ABSTRACT
Objectives
The purpose of the present study was to systematically review the literature on the surgical regenerative treatment of the peri-implantitis and to determine an effective therapeutic predictable option for their clinical management.
Material and Methods
The study searched MEDLINE and EMBASE databases from 2006 to 2016. Clinical human studies that had reported changes in probing depth (PD) and/or bleeding on probing (BOP) and/or radiologic marginal bone level (RBL) changes after peri-implantitis surgical treatment at 12-month follow-up or longer were included accordingly to PRISMA guidelines.
Results
The initial search obtained 883 citations. After screening and determination of eligibility, 18 articles were included in the review. The meta-analysis of selected studies revealed that the weighted mean RBL fill was 1.97 mm (95% confidence interval [CI] = 1.58 to 2.35 mm), PD reduction was 2.78 mm (95% CI = 2.31 to 3.25 mm), and BOP reduced by 52.5% (95% CI = 41.6 to 63.1%). Defect fill in studies using and not using barrier membranes for graft coverage was 1.86 mm (95% CI = 1.36 to 2.36 mm) and 2.12 mm (95% CI = 1.46 to 2.78 mm) correspondingly. High heterogeneity among the studies regarding defects morphology, surgical protocols, and selection of biomaterials were found.
Conclusions
All included studies underlined an improvement of clinical conditions after the surgical regenerative treatment of peri-implantitis, however, there is a lack of scientific evidence in the literature regarding the superiority of the regenerative versus non-regenerative surgical treatment. The presence of a barrier membrane or submergence in the regenerative procedure does not seem to be fundamental in order to obtain clinical success of the surgery.
Keywords: alveolar bone grafting, biocompatible materials, alveolar bone loss, bone regeneration, oral surgery, peri-implantitis
INTRODUCTION
In the recent years, dental implant rehabilitation has confronted requests for optimal functional and aesthetic outcomes which require an inflammation of the predictable surgical techniques and long-term clinical results. Besides of the successful functioning on a long-term, several types of complications have been recently encountered. The clinical condition involving dental implants, characterized by the inflammation, bleeding, suppuration of peri-implant tissues and rapid bone loss is widely known as peri-implantitis [1-6].
Peri-implantitis is associated with the presence of a sub-marginal plaque, which contains a large variety of Gram-negative anaerobic rods, fusiform bacteria, motile and curved rods and spirochetes [7]. It contains large amounts of densely packed inflammatory cells (neutrophilis, macrophages, lymphocytes and plasma cells), frequently accompanied by a crater like bone defects that surround the contaminated implant [8,9]. Several lines of evidence indicated that an accumulation of bacteria on the implant surface plays an important role in the aetiology of peri-implantitis – an inflammatory condition affecting the tissues around osseointegrated implants, leading to loss of supporting bone [10]. Nevertheless, peri-implant tissues can be kept in a healthy clinical state and some endosseous implants successfully used as prosthetic abutments for the oral rehabilitation of fully and partially edentulous patients for a prolonged period of time [11]. Besides of a numerous patient-related factors (insufficient bone quality, smoking, systemic diseases or chemotherapy), surgical trauma or bacterial contamination during implant insertion, non-fit dental implant prosthesis and abnormal masticatory load are reported to be the most important causes of early implant failure [12-14].
Peri-implantitis can be defined as a site-specific infectious disease associated to an inflammatory process involving periodontal soft tissues, and causing bone loss around an osseointegrated implant. Numerous aetiological factors may play a decisive role for the progress of peri-implant infection. The implant design, the degree of roughness, the external morphology, the abutment connection, the passivation of the prosthesis and excessive mechanical load are all related with the disease [6,15]. Diagnosis can be referred on altered clinical condition like the colour in the gingiva, bleeding on probing (BOP), increased probing depth (PD), suppuration, and gradual loss of peri-implant bone as diagnosed by decrease of radiologic bone level (RBL) in standardized radiography.
The diagnosis and therapies for the soft tissue inflammation and peri-implant bone loss is quite challenging for the clinician. Diagnostic measures, such as probing pocket depth, radiographic tools, and microbial sampling have been shifted from the periodontal area and utilized during the maintenance phase of the dental implant treatment [16]. The main aim in the treatment of peri-implantis is to arrest the progression of the disease and at the same time to keep the dental implant in function solving the inflammatory signs of bleeding and pain [17]. Peri-implant bony defects around dental implants can be treated with either non-surgical or surgical (resective or regenerative) techniques. Bone tissue regeneration is the objective therapeutic option in selected peri-implant bony defects of functioning implants if appropriate surgical techniques are utilized and the aetiologic cause is fully eradicated [18].
The purpose of this systematic review is to screen recent literature on various approaches of surgical regenerative treatment of peri-implantitis in order to give the clinicians valuable suggestions for the most appropriate treatment modality.
MATERIAL AND METHODS
Protocol and registration
The methods of the analysis and inclusion criteria were specified in advance and documented in a protocol. The review was registered in PROSPERO, an international prospective register of systematic reviews. The protocol can be accessed through the following link:
http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016033664
Registration number: CRD42016033664.
The reporting of this systematic analysis adhered to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement [19].
Focus question
The following focus questions were developed according to the population, intervention, comparison, and outcome (PICO) study design:
What are the overall treatment outcomes of reconstructive procedures in treating peri-implantitis?
Does the use of barrier membranes or submergence of the healing site provide beneficial clinical outcomes in the treatment of peri-implantitis?
Information sources
The search strategy incorporated examinations of electronic databases, supplemented by hand searches. A search of four electronic databases, including Ovid MEDLINE, PubMed, EMBASE, and Dentistry and Oral Sciences Source was carried out for relevant studies published in the English language from January 2006 to March 2016.
Additionally, a hand search performed in dental and implant-related journals limited to English language for the same period included: 1) “Journal of Periodontology”; 2) “Clinical Implant Dentistry and Related Research”; 3) “International Journal of Oral and Maxillofacial Implants”; 4) “Clinical Oral Implants Research”; 5) “Implant Dentistry”; 6) “International Journal of Oral and Maxillofacial Surgery”; 7) “Journal of Oral and Maxillofacial Surgery”; 8) “Journal of Dental Research”; 9) “Journal of Prosthetic Dentistry”; 10) “International Journal of Prosthodontics”; 11) “Journal of Oral Implantology”; 12) “Journal of Clinical Periodontology”; 13) “International Journal of Periodontics & Restorative Dentistry”; 14) “European Journal of Oral Implantology”. A hand search of the reference lists in the articles retrieved was carried out to source additional relevant publications and to improve the sensitivity of the search.
Search
The keywords used in the search of the selected electronic databases included the following: “peri-implantitis” OR “periimplantitis” OR “peri-implant” OR “periimplant” or (“implant” AND “failure”) AND “surgery” OR “surgical” OR “regeneration” OR “regenerative” OR “treatment” OR “therapy” OR “bone graft” OR “bone substitute” OR “laser” OR “lasers”.
The choice of keywords was intended to be extensive, to collect as much relevant data as possible without relying on electronic means alone to refine the search results.
Selection of studies
The resulting articles were independently subjected to clear inclusion and exclusion criteria by 2 independent reviewers as follows. Reviewers compared decisions and resolved differences through discussion, consulting a third party when consensus could not be reached. The third party was an experienced senior reviewer. The level of agreement between the reviewers regarding study inclusion was calculated using κ statistics. At the title and abstract stage, one reviewer accepted the citations that appeared to meet inclusion criteria and send them to full-text review, with a second reviewer assessing only those citations and abstracts that the first reviewer deemed ineligible. At the stage of reviewing of full-text articles, a complete independent dual review was undertaken.
Types of publications
The review included studies on humans published in the English language. Letters, editorials, case reports, literature reviews, and PhD theses were excluded.
Types of studies
The review included all human prospective and retrospective follow-up studies and clinical trials, cohort studies, case-control studies, and case series studies on surgical regenerative treatment of peri-implantitis, published between January 2006 and March 2016.
Types of participants/population
Subjects in the included studies must have had at least one osseointegrated titanium screw-shaped dental implant that presented signs of peri-implantitis.
Disease definition
The authors of this review classified the case definition of peri-implantitis of each study, if there was a clear radiographic threshold > 2 mm of continuous marginal bone loss beyond biologic peri-implant bone remodelling, presence of BOP and/or suppuration on probing with probing depth more than 6 mm [20].
Inclusion and exclusion criteria
The full text of all studies of possible relevance was obtained for assessment against the following inclusion criteria:
Investigated surgical regenerative treatment in patients with at least one osseointegrated titanium screw-shaped dental implant, that presented signs of peri-implantitis;
Studies involving at least one surgical regenerative treatment method of peri-implantitis applied;
All human prospective or retrospective follow-up studies and clinical trials, cohort studies, case-control studies, and case series studies with a minimal sample size of 10 implants and not less than 12 months follow-up after surgical regenerative treatment of peri-implantitis;
Clear report on clinical and radiographic peri-implant tissues changes, including RBL and/or PD change as primary outcome measure and/or BOP as secondary outcome measure.
The applied exclusion criteria for studies were as follows:
Animal or in vitro studies;
Studies involving patients with specific diseases, immunologic disorders, uncontrolled diabetes mellitus, osteoporosis, or other implant risk related systemic conditions;
Studies investigating ceramic or coated surface implants;
Not enough information regarding the selected topic;
No access to the title and abstract in English language.
Sequential search strategy
Following the initial literature search, all article titles were screened to eliminate irrelevant publications, considering the exclusion criteria. Next, studies were excluded based on data obtained from screening the abstracts. The final stage of screening involved reading the full texts to confirm each study’s eligibility, based on the inclusion criteria.
Data extraction
The data were independently extracted from studies in the form of variables, according to the aims and themes of the present review, as listed onwards.
Data items
Data were collected from the included articles and arranged in the following fields:
“Author (Year)” – revealed the author and year of publication.
“Type of study” – indicated the type of the study.
“Sample size” – described the number of patients examined.
“Detoxification method” – described additional implant surface detoxification measures applied in addition to the instrumental debridement, full thickness flap, and degranulation.
“Bone substitute/membrane” – described types of bone grafts and membranes used for regeneration.
“Antimicrobial” – described antimicrobial agents (e.g. systemic antibiotics, chlorhexidine mouth rinse) used adjunctive to the surgery.
“Follow-up” – described the duration of the observed outcomes after applied surgical regenerative treatment of peri-implantitis.
“Probing depth change (ΔPD)” – described probing depth difference (in mm) before and after surgical treatment.
“Bleeding on probing change (ΔBOP)” – described BOP difference (in %) before and after surgical treatment.
“Radiologic bone level change (ΔRBL)” – described the marginal bone level difference (in mm; measured from implant shoulder to the bone surface) before and after the treatment; and/or intrabony defect depth difference (in mm; measured from the bottom of the defect to the interproximal bone) before and after treatment.
Risk of bias assessment
Assessment of risk of bias was undertaken independently, and in duplicate by the two authors during the data extraction process. For the included studies, this was conducted using the Cochrane Collaboration’s two-part tool for assessing risk of bias [21].
The following possible sources of bias were addressed: random sequence generation (selection bias); allocation concealment (selection bias); blinding of participants and personnel (performance bias and detection bias); incomplete outcome data (attrition bias);
selective reporting (reporting bias); and other bias (examiner blinding, examiner calibration, standardized probing force, standardized radiographic assessment). The authors’ judgment for each source of bias item was assigned for each study in the data extraction table (Table 1). An overall risk of bias was then assigned to each trial according to Higgins et al. [21]. The degrees of bias were categorized as follows: low risk, if all the criteria were met; moderate risk, when only one criterion was missing; high risk, if two or more criteria were missing; and unclear risk, if too few details were available to make a judgement of certain risk assessment.
Table 1.
Risk of bias within the studies
| Study |
Year of publication |
Random sequence generation | Allocation concealment | Blinding | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Deppe et al. [22] | 2007 | ? | ? | ? | - | + | + |
| Roos-Jansåker et al. [23] | 2007 | ? | ? | + | - | ? | + |
| Roos-Jansåker et al. [24] | 2007 | ? | ? | ? | - | ? | + |
| Schwarz et al. [25] | 2008 | + | ? | + | - | ? | + |
| Romanos et al. [26] | 2008 | ? | ? | ? | - | - | - |
| Schwarz et al. [27] | 2009 | + | ? | + | - | ? | + |
| Schwarz et al. [28] | 2010 | ? | ? | + | - | ? | + |
| Roccuzzo et al. [29] | 2011 | ? | ? | + | + | ? | + |
| Froum et al. [30] | 2012 | ? | ? | + | - | - | - |
| Aghazadeh et al. [31] | 2012 | + | - | + | + | + | + |
| Wohlfahrt et al. [32] | 2012 | + | + | + | + | + | + |
| Wiltfang et al. [33] | 2012 | ? | ? | ? | - | - | + |
| Schwarz et al. [34] | 2013 | + | ? | + | ? | - | + |
| Matarasso et al. [35] | 2014 | ? | ? | ? | - | ? | + |
| Roos-Jansåker et al. [36] | 2014 | ? | ? | + | - | ? | + |
| Jepsen et al. [37] | 2015 | + | + | + | + | + | + |
| Froum et al. [38] | 2015 | ? | ? | + | + | + | - |
| Roccuzzo et al. [39] | 2016 | ? | ? | - | + | + | + |
+ = low risk; ? = unclear risk; - = high risk.
Statistical analysis
A meta-analysis was performed to integrate the quantitative findings from separate but similar studies and to provide a numerical estimate to the overall effect of interest. All meta-analyses were performed on studies that reported the clinical and/or radiologic outcomes of regenerative peri-implantitis treatment approach. The primary outcome measures were ΔRBL and/or ΔPD, evaluating ΔBOP as secondary outcome measure. The pooled weighted mean (WM) and the 95% confidence interval (CI) of each measure were estimated with Comprehensive Meta-analysis (Version 2, Biostat, Englewood, NJ, USA) statistical software. Parametric data were expressed as mean and standard deviation (M [SD]). The random effect model was applied when performing meta-analysis to account for methodologic differences among studies. Forest plots were produced to graphically represent WM and 95% CI for the primary and secondary outcomes, with the implant as the analysis unit. Heterogeneity was assessed with the I2 test. To evaluate the potential influences of different treatment modalities, WM and 95% CI were calculated separately for membrane use, and type of the flap manipulation (submerged/nonsubmerged healing). The reporting of this meta-analysis adhered to the PRISMA statement [19].
RESULTS
Study selection
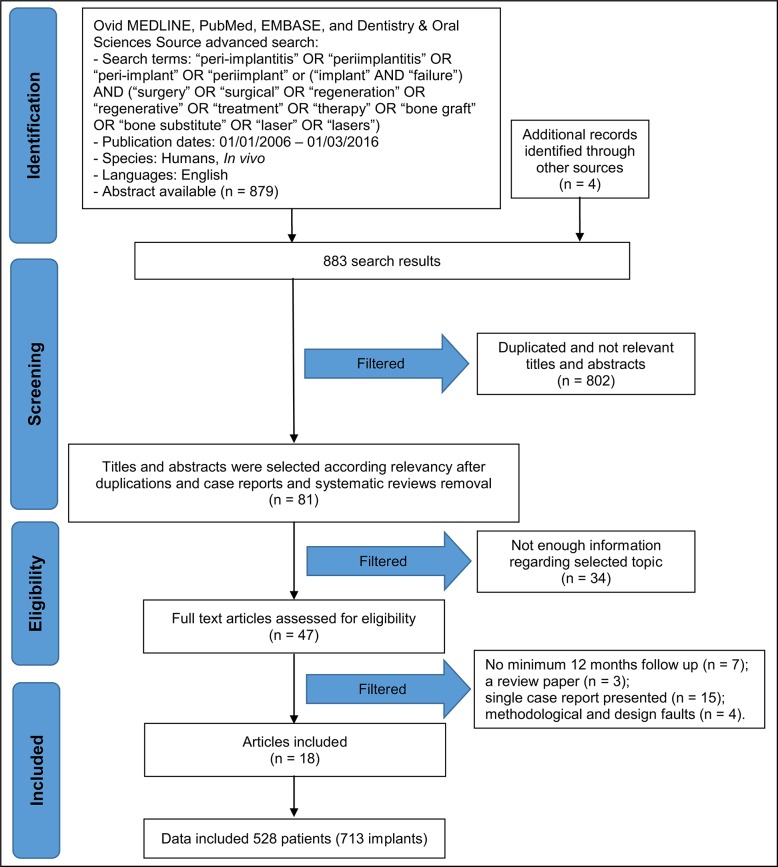
Article review and data extraction were performed according to PRISMA flow diagram (Figure 1). The initial electronic and hand search retrieved 883 citations, 802 of which were eliminated as duplicates or not relevant articles. After titles and abstracts were reviewed, additional 34 articles were filtered as having not enough information regarding selecting topic. 47 articles were identified as full-text articles, 18 of which were included in this review [22-39]. Reasons for studies exclusion after full-text assessment were as follows: no minimum 12 months follow-up (n = 7) [40-46]; a review paper (n = 3) [47-49], single case report presented (n = 15) [50-64]; methodological and design faults (n = 4) [66-69]. The κ value for the interreviewer agreement of the included publications was 0.92.
Figure 1.
PRISMA flow diagram.
Study characteristics
Eight prospective clinical studies [22,23,28,29,34,36,38,39], seven case series [24-27,30,33,35], and three randomized clinical trials (RCTs) [31,32,37] were included for the final review. A total of 528 patients (713 implants) were treated. The mean age of the patients was 61 years (ranged from 20 to 83 years) with the mean observation period of 37 months (ranging from 12 to 236 months). Eleven studies [23-25,27,29,31,32,35-37,39] reported smoking status of the patients, ranging from 14% [39] to 72% [36]. The summarized individual study characteristics are described in Table 2.
Table 2.
Selected studies
| Author | Type of study | Sample size (implants) |
Detoxification method |
Bone substitute/membrane | Antimicrobial |
Follow-up (months) |
PD Mean (SD) mm |
BOP+ Mean, % |
Radiologic bone level Mean (SD) mm |
Complications | Comments | Submerge | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IN | ΔPD | IN | ΔBOP | IN | ΔRBL | ||||||||||
| Deppe et al. [22] | Prospective clinical study | 19 (6 patients)a | No augmentation | 20 - 236 | 5.1 (1.3) | 0.8 (1.2) | No data | No data | 7.6 (1.4) | 0.3 (1.3) | 8 implants were lost in augmented bone groups due to infection and 5 implants lost in non-augmented sites. | With the respect to the long term results of augmentation procedures, the method used for decontamination seems to play a subordinate role. Augmentation with 1:1 mix of AB and βTCP can lead to reduction of the defect depth. | Y | ||
| 15 (7 patients) | AB mixed with βTCP 1:1/PTFE | 4.8 (1.4) | 2.3 (1.2) | 6.8 (1.2) | 2.1 (1.1) | ||||||||||
| 22 (10 patients)a | CO2 laser | No augmentation | 6.1 (1.6) | 2.7 (1.5) | 7.2 (1.3) | 0.4 (1.1) | |||||||||
| 17 (9 patients) | AB mixed with βTCP 1:1/PTFE | 5 (1.3) | 2.5 (1.3) | 6.7 (1.5) | 2.2 (1.3) | ||||||||||
| Roos-Jansåker et al. [23] | Prospective clinical study | 29 (17 patients) | 3% H2O2, Saline | FHA/SRM |
0.1% CHX rinse for 5 weeks.
Amx (375 mg x 3) + Metro (400 mg x 2) for 10 days. |
12 | 5.4 (1.8) | 2.9 (1) | 79.3% | 57.7% | 3.4 (1.2) | 1.5 (1.2) | Membrane exposure after 2 weeks was noted in 43.8% of the treated implants. | It is possible to treat peri-implant defects with a bone substitute, with or without a resorbable membrane. | N |
| 36 (19 patients) | FHA | 5.6 (1.8) | 3.4 (1.7) | 92.9% | 67.9% | 2.8 (0.8) | 1.4 (1.3) | Uneventful | |||||||
| Roos-Jansåker et al. [24] | Case series | 16 (12 patients) | 3% H2O2, Saline | FHA/SRM |
0.1% CHX rinse for 5 weeks.
Amx (375 mg x 3) + Metro (400 mg x 2) for 10 days. |
12 | 5.1 (1) | 4.2 (1.5) | 75% | 62.5% | 3.8 (1) | 2.3 (1.2) |
62.5% implant sites demonstrated inadequate primary healing with the presence of soft tissue craters.
Membrane exposure 75.1% |
Treatment of peri-implantitis defect using a bone graft substitute combined with a resorbable membrane and submerged healing resulted in defect fill and clinical healthier situations. | Y |
| Schwarz et al. [25] | Case series | 11 (11 patients) | Saline | nanoHA | 0.2% CHX rinse for 2 weeks. | 24 | 6.9 (0.6) | 1.5 (0.6) | 80%; | 36% | No data | Suppuration around 2 implants | Both treatment procedures have shown efficacy, however, the application of natural bone mineral in combination with a collagen membrane may result in an improved outcome of healing. | N | |
| 11 (11 patients) | BDX/CM | 7.1 (0.8) | 2.4 (0.8) | 78% | 44% | N | |||||||||
| Romanos et al. [26] | Case series | 19 (15 patients) | CO2 laser | AB or BDX/CM | 27 | 6 (2) | 3.5 (1.3) | No data | No data | No data | Bovine xenograft provided more radiographic bone fill than autogenous graft, because of autogenous graft resorption over time. | YN | |||
| Schwarz et al. [27] | Case series | 9 (9 patients) | Saline | nanoHA | 0.2% CHX rinse for 2 weeks. | 48 | 6.9 (0.6) | 1.1 (0.3) | 80% | 32% | Decreased translucency in the former peri-implant defect area noticed at 8 sites in nanoHA and 5 sites in BDX/resorbable membrane group. | Uneventful | While the application of natural bone mineral with a collagen membrane resulted in clinical improvements, a long-term outcome obtained with nanocrystaline hydroxyapatite without a barrier membrane must be considered as poor. | N | |
| 10 (10 patients) | BDX/CM | 7.1 (0.7) | 2.5 (0.9) | 79% | 51% | N | |||||||||
| Schwarz et al. [28] | Prospective clinical study | 9 (9 patients) | Saline | DBX/CM | 0.2% CHX rinse for 2 weeks. | 12 | 6.7 (0.7) | 1.6 (0.9 | 81.5% | 38.9% | No data | Uneventful | Defect configuration may have an impact on the clinical outcome following surgical regenerative therapy of peri-implantitis lesions. | N | |
| 9 (9 patients) | 7.1 (0.6) | 1.6 (0.7) | 83.3% | 25.9% | |||||||||||
| 9 (9 patients) | 7 (0.5) | 2.7 (0.7) | 85.2% | 61.1% | |||||||||||
| Roccuzzo et al. [29] | Prospective clinical study | 14 TPS implants (14 patients) | 24% EDTA gel, 1% CHX gel. Saline | BDX |
Axm and Clavulanic acid (1000 mg x 2) for 6 days.
0.2% CHX rinse for 3 weeks. |
12 | 7.2 (1.5) | 2.1 (1.2) | 91.1% | 33.9% | 3.9 (1.6) | 1.6 (0.7) | No complications |
Clinical parameters around moderately rough implants were better than around rough implants.
Surgical regenerative technique described resulted in a clinical healthier situation around many of the treated implants so that their function could be fully maintained. Complete fill of the bony defect seems not to be a predictable result. |
N |
| 12 SLA implants (12 patients) | 6.8 (1.2) | 3.4 (1.7) | 75% | 60.4% | 3 (0.9) | 1.9 (1.3) | |||||||||
| Froum et al. [30] | Case series | 19 (15 patients) | AA, Saline, Tetracycline (50 mg/mL), 0.12% CHX | DBX or MBA/Enamel matrix derivative/ /PDGF/CM or SCTG |
Amx 500 mg x 3 for 10 days.
0.12% CHX rinse for 2 weeks. |
36 - 90 | 8.8 (1.9) | 5.4 (1.5) | 100% | 78.9% | 6.4 (1.9) | 3.8 (1.5) | Regenerative approach for the treatment of peri-implantitis appear to be encouraging. | N | |
| Aghazadeh et al. [31] | Randomized controlled clinical study | 34 (22 patients) | 3% H2O2, Saline | AB/resorbable bovine collagen |
Azithromycin (250 mg x1) for 4 days.
0.1% CHX rinse for 6 weeks. |
12 | 6 (1.3) | 2 (1.2) | 87.5% | 44.8% | 5.8 (1.7) | 0.2 (1.8) | BDX provided more radiographic bone fill than AB. The success for both surgical regenerative procedures was limited. | N | |
| 37 (23 patients) | BDX/resorbable bovine collagen | 6.2 (1.4) | 3.1 (1.2) | 79.4% | 50.4% | 5.2 (1.8) | 1.1 (1.9) | ||||||||
| Wohlfahrt et al. [32] | Randomized clinical trial | 16 (16 patients) | 24% EDTA gel. Saline | Porous titanium granules |
0.2% CHX rinse for 4 weeks.
Amx (500 mg x 3) + Metro (400 mg x 2) for 10 days. |
12 | 6.5 (1.9) | 1.7 (1.7) | No data | No data | 6.8 (2.7) | 2 (1.7) | Uneventful | Reconstruction with porous titanium granules resulted in significantly better radiographic peri-implant defect fill compared with controls. | Y |
| 16 (16 patients)a | No augmentation | 6.5 (2.3) | 2 (2.3) | No data | No data | 6.8 (3.9) | 0.1 (1.9) | ||||||||
| Wiltfang et al. [33] | Case series | 36 (22 patients) | Implantoplsty, 20% Phosphoric Acid | AB mixed with BDX 1:1 | Ampicillin/sulbactam 1500 mg preoperatively. | 12 | 7.5 (1.8) | 4 (1.8) | 61% | 36% | 5.1 (2.4) | 3.5 (2.4) | One local infection 1 week after surgery causing loss of the augmentation material without loss of the implant. | Surgical regenerative treatment provided a reliable method to reduce peri-implantitis induced bone defects. | N |
| Schwarz et al. [34] | Prospective clinical study | 7 (7 patients) | Implantoplasty, Saline | BDX/CM | Non specified antibiotic medication for 5 days. | 48 | 5.5 (1.7) | 1.2 (1.9) | 100% | 85.2% | No data | Reinfection in 4 patients occurred between 24 - 36 months postoperatively. | A combined surgical respective/regenerative therapy of peri-implantitis were not influenced by the method of surface decontamination. | N | |
| 10 (10 patients) |
Implantoplasty, Er:YAG laser |
5.1 (1.5) | 1.3 (1.8) | 95.2% | 71.6% | ||||||||||
| Matarasso et al. [35] | Case series | 11 (11 patients) |
Implantoplasty, AA, Saline |
DBX/CM |
Amx 875 mg and clavulanic acid 125 mg x 2 for 5 days.
0.12% CHX rinse for 4 weeks. |
12 | 8.1 (1.8) | 4.1 (1.5) | 19.7% | 13.6% | 8 (3.7) | 2.7 (3.3) | Membrane exposure in 18% of cases. | Combined regenerative/resective surgical approach for the treatment of peri-implantitis defects yielded positive clinical and radiographic results after 12 months. | N |
| Roos-Jansåker et al. [36] | Prospective clinical study | 23 (13 patients) | 3% H2O2, Saline | FHA/SRM |
0.1% CHX rinse for 5 weeks.
Amx (375 mg x 3) + Metro (400 mg x 2) for 10 days. |
60 | 5.6 (1.9) | 3 (2.4) | 75% | 42.4% | 4.6 (1.3) | 1.5 (1.2) | Membrane exposure | The use of a resorbable membrane in combination with a bone substitute did not add to the predictability or extent of bone fill. | N |
| 22 (12 patients) | FHA | 6 (2.2) | 3.3 (2) | 94.3% | 82.9% | 4 (0.8) | 1.1 (1.2) | ||||||||
| Jepsen et al. [37] | Randomized clinical trial | 33 (33 patients) | Titanium brush, 3% H2O2, Saline | Porous titanium granules |
0.2% CHX rinse for 4 weeks.
Amx (500 mg x 3) + Metro (400 mg x 2) for 8 days. |
12 | 6.3 (1.3) | 2.8 (1.3) | 89.4% | 56.1% | 4.6 (2) | 3.6 (2) | Uneventful | Reconstructive surgery using porous titanium granules resulted in significantly enhanced radiographic defect fill compared with open flap debridement. The radiographic findings must be interpreted with caution, because it is difficult to discern biomaterial and newly formed osseous tissue. | N |
| 30 (30 patients)a | No augmentation | 6.3 (1.6) | 2.6 (1.4) | 85.8% | 44.9% | 4 (2.5) | 1.1 (1.4) | ||||||||
| Froum et al. [38] | Prospective clinical study | 168 (100 patients) | AA, Saline, Tetracycline (50 mg/mL), 0.12% CHX | DBX or MBA/Enamel matrix derivative/ /PDGF/CM or SCTG | 24 - 120 | 8.1 (2.5) | 5.1 (2.2) | 100% | 91.1% | 3.8 (2.3) | 1.8 (2) | 2 implants were lost at 6 months follow up 16.7% of cases needed additional surgeries to obtain desired result. | Regenerative protocol used in treating peri-implantitis produced positive clinical outcomes in terms of reduction in BOP and PD, bone gain, and implant survival. | N | |
| Roccuzzo et al. [39] | Prospective clinical study | 71 (71 patients) | Titanium brush, 24% EDTA gel, 1% CHX gel. Saline | DBX |
Axm and Clavulanic acid (1000 mg x 2) for 6 days.
0.2% CHX rinse for 3 weeks. |
12 | 7.2 (1.6) | 2.9 (1.7) | 71.5% | 53.2% | No data | No data | 6 implants were explanted due to persistent pus formation after 12 months. | Partial defect fill was obtained. Complete resolution does not seem a predictable outcome. | N |
aNon augmented control groups were excluded for evaluation.
NanoHA = nanocrystalline hydroxyapatite; BDX = bovine-derived xenograft; CHX = chlorhexidine solution; Saline = Sterile physiologic saline solution; FHA = fluorohydroxyapatite, Amx = Amoxicillin; Metro = Metronidazole; AB = autogenous bone; SCTG = Subepithelial connective tissue graft; PDGF = Platelet-derived growth factor; MBA = mineralized bone allograft; βTCP = beta tricalcium phosphate; CM = collagen membrane; PTFE = polytetrafluoroethylene membrane; SRM = synthetic resorbable membrane; AA = air abrasive.
Eight studies included rough surface dental implants [26,29,30,31,32,35,37,39], three studies investigated machined surface implants [23,24,36] and five studies - both rough and machined surface dental implants [22,25,27,28]. The remaining two studies did not reveal implant surface specifications [33,38].
Initial radiologic bone defect depth was measured in twelve studies [22-24,29-33,35-38]. In nine studies [22,29-33,35,37,38] the distance was measured from the implant shoulder to the first bone contact on dental radiographs with the mean from 3 to 8 mm [29,35]. In three studies [23,24,36] the measurement reference point of the radiologic defect depth was the first thread of the implant. The mean initial PD was measured clinically in all included studies, ranging from 4.8 to 8.8 mm [22,30]. After applied surgical regenerative treatment, the PD reduction was ranging from 1.1 to 5.4 mm [27,30]. All studies except three [22,26,32] provided BOP data: the mean initial BOP was ranging from 19.7 to 100% [35,30,34,38]. The mean BOP reduction from 25.9 to 91.1% was obtained after surgical regenerative treatment of peri-implantitis [28,38].
All included studies used grafting materials for peri-implant bone defect augmentation. Most commonly xenografts [25-31,34,35,38,39] were utilized as well as other grafting materials, including flourhydroxyapatites [23,24,36], autografts [26,31], allografts [30,39], porous titanium granules [32,37], and comixtures of several different grafts [22,33]. There were more study groups using barrier membranes [22-28,30,31,34-36,38], than study groups, without barrier membranes utilized to cover the grafted area [23,25,27,29,32,33,36,37,39]. Due to high heterogeneity of the studies, statistical comparisons among different grafting materials and membrane types were not intended.
Risk of bias within studies
Summarizing the risk of bias for each study, most of the studies were classified as unclear risk [22-25,27-29,34-36,38,39]. Two studies were considered as having low risk of bias [32,37], where as another one was classified as moderate risk [31], and three studies were attributed to high risk of bias [26,30,33]. The risk of bias assessment for the included studies is summarized in Table 1.
Results of meta-analysis
The results and forest plots of the meta-analyses for ΔRBL, ΔPD and ΔBOP are demonstrated in Figures 2 - 4.
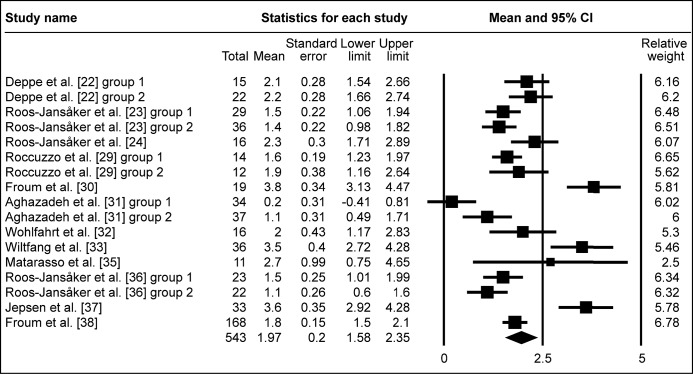
Figure 2A.
Meta-analysis for radiologic bone level change (ΔRBL).
The calculated WM was 1.97 mm (95% CI = 1.58 to 2.35 mm)
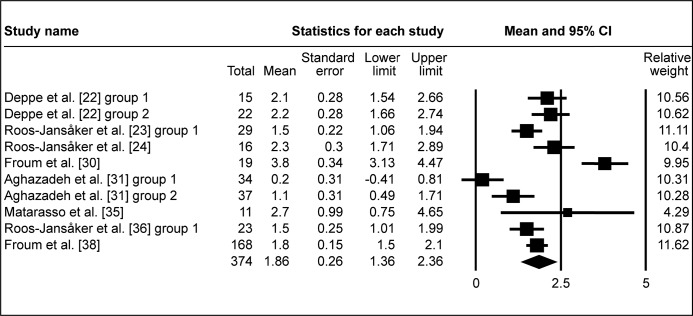
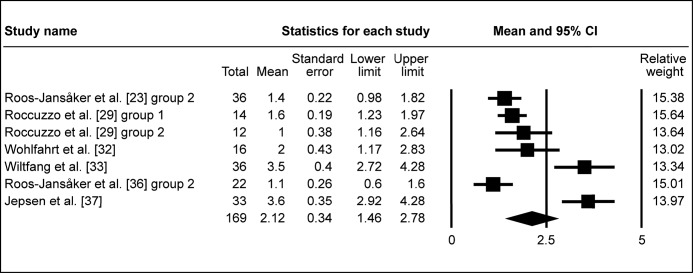
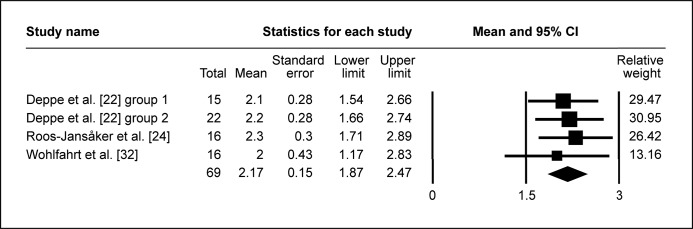
Following surgical regenerative treatment of peri-implantitis, the reported WM of ΔRBL was 1.97 mm (95% CI = 1.58 to 2.35 mm; Figure 2A). WM of ΔRBL was 1.86 mm (95% CI = 1.36 to 2.36 mm; Figure 2B) in ten study groups with membranes used, whereas WM of ΔRBL was calculated 2.12 mm (95% CI = 1.46 to 2.78 mm; Figure 2C) in seven study groups without membranes used to cover the grafted area. ΔRBL in submerged healing cases was evaluated in four study groups, with WM 2.17 mm (95% CI = 1.87 to 2.47 mm; Figure 2D), whereas thirteen groups of non-submerged healing presented WM 1.91 mm (95% CI = 1.44 to 2.39 mm; Figure 2E).
Figure 2B.
Meta-analysis for radiologic bone level change (ΔRBL) with membrane coverage of the grafted area.
The calculated WM was 1.86 mm (95% CI = 1.36 to 2.36 mm)
Figure 2C.
Meta-analysis for radiologic bone level change (ΔRBL) without membrane coverage of the grafted area.
The calculated WM was 2.12 mm (95% CI = 1.46 to 2.78 mm)
Figure 2D.
Meta-analysis for radiologic bone level change (ΔRBL) in submerged peri-implant defect healing.
The calculated WM was 2.17 mm (95% CI = 1.87 to 2.47 mm)
Figure 2E.
Meta-analysis for radiologic bone level change (ΔRBL) in non-submerged peri-implant defect healing.
The calculated WM was 1.91 mm (95% CI = 1.44 to 2.39 mm)
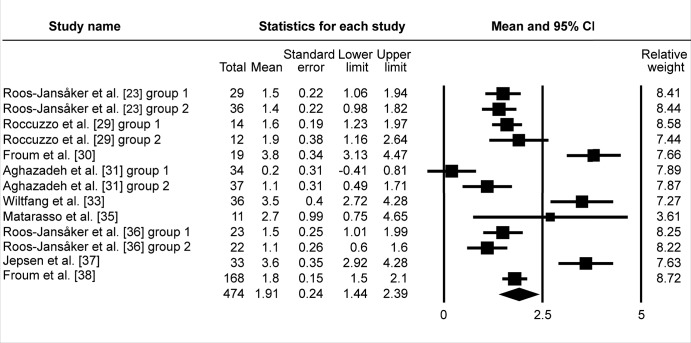
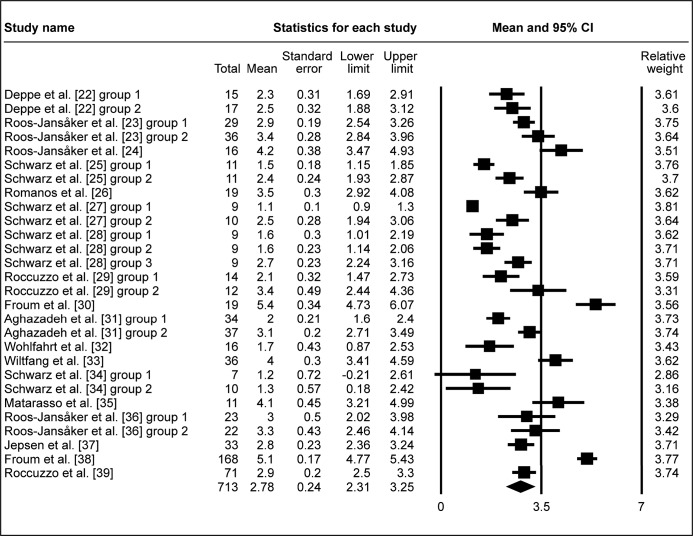
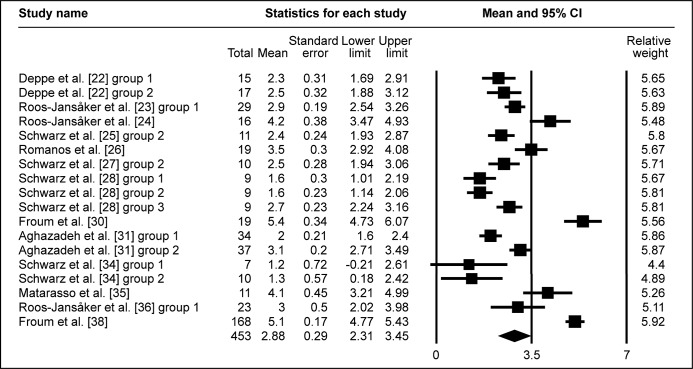
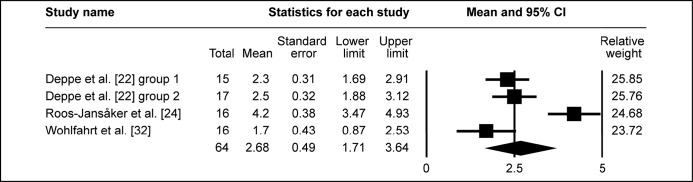
All included studies investigated probing depth change prior and post treatment. The pooled WM of ΔPD was 2.78 mm (95% CI = 2.31 to 3.25 mm; Figure 3A). WM of ΔPD 2.88 mm (95% CI = 2.31 to 3.45 mm; Figure 3B) was calculated in eighteen study groups using the barrier membrane. Similarly, WM of ΔPD 2.6 mm (95% CI = 1.9 to 3.3 mm; Figure 3C) was obtained in ten study groups, without membranes used to cover the graft. In four study groups, that evaluated submerged healing, WM of ΔPD 2.68 mm (95% CI = 1.71 to 3.64 mm; Figure 3D) was obtained, meanwhile WM of ΔPD 2.77 mm (95% CI = 2.23 to 3.3 mm; Figure 3E) was granted in twenty three study groups of non-submerged healing.
Figure 3A.
Meta-analysis for probing depth change (ΔPD).
The calculated WM was 2.78 mm (95% CI = 2.31 to 3.25 mm)
Figure 3B.
Meta-analysis for probing depth change (ΔPD) with membrane coverage of the grafted area.
The calculated WM was 2.88 mm (95% CI = 2.31 to 3.45 mm)
Figure 3C.
Meta-analysis for probing depth change (ΔPD) without membrane coverage of the grafted area.
The calculated WM was 2.6 mm (95% CI = 1.9 to 3.3 mm)
Figure 3D.
Meta-analysis for probing depth change (ΔPD) in submerged peri-implant defect healing.
The calculated WM was 2.68 mm (95% CI = 1.71 to 3.64 mm)
Figure 3E.
Meta-analysis for probing depth change (ΔPD) in non-submerged peri-implant defect healing.
The calculated WM was 2.77 mm (95% CI = 2.23 to 3.3 mm)
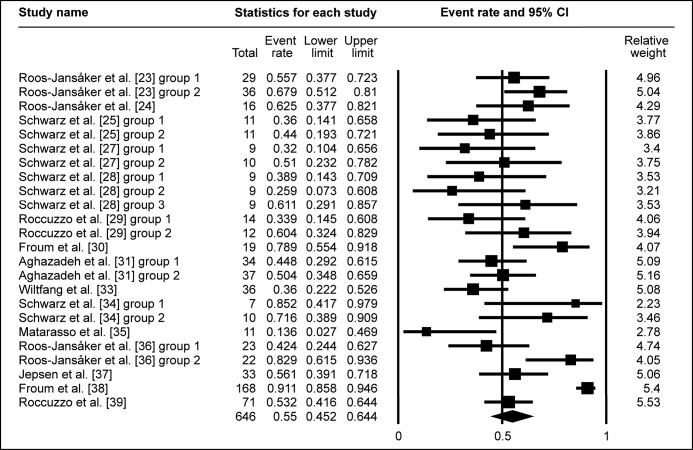
The percentage of BOP reduction was reported in fifteen studies, with the WM being 55% (95% CI = 45.2 to 64.4%; Figure 4A).
Figure 4A.
Meta-analysis for bleeding on probing change (ΔBOP).
The calculated WM was 55% (95% CI = 45.2% to 64.4%)
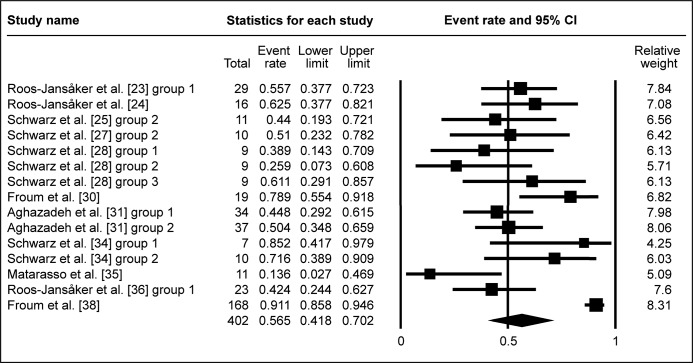
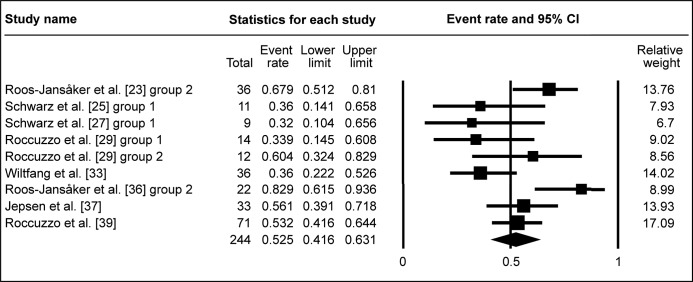
Studies, with membranes used, exhibited WM of ΔBOP 56.5% (95% CI = 41.8 to 70.2%; Figure 4B). This was similar for studies, without membranes used for graft coverage, where WM of ΔBOP was 52.5% (95% CI = 41.6 to 63.1%; Figure 4C). Due to low number of studies, investigating ΔBOP after submerged healing surgery, meta-analysis of submergence impact on ΔBOP was not conducted.
Figure 4B.
Meta-analysis for bleeding on probing change (ΔBOP) with membrane coverage of the grafted area.
The calculated WM was 56.5% (95% CI = 41.8% to 70.2%)
Figure 4C.
Meta-analysis for bleeding on probing change (ΔBOP) without membrane coverage of the grafted area.
The calculated WM was 52.5% (95% CI = 41.6% to 63.1%)
Risk of bias across studies
There were several limitations present in the current meta-analysis. Current review includes studies written in English only, which could introduce a publication bias. The included studies were of relatively short follow-up period and included relatively small numbers of patients. There were various degrees of heterogeneity in each study design, case selection, and treatment provided among studies. The absence of control groups was an important limitation. There was also too high heterogeneity of the selected studies to compare the impact of different bone grafting materials or membrane types for the final treatment outcome.
DISCUSSION
Peri-implantitis can be defined as a clinical condition characterized by an inflammatory reaction that involves the hard and soft tissue, which causes loss of supporting bone and pathological pocket formation surrounding the osseointegrated dental implants [1-18]. Several therapies options have been developed in order to manage the peri-implantitis, which could be non-surgical or surgical [6,10,47-50].
A conservative treatment option may be useful in cases without bone loss and a pocket formation limited to 3 - 4 mm. In case of evident bone loss and pocket formation over 5 mm, the surgical treatment seems to be necessary [22-39]. Surgical techniques can be divided into resective and regenerative surgery [6,50].
The resective implant treatment attempts to eliminate the aetiologic factors and maintain optimal peri-implant conditions, mainly by cleaning the surfaces of the implants; whereas regenerative therapy (using bone grafts, membranes and growth factors) aims to regenerate peri-implant bone defect and reconstruct the peri-implant unit to previously existing normal physiologic limits [6,47,49,50,66].
Romeo et al. [66] have compared the efficacy of resective surgery with that of implantoplasty. The results obtained after 3 years of therapy demonstrated that the marginal bone loss was significantly lower after implantoplasty and the surgical therapy without biomaterial results effective in case of deep peri-implantitis.
The present investigation aimed to screen what is published in the recent international literature on the overall treatment outcomes of reconstructive procedures in treating peri-implantitis. The application of bone grafting materials for peri-implant bone defect treatment was used in all the recorded studies [22-39]. The meta-analysis showed that 1.97 mm mean ΔRBL was gained after surgical regenerative treatment. The lowest bone fill (0.2 [1.8] mm) was obtained in the comparative study by Aghazadeh et al. [31] using autografts, which was similar in results of Deppe et al. [22] (0.3 [1.3] mm) and Wohlfahrt et al. [32] (0.1 [1.9] mm) study groups, where surgical non-regenerative treatment was applied. Autogenous bone, which has been often defined as the “gold standard” in bone augmentation, shows a volume loss of approximately 40% during healing time. On the other hand, synthetic bone graft substitutes show a high stability in volume, but remain nearly or completely unresorbed even several years after surgery [70]. Recently, Araujo and Lindhe [71] demonstrated, that autologous bone neither stimulate nor retard new bone formation in beagle dog extraction socket model. This raises an open dispute, if autografts, despite of their osteoinductive and osteogenic properties, still might be described as “gold standard” in situations, where slow resorption and adequate space maintaining biomaterials are desired. In contrary, regeneration of peri-implant defects with autografts mixed with bovine bovine-derived xenograft [33] or beta tricalcium phosphate [22] exhibited 2.1 (1.1) mm and 3.5 (2.4) mm peri-implant bone gain correspondingly. Decreased resorption rate and increased osteoconductivity of composite autograft mixture with synthetic or xenogenic matrials compared of autogratfs alone could influence this better outcome. However, it is important to note, that visual bone fill on the radiographs per se is barely sufficient to claim a successful long-term outcome after peri-implantitis treatment [72].
The highest ΔRBL aproximatelly 3.5 to 3.8 mm was reported in three studies (Froum et al. [30]; Wiltfang et al. [33]; Jepsen et al. [37]). Froum et al. [30] used enamel matrix derivative and platelet derived growth factor (PDGF) to enhance regenerative outcome. This might influence the increased bone fill, however well designed studies and long-term RCTs, comparing effectiveness of these bioactive materials on peri-implant bone regeneration, currently are still lacking. Wilfang el al. [33] and Jepsen et al. [37] used bovine-derived xenograft and porous titanium granules correspondingly to fill peri-implant bone defects. The above average ΔRBL, obtained in the latter two studies, should be also interpreted with caution, because both xenograft and porous titanium granules are highly radiopaque materials, and it is difficult to discern biomaterial and newly formed osseous tissue.
There were numerous surgical techniques described in the included articles of this review to regenerate the peri-implant bone defects, including various submergence protocols (e.g. submerged or non-submerged healing), polytetrafluoroethylene (PTFE), collagen, synthetic membranes or no membrane used to cover the grafted area. Submergence of the healing site was employed in four study groups of the included articles [22,24,32]. The rest study groups included in this review applied non-submerged healing. The meta-analysis and comparison between submerged and non-submerged sites provided similar clinical outcomes, having mean ΔRBL 2.17 mm and 1.91 mm alongside to the mean ΔPD 2.68 mm and 2.77 mm in submerged and non-submerged sites correspondingly.
The use of barrier membrane to cover the grafted area is often an operator dependent decision. Barrier membranes are intended to stabilize the graft and to prevent epithelial downgrowth and fibroblast transgrowth into the grafted area, thereby favouring the population of bone cells in a bony defect. In agreement to findings in this review, Sahrmann et al. [73] concluded that guided bone regeneration using both bone graft substitute and membrane represents the major part of published surgical regenerative treatment of peri-implantitis cases. Schwarz et al. [27] stated that combination of biomaterial (bovine-derived xenograft) together with collagen membrane resulted in improved clinical outcomes compared to biomaterial (nanocrystalline hydroxyapatite) alone. However, in this study different biomaterials were used, therefore comparisons should be interpreted with caution. Another study of Schwarz et al. [28] have shown that the use of a bone graft with the additional placement of a membrane may be more efficient in specific bone lesions around implants, however well-defined crater-like defect may improve the retention of the bone graft without additional use of the barrier membrane thereby favouring the healing [27,36]. Further studies of Roos-Jansaker et al. [36,74] and systematic reviews by Figuero et al. and Chan et al. [75,76] concluded that the application of a barrier membrane is costly, time consuming, technique sensitive, related to high membrane exposure risk, and its application did not provided improved clinical outcome nor in the terms of RBL, neither PD or BOP. This is in agreement with findings of meta-analysis in this review, where mean ΔRBL was 1.86 mm versus 2.12 mm, mean ΔPD 2.88 mm versus 2.6 mm, and mean ΔBOP 56.5% versus 52.5% in case with and without membrane used for graft coverage correspondingly. These findings suggest that barrier membrane placement might be not necessary in well-contained peri-implant bone defects.
The meta-analysis revealed reduction of PD and BOP after surgical regenerative treatment of peri-implantitis in all included studies, with mean ΔPD 2.78 mm and mean ΔBOP 55%. Although surgical regenerative treatment resulted in a clinical healthier situation around treated implants, most of the included studies [23,24,28,29,31-33,35,37,39] had minimal 12 months postoperative follow-up period to meet the borderline of the inclusion criteria. At the time there is very limited number of long-term RCTs, investigating the outcomes of surgical regenerative treatment of peri-implantitis [39,48]. Nevertheless, in real clinical situations when every treatment modality should be well applied and effective to treat peri-implantis, the ethical concern is an important issue. Constantly, there were only three RCTs included in this systematic review with the longest 12 months follow-up [31,32,37]. Future years of observation are necessary to verify whether an osseous defect fill is adequate to ensure favourable long-term maintenance. A 6 months case series study published by of Schwarz et al. [40] concluded that both the application of alloplastic material (nanocrystalline hydroxyapatite) or bovine-derived xenograft covered with collagen membrane induce significant improvement in clinical parameters (probing depth, clinical attachment level) at the healing control. A continuing follow-up of the same study group in a 2-years outcomes published by Schwarz et al. [25] also underlined the successful bone filling of the peri-implant defect applying both with nanocrystalline hydroxyapatite and bovine-derived xenograft in combination with collagen membrane that provided a significant reduction of the PD and gain in clinical attachment level. However, in the 4-year follow-up [27], the combination of bovine-derived xenograft and collagen membrane were more clinically efficacious as compared to nanocrystalline hydroxyapatite alone. This difference might be influenced by different long-term physicochemical properties of the applied bone graft substitute. In this systematic review high heterogeneity among the studies regarding surgical protocols, and selection of biomaterials were found, therefore at the time no clear recommendations could be drawn, how various treatment modalities or grafting materials could influence the clinical outcomes on the long-term basis.
Limitations
Even if a comprehensive and complete investigation of the effects of surgical therapies has been performed, there were some limitations to this systematic review. Surgical regenerative treatment of peri-implantitis is a complex procedure, depending on various multiple factors, including patient general health condition, oral hygiene habits, defect configuration, implant surface characteristics, decontamination procedure, surgical technique, postoperative maintenance program, and various other factors which are not possible to fit within the frames of systematic literature review and meta-analysis.
CONCLUSIONS
Within the limits of this systematic review, surgical regenerative treatment is a predictable option in managing peri-implantitis and improving clinical parameters of peri-implant tissues. There is no fundamental advantage of membrane use for bone graft coverage or submergence of the healing site on the final outcome of peri-implant defect regeneration.
Due to the limited number of randomized clinical trials, at the time there is a lack of scientific evidence in the literature regarding the superiority of the regenerative versus non-regenerative surgical treatment. No conclusions could be drawn regarding the choice of biomaterials for peri-implant bone regeneration due to high heterogeneity among the present studies. Further well designed long-term randomized clinical trials, investigating the impact of peri-implant defect configuration, application of different grafting materials and bioactive modifiers, implant surface decontamination methods, and various surgical protocols on the final outcome of the regenerative procedure are needed.
Acknowledgments
ACKNOWLEDGMENTS AND DISCLOSURE STATEMENTS
The authors report no conflicts of interest related to this study. The authors would like to thank Irena Nedzelskiene (Clinic of Dental and Oral Pathology, Lithuanian University of Health Sciences, Kaunas, Lithuania) for her help in statistical analysis.
REFERENCES
- 1.Swart LC, Dreyer WP, van Zyl PP, Blignaut RJ. Early loading of mandibular implants placed immediately after extraction: a 10-year prospective study of eight patients. Int J Oral Maxillofac Implants. 2014 Nov-Dec;29(6):1388-96. [DOI] [PubMed]
- 2.Buser D, Bornstein MM, Weber HP, Grütter L, Schmid B, Belser UC. Early implant placement with simultaneous guided bone regeneration following single tooth extraction in the esthetic zone: A cross-sectional, retrospective study in 45 patients with a 2- to 4-year follow-up. J Periodontol 2008;79:1773–1781. [DOI] [PubMed]
- 3.Simion M, Jovanovic SA, Tinti C, Benfenati SP. Long-term evaluation of osseointegrated implants inserted at the time or after vertical ridge augmentation. A retrospective study on 123 implants with 1-5 year follow-up. Clin Oral Implants Res 2001;12:35-45. [DOI] [PubMed]
- 4.Donati M, Ekestubbe A, Lindhe J, Wennstrom JL. Implant-supported single-tooth restorations. A 12-year prospective study. Clin Oral Implants Res. 2015 Nov 18. [DOI] [PubMed]
- 5.Balshi TJ, Wolfinger GJ, Stein BE, Balshi SF. A long-term retrospective analysis of survival rates of implants in the mandible. Int J Oral Maxillofac Implants. 2015 Nov-Dec;30(6):1348-54. [DOI] [PubMed]
- 6.Lang NP, Wilson TG, Corbet EF. Biological complications with dental implants: their prevention, diagnosis and treatment. Clin Oral Implants Res. 2000;11 Suppl 1:146-55. [DOI] [PubMed]
- 7.Mombelli A, van Oosten MA, Schurch E Jr, Lang NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol. 1987 Dec;2(4):145-51. [DOI] [PubMed]
- 8.Westfelt E, Rylander H, Dahlén G, Lindhe J. The effect of supragingival plaque control on the progression of advanced periodontal disease. J Clin Periodontol. 1998 Jul;25(7):536-41. [DOI] [PubMed]
- 9.Leonhardt A, Renvert S, Dahlén G. Microbial findings at failing implants. Clin Oral Implants Res. 1999 Oct;10(5):339-45. [DOI] [PubMed]
- 10.Mombelli A, Lang NP. The diagnosis and treatment of peri-implantitis. Periodontol 2000. 1998 Jun;17:63-76. [DOI] [PubMed]
- 11.van Steenberghe D, Naert I, Jacobs R, Quirynen M. Influence of inflammatory reactions vs. occlusal loading on peri-implant marginal bone level. Adv Dent Res. 1999 Jun;13:130-5. [DOI] [PubMed]
- 12.Esposito M, Thomsen P, Ericson LE, Lekholm U. Histopathologic observations on early oral implant failures. Int J Oral Maxillofac Implants. 1999 Nov-Dec;14(6):798-810. [PubMed]
- 13.Quirynen M, Peeters W, Naert I, Coucke W, van Steenberghe D. Peri-implant health around screw-shaped c.p. titanium machined implants in partially edentulous patients with or without ongoing periodontitis.Clin Oral Implants Res. 2001 Dec;12(6):589-94. [DOI] [PubMed]
- 14.Cicciù M, Risitano G, Maiorana C, Franceschini G. Parametric analysis of the strength in the ''Toronto'' osseous-prosthesis system. Minerva Stomatol. 2009 Jan-Feb;58(1-2):9-23. [PubMed]
- 15.Renvert S, Lessem J, Dahlen G, Renvert H, Lindahl C. Mechanical and repeated antimicrobial therapy using a local drug delivery system in the treatment of peri implantitis: a randomized clinical trial. J Periodontol. 2008;79:836-44. [DOI] [PubMed]
- 16.Jovanovic SA. Diagnosis and treatment of peri-implant disease. Curr Opin Period 1994:194-204. [PubMed]
- 17.Jovanovic SA. The management of peri-implant breakdown around functioning osseointegrated dental implants. J Periodontol. 1993 Nov; 64(11 Suppl):1176-83. [DOI] [PubMed]
- 18.Artzi Z, Tal H, Chweidan H. Bone regeneration for reintegration in peri-implant destruction. Compend Contin Educ Dent. 1998 Jan;19(1):17-20, 22-3, 26-8; quiz 30. [PubMed]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009 Oct;62(10):1006-12. [DOI] [PubMed]
- 20.American Academy of Periodontology. Academy report: Peri-implant mucositis and peri-implantitis: a current understanding of their diagnoses and clinical implications. J Periodontol. 2013 Apr;84(4):436-43. [DOI] [PubMed]
- 21.Higgins JP, Altman DG, Gotzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928. [DOI] [PMC free article] [PubMed]
- 22.Deppe H, Horch HH, Neff A. Conventional versus CO2 laser-assisted treatment of peri-implant defects with the concomitant use of pure-phase beta-tricalcium phosphate: a 5-year clinical report. Int J Oral Maxillofac Implants. 2007 Jan-Feb;22(1):79-86. [PubMed]
- 23.Roos-Jansåker AM, Renvert H, Lindahl C, Renvert S. Surgical treatment of peri-implantitis using a bone substitute with or without a resorbable membrane: a prospective cohort study. J Clin Periodontol. 2007 Jul;34(7):625-32. [DOI] [PubMed]
- 24.Roos-Jansåker AM, Renvert H, Lindahl C, Renvert S. Submerged healing following surgical treatment of peri-implantitis: a case series. J Clin Periodontol. 2007 Aug;34(8):723-7. Epub 2007 May 29. [DOI] [PubMed]
- 25.Schwarz F, Sculean A, Bieling K, Ferrari D, Rothamel D, Becker J. Two-year clinical results following treatment of peri-implantitis lesions using a nanocrystalline hydroxyapatite or a natural bone mineral in combination with a collagen membrane. J Clin Periodontol. 2008 Jan;35(1):80-7. [DOI] [PubMed]
- 26.Romanos GE, Nentwig GH. Regenerative therapy of deep peri-implant infrabony defects after CO2 laser implant surface decontamination. Int J Periodontics Restorative Dent. 2008 Jun;28(3):245-55. [PubMed]
- 27.Schwarz F, Sahm N, Bieling K, Becker J. Surgical regenerative treatment of peri-implantitis lesions using a nanocrystalline hydroxyapatite or a natural bone mineral in combination with a collagen membrane: a four-year clinical follow-up report. J Clin Periodontol. 2009 Sep;36(9):807-14. [DOI] [PubMed]
- 28.Schwarz F, Sahm N, Schwarz K, Becker J. Impact of defect configuration on the clinical outcome following surgical regenerative therapy of peri-implantitis. J Clin Periodontol. 2010 May;37(5):449-55. [DOI] [PubMed]
- 29.Roccuzzo M, Bonino F, Bonino L, Dalmasso P. Surgical therapy of peri-implantitis lesions by means of a bovine-derived xenograft: comparative results of a prospective study on two different implant surfaces. J Clin Periodontol. 2011 Aug;38(8):738-45. [DOI] [PubMed]
- 30.Froum SJ, Froum SH, Rosen PS. Successful management of peri-implantitis with a regenerative approach: a consecutive series of 51 treated implants with 3- to 7.5-year follow-up. Int J Periodontics Restorative Dent. 2012 Feb;32(1):11-20. [PubMed]
- 31.Aghazadeh A, Rutger Persson G, Renvert S. A single-centre randomized controlled clinical trial on the adjunct treatment of intra-bony defects with autogenous bone or a xenograft: results after 12 months. J Clin Periodontol. 2012 Jul;39(7):666-73. [DOI] [PubMed]
- 32.Wohlfahrt JC, Lyngstadaas SP, Rønold HJ, Saxegaard E, Ellingsen JE, Karlsson S, Aass AM. Porous titanium granules in the surgical treatment of peri-implant osseous defects: a randomized clinical trial. Int J Oral Maxillofac Implants. 2012 Mar-Apr;27(2):401-10. [PubMed]
- 33.Wiltfang J, Zernial O, Behrens E, Schlegel A, Warnke PH, Becker ST. Regenerative treatment of peri-implantitis bone defects with a combination of autologous bone and a demineralized xenogenic bone graft: a series of 36 defects. Clin Implant Dent Relat Res. 2012 Jun;14(3):421-7. [DOI] [PubMed]
- 34.Schwarz F, Hegewald A, John G, Sahm N, Becker J. Four-year follow-up of combined surgical therapy of advanced peri-implantitis evaluating two methods of surface decontamination. J Clin Periodontol. 2013 Oct;40(10):962-7. [DOI] [PubMed]
- 35.Matarasso S, Iorio Siciliano V, Aglietta M, Andreuccetti G, Salvi GE. Clinical and radiographic outcomes of a combined resective and regenerative approach in the treatment of peri-implantitis: a prospective case series. Clin Oral Implants Res. 2014 Jul;25(7):761-7. [DOI] [PubMed]
- 36.Roos-Jansåker AM, Persson GR, Lindahl C, Renvert S. Surgical treatment of peri-implantitis using a bone substitute with or without a resorbable membrane: a 5-year follow-up. J Clin Periodontol. 2014 Nov;41(11):1108-14. [DOI] [PubMed]
- 37.Jepsen K, Jepsen S, Laine ML, Anssari Moin D, Pilloni A, Zeza B, Sanz M, Ortiz-Vigon A, Roos-Jansåker AM, Renvert S. Reconstruction of peri-implant osseous defects: a multicenter randomized trial. J Dent Res. 2016 Jan;95(1):58-66. [DOI] [PubMed]
- 38.Froum SJ, Froum SH, Rosen PS. A regenerative approach to the successful treatment of peri-implantitis: a consecutive series of 170 implants in 100 patients with 2- to 10-year follow-up. Int J Periodontics Restorative Dent. 2015 Nov-Dec;35(6):857-63. [DOI] [PubMed]
- 39.Roccuzzo M, Gaudioso L, Lungo M, Dalmasso P. Surgical therapy of single peri-implantitis intrabony defects, by means of deproteinized bovine bone mineral with 10% collagen. J Clin Periodontol. 2016 Mar;43(3):311-8. [DOI] [PubMed]
- 40.Schwarz F, Bieling K, Latz T, Nuesry E, Becker J. Healing of intrabony peri-implantitis defects following application of a nanocrystalline hydroxyapatite (Ostim) or a bovine-derived xenograft (Bio-Oss) in combination with a collagen membrane (Bio-Gide). A case series. J Clin Periodontol. 2006 Jul;33(7):491-9. [DOI] [PubMed]
- 41.Caccianiga G, Rey G, Baldoni M, Paiusco A. Clinical, radiographic and microbiological evaluation of high level laser therapy, a new photodynamic therapy protocol, in peri-implantitis treatment; a pilot experience. Biomed Res Int. 2016;2016:6321906. [DOI] [PMC free article] [PubMed]
- 42.Mijiritsky E, Yatzkaier G, Mazor Z, Lorean A, Levin L. The use of porous titanium granules for treatment of peri-implantitis lesions: preliminary clinical and radiographic results in humans. Br Dent J. 2013 Mar;214(5):E13. [DOI] [PubMed]
- 43.Schwarz F, Sahm N, Iglhaut G, Becker J. Impact of the method of surface debridement and decontamination on the clinical outcome following combined surgical therapy of peri-implantitis: a randomized controlled clinical study. J Clin Periodontol. 2011 Mar;38(3):276-84. [DOI] [PubMed]
- 44.Froum SJ, Rosen PS. Reentry evaluation following treatment of peri-implantitis with a regenerative approach. Int J Periodontics Restorative Dent. 2014 Jan-Feb;34(1):47-59. [DOI] [PubMed]
- 45.Schwarz F, Sahm N, Becker J. Combined surgical therapy of advanced peri-implantitis lesions with concomitant soft tissue volume augmentation. A case series. Clin Oral Implants Res. 2014 Jan;25(1):132-6. [DOI] [PubMed]
- 46.Behneke A, Behneke N, d'Hoedt B. Int J Oral Maxillofac Implants. Treatment of peri-implantitis defects with autogenous bone grafts: six-month to 3-year results of a prospective study in 17 patients. 2000 Jan-Feb;15(1):125-38. [PubMed]
- 47.Mahato N, Wu X, Wang L. Management of peri-implantitis: a systematic review, 2010-2015. Springerplus. 2016 Feb 1;5:105. [DOI] [PMC free article] [PubMed]
- 48.Esposito M, Grusovin MG, Worthington HV. Cochrane Interventions for replacing missing teeth: treatment of peri-implantitis. Database Syst Rev. 2012 Jan 18;1:CD004970. [DOI] [PMC free article] [PubMed]
- 49.Heitz-Mayfield LJ, Mombelli A. The therapy of peri-implantitis: a systematic review. Int J Oral Maxillofac Implants. 2014;29 Suppl:325-45. [DOI] [PubMed]
- 50.Ramanauskaite A, Daugela P, Juodzbalys G. Treatment of peri-implantitis: Meta-analysis of findings in a systematic literature review and novel protocol proposal. Quintessence Int. 2016;47(5):379-93. [DOI] [PubMed]
- 51.Materni A. Managing an extreme peri-implantitis. Minerva Stomatol. 2013 Sep;62(9):295-305. [PubMed]
- 52.Slotte C, Lindfors N, Nannmark U. Surgical reconstruction of peri-implant bone defects with prehydrated and collagenated porcine bone and collagen barriers: case presentations. Clin Implant Dent Relat Res. 2013 Oct;15(5):714-23. [DOI] [PubMed]
- 53.Tozum TF, Keçeli HG. Treatment of peri-implant defect with modified sandwich bone augmentation. Case report and follow-up. N Y State Dent J. 2008 Jun-Jul;74(4):52-7. [PubMed]
- 54.Nicoli LG, Pigossi SC, Marcantonio C, Leal Zandim-Barcelos D, Marcantonio E Jr. Surgical treatment of implants affected by periimplantitis after 15 years of loading: a case report. Implant Dent. 2016 Apr;25(2):288-92. [DOI] [PubMed]
- 55.Lombardo G, Corrocher G, Rovera A, Pighi J, Marincola M, Lehrberg J, Nocini PF. Decontamination using a desiccant with air powder abrasion followed by biphasic calcium sulfate grafting: a new treatment for peri-implantitis. Case Rep Dent. 2015;2015:474839. [DOI] [PMC free article] [PubMed]
- 56.Lorenz B, Kang T. Treating peri-implantitis using a combined regenerative/resective procedure: a case report. Compend Contin Educ Dent. 2013 Apr;34(4):e57-61. [PubMed]
- 57.Kim JE, Kim HY, Huh JB, Lee JY, Shin SW. A two-stage surgical approach to the treatment of severe peri-implant defect: a 30-month clinical follow-up report. J Oral Implantol. 2014 Jun;40(3):299-305. [DOI] [PubMed]
- 58.Park JB. Treatment of peri-implantitis with deproteinised bovine bone and tetracycline: a case report. Gerodontology. 2012 Jun;29(2):145-9. [DOI] [PubMed]
- 59.Azzeh MM. Er,Cr:YSGG laser-assisted surgical treatment of peri-implantitis with 1-year reentry and 18-month follow-up. J Periodontol. 2008 Oct;79(10):2000-5. [DOI] [PubMed]
- 60.Lu SY, Huang CC. Resolution of an active peri-implantitis in a chronic steroid user by bone augmentation with PepGen P-15 and a barrier membrane. J Oral Implantol. 2007;33(5):280-7. [DOI] [PubMed]
- 61.Bassi F, Poli PP, Rancitelli D, Signorino F, Maiorana C. Surgical Treatment of Peri-Implantitis: A 17-Year Follow-Up Clinical Case Report. Case Rep Dent. 2015;2015:574676. [DOI] [PMC free article] [PubMed]
- 62.Schwarz F, John G, Sahm N, Becker J. Combined surgical resective and regenerative therapy for advanced peri-implantitis with concomitant soft tissue volume augmentation: a case report. Int J Periodontics Restorative Dent. 2014 Jul-Aug;34(4):489-95. [DOI] [PubMed]
- 63.Malchiodi L, Cucchi A, Ghensi P, Bondì V. A case of rapidly progressive peri-implantitis around a short sintered porous-surfaced implant. J Indiana Dent Assoc. 2009-2010 Winter;88(4):33-5. [PubMed]
- 64.Santos VR, Duarte PM. Surgical anti-infective mechanical therapy for peri-implantitis: a clinical report with a 12-month follow-up. Gen Dent. 2009 May-Jun;57(3):230-5; quiz 236-7. [PubMed]
- 65.Shabestari GO, Shayesteh YS, Khojasteh A, Alikhasi M, Moslemi N, Aminian A, Masaeli R, Eslami B, Treister NS. Implant placement in patients with oral bisphosphonate therapy: a case series. Clin Implant Dent Relat Res. 2010 Sep;12(3):175-80. [DOI] [PubMed]
- 66.Romeo E, Lops D, Chiapasco M, Ghisolfi M, Vogel G. Therapy of peri-implantitis with resective surgery. A 3-year clinical trial on rough screw-shaped oral implants. Part II: radiographic outcome. Clin Oral Implant Res. 2007;18(2):179–187. [DOI] [PubMed]
- 67.Lagervall M, Jansson LE. Treatment outcome in patients with peri-implantitis in a periodontal clinic: a retrospective study. J Periodontol. 2013 Oct;84(10):1365-73. [DOI] [PubMed]
- 68.Schwarz F, John G, Becker J. Reentry after combined surgical resective and regenerative therapy of advanced peri-implantitis: a retrospective analysis of five cases. Int J Periodontics Restorative Dent. 2015 Sep-Oct;35(5):647-53. [DOI] [PubMed]
- 69.Yoshino T, Yamamoto A, Ono Y. Innovative regeneration technology to solve peri-implantitis by Er:YAG laser based on the microbiologic diagnosis: a case series. Int J Periodontics Restorative Dent. 2015 Jan-Feb;35(1):67-73. [DOI] [PubMed]
- 70.Iezzi G, Degidi M, Scarano A, Petrone G, Piattelli A. Anorganic bone matrix retrieved 14 years after a sinus augmentation procedure: a histologic and histomorphometric evaluation. J Periodontol. 2007 Oct;78(10):2057-61. [DOI] [PubMed]
- 71.Araújo MG, Lindhe J. Socket grafting with the use of autologous bone: an experimental study in the dog. Clin Oral Implants Res. 2011 Jan;22(1):9-13. [DOI] [PubMed]
- 72.Salvi GE, Lang NP. Diagnostic parameters for monitoring peri-implant conditions. Int J Oral Maxillofac Implants. 2004;19 Suppl:116-27. [PubMed]
- 73.Sahrmann P, Attin T, Schmidlin PR. Regenerative treatment of peri-implantitis using bone substitutes and membrane: a systematic review. Clin Implant Dent Relat Res. 2011 Mar;13(1):46-57. [DOI] [PubMed]
- 74.Roos-Jansåker AM, Lindahl C, Persson GR, Renvert S. Long-term stability of surgical bone regenerative procedures of peri-implantitis lesions in a prospective case-control study over 3 years. J Clin Periodontol. 2011 Jun;38(6):590-7. [DOI] [PubMed]
- 75.Figuero E, Graziani F, Sanz I, Herrera D, Sanz M. Management of peri-implant mucositis and peri-implantitis. Periodontol 2000. 2014 Oct;66(1):255-73. [DOI] [PubMed]
- 76.Chan HL, Lin GH, Suarez F, MacEachern M, Wang HL. Surgical management of peri-implantitis: a systematic review and meta-analysis of treatment outcomes. J Periodontol. 2014 Aug;85(8):1027-41. [DOI] [PubMed]