 2010;203(1):70 e1–8. [DOI] [PubMed] [Google Scholar]
- 8. Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: circulating vitamin D and ovarian cancer risk. Gynecol Oncol 2011;121(2):369–75. [DOI] [PubMed] [Google Scholar]
- 9. Webb PM, de Fazio A, Protani MM, et al. Circulating 25-hydroxyvitamin D and survival in women with ovarian cancer. Am J Clin Nutr 2015;102(1):109–14. [DOI] [PubMed] [Google Scholar]
- 10. Zheng W, Danforth KN, Tworoger SS, et al. Circulating 25-hydroxyvitamin D and risk of epithelial ovarian cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol 2010;172(1):70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prescott J, Bertrand KA, Reid BM, et al. Evidence of differential effects of vitamin d receptor variants on epithelial ovarian cancer risk by predicted vitamin d status. Frontiers in Oncology 2014;4:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bakhru A, Mallinger JB, Buckanovich RJ, Griggs JJ. Casting light on 25-hydroxyvitamin D deficiency in ovarian cancer: a study from the NHANES. Gynecol Oncol 2010;119(2):314–18. [DOI] [PubMed] [Google Scholar]
- 13. Holick CN, Stanford JL, Kwon EM, Ostrander EA, Nejentsev S, Peters U. Comprehensive association analysis of the vitamin D pathway genes, VDR, CYP27B1, and CYP24A1, in prostate cancer. Cancer Epidemiol Biomarkers Prev 2007;16(10):1990–9. [DOI] [PubMed] [Google Scholar]
- 14. Wranicz J, Szostak-Wegierek D. Health outcomes of vitamin D. Part II. Role in prevention of diseases. Rocz Panstw Zakl Hig 2014;65(4):273–9. [PubMed] [Google Scholar]
- 15. Malloy PJ, Feldman D. Genetic disorders and defects in vitamin d action. Endocrinol Metab Clin North Am 2010;39(2):333–46, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Walentowicz-Sadlecka M, Sadlecki P, Walentowicz P, Grabiec M. [The role of vitamin D in the carcinogenesis of breast and ovarian cancer]. Ginekol Pol 2013;84(4):305–8. [DOI] [PubMed] [Google Scholar]
- 17. Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol 2011;51:311–36. [DOI] [PubMed] [Google Scholar]
- 18. Toriola AT, Surcel HM, Agborsangaya C, et al. Serum 25-hydroxyvitamin D and the risk of ovarian cancer. Eur J Cancer 2010;46(2):364–9. [DOI] [PubMed] [Google Scholar]
- 19. Arslan AA, Clendenen TV, Koenig KL, et al. Circulating vitamin d and risk of epithelial ovarian cancer. J Oncol 2009;2009:672492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tworoger SS, Lee IM, Buring JE, Rosner B, Hollis BW, Hankinson SE. Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of incident ovarian cancer. Cancer Epidemiol Biomarkers Prev 2007;16(4):783–8. [DOI] [PubMed] [Google Scholar]
- 21. Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res 2015. doi : 10.1177/0962280215597579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sheehan NA, Didelez V, Burton PR, Tobin MD. Mendelian randomisation and causal inference in observational epidemiology. PLoS Med 2008;5(8):e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pharoah PD, Tsai YY, Ramus SJ, et al. GWAS meta-analysis and replication identifies three new susceptibility loci for ovarian cancer. Nat Genet 2013;45(4):362–70, 70e1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet 2010;19(13):2739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berry DJ, Vimaleswaran KS, Whittaker JC, Hingorani AD, Hypponen E. Evaluation of genetic markers as instruments for Mendelian randomization studies on vitamin D. PLoS One 2012;7(5):e37465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mokry LE, Ross S, Ahmad OS, et al. Vitamin D and risk of multiple sclerosis: a mendelian randomization study. PLoS Med 2015;12(8):e1001866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Theodoratou E, Palmer T, Zgaga L, et al. Instrumental variable estimation of the causal effect of plasma 25-hydroxy-vitamin D on colorectal cancer risk: a Mendelian randomization analysis. PLoS One 2012;7(6):e37662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Trummer O, Pilz S, Hoffmann MM, et al. Vitamin D and mortality: a Mendelian randomization study. Clin Chem 2013;59(5):793–7. [DOI] [PubMed] [Google Scholar]
- 29. Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 2010;376(9736):180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bratke K, Wendt A, Garbe K, et al. Vitamin D binding protein and vitamin D in human allergen-induced endobronchial inflammation. Clin Exp Immunol 2014;177(1):366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Afzal S, Brondum-Jacobsen P, Bojesen SE, Nordestgaard BG. Genetically low vitamin D concentrations and increased mortality: Mendelian randomisation analysis in three large cohorts. BMJ 2014;349:g6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marchini J, Howie B, Myers S, McVean G, Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet 2007;39(7):906–13. [DOI] [PubMed] [Google Scholar]
- 33. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 2013;37(7):658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Afzal S, Brondum-Jacobsen P, Bojesen SE, Nordestgaard BG. Vitamin D concentration, obesity, and risk of diabetes: a Mendelian randomisation study. Lancet Diabetes Endocrinol 2014;2(4):298–306. [DOI] [PubMed] [Google Scholar]
- 35. Uhlen M, Oksvold P, Fagerberg L, et al. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol 2010;28(12):1248–50. [DOI] [PubMed] [Google Scholar]
- 36. Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci U S A 2004;101(20):7711–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG, Consortium E-I. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol 2015;30(7):543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med 2013;369(21):1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Taylor AE, Burgess S, Ware JJ, et al. Investigating causality in the association between 25(OH)D and schizophrenia. Sci Rep 2016;6:26496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 2015;44(2):512–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fleet JC, DeSmet M, Johnson R, Li Y. Vitamin D and cancer: a review of molecular mechanisms. Biochem J 2012;441(1):61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Holick MF. Vitamin D, sunlight and cancer connection. Anticancer Agents Med Chem 2013;13(1):70–82. [PubMed] [Google Scholar]
- 43. Ingraham BA, Bragdon B, Nohe A. Molecular basis of the potential of vitamin D to prevent cancer. Curr Med Res Opin 2008;24(1):139–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


