Abstract
Context
Patients with hepatic dysfunction suffer from many problems and associated complications in organs other than the liver. Therefore, it is very important to investigate the effects of different drugs in the treatment of these patients. Due to the high consumption of non-steroidal anti-inflammatory drugs (NSAIDs), studying the effects of these drugs in patients with hepatic dysfunction is particularly important.
Evidence Acquisition
Research studies published from 1958 to 2014 were investigated in the present study. The literature search was conducted based on the following keywords: non-steroidal anti-inflammatory drugs (NSAIDs), liver dysfunction, cirrhosis, pharmaceutical complications, drug-induced liver injury (DILI), and similar words from reliable resources. In total, 63 articles and two books (out of 179 initially identified resources) were included in the study.
Results
In addition to significant hemostatic disorders and cardiovascular disorders, disorders of the renal, respiratory, and gastrointestinal systems, as well as disorders of the central nervous system, occur in patients with hepatic dysfunction. The various NSAIDs have different effects on different bodily systems. Therefore, the appropriate drug should be chosen based on both the condition of the disease and the severity of the dysfunction.
Conclusions
Due to the potential adverse effects of NSAIDs in patients with hepatic disease, their impact on all bodily systems should be emphasized when determining whether their use is necessary. Further, the appropriate medication should be selected after a careful assessment of the severity of the disease and any associated complications. It is logical that medicines should only be prescribed by a qualified physician.
Keywords: NSAIDs, Central Nervous System, Cirrhosis, Liver Disease
1. Context
The liver is one of the most vital organs of the body (1). It has a role in the metabolism of carbohydrates, lipids, cholesterol, and proteins, the metabolism of drugs and toxins, and many other biological processes, some of which are still unknown (2, 3). Due to the various functions of the liver, hepatic impairments are complicated. Liver diseases are classified into two types: acute and chronic. In terms of etiology, the most important causes of acute liver disease worldwide are viral infections, alcohol, and pharmaceutical poisonings (3). Viral hepatitis (types B and C), alcohol, autoimmune hepatitis, and genetic disorders are all important and common causes of chronic hepatic failure (1, 3). The long-term process of the illness has led to increasing enhancement in a number of people with liver disease. Due to the numerous roles of the liver, patients with advanced chronic hepatic failure (cirrhotic patients) also suffer from numerous disorders in many other organs. For instance, coagulation disorders, gastrointestinal bleeding, gynecomastia and testicular atrophy, metabolic disorders, thrombocytopenia and leukopenia, anemia, hyponatremia, renal dysfunction (hepatorenal syndrome), pulmonary dysfunction (hepatopulmonary syndrome), nausea and anorexia, and spontaneous bacterial peritonitis are among the additional problems experienced by such patients (4-6). Accordingly, multiple medications are used for the treatment and control of patients with hepatic disorder. Cirrhotic patients periodically undergo multiple surgical and endoscopic procedures; hence, multiple medications are also used for these patients. In fact, the issue of multiple medication use is so important that there is a dedicated field of study entitled drug-induced liver injury or DILI (7). Another serious problem is that, unlike other organs such as the kidney, there are no proper numerical standards (e.g., glomerular filtration rate) or specific guidelines concerning the liver (3, 7). Most prior research studies have been conducted based on the pharmacokinetic properties of drugs (at low doses), and little information is currently available regarding the potential harm caused by most medications (in fact, comprehensive pharmacodynamic information is not available) (8). Anti-tuberculosis and anti-retroviral drugs account for a major proportion of the drugs identified as harmful in the field of DILI; however, statistics actually show that the majority of known liver damage is related to the use of acetaminophen and over-the-counter (OTC) drugs (8, 9). Unfortunately, these medications remain “unknown common murderers,” since few research studies (aside from the abovementioned studies involving drugs such as anti-tuberculosis drugs) have been conducted in relation to their harmful and pharmacokinetic characteristics (as associated with DILI) (7, 9).
According to the world health organization (WHO) as well as statistics from various countries, acetaminophen is the most widely used drug worldwide, followed by non-steroidal anti-inflammatory drugs (NSAID) such as aspirin and ibuprofen (whether obtained on prescription or OTC) (10). Therefore, studying the effects of these drugs in many patients, including patients with hepatic disease, is essential (http: /mohme.gov.ir). However, the use of these drugs is uncontrolled and this, coupled with the fact that relatively few studies have been conducted in this area, has led NSAIDs being prescribed for even a simple headache (7, 11). Meanwhile, some studies has shown that liver damage due to the administration of vitamin A is more significant than that due to paracetamol or ibuprofen (11-13). Further, a study regarding the relationship between naproxen, diclofenac, and ibuprofen and gastrointestinal bleeding showed that the possibility of gastrointestinal bleeding is very insignificant for those aged less than 45 years (in both sexes) (14).
Non-steroidal anti-inflammatory drugs have been known and used for many years. Indeed, the ancient Egyptians used salicylate compounds for pain relief in pregnant women. Charles Frederic Gerhardt succeeded in producing aspirin (the first NSAID) in 1859. Thereafter, the old generation drugs and, later, specific inhibitors of cyclooxygenase-2 (COX-2), as well as synthetic drugs, were produced (15, 16). These drugs are generally divided on the basis of their effect on the cyclooxygenase enzymes, which have a role in arachidonic acid metabolism. Further, all their positive and negative effects occur based on their impact on these enzymes. Arachidonic acid obtained from cellular membrane lipids is converted into important eicosanoids of the body (i.e., prostaglandins, prostacyclins, thromboxanes, and leukotrienes), with two isomers of the cyclooxygenase enzymes playing a pivotal role in this route (17-19). Accordingly, the NSAIDS are classically divided into three categories: salicylates, old NSAIDs (non-specific inhibitors), and COX-2 (specific inhibitors). In this study, the effects of the three categories will be discussed and reviewed in patients with hepatic dysfunction.
NSAIDs typically lead to the exacerbation of renal dysfunction and gastrointestinal bleeding, as well as reducing the effects of diuretics in cirrhotic patients (severe hepatic dysfunction) (20, 21). The widespread negative effects of these drugs coupled with the extensive metabolic disorders seen in patients with hepatic dysfunction have led to the recommendation “use with caution” being applied to NSAIDs. The various side effects of NSAIDs and the extended drug load in patients with hepatic disease have exacerbated this problem (8, 22). Although liver injury induced by these drugs occurs only rarely, their high level of consumption worldwide renders this issue very important. Indeed, NSAID-related DILI is the second most common cause of liver injury in France (2002) and Iceland (2013), as well as the third most common cause in Spain (2005) (23-25). Evidence shows that all NSAIDs have the potential for hepatotoxicity to different degrees (26, 27), which has been suggested as an important reason for withdrawing large numbers of these drugs from the market (e.g., rofecoxib). In most previous research studies and scientific reports, NSAID-induced liver injury has been reported in relation to the chronic use of the drug, while renal injury, gastrointestinal hemorrhage, and respiratory symptoms can occur even in a non-toxic dose (28-31). The mechanism of injury has been cited as hepato-cellular, and it is based on autoimmune issues, mitochondrial injury, and metabolic errors (32). The lesions seen in histological studies are similar to those related to acetaminophen and centrilobular necrosis in severe cases (33). The female gender, being aged over 50 years, and the presence of immune disorders have all been reported as important risk factors in various studies (34). Further, a cohort study found that the risk of liver injury is increased tenfold in patients with rheumatoid arthritis when compared to patients with osteoarthritis (29).
2. Evidence Acquisition
Resources published between 1958 and 2014 that are available in various reliable databases (e.g., PubMed, Medline (Ovid), Cochrane) were searched in the present study. A total of 65 papers and two books were investigated (Table 1).
Table 1. Data Sources for the Study.
| Drug | Study | Year |
|---|---|---|
| Aspirin | Lewis et al. (36) | 1983 |
| Julian et al. (37) | 1996 | |
| Levy et al. (38) | 1972 | |
| Gaudreault et al. (39) | 1982 | |
| He et al. (40) | 1998 | |
| Knight et al. (41) | 2009 | |
| Hurwitz et al. (42) | 1989 | |
| Gosalakkal et al. (43) | 2008 | |
| Katzung et al. (17) | 2012 | |
| Amino salicylic acid | Lewis et al. (36) | 1983 |
| Julian et al. (37) | 1996 | |
| Magnesium salicylate | Lewis et al. (36) | 1983 |
| Julian et al. (37) | 1996 | |
| Methyl salicylate | Lewis et al. (36) | 1983 |
| Julian et al. (37) | 1996 | |
| Toro-lamina | Lewis et al. (36) | 1983 |
| Julian et al. (37) | 1996 | |
| Ibuprofen | Sneader et al. (15) | 2005 |
| Katzung et al. (17) | 2012 | |
| Bushra et al. (44) | 2010 | |
| Casper et al. (45) | 2000 | |
| Durkin et al. (46) | 2006 | |
| Peter et al. (3) | 2012 | |
| Bateman et al. (67) | 1994 | |
| Ayres et al. (47) | 1987 | |
| Gaziano et al. (48) | 2006 | |
| Gladding et al. (49) | 2008 | |
| Dooley et al. (50) | 2007 | |
| Pargal et al. (51) | 1996 | |
| Gutthane et al. (14) | 1997 | |
| Diclofenac | Sneader et al. (15) | 2005 |
| Katzung et al. (17) | 2012 | |
| Naidoo et al. (52) | 2008 | |
| Solomon et al. (53) | 2006 | |
| O’Connor et al. (20) | 2003 | |
| Bessone et al. (26) | 2010 | |
| Peter et al. (3) | 2012 | |
| Swan et al. (54) | 2006 | |
| Indomethacin | Sneader et al. (15) | 2005 |
| Katzung et al. (17) | 2012 | |
| Sehgal et al. (55) | 2013 | |
| Giles et al. (56) | 2007 | |
| Akbarpour et al. (57) | 1985 | |
| Peter et al. (3) | 2012 | |
| Naproxen | JFC. (58) | 2013 |
| Katzung et al. (17) | 2012 | |
| Piroxicam | Katzung et al. (17) | 2012 |
| Peter et al. (3) | 2012 | |
| Weintraub et al. (59) | 1977 | |
| Ketorolac | Sneader et al. (15) | 2005 |
| Katzung et al. (17) | 2012 | |
| Sehgal et al. (53) | 2013 | |
| Goldkind et al. (32) | 2006 | |
| Mefenamic acid | Katzung et al. (17) | 2012 |
| Peter et al. (3) | 2012 | |
| Pringsheim et al. (60) | 2008 | |
| Trinus et al. (61) | 1977 | |
| Rofecoxib | Sneader et al. (15) | 2005 |
| Katzung et al. (17) | 2012 | |
| Celecoxib | McCormack et al. (63) | 2011 |
| Penning et al. (62) | 1997 | |
| Derry et al. (64) | 2012 | |
| Mathew et al. (65) | 2011 | |
| Mukherjee et al. (66) | 2001 | |
| Katzung et al. (17) | 2012 | |
| Peter et al. (3) | 2012 |
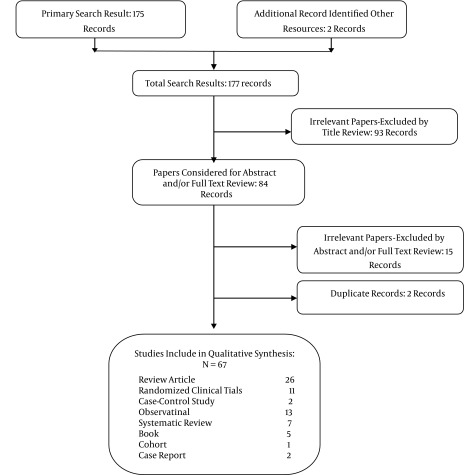
Searching was conducted based on the following keywords: NSAIDs, hepatic dysfunction, cirrhosis, pharmaceutical complication, DILI (drug-induced liver injury), and similar words used in reliable sources. Our primary search identified 169 articles and six books. All irrelevant data, including 110 irrelevant abstracts or full-text reviews, duplicate records, and articles or books where access to the full text was lacking, were excluded. The final resources included 11 clinical trials, two case-control studies, two case reports, 13 observational studies, seven systematic reviews, five books, one cohort study, and 26 review articles (Figure 1).
Figure 1. Study Selection.
3. Results
Due to the significant differences between the three categories of NSAIDs, this study will analyze these drugs according to the classical division.
3.1. Salicylates
Aspirin is the main salicylate, although other derivatives of salicylic acid such as amino salicylic acid, magnesium salicylate, methyl salicylate, and toro-lamina, as well as synthetic painkillers derived from aspirin, are included in this category. The most common and important uses of aspirin include the relief of pain and fever, anticoagulation, and preventive treatment of cardiovascular and cerebrovascular diseases (35, 36). The drug is offered in various pharmaceutical forms, is quickly absorbed, and has a bioavailability of 50% to 75%. Aspirin is conjugated in the liver, enters many body fluids and tissues, and is eventually excreted through the kidneys. Although 50% to 100% of aspirin is removed during dialysis, it is not recommended to be prescribed for patients with severe kidney injury (37).
The mechanism of the drug’s effect is based on an inhibitive effect on both the COX enzymes, and unlike the effects of other NSAIDs, this effect is permanent. The antiplatelet effects of aspirin are also induced by this irreversible effect on prostaglandins and thromboxane A2 and the inherent inability of the platelets in the production of new proteins (37). Research studies concerning the association between the serum level of aspirin and its favorable impacts or adverse effects show that its analgesic, antipyretic, and antiplatelet effects can be observed in a serum level of approximately 100 µg/ml. Gastrointestinal intolerance or complications (but not both), GI hemorrhage, sensitivity to the drug, as well as hemostasis disorders can also be seen at this dose (albeit with less prevalence). A higher level (about 150 to 400 µg/ml) is required to obtain aspirin’s anti-inflammatory effects, as well as for its use in the treatment of rheumatic fever, with the initial symptoms of salicylism being seen at this dosage. Higher serum levels can lead to poisoning and the occurrence of severe salicylism (38, 39).
Another important issue related to the use of aspirin is its significant association with Reye’s syndrome. This syndrome, which mostly occurs in childhood following the use of aspirin to control a fever associated with viral infections (especially influenza and varicella), is very dangerous and often fatal despite its low prevalence (40). Hence, many health organizations (including the WHO and the US food and drug administration) advise against using aspirin in subjects younger than 19 years (or 16 years in some cases) (41). Further, the avoidance of aspirin following influenza vaccination has been advised (42). Although the exact mechanism and cause of Reye’s syndrome is not yet known, the alternation in hepatocytes and genetic enzymatic disorder related to fatty acids oxidation can provide important information on the usage of aspirin in different patients (40, 42).
In addition, studies have shown that the simultaneous use of aspirin and other NSAIDs (particularly floctafenine and ketorolac) is not appropriate. Aspirin increases the effects of medications such as anticoagulants, systemic corticosteroids, sulfonylureas, and iodine, as well as reducing the effectiveness of ACE inhibitors and loop diuretics (17). Tricyclic antidepressants and SSRIs, antiplatelets, calcium channel blockers, loop diuretics, and other NSAIDs increase both the serum level and the effectiveness of aspirin (hence its toxicity equally) (17, 40).
The use of aspirin in patients with hepatic disease has been discussed in several aspects. The first issue is a detailed examination of the disease’s severity, as well as other underlying disorders in the individual. For example, the risk of Reye’s syndrome (caused by aspirin) in children with viral infections is increased when the individual has an underlying disorder affecting the metabolism of fatty acids (40). Renal failure in patients with hepatic disease (with or without hepatic cause) is among the other contraindications of aspirin, which again shows that paying attention to the underlying disorder is particularly important in these patients. Research suggests that the concurrent use of alcoholic beverages and aspirin also shows the possibility of damage to the gastrointestinal mucosa. Therefore, avoiding the administration of aspirin seems reasonable in patients whose hepatic disorder is caused by alcoholism (39, 40). Additionally, aspirin-related pharmaceutical interventions alongside the medications used to treat patients with hepatic disease should be considered. Several studies have suggested that this drug reduces the effects of loop diuretics (one of the most important drugs used in the treatment of ascites) (17). However, it is important to consider that the use of aspirin (in cases without any other underlying problem) is not contraindicated in patients with mild hepatic dysfunction. In fact, aspirin can be prescribed for patients who show only some degree of increase in liver enzymes without hemostatic disorder. Yet, based on the abovementioned issues and the results of several research studies, a recommendation for the prescribed (and not OTC) use of aspirin seems reasonable (35).
3.2. Nonspecific Inhibitors
There are numerous medications included in this category, with new types being introduced annually and entering into the cycle of use. However, in the interests of both brevity and clarity, this study will only discuss the most widely used medications.
3.2.1. Ibuprofen
In 1961, researchers attempted to find a new drug that could replace aspirin and have fewer complications. They achieved a new combination known as 2-(4-isobutylphenyl) propanoic acid, which was later known as ibuprofen (15). This drug is non-specifically effective on both cyclooxygenase isomers. It enters into the bloodstream and more than 90% of its protein is bound. Most of the absorbed ibuprofen is metabolized in the liver, although a small portion is excreted unchanged. It is either excreted in a modified form (metabolites) or conjugated through urine. Ibuprofen is entirely excreted within 24 hours from the time of use (17). Considering that ibuprofen is available in many countries without a physician’s prescription (i.e., OTC), it is one of the most widely used drugs worldwide. This fact has led to several research studies being conducted regarding the effects of ibuprofen. However, considering its very high level of consumption, special attention should be paid to its side effects. Ibuprofen is commonly used to control pain (especially in rheumatic diseases), fever, headache, toothache, and dysmenorrhea. Recent research studies have suggested that this drug can be effective in the treatment of patent arteriosus ductus and the prevention of orthostatic hypotension (43, 44). It has also been suggested that ibuprofen can be effective in preventing Alzheimer’s disease, Parkinson’s disease, and breast cancer (44). However, the use of ibuprofen is also associated with some complications. For instance, digestive disorders with different intensities stemming from stomach pain discomfort to ulcers, and gastrointestinal bleeding are among the most important and common side effects of ibuprofen. Research has shown that 25% of people whose use of ibuprofen could be described as chronic suffer from different degrees of gastric ulcers (mostly asymptomatic) (45). This percentage indicates a considerable risk when considered in relation to the very high level of use of the drug worldwide. Indeed, the risk is such that internists often prescribe an H2 blocker in addition to ibuprofen. Moreover, the concurrent use of ibuprofen with another non-specific NSAIDs increases this risk to such an extent that the use of proton pump inhibitors (instead of an H2 blocker) has been recommended when concurrent use cannot be avoided (3). Furthermore, renal failure and hyperkalemia, cardiac failure, epistaxis, and bronchospasm are among the dangerous complications that have been reported following the administration of ibuprofen (46). Asthma exacerbation is another rare complication. However, this effect has only been reported in patients with severe asthma (45). Lithium, warfarin, oral hypoglycemic agents, antihypertensives, beta blockers, and diuretics (as with other NSAIDs) interact with ibuprofen and so their effect must be considered (17). It has also been found that the concurrent use of ibuprofen with aspirin leads to a reduction in the aspirin’s antiplatelet effects (due to the full occupation of the receptor site on the platelet) (47). Ibuprofen significantly increases systolic and diastolic pressure, leading to a reduction in urine volume, insulin clearance, and sodium excretion. When used concurrently with naproxen, ibuprofen results in its separation from binding proteins because of a competitive effect and, in fact, also reduces naproxen’s half-life and expected effects (48). Furthermore, research has shown that the concurrent administration of ibuprofen and caffeine may increase the desired effects of ibuprofen (particularly the analgesic effect) (49). Taking the drug after a meal leads to a significant increase in the duration of its effect while not necessarily impacting its uptake and efficacy (50). Some studies have shown that the concurrent use and combination of ibuprofen, aspirin, naproxen, and ketoprofen with acetaminophen leads to an increased possibility of hepatotoxicity and GI complications, with this possibility increasing significantly in alcoholics (47). After taking into consideration all its aspects, various studies conducted in Great Britain have shown that ibuprofen is the safest NSAID (43). In a study comparing the effects of ibuprofen, naproxen, and diclofenac, a minimal association with gastrointestinal bleeding was reported in relation to the use of ibuprofen (for patients of all ages) (14).
3.2.2. Diclofenac
In 1973, British scientists introduced a medication known as 2-(2, 6-dichloranilino) phenylacetic acid, which later became known as diclofenac (15). This drug non-specifically inhibits both the cyclooxygenase enzymes in the same way as ibuprofen, while about 99% is protein bound and it exhibits hepatic metabolism (17). About 40% is subject to biliary excretion, while 60% is excreted in urine (17). The benefits and side effects of diclofenac are similar to those of ibuprofen, and the contraindications are almost the same in all cases save for the fact that the analgesic and side effects of diclofenac are substantially are higher (51). Like other non-specific NSAIDs, diclofenac increases the risk of cardiac arrest, and this risk is substantially higher when compared to other drugs (52). Research has shown that the hepatotoxic effects of this drug are significant, and a minimal dose should be administered with caution in patients with hepatic disease (especially hepatic porphyria) (20). In terms of the amount of liver injury and hepatopathy caused by the NSAIDs, diclofenac is second only to nimesulide (26). However, it has been shown that diclofenac-induced liver injury is reversible in most patients. Liver injury is more commonly reported, as well as being more severe, in patients with rheumatoid arthritis than in patients with osteoarthritis. Hence, it is recommended that the level of liver enzymes should be evaluated after about four to eight weeks from the initiation of treatment with diclofenac (3, 20). Regarding the effects of this drug on the gastrointestinal tract, it has been shown that the incidence of gastrointestinal bleeding and peptic ulcer caused by diclofenac is less than that of aspirin, although the effect of diclofenac is also significant (53). In addition, ibuprofen’s bronchospasm, allergic, and renal effects have also been reported to be more severe, and its use should be avoided in cases of renal failure and severe asthma (3).
3.2.3. Indomethacin
Indomethacin was produced in 1963 by American researchers, and it entered the consumer market in 1965 (15). This drug also inhibits both the cyclooxygenase enzymes similarly to the two previously described drugs (17). Due to the very powerful effects of this drug in terms of reducing inflammation and pain, but bearing in mind its extensive side effects, it is recommended that indomethacin not be used for mild degrees of pain and instead be used for lowering inflammation (54). A reduction of the amniotic fluid is among the specific effects of this drug, which can be effective in the treatment of polyhydramnios (55). The side effects of indomethacin are similar to those of ibuprofen in terms of the impact on the digestive system, liver, and kidneys; however, unlike ibuprofen, this drug may aggravate Parkinson’s disease. Indomethacin also increases the risk of cardiac arrest in the same way as other non-specific NSAIDs (3, 56). In addition to checking the hepatic enzymes, the function of the kidneys, the coagulation system, and the condition of the electrolytes should carefully be studied in the long-term use of this drug (3). Therefore, all the above mentioned conditions should be considered when treating patients with hepatic disease.
3.2.4. Naproxen
Naproxen has a similar molecular structure and pharmacological properties to ibuprofen, with the difference being that it is the safest NSAID for cardiac patients due to the low risk of cardiac arrest reported for this drug (17, 57). Hence, it has a particular importance among the other NSAIDs from this point of view. Cardiovascular problems are one of the most common and important issues encountered in patients with hepatic disease, especially cirrhotic patients. Therefore, providing a drug with minimal cardiac complications seems ideal.
3.2.5. Piroxicam
Piroxicam has the highest number of unwanted side effects of all the non-specific NSAID, which has caused it to be increasingly removed from the use cycle. The half-life of drug is very high (approximately 50 hours) (17). The associated hepatic, renal, and gastrointestinal complications, as well as the negative effects on many bodily systems, have minimized the use of this drug. It has been strongly recommended in most countries that doctors avoid prescribing piroxicam, especially in elderly patients. Therefore, it is better to avoid the prescription of piroxicam for patients with any degree of renal or hepatic failure as well as elderly patients (especially women of menopause age) (3, 58).
3.2.6. Ketorolac
Ketorolac was introduced in 1989 (15). Despite having a similar name to ketoprofen (structures derived from propionic acid, with ibuprofen also included in this category), ketorolac is structurally and functionally more similar to indomethacin and so is used for treating moderate to severe pain (17). Thus, the hepatic and renal complications of this drug and its therapeutic approach should also be similar to those of indomethacin (although studies on this issue are scarce) (54). The most commonly used form of ketorolac is topical (ocular), and other forms are only used in hospitals in many countries (32). Its adverse effects are similar to those of indomethacin, and the results of a few studies on the pharmacokinetics of ketorolac have led to the limited consumption of this drug (especially in Iran, due to the very limited supply).
3.2.7. Mefenamic Acid
Unlike other NSAIDs, mefenamic acid has active metabolites after it has been affected by liver enzymes, which leads to the clearance and excretion of this drug. Thus, liver activity is significant and important in the determination of mefenamic acid’s serum and clearance levels (17). An important point in the excretion of this drug is that about 20% to 25% of it is excreted through the gastrointestinal tract, which should be considered in patients with inflammatory bowel diseases. It is a non-specific inhibitor, and it is used as a mild to moderate anti-inflammatory and analgesic drug (3, 17). Mefenamic acid affects the uterus through an unknown mechanism, leading to a reduction in inflammation induced by the menstrual process, which has led gynecologists to be particularly interested this drug (59, 60).
3.3. New Generation Drugs (Specific Inhibitors of COX-2)
After examining the mechanism of the NSAIDs’ effect on the cyclooxygenase enzymes, researchers sought compounds with less gastrointestinal complications and an inhibitory effect on only the COX-2 enzyme. The drugs in the coxib category are obtained from the results of these research endeavors. However, contrary to expectations, the high risk of cardiac diseases led to this class being restricted and, hence, to the elimination of rofecoxib. Currently, the maximum amount of usage is related to celecoxib that was first introduced in 1997 and by elimination of rofecoxib, the public interest toward it become more (15). This drug as well as other drugs in the coxib category specifically affect COX-2 and, correspondingly, have special features (17). Celecoxib is currently only available orally and, unlike other NSAIDs, it has less bioavailability, being mostly excreted through feces (less than 30% of it is excreted through urine, while nearly 60% is excreted through feces). It is bound to the plasma proteins, and it has a hepatic metabolism like other NSAIDs (17, 61). Due to its structural and molecular similarity to sulfonamides, celecoxib is very important in terms of a history of pharmaceutical allergies. In fact, it is contraindicated in both a history of allergy to aspirin and other NSAIDs and a history of allergy to sulfonamides (17, 62). Celecoxib can be used in the treatment of hepatic dysfunction and no evidence of serious liver injury has been reported; hence, it can even be administered in patients with mild and moderate hepatic insufficiency (3, 63). However, the increased risk of vascular diseases, especially cardiac arrest, is a serious problem for this pharmaceutical. Furthermore, celecoxib also exacerbates insufficiency in patients with renal failure, and it is contraindicated in patients with any degree of renal insufficiency in countries such as the United States so that its use should be discontinued with any signs of azotemia (64-66). In fact, as this pharmaceutical class is much newer than the other NSAIDs, not enough research has so far been conducted on its complications. Thus, it is recommended to be used with caution.
4. Conclusions
An important issue related to the use of NSAIDs is the fact that many of these medicines are available without a prescription and at relatively low prices. Hence, despite having less complications when compared to other drugs, the prevalence of NSAID-related complications is high due to their very high level of use worldwide. Hepatic complications and the harmful effects of these drugs in chronic users have been cited in many of the reviewed articles. In fact, it seems that the occasional use of such drugs in low doses is uncomplicated in many situations (only in terms of hepatic injury). The most important issue identified in this study is that in the case of patients with hepatic disease, the patients must be clinically and paraclinically assessed from all aspects in order to determine the severity of the disease. Further, if the use of NSAIDs becomes necessary, the most appropriate option must be selected for the individual patient. While the administration of many NSAIDs in low doses and for only a short period may have no undesirable effects, the cause of this issue is attributed to the high prevalence of possible complications in these patients. In fact, not all complications occur in all patients, and given the chronicity of the disease, more co-morbid conditions exist for patients, which makes the selection of an appropriate drug more difficult. For example, cirrhotic patients show different degrees of respiratory, cardiovascular, renal, hemostatic, and coagulation disorders, as well as hepatic dysfunction. Due to the fact that most NSAIDs exhibit hepatic metabolism, bind to albumin, are subject to renal excretion, and mostly affect platelets, a very comprehensive examination is required to determine the prescription of even a single dose for the patient. Another important issue is the concurrent use of drugs and possible pharmaceutical interventions. Appropriate pharmaceutical support for the gastrointestinal tract with H2 blockers and/or proton pump inhibitors as well as nutritional recommendations are essential. Avoiding the use of nimesulide and diclofenac, as well as the use of NSAIDs in combination with caffeine and, in some circumstances, paracetamol, can be considered in these patients. An investigation of the patient’s age, the presence or absence of cardiac disease, and coagulation problems is important in the use of aspirin and naproxen. Finally, the best recommendation in terms of NSAID prescription for patients with hepatic disease is that they should be discouraged from considering OTC medicines and instead strongly recommended to seek the opinion of a physician regarding the use of these drugs.
Acknowledgments
We would like to express our sincere gratitude to Dr. Ata Mahmoodpoor, department of anesthesiology and critical care, Tabriz University of Medical Sciences, Tabriz, Iran, who gave us significant help in finishing this review within the limited time available.
Footnotes
Authors’ Contribution:Study concept and design, Seyed Moayed Alavian and Maryam Soleimanpour; supervision, Farnad Imani; drafting of the manuscript, Saeid Safari, Sarvin Sanaie, Hoorolnesa Ameli and Hassan Soleimanpour.
Financial Disclosure:The authors declare they have no financial issues to disclose.
Funding/Support:This article was not supported by any funding organization. There was no sponsor for this work.
References
- 1.Kamath PS, Kim WR, Advanced Liver Disease Study G. The model for end-stage liver disease (MELD). Hepatology. 2007;45(3):797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 2.Murray JF, Dawson AM, Sherlock S. Circulatory changes in chronic liver disease. Am J Med. 1958;24(3):358–67. doi: 10.1016/0002-9343(58)90322-x. [DOI] [PubMed] [Google Scholar]
- 3.Longo D, Fauci A, Kasper D, Hauser S, Jameson J, Loscalzo J. Principles of Internal Medicine. In: Peter K, editor. 18 ed. New York: McGraw-Hill; 2012. [Google Scholar]
- 4.Parker BM, Bhatia S, Younossi Z, Henderson JM, Tetzlaff JE. Autonomic dysfunction in end-stage liver disease manifested as defecation syncope: impact of orthotopic liver transplantation. Liver Transpl Surg. 1999;5(6):497–501. doi: 10.1002/lt.500050603. [DOI] [PubMed] [Google Scholar]
- 5.De Wolf AM. 6/2/06 Perioperative assessment of the cardiovascular system in ESLD and transplantation. Int Anesthesiol Clin. 2006;44(4):59–78. doi: 10.1097/01.aia.0000210818.85287.de. [DOI] [PubMed] [Google Scholar]
- 6.Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365(2):147–56. doi: 10.1056/NEJMra1011170. [DOI] [PubMed] [Google Scholar]
- 7.Njoku DB. Drug-induced hepatotoxicity: metabolic, genetic and immunological basis. Int J Mol Sci. 2014;15(4):6990–7003. doi: 10.3390/ijms15046990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis JH, Stine JG. Review article: prescribing medications in patients with cirrhosis - a practical guide. Aliment Pharmacol Ther. 2013;37(12):1132–56. doi: 10.1111/apt.12324. [DOI] [PubMed] [Google Scholar]
- 9.Suchy FJ, Sokol RJ, Balistreri WF. Liver disease in children. 2 ed. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 10.Perrott DA, Piira T, Goodenough B, Champion GD. Efficacy and safety of acetaminophen vs ibuprofen for treating children's pain or fever: a meta-analysis. Arch Pediatr Adolesc Med. 2004;158(6):521–6. doi: 10.1001/archpedi.158.6.521. [DOI] [PubMed] [Google Scholar]
- 11.Bosilkovska M, Walder B, Besson M, Daali Y, Desmeules J. Analgesics in patients with hepatic impairment: pharmacology and clinical implications. Drugs. 2012;72(12):1645–69. doi: 10.2165/11635500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Khalid SK, Lane J, Navarro V, Garcia-Tsao G. Use of over-the-counter analgesics is not associated with acute decompensation in patients with cirrhosis. Clin Gastroenterol Hepatol. 2009;7(9):994–9. doi: 10.1016/j.cgh.2009.04.015. quiz 913-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenkel JM, Coron RN, Daskalakis C, Vega M, Rossi S, Herrine SK, et al. Over-the-counter analgesics in cirrhotic patients: a case-control study examining the risk of hospitalization for liver-associated events. Scand J Gastroenterol. 2010;45(9):1101–9. doi: 10.3109/00365521003663704. [DOI] [PubMed] [Google Scholar]
- 14.Perez-Gutthann S, Garcia-Rodriguez LA, Duque-Oliart A, Varas-Lorenzo C. Low-dose diclofenac, naproxen, and ibuprofen cohort study. Pharmacotherapy. 1999;19(7):854–9. doi: 10.1592/phco.19.10.854.31550. [DOI] [PubMed] [Google Scholar]
- 15.Sneader W. Drug Discovery: A History. U.S.A.: John wiley and Sons; 2005. [DOI] [Google Scholar]
- 16.Bellis M. History of aspirin. U.S.A: about.com Inventors; 2010. Available from: http://inventors.about.com/library/inventors/blaspirin.htm. [Google Scholar]
- 17.Katzung BG, Masters SB, Trevor AJ. Basic and clinical pharmacology. 12 ed. New York: Mc Graw Hill Lange; 2012. [Google Scholar]
- 18.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231(25):232–5. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 19.Tohgi H, Konno S, Tamura K, Kimura B, Kawano K. Effects of low-to-high doses of aspirin on platelet aggregability and metabolites of thromboxane A2 and prostacyclin. Stroke. 1992;23(10):1400–3. doi: 10.1161/01.str.23.10.1400. [DOI] [PubMed] [Google Scholar]
- 20.O'Connor N, Dargan PI, Jones AL. Hepatocellular damage from non-steroidal anti-inflammatory drugs. QJM. 2003;96(11):787–91. doi: 10.1093/qjmed/hcg138. [DOI] [PubMed] [Google Scholar]
- 21.Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014;89(1):95–106. doi: 10.1016/j.mayocp.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Gines P, Arrovo V, Rodes J. Pharmacotherapy of ascites associated with cirrhosis. Drugs. 1992;43(3):316–32. doi: 10.2165/00003495-199243030-00003. [DOI] [PubMed] [Google Scholar]
- 23.Sgro C, Clinard F, Ouazir K, Chanay H, Allard C, Guilleminet C, et al. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology. 2002;36(2):451–5. doi: 10.1053/jhep.2002.34857. [DOI] [PubMed] [Google Scholar]
- 24.Bjornsson ES, Bergmann OM, Bjornsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144(7):1419-25. doi: 10.1053/j.gastro.2013.02.006. quiz e19-20. [DOI] [PubMed] [Google Scholar]
- 25.Andrade RJ, Lucena MI, Fernandez MC, Pelaez G, Pachkoria K, Garcia-Ruiz E, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129(2):512–21. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Bessone F. Non-steroidal anti-inflammatory drugs: What is the actual risk of liver damage? World J Gastroenterol. 2010;16(45):5651–61. doi: 10.3748/wjg.v16.i45.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imani F, Motavaf M, Safari S, Alavian SM. The therapeutic use of analgesics in patients with liver cirrhosis: a literature review and evidence-based recommendations. Hepat Mon. 2014;14(10):23539. doi: 10.5812/hepatmon.23539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjornsson E. Review article: drug-induced liver injury in clinical practice. Aliment Pharmacol Ther. 2010;32(1):3–13. doi: 10.1111/j.1365-2036.2010.04320.x. [DOI] [PubMed] [Google Scholar]
- 29.Hussaini SH, Farrington EA. Idiosyncratic drug-induced liver injury: an overview. Expert Opin Drug Saf. 2007;6(6):673–84. doi: 10.1517/14740338.6.6.673. [DOI] [PubMed] [Google Scholar]
- 30.Soleimanpour H, Safari S, Rahmani F, Ameli H, Alavian SM. The role of inhalational anesthetic drugs in patients with hepatic dysfunction: a review article. Anesth Pain Med. 2015;5(1):23409. doi: 10.5812/aapm.23409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soleimanpour H, Safari S, Rahmani F, Jafari Rouhi A, Alavian SM. Intravenous hypnotic regimens in patients with liver disease; a review article. Anesth Pain Med. 2015;5(1):23923. doi: 10.5812/aapm.23923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldkind L, Laine L. A systematic review of NSAIDs withdrawn from the market due to hepatotoxicity: lessons learned from the bromfenac experience. Pharmacoepidemiol Drug Saf. 2006;15(4):213–20. doi: 10.1002/pds.1207. [DOI] [PubMed] [Google Scholar]
- 33.Garcia Rodriguez LA, Williams R, Derby LE, Dean AD, Jick H. Acute liver injury associated with nonsteroidal anti-inflammatory drugs and the role of risk factors. Arch Intern Med. 1994;154(3):311–6. doi: 10.1001/archinte.1994.00420030117012. [DOI] [PubMed] [Google Scholar]
- 34.Manoukian AV, Carson JL. Nonsteroidal anti-inflammatory drug-induced hepatic disorders. Incidence and prevention. Drug Saf. 1996;15(1):64–71. doi: 10.2165/00002018-199615010-00005. [DOI] [PubMed] [Google Scholar]
- 35.Walker AM. Quantitative studies of the risk of serious hepatic injury in persons using nonsteroidal antiinflammatory drugs. Arthritis Rheum. 1997;40(2):201–8. doi: 10.1002/art.1780400204. [DOI] [PubMed] [Google Scholar]
- 36.Lewis HJ, Davis JW, Archibald DG, Steinke WE, Smitherman TC, Doherty J, et al. Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1983;309(7):396–403. doi: 10.1056/NEJM198308183090703. [DOI] [PubMed] [Google Scholar]
- 37.Julian DG, Chamberlain DA, Pocock SJ. A comparison of aspirin and anticoagulation following thrombolysis for myocardial infarction (the AFTER study): a multicentre unblinded randomised clinical trial. BMJ. 1996;313(7070):1429–31. doi: 10.1136/bmj.313.7070.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levy G, Tsuchiya T. Salicylate accumulation kinetics in man. N Engl J Med. 1972;287(9):430–2. doi: 10.1056/NEJM197208312870903. [DOI] [PubMed] [Google Scholar]
- 39.Gaudreault P, Temple AR, Lovejoy FH. The relative severity of acute versus chronic salicylate poisoning in children: a clinical comparison. Pediatrics. 1982;70(4):566–9. [PubMed] [Google Scholar]
- 40.He J, Whelton PK, Vu B, Klag MJ. Aspirin and risk of hemorrhagic stroke: a meta-analysis of randomized controlled trials. JAMA. 1998;280(22):1930–5. doi: 10.1001/jama.280.22.1930. [DOI] [PubMed] [Google Scholar]
- 41.Progeria Research Foundation. The Progeria Handbook. London: Progeria Research Foundation; 2010. [Google Scholar]
- 42.Hurwitz ES. Reye's syndrome. Epidemiol Rev. 1989;11:249–53. doi: 10.1093/oxfordjournals.epirev.a036043. [DOI] [PubMed] [Google Scholar]
- 43.Gosalakkal JA, Kamoji V. Reye syndrome and reye-like syndrome. Pediatr Neurol. 2008;39(3):198–200. doi: 10.1016/j.pediatrneurol.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Bushra R, Aslam N. An overview of clinical pharmacology of Ibuprofen. Oman Med J. 2010;25(3):155–1661. doi: 10.5001/omj.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Casper D, Yaparpalvi U, Rempel N, Werner P. Ibuprofen protects dopaminergic neurons against glutamate toxicity in vitro. Neurosci Lett. 2000;289(3):201–4. doi: 10.1016/s0304-3940(00)01294-5. [DOI] [PubMed] [Google Scholar]
- 46.Durkin E, Moran AP, Hanson PJ. Apoptosis induction in gastric mucous cells in vitro: lesser potency of Helicobacter pylori than Escherichia coli lipopolysaccharide, but positive interaction with ibuprofen. J Endotoxin Res. 2006;12(1):47–56. doi: 10.1179/096805106X89080. [DOI] [PubMed] [Google Scholar]
- 47.Ayres JG, Fleming DM, Whittington RM. Asthma death due to ibuprofen. Lancet. 1987;1(8541):1082. doi: 10.1016/s0140-6736(87)90499-5. [DOI] [PubMed] [Google Scholar]
- 48.Gaziano JM, Gibson CM. Potential for drug-drug interactions in patients taking analgesics for mild-to-moderate pain and low-dose aspirin for cardioprotection. Am J Cardiol. 2006;97(9A):23–9. doi: 10.1016/j.amjcard.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 49.Gladding PA, Webster MW, Farrell HB, Zeng IS, Park R, Ruijne N. The antiplatelet effect of six non-steroidal anti-inflammatory drugs and their pharmacodynamic interaction with aspirin in healthy volunteers. Am J Cardiol. 2008;101(7):1060–3. doi: 10.1016/j.amjcard.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 50.Dooley JM, Gordon KE, Wood EP, Brna PM, MacSween J, Fraser A. Caffeine as an adjuvant to ibuprofen in treating childhood headaches. Pediatr Neurol. 2007;37(1):42–6. doi: 10.1016/j.pediatrneurol.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 51.Pargal A, Kelkar MG, Nayak PJ. The effect of food on the bioavailability of ibuprofen and flurbiprofen from sustained release formulations. Biopharm Drug Dispos. 1996;17(6):511–9. doi: 10.1002/(SICI)1099-081X(199608)17:6<511::AID-BDD969>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 52.Naidoo V, Swan GE. Diclofenac toxicity in Gyps vulture is associated with decreased uric acid excretion and not renal portal vasoconstriction. Comp Biochem Physiol C Toxicol Pharmacol. 2009;149(3):269–74. doi: 10.1016/j.cbpc.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 53.Solomon DH, Avorn J, Sturmer T, Glynn RJ, Mogun H, Schneeweiss S. Cardiovascular outcomes in new users of coxibs and nonsteroidal antiinflammatory drugs: high-risk subgroups and time course of risk. Arthritis Rheum. 2006;54(5):1378–89. doi: 10.1002/art.21887. [DOI] [PubMed] [Google Scholar]
- 54.Swan G, Naidoo V, Cuthbert R, Green RE, Pain DJ, Swarup D, et al. Removing the threat of diclofenac to critically endangered Asian vultures. PLoS Biol. 2006;4(3):66. doi: 10.1371/journal.pbio.0040066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sehgal N, Colson J, Smith HS. Chronic pain treatment with opioid analgesics: benefits versus harms of long-term therapy. Expert Rev Neurother. 2013;13(11):1201–20. doi: 10.1586/14737175.2013.846517. [DOI] [PubMed] [Google Scholar]
- 56.Giles W, Bisits A. Preterm labour. The present and future of tocolysis. Best Pract Res Clin Obstet Gynaecol. 2007;21(5):857–68. doi: 10.1016/j.bpobgyn.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 57.Akbarpour F, Afrasiabi A, Vaziri ND. Severe hyperkalemia caused by indomethacin and potassium supplementation. South Med J. 1985;78(6):756–7. doi: 10.1097/00007611-198506000-00039. [DOI] [PubMed] [Google Scholar]
- 58.Joint Formulary Committee. British National Formulary. London: British National Formulary; 2013. p. 665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weintraub M, Jacox RF, Angevine CD, Atwater EC. Piroxicam (CP 16171) in rheumatoid arthritis: a controlled clinical trial with novel assessment techniques. J Rheumatol. 1977;4(4):393–404. [PubMed] [Google Scholar]
- 60.Pringsheim T, Davenport WJ, Dodick D. Acute treatment and prevention of menstrually related migraine headache: evidence-based review. Neurology. 2008;70(17):1555–63. doi: 10.1212/01.wnl.0000310638.54698.36. [DOI] [PubMed] [Google Scholar]
- 61.Trinus FP, Mokhort NA, Yagupol'skii LM, Fadeicheva AG, Danilenko VS, Ryabukha TK, et al. Mefenamic acid ? A nonsteroid antiinflammatory agent. Pharm Chemistry J. 1977;11(12):1706–11. doi: 10.1007/bf00778304. [DOI] [Google Scholar]
- 62.Penning TD, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, Docter S, et al. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benze nesulfonamide (SC-58635, celecoxib). J Med Chem. 1997;40(9):1347–65. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- 63.McCormack PL. Celecoxib: a review of its use for symptomatic relief in the treatment of osteoarthritis, rheumatoid arthritis and ankylosing spondylitis. Drugs. 2011;71(18):2457–89. doi: 10.2165/11208240-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 64.Derry S, Moore RA. Single dose oral celecoxib for acute postoperative pain in adults. Cochrane Database Syst Rev. 2013;(10):CD004233. doi: 10.1002/14651858.CD004233.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mathew ST, Devi SG, Prasanth VV, Vinod B. Efficacy and Safety of COX-2 Inhibitors in the Clinical Management of Arthritis: Mini Review. ISRN Pharmacol. 2011;2011:480291. doi: 10.5402/2011/480291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286(8):954–9. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 67.Bateman DN. NSAIDs: time to re-evaluate gut toxicity. Lancet. 1994;343(8905):1051–2. doi: 10.1016/s0140-6736(94)90175-9. [DOI] [PubMed] [Google Scholar]