Abstract
We analyzed birth order differences in means and variances of height and body mass index (BMI) in monozygotic (MZ) and dizygotic (DZ) twins from infancy to old age. The data were derived from the international CODATwins database. The total number of height and BMI measures from 0.5 to 79.5 years of age was 397,466. As expected, first-born twins had greater birth weight than second-born twins. With respect to height, first-born twins were slightly taller than second-born twins in childhood. After adjusting the results for birth weight, the birth order differences decreased and were not statistically significant anymore. First-born twins had greater BMI than the second-born twins over childhood and adolescence. After adjusting the results for birth weight, birth order was still associated with BMI until 12 years of age. No interaction effect between birth order and zygosity was found. Only limited evidence was found that birth order influenced variances of height or BMI. The results were similar among boys and girls and also in MZ and DZ twins. Overall, the differences in height and BMI between first and second born twins were modest even in early childhood, while adjustment for birth weight reduced the birth order differences but did not remove them for BMI.
Keywords: birth order, BMI, height, zygosity
It is well known that growth patterns of twins during the third trimester of pregnancy differ from those of singletons. In addition to having two fetuses in utero, there are twin-specific factors, such as birth order (Glinianaia et al., 2000; Gielen, et al., 2007), zygosity (Loos,et al., 2005; Daw, et al., 1975; Buckler & Green, 2008) and chorionicity (Gruenwald, 1970; Ananth, et al., 1998; Naeye, et al., 1966; Bleker, et al., 1979; Gielen M, et al., 2009; van Beijsterveldt et al. 2015), which are associated with intrauterine twin growth. Previous studies of twins have reported that the second-born twin is, on average, lighter than the first-born twin at birth (Glinianaia et al., 2000; Gielen, et al., 2007; van Baal and Boomsma, 1998). The factors determining birth order have a greater influence on birth weight than zygosity or chorionicity (Gielen, et al., 2007; Sheay, et al., 2004).
The lower birth weight for second-born twins could be due to the fact that first-born twins have higher placental weights and have more often a central insertion of the umbilical cord, which are both positively correlated with birth weight. Possibly, first-born twins are also more optimally positioned with respect to nutrients intake (Gielen et al., 2006; Heinonen, et al, 1996). In addition, previous studies have shown that first-born twins are, on average, taller and heavier than second-born twins until adolescence (Silventoinen et al., 2007; Pietiläinen et al., 2002). Second-born twins have also higher morbidity and mortality (Armson, et al., 2006; Smith, et al., 2007; Shinwell et al., 2004; Luo, et al., 2014).
The persistence of the birth-order association suggests that prenatal factors can have long-lasting effects on body size. However, it is not known how these associations may change over the life course. Studies on age-dependent birth order differences in height and body mass index (BMI) are scarce, and small sample sizes make comparisons of the existing results difficult. Further, it is not known whether the factors behind birth order differences in height and BMI also induce variance differences. In this study, we aim to analyze birth-order differences in means and variances of height and BMI among MZ and DZ twins from infancy to old age and to test whether they can be explained by differences in birth weight. The data were derived from the large international CODATwins database, which was intended to collect together height and weight measurements from all twin cohorts in the world.
Data and methods
In the CODATwins database (Silventoinen, et al., 2015), there are 960,859 height and weight measures from twins at ages ranging from 0.5 to 103 years. Information on birth order, height and weight measures were self-reported (67%), parentally reported (19%), or based on measures by nurses and clinicians (14%). In this study, we included the following cohorts with information on birth order: Australian Twin Registry, Boston University Twin Project, Carolina African American Twin Study of Aging, FinnTwin12, FinnTwin16, Gemini Study, Guangzhou Twin Eye Study, Guinea-Bissau Twin Study, Hungarian Twin Registry, Japanese Twin Cohort, Korean Twin-Family Register, Longitudinal Israeli Study of Twins, Michigan Twins Study, Murcia Twin Registry, Norwegian Twin Registry, Peri/Postnatal Epigenetic Twins Study, Qingdao Twin Registry of Children, Quebec Newborn Twin Study, Queensland Twin Register, Swedish Young Male Twins Study of Adults, Swedish Young Male Twins Study of Children, South Korea Twin Registry, Swedish Twin Cohorts, Twins Early Developmental Study, West Japan Twins and Higher Order Multiple Births Registry and Young Netherlands Twin Registry. Height and weight measurement protocols, sample frames and other basic information of these cohorts have been described elsewhere (Silventoinen, et al., 2015). Age was classified to 1-year age groups from age 1 to 19 years (e.g., age 1 refers to 0.5–1.5 years range), and 10-year age groups from age 20 to 79 years (e.g., 20–29, . . . , and age 70–79 years). Since the number of twin participants at 80 years of age or older was small, this group was excluded from the analyses.
In total, we had 429,587 height and BMI measurements at ages 0.5–79.5 years with information on birth order. Additionally we had information on birth weight from 107,782 and birth length from 54,941 twin individuals. BMI was calculated as weight (kg)/square of height (m2). Outliers were checked by visual inspection of histograms for each age and sex group. They were removed to obtain an approximately normal distribution of height, whereas the distribution of BMI was allowed to be positively skewed. The number of observations removed (n=1134) represented less than 0.3% of the whole database. We also excluded extreme birth length (<25cm or >65 cm) or birth weight (<500g or >6000g) values. For the purpose of this study, we restricted the analyses to one observation per individual in each age group. The total number of height and BMI measures in this study was 397,466; in 307,606 of these cases we had information also on birth weight.
Equality of mean values between first- and second- born twins by zygosity, age group and sex was tested using fixed effects regression analysis corrected for clustering of twin pairs. Equality of variances was tested using the Levene’s clustered test based on the 10% trimmed mean (Iachine et al. 2010). This clustered version of the Levene’s test is robust under the non-normality of outcomes. The interaction effects between zygosity and birth order were tested using Bonferroni correction of multiple testing with alpha level 0.0005 (0.05/100 tests). Percentage difference (%) between first- and second-born twins in mean values [(first born mean-second born mean) / second born mean] * 100 and standard deviations (SD) [(first born SD-second born SD) / second born SD] * 100 of height and BMI were calculated by sex. We also tested how the adjustment for birth weight affected the birth order difference on height and BMI in the cohorts having this information available using the fixed effects multiple regression model in each age groups. Statistical analyses were conducted using the Stata statistical software package (version 12.0; StataCorp, College Station, Texas, USA).
The pooled analysis was approved by the ethical board of the Department of Public Health, University of Helsinki. The data collections procedures of participating twin cohorts were approved by local ethical boards following the regulation in each country. Only anonymized data were delivered to the data management center at University of Helsinki.
Results
Table 1 provides the mean birth length and birth weight according to birth order, sex and zygosity. In MZ twins, the first-born male twins had greater length than the second-born male twins. However, in DZ twins, average birth length was not significantly different between the first-born and the second-born twins. In MZ and DZ twins, the first-born twins had greater birth weight than the second-born twins. The SDs of birth weight in the first-born and the second-born twins in MZ and DZ twins were significantly different except in MZ boys.
Table 1.
Number of twin individuals, mean and standard deviation of birth length (cm) and birth weight (kg) by birth order, sex and zygosity
| Birth order | Boys
|
Girls
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | p valuea | SD | p valueb | N | Mean | p valuea | SD | p valueb | |||
| Monozygotic twins | Birth length | 1 | 5304 | 47.0 | 0.002 | 3.58 | 0.001 | 5101 | 46.3 | 0.132 | 3.58 | 0.085 |
| 2 | 5293 | 46.8 | 3.69 | 5070 | 46.2 | 3.64 | ||||||
| Birth weight (kg) | 1 | 9617 | 2.54 | <0.001 | 0.56 | 0.067 | 10383 | 2.43 | <0.001 | 0.54 | <0.001 | |
| 2 | 9524 | 2.51 | 0.57 | 10330 | 2.39 | 0.55 | ||||||
| Dizygotic twins | Birth length | 1 | 9160 | 47.4 | 0.207 | 3.55 | 0.009 | 7967 | 46.8 | 0.191 | 3.60 | 0.018 |
| 2 | 8914 | 47.4 | 3.65 | 8132 | 46.7 | 3.64 | ||||||
| Birth weight (kg) | 1 | 17468 | 2.63 | <0.001 | 0.57 | 0.005 | 16562 | 2.53 | <0.001 | 0.55 | 0.001 | |
| 2 | 17098 | 2.58 | 0.58 | 16800 | 2.47 | 0.56 | ||||||
p valuea: p value for equality of means, p valueb: p value for equality of variances, SD: standard deviation.
Descriptive statistics by birth order, age, and sex in MZ and DZ twins are presented in Table 2 and 3 for height, respectively. Sample size for each birth order, age, and sex group ranged between 421 and 5,407 individuals from age 1 through 19 years, and between 117 and 4,398 individuals in adulthood (≥20 years). The 6 and ≥70-year age groups in MZ twins had the smallest sample sizes. In MZ twins, significantly taller height in the first-born than in the second-born twins were observed at the age of one, three, five, eight and 10 years in men and from the age of one to three, seven and 12 years in women (Table 2). However, in DZ twins, average height was not significantly different between the first-born and the second-born twins (Table 3). The SDs of height in the first-born and the second-born twins in MZ and DZ twin were not significantly different in the majority of age groups. Results were similar in men and women.
Table 2.
Number of twin individuals, mean and standard deviation of height (cm) by birth order, age and sex in monozygotic twins
| Men
|
Women
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Birth order | N | Mean | p valuea | SD | p valueb | N | Mean | p valuea | SD | p valueb | |
| Age 1 | 1 | 2843 | 73.6 | 0.029 | 4.54 | 0.176 | 2994 | 72.4 | 0.014 | 4.55 | 0.267 |
| 2 | 2838 | 73.5 | 4.59 | 2991 | 72.3 | 4.55 | |||||
| Age 2 | 1 | 2310 | 86.5 | 0.121 | 4.41 | 0.047 | 2339 | 85.4 | 0.020 | 4.42 | 0.307 |
| 2 | 2293 | 86.4 | 4.46 | 2323 | 85.3 | 4.44 | |||||
| Age 3 | 1 | 2830 | 95.8 | 0.044 | 4.45 | 0.264 | 3140 | 94.9 | 0.002 | 4.39 | 0.775 |
| 2 | 2834 | 95.7 | 4.47 | 3125 | 94.8 | 4.38 | |||||
| Age 4 | 1 | 1600 | 102.1 | 0.181 | 5.17 | 0.045 | 1594 | 101.0 | 0.116 | 5.13 | 0.118 |
| 2 | 1588 | 101.9 | 5.33 | 1595 | 100.9 | 5.29 | |||||
| Age 5 | 1 | 1272 | 110.8 | 0.018 | 5.91 | 0.770 | 1341 | 110.2 | 0.299 | 6.16 | 0.496 |
| 2 | 1266 | 110.7 | 5.93 | 1328 | 110.1 | 6.06 | |||||
| Age 6 | 1 | 528 | 114.1 | 0.279 | 6.45 | 0.856 | 427 | 112.9 | 0.834 | 5.66 | 0.180 |
| 2 | 512 | 113.7 | 6.40 | 421 | 112.9 | 5.88 | |||||
| Age 7 | 1 | 2345 | 123.6 | 0.216 | 6.62 | 0.134 | 2540 | 122.9 | 0.020 | 6.49 | 0.973 |
| 2 | 2330 | 123.5 | 6.72 | 2536 | 122.8 | 6.53 | |||||
| Age 8 | 1 | 1057 | 127.6 | 0.016 | 6.35 | 0.322 | 1020 | 127.0 | 0.077 | 6.42 | 0.209 |
| 2 | 1042 | 127.4 | 6.23 | 1021 | 126.8 | 6.56 | |||||
| Age 9 | 1 | 1042 | 133.0 | 0.143 | 6.97 | 0.509 | 1005 | 132.0 | 0.232 | 6.93 | 0.590 |
| 2 | 997 | 132.8 | 7.04 | 986 | 131.8 | 6.92 | |||||
| Age 10 | 1 | 1988 | 140.0 | 0.044 | 7.18 | 0.737 | 2088 | 139.8 | 0.115 | 7.41 | 0.199 |
| 2 | 1924 | 139.9 | 7.19 | 2048 | 139.7 | 7.47 | |||||
| Age 11 | 1 | 1530 | 143.4 | 0.470 | 7.15 | 0.959 | 1588 | 144.2 | 0.180 | 7.30 | 0.003 |
| 2 | 1470 | 143.3 | 7.12 | 1530 | 144.0 | 7.48 | |||||
| Age 12 | 1 | 2032 | 151.4 | 0.434 | 8.30 | 0.884 | 2127 | 152.3 | 0.036 | 8.01 | 0.030 |
| 2 | 1955 | 151.3 | 8.24 | 2053 | 152.1 | 8.18 | |||||
| Age 13 | 1 | 692 | 157.9 | 0.693 | 9.30 | 0.301 | 642 | 157.3 | 0.269 | 7.40 | 0.494 |
| 2 | 620 | 157.6 | 9.39 | 591 | 157.2 | 7.54 | |||||
| Age 14 | 1 | 1328 | 165.4 | 0.815 | 8.95 | 0.808 | 1497 | 161.8 | 0.441 | 6.68 | 0.066 |
| 2 | 1280 | 165.4 | 9.09 | 1468 | 161.8 | 6.85 | |||||
| Age 15 | 1 | 658 | 171.5 | 0.433 | 8.68 | 0.968 | 639 | 164.5 | 0.615 | 7.32 | 0.029 |
| 2 | 639 | 171.7 | 8.70 | 606 | 165.1 | 7.00 | |||||
| Age 16 | 1 | 1074 | 175.5 | 0.731 | 7.61 | 0.546 | 1311 | 164.4 | 0.398 | 6.56 | 0.067 |
| 2 | 1028 | 175.5 | 7.56 | 1257 | 164.5 | 6.34 | |||||
| Age 17 | 1 | 1100 | 177.7 | 0.315 | 7.29 | 0.608 | 1411 | 165.6 | 0.710 | 6.62 | 0.601 |
| 2 | 1074 | 177.8 | 7.47 | 1388 | 165.8 | 6.59 | |||||
| Age 18 | 1 | 1253 | 178.9 | 0.207 | 7.05 | 0.085 | 826 | 166.1 | 0.450 | 6.48 | 0.495 |
| 2 | 1253 | 178.9 | 6.89 | 812 | 166.1 | 6.64 | |||||
| Age 19 | 1 | 639 | 179.2 | 0.345 | 7.04 | 0.250 | 717 | 166.1 | 0.255 | 6.98 | 0.279 |
| 2 | 607 | 179.3 | 6.85 | 702 | 165.9 | 6.83 | |||||
| Age 20–29 | 1 | 2890 | 179.2 | 0.117 | 6.90 | 0.412 | 3488 | 164.5 | 0.881 | 6.50 | 0.839 |
| 2 | 2878 | 179.0 | 6.98 | 3478 | 164.6 | 6.47 | |||||
| Age 30–39 | 1 | 2305 | 178.1 | 0.360 | 7.04 | 0.494 | 3378 | 164.0 | 0.233 | 6.67 | 0.554 |
| 2 | 2290 | 177.9 | 6.92 | 3349 | 164.0 | 6.62 | |||||
| Age 40–49 | 1 | 1420 | 177.2 | 0.295 | 7.04 | 0.370 | 1886 | 163.2 | 0.924 | 6.65 | 0.436 |
| 2 | 1355 | 177.1 | 7.03 | 1844 | 163.1 | 6.58 | |||||
| Age 50–59 | 1 | 1038 | 176.4 | 0.805 | 7.02 | 0.181 | 1601 | 162.5 | 0.613 | 6.50 | 0.047 |
| 2 | 1029 | 176.3 | 7.22 | 1609 | 162.4 | 6.70 | |||||
| Age 60–69 | 1 | 506 | 174.9 | 0.386 | 6.40 | 0.006 | 880 | 161.7 | 0.660 | 6.30 | 0.297 |
| 2 | 494 | 174.4 | 6.99 | 878 | 161.9 | 6.47 | |||||
| Age 70–79 | 1 | 126 | 173.7 | 0.412 | 7.09 | 0.199 | 273 | 161.3 | 0.378 | 6.91 | 0.162 |
| 2 | 117 | 173.1 | 7.48 | 268 | 160.8 | 6.61 | |||||
p valuea: p value for equality of means, p valueb: p value for equality of variances, SD: standard deviation.
Table 3.
Number of twin individuals, mean and standard deviation of height (cm) by birth order, age and sex in dizygotic twins
| Men
|
Women
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Birth order | N | Mean | p valuea | SD | p valueb | N | Mean | p valuea | SD | p valueb | |
| Age 1 | 1 | 5088 | 74.8 | 0.051 | 4.11 | 0.531 | 4759 | 73.4 | 0.113 | 4.06 | 0.359 |
| 2 | 4962 | 74.6 | 4.11 | 4821 | 73.2 | 4.14 | |||||
| Age 2 | 1 | 4184 | 87.4 | 0.340 | 4.22 | 0.265 | 3815 | 86.3 | 0.141 | 4.34 | 0.189 |
| 2 | 4102 | 87.2 | 4.28 | 3871 | 86.1 | 4.27 | |||||
| Age 3 | 1 | 5407 | 96.6 | 0.074 | 4.43 | 0.822 | 5085 | 95.6 | 0.159 | 4.55 | 0.257 |
| 2 | 5266 | 96.4 | 4.42 | 5208 | 95.4 | 4.62 | |||||
| Age 4 | 1 | 2993 | 102.4 | 0.873 | 5.27 | 0.872 | 2818 | 101.3 | 0.745 | 5.21 | 0.119 |
| 2 | 2954 | 102.5 | 5.31 | 2848 | 101.1 | 5.25 | |||||
| Age 5 | 1 | 2349 | 112.0 | 0.126 | 6.07 | 0.478 | 2099 | 111.1 | 0.143 | 6.37 | 0.602 |
| 2 | 2259 | 111.5 | 6.13 | 2189 | 110.7 | 6.41 | |||||
| Age 6 | 1 | 583 | 114.5 | 0.819 | 6.88 | 0.311 | 455 | 114.2 | 0.261 | 7.55 | 0.180 |
| 2 | 552 | 114.8 | 7.15 | 469 | 113.6 | 7.39 | |||||
| Age 7 | 1 | 3986 | 124.8 | 0.232 | 6.59 | 0.266 | 3877 | 123.8 | 0.359 | 6.71 | 0.239 |
| 2 | 3981 | 124.5 | 6.72 | 3864 | 123.8 | 6.65 | |||||
| Age 8 | 1 | 1478 | 129.2 | 0.533 | 6.42 | 0.154 | 1279 | 128.3 | 0.192 | 6.82 | 0.721 |
| 2 | 1433 | 128.9 | 6.65 | 1318 | 127.9 | 6.87 | |||||
| Age 9 | 1 | 1445 | 134.5 | 0.664 | 7.30 | 0.271 | 1354 | 133.9 | 0.994 | 7.25 | 0.830 |
| 2 | 1449 | 134.2 | 7.14 | 1316 | 133.9 | 7.38 | |||||
| Age 10 | 1 | 3171 | 141.8 | 0.097 | 7.02 | 0.226 | 2994 | 141.2 | 0.578 | 7.35 | 0.539 |
| 2 | 3148 | 141.4 | 7.21 | 2973 | 141.0 | 7.39 | |||||
| Age 11 | 1 | 2385 | 145.2 | 0.130 | 7.23 | 0.505 | 2146 | 145.1 | 0.691 | 7.77 | 0.279 |
| 2 | 2288 | 144.6 | 7.41 | 2153 | 145.4 | 7.92 | |||||
| Age 12 | 1 | 3152 | 152.5 | 0.945 | 7.82 | 0.617 | 3031 | 153.3 | 0.813 | 8.36 | 0.444 |
| 2 | 3021 | 152.3 | 7.92 | 3048 | 153.3 | 8.24 | |||||
| Age 13 | 1 | 1035 | 158.8 | 0.887 | 8.77 | 0.051 | 898 | 158.4 | 0.938 | 7.80 | 0.964 |
| 2 | 965 | 158.7 | 9.36 | 896 | 158.7 | 7.98 | |||||
| Age 14 | 1 | 2353 | 165.6 | 0.888 | 8.94 | 0.031 | 2332 | 162.8 | 0.358 | 6.74 | 0.529 |
| 2 | 2266 | 165.8 | 8.62 | 2370 | 162.5 | 6.87 | |||||
| Age 15 | 1 | 1143 | 172.2 | 0.878 | 8.84 | 0.729 | 1042 | 165.4 | 0.547 | 7.18 | 0.358 |
| 2 | 1078 | 172.3 | 8.87 | 1013 | 165.7 | 7.03 | |||||
| Age 16 | 1 | 1990 | 176.0 | 0.855 | 7.52 | 0.827 | 2086 | 165.4 | 0.952 | 6.61 | 0.174 |
| 2 | 1940 | 175.8 | 7.46 | 2090 | 165.5 | 6.43 | |||||
| Age 17 | 1 | 2159 | 178.5 | 0.317 | 7.30 | 0.415 | 2230 | 166.4 | 0.758 | 6.32 | 0.035 |
| 2 | 2092 | 178.0 | 7.16 | 2224 | 166.4 | 6.70 | |||||
| Age 18 | 1 | 1680 | 179.4 | 0.444 | 6.95 | 0.573 | 1281 | 166.8 | 0.434 | 6.77 | 0.267 |
| 2 | 1614 | 179.2 | 6.91 | 1320 | 166.3 | 6.49 | |||||
| Age 19 | 1 | 1044 | 180.4 | 0.973 | 6.72 | 0.362 | 1099 | 167.2 | 0.662 | 6.60 | 0.187 |
| 2 | 1049 | 179.8 | 6.86 | 1047 | 167.3 | 6.47 | |||||
| Age 20–29 | 1 | 4036 | 179.9 | 0.191 | 6.69 | 0.624 | 4398 | 165.8 | 0.255 | 6.56 | 0.167 |
| 2 | 4121 | 179.4 | 6.74 | 4270 | 165.5 | 6.50 | |||||
| Age 30–39 | 1 | 3221 | 179.2 | 0.674 | 6.82 | 0.209 | 4120 | 165.2 | 0.594 | 6.58 | 0.069 |
| 2 | 3351 | 178.9 | 6.64 | 3887 | 165.0 | 6.43 | |||||
| Age 40–49 | 1 | 2403 | 178.9 | 0.791 | 6.69 | 0.775 | 2877 | 164.6 | 0.836 | 6.36 | 0.961 |
| 2 | 2411 | 178.9 | 6.70 | 2775 | 164.9 | 6.33 | |||||
| Age 50–59 | 1 | 2678 | 178.0 | 0.532 | 6.73 | 0.281 | 3261 | 164.1 | 0.898 | 6.20 | 0.081 |
| 2 | 2634 | 177.7 | 6.56 | 3086 | 164.3 | 6.05 | |||||
| Age 60–69 | 1 | 1265 | 175.9 | 0.927 | 6.73 | 0.657 | 1560 | 162.9 | 0.813 | 6.13 | 0.280 |
| 2 | 1276 | 175.8 | 6.85 | 1532 | 162.9 | 6.27 | |||||
| Age 70–79 | 1 | 310 | 175.3 | 0.586 | 6.53 | 0.287 | 435 | 162.1 | 0.543 | 6.78 | 0.064 |
| 2 | 329 | 175.0 | 6.82 | 390 | 161.4 | 6.05 | |||||
p valuea: p value for equality of means, p valueb: p value for equality of variances, SD: standard deviation.
Table 4 and 5 shows the respective results for BMI. The sample sizes are same as for height. In MZ twins, the first-born twins had greater BMI than the second-born twins except the 18 and ≥50-year age groups in men, and the 40–49 year age group in women. Statistical significance was attained in the majority of age groups until 12 years age groups (Table 4). In DZ twins, first-borns had greater BMI than the second-born twins except the 14 and 60–69 years old men. The differences were also statistically significant particularly until 5 years of age. The SDs of BMI in the first-born and the second-born twins in MZ twins were not significantly different. However, the SDs of BMI in the first-born and the second-born twins in DZ twins were significantly different at the age of one, three, 10, 15, 17, and 18 years in men, and the age of 16, 18, and 20–29 years in women (Table 5).
Table 4.
Number of twin individuals, mean and standard deviation of birth weight (kg) and BMI (kg/m2) by birth order, age and sex in monozygotic twins
| Men
|
Women
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Birth order | N | Mean | p valuea | SD | p valueb | N | Mean | p valuea | SD | p valueb | |
| Age 1 | 1 | 2843 | 17.21 | <0.001 | 1.41 | 0.798 | 2994 | 16.84 | <0.001 | 1.42 | 0.755 |
| 2 | 2838 | 17.11 | 1.42 | 2991 | 16.74 | 1.42 | |||||
| Age 2 | 1 | 2310 | 16.62 | <0.001 | 1.40 | 0.921 | 2339 | 16.17 | <0.001 | 1.38 | 0.915 |
| 2 | 2293 | 16.51 | 1.39 | 2323 | 16.04 | 1.38 | |||||
| Age 3 | 1 | 2830 | 16.02 | <0.001 | 1.41 | 0.276 | 3140 | 15.68 | <0.001 | 1.50 | 0.217 |
| 2 | 2834 | 15.88 | 1.39 | 3125 | 15.58 | 1.47 | |||||
| Age 4 | 1 | 1600 | 15.90 | 0.023 | 1.78 | 0.506 | 1594 | 15.71 | 0.001 | 2.01 | 0.355 |
| 2 | 1588 | 15.84 | 1.81 | 1595 | 15.59 | 1.98 | |||||
| Age 5 | 1 | 1272 | 15.34 | <0.001 | 1.54 | 0.671 | 1341 | 15.13 | <0.001 | 1.64 | 0.360 |
| 2 | 1266 | 15.20 | 1.51 | 1328 | 15.01 | 1.64 | |||||
| Age 6 | 1 | 528 | 15.54 | 0.265 | 1.77 | 0.735 | 427 | 15.25 | 0.084 | 1.76 | 0.735 |
| 2 | 512 | 15.48 | 1.77 | 421 | 15.14 | 1.74 | |||||
| Age 7 | 1 | 2345 | 15.40 | 0.002 | 1.69 | 0.419 | 2540 | 15.44 | <0.001 | 1.93 | 0.314 |
| 2 | 2330 | 15.31 | 1.71 | 2536 | 15.31 | 1.93 | |||||
| Age 8 | 1 | 1057 | 15.64 | 0.026 | 1.72 | 0.331 | 1020 | 15.65 | <0.001 | 1.97 | 0.240 |
| 2 | 1042 | 15.54 | 1.67 | 1021 | 15.50 | 1.93 | |||||
| Age 9 | 1 | 1042 | 16.29 | 0.057 | 2.10 | 0.318 | 1005 | 16.33 | 0.152 | 2.38 | 0.556 |
| 2 | 997 | 16.23 | 2.17 | 986 | 16.22 | 2.39 | |||||
| Age 10 | 1 | 1988 | 16.64 | 0.005 | 2.30 | 0.042 | 2088 | 16.67 | <0.001 | 2.45 | 0.502 |
| 2 | 1924 | 16.54 | 2.17 | 2048 | 16.56 | 2.40 | |||||
| Age 11 | 1 | 1530 | 17.32 | <0.001 | 2.56 | 0.004 | 1588 | 17.37 | 0.035 | 2.72 | 0.374 |
| 2 | 1470 | 17.10 | 2.44 | 1530 | 17.30 | 2.80 | |||||
| Age 12 | 1 | 2032 | 17.87 | <0.001 | 2.72 | 0.197 | 2127 | 17.92 | <0.001 | 2.74 | 0.999 |
| 2 | 1955 | 17.72 | 2.67 | 2053 | 17.77 | 2.71 | |||||
| Age 13 | 1 | 692 | 18.45 | 0.092 | 2.89 | 0.051 | 642 | 18.94 | 0.094 | 3.19 | 0.383 |
| 2 | 620 | 18.30 | 2.79 | 591 | 18.82 | 3.27 | |||||
| Age 14 | 1 | 1328 | 19.28 | 0.132 | 2.86 | 0.422 | 1497 | 19.67 | 0.007 | 3.02 | 0.956 |
| 2 | 1280 | 19.23 | 2.82 | 1468 | 19.59 | 3.01 | |||||
| Age 15 | 1 | 658 | 19.64 | 0.194 | 2.92 | 0.416 | 639 | 19.95 | 0.168 | 3.20 | 0.503 |
| 2 | 639 | 19.59 | 2.93 | 606 | 19.80 | 3.15 | |||||
| Age 16 | 1 | 1074 | 20.68 | 0.138 | 2.99 | 0.106 | 1311 | 20.64 | 0.098 | 2.98 | 0.172 |
| 2 | 1028 | 20.55 | 2.87 | 1257 | 20.57 | 2.95 | |||||
| Age 17 | 1 | 1100 | 20.94 | 0.387 | 2.72 | 0.850 | 1411 | 20.73 | 0.112 | 2.87 | 0.982 |
| 2 | 1074 | 20.88 | 2.68 | 1388 | 20.60 | 2.85 | |||||
| Age 18 | 1 | 1253 | 21.32 | 0.479 | 2.57 | 0.098 | 826 | 21.00 | 0.286 | 2.82 | 0.170 |
| 2 | 1253 | 21.33 | 2.49 | 812 | 20.81 | 2.74 | |||||
| Age 19 | 1 | 639 | 21.66 | 0.513 | 2.51 | 0.900 | 717 | 21.00 | 0.892 | 2.76 | 0.300 |
| 2 | 607 | 21.59 | 2.59 | 702 | 20.95 | 2.83 | |||||
| Age 20–29 | 1 | 2890 | 23.09 | 0.010 | 2.96 | 0.554 | 3488 | 21.85 | 0.010 | 3.69 | 0.340 |
| 2 | 2878 | 22.94 | 2.97 | 3478 | 21.73 | 3.57 | |||||
| Age 30–39 | 1 | 2305 | 24.65 | 0.461 | 3.39 | 0.215 | 3378 | 22.86 | 0.712 | 4.03 | 0.309 |
| 2 | 2290 | 24.52 | 3.30 | 3349 | 22.85 | 4.07 | |||||
| Age 40–49 | 1 | 1420 | 25.44 | 0.137 | 3.59 | 0.042 | 1886 | 23.75 | 0.676 | 4.20 | 0.428 |
| 2 | 1355 | 25.25 | 3.33 | 1844 | 23.77 | 4.33 | |||||
| Age 50–59 | 1 | 1038 | 25.43 | 0.668 | 3.21 | 0.523 | 1601 | 24.65 | 0.384 | 4.30 | 0.368 |
| 2 | 1029 | 25.56 | 3.18 | 1609 | 24.55 | 4.13 | |||||
| Age 60–69 | 1 | 506 | 25.59 | 0.852 | 3.20 | 0.264 | 880 | 25.24 | 0.168 | 4.26 | 0.312 |
| 2 | 494 | 25.73 | 3.28 | 878 | 25.02 | 4.17 | |||||
| Age 70–79 | 1 | 126 | 24.74 | 0.925 | 3.20 | 0.532 | 273 | 24.95 | 0.675 | 4.30 | 0.213 |
| 2 | 117 | 24.90 | 3.27 | 268 | 24.58 | 3.71 | |||||
p valuea: p value for equality of means, p valueb: p value for equality of variances, SD: standard deviation.
Table 5.
Number of twin individuals, mean and standard deviation of birth weight (kg) and BMI (kg/m2) by birth order, age and sex in dizygotic twins
| Birth order | Men
|
Women
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | p valuea | SD | p valueb | N | Mean | p valuea | SD | p valueb | ||
| Age 1 | 1 | 5088 | 17.20 | <0.001 | 1.39 | 0.037 | 4759 | 16.81 | 0.004 | 1.35 | 0.728 |
| 2 | 4962 | 17.04 | 1.34 | 4821 | 16.67 | 1.35 | |||||
| Age 2 | 1 | 4184 | 16.57 | <0.001 | 1.41 | 0.930 | 3815 | 16.20 | 0.006 | 1.38 | 0.423 |
| 2 | 4102 | 16.40 | 1.40 | 3871 | 16.04 | 1.39 | |||||
| Age 3 | 1 | 5407 | 16.03 | 0.003 | 1.54 | 0.003 | 5085 | 15.74 | 0.010 | 1.53 | 0.314 |
| 2 | 5266 | 15.84 | 1.47 | 5208 | 15.63 | 1.57 | |||||
| Age 4 | 1 | 2993 | 16.01 | 0.047 | 1.87 | 0.808 | 2818 | 15.81 | 0.044 | 1.89 | 0.656 |
| 2 | 2954 | 15.82 | 1.89 | 2848 | 15.68 | 1.93 | |||||
| Age 5 | 1 | 2349 | 15.33 | 0.020 | 1.59 | 0.806 | 2099 | 15.24 | 0.049 | 1.68 | 0.195 |
| 2 | 2259 | 15.17 | 1.60 | 2189 | 15.09 | 1.71 | |||||
| Age 6 | 1 | 583 | 15.65 | 0.867 | 2.02 | 0.153 | 455 | 15.65 | 0.164 | 2.29 | 0.574 |
| 2 | 552 | 15.59 | 2.21 | 469 | 15.40 | 2.22 | |||||
| Age 7 | 1 | 3986 | 15.50 | 0.018 | 1.85 | 0.077 | 3877 | 15.59 | 0.178 | 2.09 | 0.144 |
| 2 | 3981 | 15.35 | 1.92 | 3864 | 15.41 | 2.03 | |||||
| Age 8 | 1 | 1478 | 15.75 | 0.296 | 2.09 | 0.625 | 1279 | 15.96 | 0.194 | 2.26 | 0.953 |
| 2 | 1433 | 15.63 | 2.04 | 1318 | 15.76 | 2.26 | |||||
| Age 9 | 1 | 1445 | 16.61 | 0.660 | 2.61 | 0.161 | 1354 | 16.77 | 0.181 | 2.80 | 0.765 |
| 2 | 1449 | 16.41 | 2.49 | 1316 | 16.58 | 2.82 | |||||
| Age 10 | 1 | 3171 | 16.70 | 0.060 | 2.43 | 0.002 | 2994 | 16.98 | 0.107 | 2.58 | 0.422 |
| 2 | 3148 | 16.48 | 2.32 | 2973 | 16.77 | 2.68 | |||||
| Age 11 | 1 | 2385 | 17.56 | 0.326 | 2.77 | 0.992 | 2146 | 17.77 | 0.710 | 3.01 | 0.465 |
| 2 | 2288 | 17.40 | 2.80 | 2153 | 17.65 | 3.04 | |||||
| Age 12 | 1 | 3152 | 18.06 | 0.237 | 2.99 | 0.853 | 3031 | 18.26 | 0.294 | 3.05 | 0.391 |
| 2 | 3021 | 17.89 | 2.99 | 3048 | 18.02 | 3.02 | |||||
| Age 13 | 1 | 1035 | 18.59 | 0.875 | 3.25 | 0.572 | 898 | 18.95 | 0.853 | 3.35 | 0.876 |
| 2 | 965 | 18.52 | 3.20 | 896 | 18.85 | 3.35 | |||||
| Age 14 | 1 | 2353 | 19.58 | 0.315 | 3.24 | 0.741 | 2332 | 19.92 | 0.339 | 3.16 | 0.539 |
| 2 | 2266 | 19.58 | 3.16 | 2370 | 19.70 | 3.11 | |||||
| Age 15 | 1 | 1143 | 20.03 | 0.106 | 3.28 | 0.007 | 1042 | 20.23 | 0.898 | 3.26 | 0.911 |
| 2 | 1078 | 19.62 | 2.90 | 1013 | 20.24 | 3.37 | |||||
| Age 16 | 1 | 1990 | 20.87 | 0.290 | 3.03 | 0.800 | 2086 | 21.05 | 0.109 | 3.34 | 0.046 |
| 2 | 1940 | 20.77 | 3.01 | 2090 | 20.75 | 3.11 | |||||
| Age 17 | 1 | 2159 | 21.39 | 0.644 | 2.85 | 0.038 | 2230 | 21.09 | 0.158 | 2.98 | 0.500 |
| 2 | 2092 | 21.30 | 3.04 | 2224 | 20.79 | 2.93 | |||||
| Age 18 | 1 | 1680 | 21.79 | 0.597 | 2.86 | 0.012 | 1281 | 21.29 | 0.148 | 3.02 | 0.024 |
| 2 | 1614 | 21.83 | 3.01 | 1320 | 20.93 | 2.76 | |||||
| Age 19 | 1 | 1044 | 21.91 | 0.785 | 2.61 | 0.969 | 1099 | 21.38 | 0.212 | 3.05 | 0.082 |
| 2 | 1049 | 21.83 | 2.67 | 1047 | 21.12 | 2.84 | |||||
| Age 20–29 | 1 | 4036 | 23.39 | 0.398 | 3.04 | 0.507 | 4398 | 22.07 | 0.171 | 3.58 | 0.040 |
| 2 | 4121 | 23.37 | 3.11 | 4270 | 21.82 | 3.42 | |||||
| Age 30–39 | 1 | 3221 | 24.81 | 0.813 | 3.42 | 0.987 | 4120 | 23.19 | 0.619 | 4.30 | 0.125 |
| 2 | 3351 | 24.75 | 3.41 | 3887 | 23.02 | 4.17 | |||||
| Age 40–49 | 1 | 2403 | 25.48 | 0.932 | 3.35 | 0.553 | 2877 | 24.07 | 0.929 | 4.23 | 0.975 |
| 2 | 2411 | 25.39 | 3.40 | 2775 | 23.99 | 4.25 | |||||
| Age 50–59 | 1 | 2678 | 25.69 | 0.970 | 3.35 | 0.673 | 3261 | 24.75 | 0.525 | 4.00 | 0.065 |
| 2 | 2634 | 25.63 | 3.28 | 3086 | 24.53 | 3.89 | |||||
| Age 60–69 | 1 | 1265 | 25.49 | 0.897 | 3.19 | 0.595 | 1560 | 25.15 | 0.769 | 4.19 | 0.373 |
| 2 | 1276 | 25.64 | 3.29 | 1532 | 25.07 | 4.09 | |||||
| Age 70–79 | 1 | 310 | 25.34 | 0.909 | 3.35 | 0.279 | 435 | 24.99 | 0.905 | 3.86 | 0.610 |
| 2 | 329 | 25.31 | 3.20 | 390 | 24.87 | 4.03 | |||||
p valuea: p value for equality of means, p valueb: p value for equality of variances, SD: standard deviation.
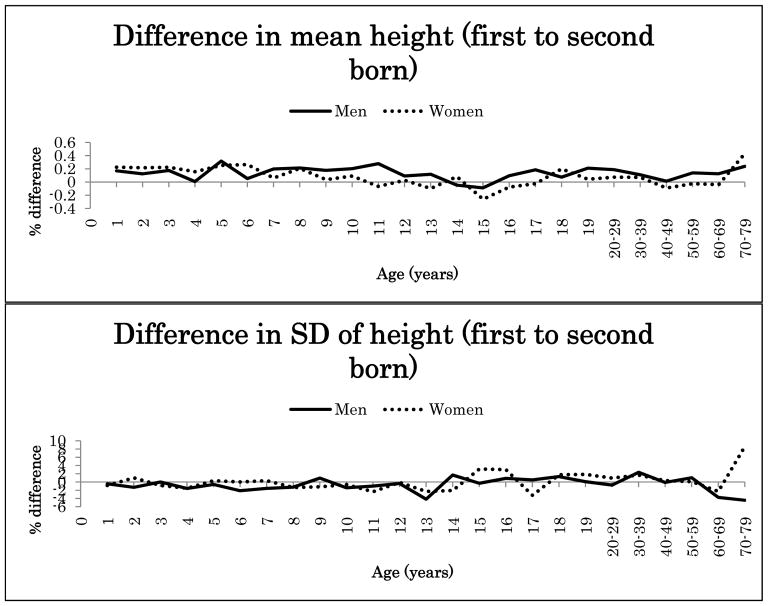
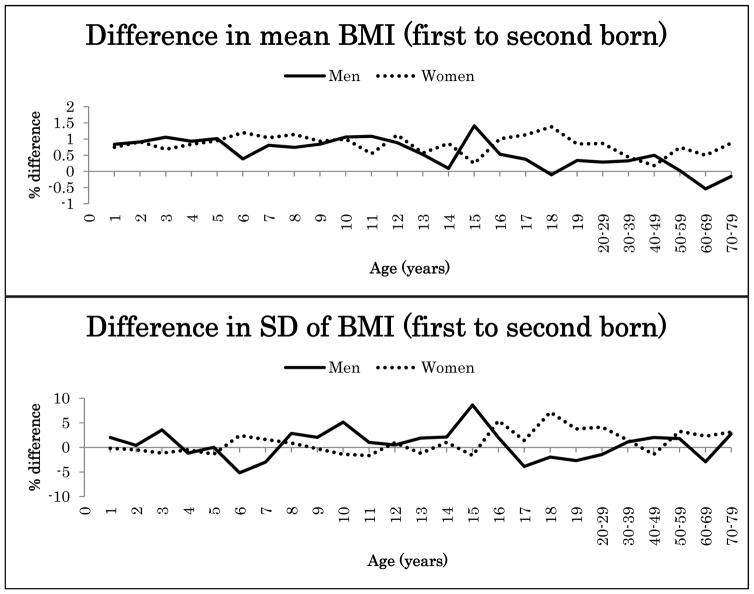
Because the interaction effects between birth order and zygosity were not statistically significant after Bonferroni correction for height or BMI (nominal p-values 0.047–0.008), data from MZ and DZ twins were combined in the further analyses. Figure 1 illustrates the percentage difference (%) in the mean and SD of height between the first-born and the second-born twins in men and women in the pooled data of MZ and DZ twins. Figure 2 presents the same results for BMI. Both for height and BMI, the first-born twins showed almost always higher mean values than the second-born twins. The mean differences in height between the first-born and the second-born twins ranged from −0.1% to 0.3% in men (at the age of 15 years and 5 years) and from −0.3% to 0.4% in women (at the 15 and ≥70-year age groups). The first-born male twins presented up to 1.4% greater BMI than the second-born male twins until 17 years of age and decreased with age in adulthood. The mean differences between the first-born and the second-born twins ranged from 0.2% to 1.4% in women (at the age of 15 years and 18 years). For SD the differences were small and did not show any systematic pattern varying from negative to positive.
Figure 1.
Mean and standard deviation differences (%) in height between first- and second- born twins across ages.
Figure 2.
Mean and standard deviation differences (%) in BMI between first- and second- born twins across ages.
Table 6 shows the results of fixed effects regression analysis of height and BMI at each age in the sub-cohort with information on birth weight. Adjustment for birth weight decreased the birth order differences in height and BMI. After adjusting for birth weight, birth order was associated with height at the age of one, three, five, seven and 10 years in men, whereas birth order was not associated with height in women. Moreover, as adjusting for birth weight, birth order was associated with BMI from the age of one to five, seven, 10, 11 and 12 years in men, and from the age of one to five, seven, 10 and 12 years in women.
Table 6.
Fixed effects regression coefficients for the association of birth order with height and BMI
| Height
|
BMI
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | ||||||
| B | p value | B | p value | B | p value | B | p value | ||
| Men | Age 1 | −0.14 | 0.004 | −0.02 | 0.565 | −0.15 | <0.001 | −0.10 | <0.001 |
| Age 2 | −0.10 | 0.120 | 0.00 | 0.980 | −0.16 | <0.001 | −0.11 | <0.001 | |
| Age 3 | −0.18 | 0.008 | −0.09 | 0.162 | −0.15 | <0.001 | −0.12 | <0.001 | |
| Age 4 | −0.07 | 0.510 | 0.00 | 0.978 | −0.11 | 0.005 | −0.08 | 0.025 | |
| Age 5 | −0.30 | 0.017 | −0.17 | 0.167 | −0.16 | <0.001 | −0.11 | 0.005 | |
| Age 6 | −0.01 | 0.952 | 0.13 | 0.510 | −0.03 | 0.635 | 0.00 | 0.981 | |
| Age 7 | −0.20 | 0.045 | −0.12 | 0.238 | −0.13 | 0.001 | −0.10 | 0.004 | |
| Age 8 | −0.21 | 0.169 | −0.11 | 0.455 | −0.10 | 0.062 | −0.06 | 0.250 | |
| Age 9 | −0.10 | 0.572 | −0.03 | 0.852 | −0.07 | 0.376 | −0.04 | 0.584 | |
| Age 10 | −0.36 | 0.008 | −0.24 | 0.064 | −0.16 | 0.003 | −0.13 | 0.015 | |
| Age 11 | −0.29 | 0.089 | −0.19 | 0.251 | −0.17 | 0.015 | −0.14 | 0.044 | |
| Age 12 | −0.07 | 0.678 | 0.03 | 0.839 | −0.18 | 0.004 | −0.15 | 0.020 | |
| Age 13 | −0.16 | 0.658 | −0.08 | 0.813 | −0.08 | 0.498 | −0.04 | 0.708 | |
| Age 14 | 0.04 | 0.895 | 0.11 | 0.699 | −0.08 | 0.415 | −0.05 | 0.608 | |
| Age 15 | 0.03 | 0.932 | 0.09 | 0.825 | −0.29 | 0.042 | −0.28 | 0.058 | |
| Age 16 | −0.03 | 0.900 | 0.05 | 0.845 | −0.16 | 0.172 | −0.13 | 0.249 | |
| Age 17 | −0.28 | 0.239 | −0.19 | 0.411 | −0.05 | 0.663 | −0.04 | 0.735 | |
| Age 18 | −0.29 | 0.135 | −0.10 | 0.607 | −0.07 | 0.475 | −0.04 | 0.638 | |
| Age 19 | −0.21 | 0.536 | −0.02 | 0.962 | −0.11 | 0.477 | −0.09 | 0.563 | |
| Age 20–29 | −0.30 | 0.068 | −0.12 | 0.447 | −0.08 | 0.341 | −0.07 | 0.445 | |
| Age 30–39 | −0.59 | 0.095 | −0.39 | 0.257 | 0.06 | 0.771 | 0.10 | 0.616 | |
| Age 40–49 | 0.17 | 0.770 | 0.22 | 0.697 | −0.18 | 0.646 | −0.17 | 0.660 | |
| Age 50–59 | 0.10 | 0.917 | 0.13 | 0.886 | −0.01 | 0.990 | −0.01 | 0.990 | |
| Age 60–69 | −0.35 | 0.795 | −0.33 | 0.784 | −0.56 | 0.571 | −0.56 | 0.577 | |
| Age 70–79 | −4.00 | 0.753 | −3.88 | 0.807 | 1.58 | 0.691 | 1.88 | 0.698 | |
| Women | Age 1 | −0.13 | 0.005 | 0.00 | 0.970 | −0.13 | <0.001 | −0.07 | 0.002 |
| Age 2 | −0.15 | 0.015 | 0.00 | 0.959 | −0.14 | <0.001 | −0.08 | 0.002 | |
| Age 3 | −0.18 | 0.007 | −0.08 | 0.185 | −0.12 | <0.001 | −0.09 | <0.001 | |
| Age 4 | −0.11 | 0.288 | −0.05 | 0.618 | −0.15 | <0.001 | −0.13 | 0.002 | |
| Age 5 | −0.24 | 0.056 | −0.12 | 0.330 | −0.15 | <0.001 | −0.11 | 0.008 | |
| Age 6 | −0.35 | 0.188 | −0.13 | 0.628 | −0.18 | 0.033 | −0.11 | 0.204 | |
| Age 7 | −0.19 | 0.048 | −0.12 | 0.223 | −0.13 | 0.001 | −0.10 | 0.006 | |
| Age 8 | −0.35 | 0.031 | −0.18 | 0.273 | −0.18 | 0.003 | −0.11 | 0.059 | |
| Age 9 | −0.06 | 0.742 | 0.07 | 0.668 | −0.16 | 0.046 | −0.11 | 0.190 | |
| Age 10 | −0.15 | 0.235 | −0.04 | 0.729 | −0.18 | 0.001 | −0.13 | 0.016 | |
| Age 11 | −0.04 | 0.820 | 0.01 | 0.972 | −0.09 | 0.208 | −0.07 | 0.306 | |
| Age 12 | −0.18 | 0.269 | −0.12 | 0.450 | −0.17 | 0.007 | −0.14 | 0.030 | |
| Age 13 | −0.03 | 0.925 | 0.07 | 0.833 | −0.12 | 0.344 | −0.09 | 0.504 | |
| Age 14 | −0.25 | 0.191 | −0.17 | 0.369 | −0.20 | 0.033 | −0.18 | 0.053 | |
| Age 15 | 0.17 | 0.588 | 0.32 | 0.293 | −0.11 | 0.506 | −0.08 | 0.630 | |
| Age 16 | −0.08 | 0.697 | 0.02 | 0.924 | −0.24 | 0.036 | −0.21 | 0.066 | |
| Age 17 | −0.12 | 0.538 | −0.01 | 0.951 | −0.19 | 0.045 | −0.17 | 0.071 | |
| Age 18 | −0.32 | 0.226 | −0.15 | 0.571 | −0.28 | 0.047 | −0.24 | 0.081 | |
| Age 19 | −0.01 | 0.980 | 0.10 | 0.728 | −0.21 | 0.180 | −0.18 | 0.245 | |
| Age 20–29 | −0.20 | 0.214 | −0.07 | 0.647 | −0.18 | 0.097 | −0.16 | 0.159 | |
| Age 30–39 | −0.16 | 0.457 | −0.01 | 0.953 | −0.11 | 0.475 | −0.09 | 0.565 | |
| Age 40–49 | 0.04 | 0.914 | 0.10 | 0.790 | 0.08 | 0.778 | 0.08 | 0.788 | |
| Age 50–59 | −0.25 | 0.564 | −0.17 | 0.700 | 0.05 | 0.889 | 0.04 | 0.921 | |
| Age 60–69 | 0.22 | 0.675 | 0.40 | 0.438 | 0.09 | 0.833 | 0.12 | 0.802 | |
| Age 70–79 | −0.73 | 0.554 | −0.41 | 0.736 | 0.00 | 0.999 | 0.08 | 0.940 | |
B: Unstandardized regression coefficient
Model 1: Unadjusted, Model 2: Adjusted for birth weight
Discussion
The CODATwins study established database with data on body size from twin cohorts in different countries from infancy to old age. Previous studies in twins have reported that the second-born twin was lighter than the first-born twin at birth (Glinianaia et al., 2000; Gielen M, et al., 2007) and our results from this very large international database are consistent with these studies. First-born twins were slightly taller than second-born twins in MZ pairs at some ages until 12 years of age. After adjustment for birth weight, birth order was associated with height in males only at some ages until 10 years of age. We did not find any strong evidence that birth order differences varied according to zygosity since the interaction effects between zygosity and birth order were not statistically significant after Bonferroni correction. These results suggest that birth order has a slight influence on height in twins during childhood mainly explained by birth weight.
Meanwhile, the current study revealed birth order differences in mean BMI until 12 years of age in MZ pairs, and in mean values of BMI until 5 years of age in DZ twin pairs. These birth order differences in mean values of BMI were generally modest but still statistically significant. Adjustment for birth weight reduced these differences, but a significant association of birth order with BMI remained until 12 years of age. Jelenkovic et al. (2015) reported that DZ twins are consistently taller than MZ twins, but mean BMI is not significantly different between MZ and DZ twins at young ages. Based on our study, birth order difference seems to associate more strongly with BMI than zygosity difference. For SDs of height and BMI the results were not statistically significant and did not show any systematic pattern either. Thus, it seems that the factors behind the mean differences between first-born and second-born twins were not associated with variances.
According to the Developmental Origins of Health and Disease hypothesis, birth weight is associated with disease risk later in life and is a determinant of adult health (Barker, 1998; Brodsky and Christou, 2004). This appears more salient for twins, who are born earlier and weigh less as compared to singletons (Loos, et al., 2005). However, it is indicated that growth of twins is not equal to growth of singletons after 29 weeks of gestation (Loos, et al., 2005), and the optimal intrauterine growth and lowest morbidity is achieved earlier in gestation for twins than for singletons (Luke, et al., 1993; Soucie, et al., 2006). In addition, previous studies in twins have reported that the second-born twin was lighter than the first-born twin at birth (Glinianaia et al., 2000; Gielen M, et al., 2007). Moreover, low birth weight predicts lower adult BMI in twins (Johansson & Rasmussen, 2001; Pietiläinen et al., 2001). Whitfield et al. (2001) reported that the correlation between birth weight and BMI in adulthood was positive, and the correlation is due to genetic factors and non-shared environmental factors. Gielen et al. (2007) indicated that the factors determining birth order, which is one of twin-specific factors, have a greater influence on birth weight than zygosity, chorionicity and fusion of the placentas. However it was not known how the birth order differences change over the life course. We found residual differences in BMI between the first-born and the second-born twins until 12 years of age in boys and girls after adjusting for birth weight. Our findings are in accordance with a previous Dutch study showing that the first-born twins were slightly heavier form three to 12 years of age (Silventoinen et al., 2007).
The reasons for the birth order difference in BMI in twins are not clear. It is possible that vascular and placental circumstances are important. Twins offer an opportunity to distinguish between maternal factors (smoking, alcohol, and total placental weight) affecting both twins and factors unique to each twin, such as individual placental weight and site of the insertion of the umbilical cord. Gielen et al. (2007) reported that the individual placental weight has a stronger association with birth weight than the total placental weight. This suggests that the unique factors are more important than the maternal factors. While placental factors appear to be important, consideration of chorionicity is also necessary.
There are three types of MZ twins based on chorionicity. MZ twins can either share one chorion and one amnion, each twin can have own amnion, or MZ twins can, like all DZ twins, each have their own chorion and amnion. Sharing the same chorion could create either a more similar or a more dissimilar prenatal environment (van Beijsterveldt et al. 2015). Kent et al. (2011) reported that monochorionic twins had higher rates of marginal and velamentous placental cord insertion, and noncentral cord insertion contributed to birth weight discordance in monochorionic twin pregnancies. Antoniou et al. (2011), who examine the genetic and environmental etiology of the umbilical cord in a large population of twins, indicated that partly genetic and unique environmental factors influence a number of the morphological characteristics of the overall umbilical cord development. Thus, even in the very early stages of life, twins can experience unique environmental influences (Antoniou et al., 2011). Unfortunately, our database does not have information on chorionicity, which is gathered reliably in few cohorts.
The birth order of twins is determined in early pregnancy, and the first twin at the beginning of pregnancy intrinsically remains in this position (Bronshtein et al., 1998). However, the relationship between the amniotic sac and the cervix, which remains relatively constant throughout gestation, may change near term or during labor in about 10% of twin pairs discordant for sex leading to a change in the anticipated twin order (Bronshtein et al., 1998). Since we did not find the interaction effects between birth order and zygosity, potential prenatal environmental differences between MZ and DZ twins do not seem to modify the birth order differences in BMI or height. However, we observed that birth order effects on BMI appear to last longer in MZ twins than in DZ twins, which may reflect the differences in chorionicity. The differences of intrauterine environment between the first-born and the second-born twins need more detailed research.
Birth order differences have been analyzed previously for perinatal and neonatal outcomes. Previous studies (Smith, et al., 2002; Sheay, et al., 2004; Smith, et al., 2005; Armson, et al., 2006; Smith, et al., 2007) have reported an increased risk of perinatal death of second-born compared with first-born twins. It has been proposed that this may be mainly a problem of more stillbirths in the second twins (Smith, et al., 2002). Luo et al. (2014) reported that perinatal mortality risk differences in second vs first twins depended on their relative birth size, and vaginal delivery at term was associated with a substantially greater risk of perinatal mortality in second twins. These early deaths would have resulted in such pairs not being included in our database.
With regard to neonatal morbidity, the studies of Hacking et al. (2001) and Donovan et al. (1998) both found that second-born twins have increased risk for respiratory distress syndrome. Shinwell et al. (2004) reported that very low birthweight (< 1500g) second twins were at increased risk for acute and chronic lung disease and neonatal mortality, irrespective of mode of delivery. Moreover, Marttila et al. (2004), who analyzed respiratory distress syndrome by gestational age in singletons, first-born and second-born twins, indicated that first-born twins had a significantly lower incidence of respiratory distress syndrome compared with second-born twins and singletons, except at less than 28 weeks of gestation. By being delivered by the same mother at the same time and gestational age, these factors cannot influence postnatal difference in growth, perinatal mortality, and neonatal morbidity risk between the second-born and the first-born twins. So, any such differences are likely to be attributable to birthweight, fetal growth, and intrauterine environment. Our study suggests that the impact of early morbidity, to the degree that it is indexed by birth order, is limited in time to early childhood, and more so on BMI than height.
The major strength of the present study is the large sample size of our international database of twin cohorts with height and weight measures covering almost the whole lifespan. However, a limitation is that countries or geographical regions are not equally represented, and the database is heavily weighted toward Caucasian populations. Accordingly, we could not study in detail possible ethnic differences in birth order differences. In addition, the number of twin participants in the oldest age groups is small. Multiple testing may have resulted in false-positive differences between the first-born and the second-born twins. However, mean values showed a consistent pattern across age and sex groups in both MZ and DZ twins, which provides considerable robustness to the results. Finally a large majority of height, weight and birth order measures are self- or maternally reported. This probably increases random error which lead to decreasing effect sizes. Our results may thus be underestimations of the real birth order differences.
In conclusion, the first-born twins had greater BMI and are slightly taller than the second-born twins. Birth order showed a significant association with BMI before 12 year of age. However, in generally, the differences in BMI and height between first-born and second- born twins were very modest even in early childhood. Adjustment for birth weight reduced the birth order differences but did not fully remove them for BMI. Evidence that birth order affects variances of height and BMI was limited.
Acknowledgments
The Australian Twin Registry is supported by a Centre of Research Excellence (grant ID 1079102) from the National Health and Medical Research Council administered by the University of Melbourne. The Boston University Twin Project is funded by grants (#R01 HD068435 #R01 MH062375) from the National Institutes of Health to K. Saudino. The Carolina African American Twin Study of Aging (CAATSA) was funded by a grant from the National Institute on Aging (grant 1RO1-AG13662-01A2) to K. E. Whitfield. Data collection and analyses in Finnish twin cohorts have been supported by ENGAGE – European Network for Genetic and Genomic Epidemiology, FP7-HEALTH-F4-2007, grant agreement number 201413, National Institute of Alcohol Abuse and Alcoholism (grants AA-12502, AA-00145, and AA-09203 to R J Rose, the Academy of Finland Center of Excellence in Complex Disease Genetics (grant numbers: 213506, 129680), and the Academy of Finland (grants 100499, 205585, 118555, 141054, 265240, 263278 and 264146 to J Kaprio). Gemini was supported by a grant from Cancer Research UK (C1418/A7974). Guangzhou Twin Eye Study is supported by National Natural Science Foundation of China (grant #81125007). Anthropometric measurements of the Hungarian twins were supported by Medexpert Ltd., Budapest, Hungary. Korean Twin-Family Register was supported by the Global Research Network Program of the National Research Foundation (NRF 2011-220-E00006). Longitudinal Israeli Study of Twins was funded by the Starting Grant no. 240994 from the European Research Council (ERC) to Ariel Knafo. The Michigan State University Twin Registry has been supported by Michigan State University, as well as grants R01-MH081813, R01-MH0820-54, R01-MH092377-02, R21-MH070542-01, R03-MH63851-01 from the National Institute of Mental Health (NIMH), R01-HD066040 from the Eunice Kennedy Shriver National Institute for Child Health and Human Development (NICHD), and 11-SPG-2518 from the MSU Foundation. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH, the NICHD, or the National Institutes of Health. PETS was supported by grants from the Australian National Health and Medical Research Council (grant numbers 437015 and 607358 to JC, and RS), the Bonnie Babes Foundation (grant number BBF20704 to JMC), the Financial Markets Foundation for Children (grant no. 032-2007 to JMC), and by the Victorian Government’s Operational Infrastructure Support Program. The Quebec Newborn Twin Study acknowledges financial support from the Fonds Québécois de la Recherche sur la Société et la Culture, the Fonds de la Recherche en Santé du Québec, the Social Science and Humanities Research Council of Canada, the National Health Research Development Program, the Canadian Institutes for Health Research, Sainte-Justine Hospital’s Research Center, and the Canada Research Chair Program (Michel Boivin). South Korea Twin Registry is supported by National Research Foundation of Korea (NRF-371-2011-1 B00047). The Twins Early Development Study (TEDS) is supported by a program grant (G0901245) from the UK Medical Research Council and the work on obesity in TEDS is supported in part by a grant from the UK Biotechnology and Biological Sciences Research Council (31/D19086). The West Japan Twins and Higher Order Multiple Births Registry was supported by Grant-in-Aid for Scientific Research (B) (grant number 15H05105) from the Japan Society for the Promotion of Science. The Murcia Twin Registry is supported by Fundación Séneca, Regional Agency for Science and Technology, Murcia, Spain (08633/PHCS/08, 15302/PHCS/10 & 19479/PI/14) and Ministry of Science and Innovation, Spain (PSI2009-11560 & PSI2014-56680-R).
References
- Ananth CV, Vintzileos AM, Shen-Schwarz S, Smulian JC, Lai YL. Standards of birth weight in twin gestations stratified by placental chorionicity. Obstet Gynecol. 1998;91:917–924. doi: 10.1016/s0029-7844(98)00052-0. [DOI] [PubMed] [Google Scholar]
- Antoniou EE, Derom C, Thiery E, Fowler T, Southwood TR, Zeegers MP. The Influence of genetic and environmental factors on the etiology of the human umbilical cord: the east flanders prospective twin survey. Biology of Reproduction. 2011;85:137–143. doi: 10.1095/biolreprod.110.088807. [DOI] [PubMed] [Google Scholar]
- Armson BA, O’Connell C, Persad V, Joseph KS, Young DC, Baskett TF. Determinants of perinatal mortality and serious neonatal morbidity in the second twin. Obstet Gynecol. 2006;108(3 Pt 1):556–64. doi: 10.1097/01.AOG.0000227747.37184.0a. [DOI] [PubMed] [Google Scholar]
- Ananth CV, Demissie K, Hanley ML. Birth weight discordancy and adverse perinatal outcomes among twin gestations in the United States: the effect of placental abruption. Am J Obstet Gynecol. 2003;188:954–60. doi: 10.1067/mob.2003.210. [DOI] [PubMed] [Google Scholar]
- Baal GCM, Boomsma D. Etiology of individual differences in birth weight of twins as a function of maternal smorking during pregnancy. Twin Research. 1998;1:123–130. doi: 10.1375/136905298320566258. [DOI] [PubMed] [Google Scholar]
- Bleker OP, Breur W, Huidekoper BL. A study of birth weight, placental weight and mortality of twins as compared to singletons. Br J Obstet Gynaecol. 1979;86:111–118. doi: 10.1111/j.1471-0528.1979.tb10577.x. [DOI] [PubMed] [Google Scholar]
- Barker DJP. Mothers, Babies and Health in Later Life. Edinburgh: Churchill Livingstone; 1998. [Google Scholar]
- Brodsky D, Christou H. Current concepts in intrauterine growth restriction. J Intensive Care Med. 2004;19:307–319. doi: 10.1177/0885066604269663. [DOI] [PubMed] [Google Scholar]
- Bronshtein M, Bar-Have I, Ben-Rafeal Z, Orvieto R, Ofir H, Itskovitz J. Twin gestation: is there a correlation n between the location of the gestational sacs at the beginning of pregnancy, and the order of delivery. Eur J Obstet Gynecol. 2006;108:556–564. doi: 10.1016/s0301-2115(97)00258-3. [DOI] [PubMed] [Google Scholar]
- Buckler JM, Green M. The growth of twins between the ages of 2 and 9 years. Ann Hum Biol. 2008;35(1):75–92. doi: 10.1080/03014460701809000. [DOI] [PubMed] [Google Scholar]
- Daw E, Walker J. Biological aspects of twin pregnancy in Dundee. Br J Obstet Gynaecol. 1975;82:29–34. doi: 10.1111/j.1471-0528.1975.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Donovan EF, Ehrenkrantz RA, Shankaran S, et al. Outcomes of very low birth weight twins cared for in the National Institute of Child Health and Human Development Neonatal Research Network’s intensive care units. Am J Obstet Gynecol. 1998;179:742–9. doi: 10.1016/s0002-9378(98)70075-4. [DOI] [PubMed] [Google Scholar]
- Gielen M, Derom C, Derom R, Vietinck R, Zeegers MP. Can birthweight discordancy within monozygotic twin pairs be used as an indicator of chorionicity? Twin Res Hum Genet. 2009;12(2):169–74. doi: 10.1375/twin.12.2.169. [DOI] [PubMed] [Google Scholar]
- Gielen MLP, Lindsey P, Derom C, Loos RJF, Derom R, Nijhuis JG, Vlietinck R. Twin Birth Weight Standards. Neonatology. 2007;92:164–173. doi: 10.1159/000102055. [DOI] [PubMed] [Google Scholar]
- Gielen MLP, Derom C, Loos RJF, Derom R, Vlietinck R. Curves of placental weights of live born twins. Twin Res Hum Genet. 2006;9:664–672. doi: 10.1375/183242706778553471. [DOI] [PubMed] [Google Scholar]
- Glinianaia SV, Skjaerven R, Magnus P. Birth weight percentiles by gestational age in multiple births. A population-based study of Norwegian twins and triplets. Acta Obstet Gynecol Scand. 2000;79:450–458. [PubMed] [Google Scholar]
- Gruenwald P. Environmental influences on twins apparent at birth. A preliminary study. Biol Neonate. 1970;15:79–93. doi: 10.1159/000240213. [DOI] [PubMed] [Google Scholar]
- Hacking D, Watkins A, Fraser S, Wolfe R, Nolan T. Respiratory distress syndrome and birth order in premature twins. Arch Dis Child Fetal Neonatal Ed. 2001;84:F117–21. doi: 10.1136/fn.84.2.F117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley RS, Hitti J, Emanuel I. Size-discordant twin pairs have higher perinatal mortality rates than nondiscordant pairs. Am J Obstet Gynecol. 2002;187:1173–8. doi: 10.1067/mob.2002.126961. [DOI] [PubMed] [Google Scholar]
- Heinonen S, Ryynanen M, Kirkinen P, Saarikoski S. Perinatal diagnostic evaluation of velamentous umbilical cord insertion: clinical, doppler, and ultrasonic findings. Obstet Gynecol. 1996;87:112–117. doi: 10.1016/0029-7844(95)00339-8. [DOI] [PubMed] [Google Scholar]
- Jelenkovic A, Yokoyama Y, Sund R, Honda C, Bogl LH, Aaltonen S, Ji F, Ning F, Pang Z, Ordoñana JR, Sánchez-Romera JF, Colodro-Conde L, Burt SA, Klump KL, Medland SE, Montgomery GW, Kandler C, McAdams TA, Eley TC, Gregory AM, Saudino KJ, Dubois L, Boivin M, Tarnoki AD, Tarnoki DL, Haworth CM, Plomin R, Öncel SY, Aliev F, Stazi MA, Fagnani C, D’Ippolito C, Craig JM, Saffery R, Siribaddana SH, Hotopf M, Sumathipala A, Rijsdijk F, Spector T, Mangino M, Lachance G, Gatz M, Butler DA, Bayasgalan G, Narandalai D, Freitas DL, Maia JA, Harden KP, Tucker-Drob EM, Kim B, Chong Y, Hong C, Shin HJ, Christensen K, Skytthe A, Kyvik KO, Derom CA, Vlietinck RF, Loos RJ, Cozen W, Hwang AE, Mack TM, He M, Ding X, Chang B, Silberg JL, Eaves LJ, Maes HH, Cutler TL, Hopper JL, Aujard K, Magnusson PK, Pedersen NL, Aslan AK, Song YM, Yang S, Lee K, Baker LA, Tuvblad C, Bjerregaard-Andersen M, Beck-Nielsen H, Sodemann M, Heikkilä K, Tan Q, Zhang D, Swan GE, Krasnow R, Jang KL, Knafo-Noam A, Mankuta D, Abramson L, Lichtenstein P, Krueger RF, McGue M, Pahlen S, Tynelius P, Duncan GE, Buchwald D, Corley RP, Huibregtse BM, Nelson TL, Whitfield KE, Franz CE, Kremen WS, Lyons MJ, Ooki S, Brandt I, Nilsen TS, Inui F, Watanabe M, Bartels M, van Beijsterveldt TC, Wardle J, Llewellyn CH, Fisher A, Rebato E, Martin NG, Iwatani Y, Hayakawa K, Sung J, Harris JR, Willemsen G, Busjahn A, Goldberg JH, Rasmussen F, Hur YM, Boomsma DI, S⊘rensen TI, Kaprio J, Silventoinen K. Zygosity Differences in Height and Body Mass Index of Twins From Infancy to Old Age: A Study of the CODATwins Project. Twin Res Hum Genet. 2015;4:1–14. doi: 10.1017/thg.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent EM1, Breathnach FM, Gillan JE, McAuliffe FM, Geary MP, Daly S, Higgins JR, Dornan J, Morrison JJ, Burke G, Higgins S, Carroll S, Dicker P, Manning F, Malone FD. Placental cord insertion and birthweight discordance in twin pregnancies: results of the national prospective ESPRiT Study. American Journal of Obstetrics and Gynecology. 2011;205(4):376.e1–7. doi: 10.1016/j.ajog.2011.06.077. [DOI] [PubMed] [Google Scholar]
- Lajunen HR, Kaprio J, Keski-Rahkonen A, Rose RJ, Pulkkinen L, Rissanen A, Silventoinen K. Genetic and environmental effects on body mass index during adolescence: A prospective study among Finnish twins. International Journal of Obesity. 2009;33:559–567. doi: 10.1038/ijo.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos RJ, Derom C, Derom R, Vlietinck R. Determinants of birthweight and intrauterine growth in liveborn twins. Paediatr Perinat Epidemiol. 2005;19(suppl 1):15–22. doi: 10.1111/j.1365-3016.2005.00611.x. [DOI] [PubMed] [Google Scholar]
- Loos RJ, Derom C, Derom R, Vlietinck R. Birthweight in liveborn twins: the influence of the umbilical cord insertion and fusion of placentas. Br J Obstet Gynaecol. 2001;108:943–948. doi: 10.1111/j.1471-0528.2001.00220.x. [DOI] [PubMed] [Google Scholar]
- Luke B, Minogue J, Witter FR, Keith LG, Johnson TR. The ideal twin pregnancy: patterns of weight gain, discordancy, and length of gestation. Am J Obstet Gynecol. 1993;169:588–597. doi: 10.1016/0002-9378(93)90628-v. [DOI] [PubMed] [Google Scholar]
- Luo ZC, Ouyang F, Zhang J, Klebanoff M. Perinatal mortality in second- vs firstborn twins: a matter of birth size or birth order? Am J Obstet Gynecol. 2014;211:153.e1–8. doi: 10.1016/j.ajog.2014.02.024. [DOI] [PubMed] [Google Scholar]
- Marttila R, Kaprio J, Hallman M. Respiratory distress syndrome in twin infants compared with singletons. Am J Obstet Gynecol. 2004;191:271–6. doi: 10.1016/j.ajog.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Naeye RL, Benirschke K, Hagstrom JW, Marcus CC. Intrauterine growth of twins as estimated from liveborn birth-weight data. Pediatrics. 1966;37:409–416. [PubMed] [Google Scholar]
- Pietiläinen KH, Kaprio J, Rasanen M, Rissanen A, Rose RJ. Genetic and environmental influences on the tracking of body size from birth to early adulthood. Obesity Research. 2002;10:875–884. doi: 10.1038/oby.2002.120. [DOI] [PubMed] [Google Scholar]
- Rietveld MJ, Posthuma ID, Dolan CV, Boomsma DI. ADHD: Sibling interaction or dominance: An evaluation of statistical power. Behavior Genetics. 2003;33:247–55. doi: 10.1023/a:1023490307170. [DOI] [PubMed] [Google Scholar]
- Schousboe K, Willemsen G, Kyvik KO, Mortensen J, Boomsma DI, Cornes BK, Davis CJ, Fagnani C, Hjelmborg J, Kaprio J, Lange M, Luciano M, Martin NG, Pedersen N, Pietilainen KH, Rissanen A, Saarni S, Sorensen TIA, Baa GCM, Harris JR. Sex differences in heritability of BMI: A comparative study of results from twin studies in eight countries. Twin Research. 2003;6:409–421. doi: 10.1375/136905203770326411. [DOI] [PubMed] [Google Scholar]
- Silventoinen K, Bartels M, Posthuma D, Estourgie-van Burk GF, Willemsen G, van Beijsterveldt TC, Boomsma DI. Genetic regulation of growth in height and weight from 3 to 12 years of age: A longitudinal study of Dutch twin children. Twin Research and Human. 2007a;10(2):354–363. doi: 10.1375/twin.10.2.354. [DOI] [PubMed] [Google Scholar]
- Silventoinen K, Jelenkovic A, Sund R, Honda C, Bogl LH, Aaltonen S, Yokoyama Y, Tarnoki AD, Tarnoki DL, Ning F, Ji F, Pang Z, Ordoñana JR, Sánchez-Romera JF, Burt SA, Colodro-Conde L, Klump KL, Medland SE, Montgomery GW, Kandler C, McAdams TA, Eley TC, Gregory AM, Saudino KJ, Dubois L, Boivin M, Haworth CM, Plomin R, Öncel SY, Aliev F, Stazi MA, Fagnani C, D’Ippolito C, Craig JM, Saffery R, Siribaddana SH, Hotopf M, Sumathipala A, Rijsdijk F, Spector T, Mangino M, Lachance G, Gatz M, Butler DA, Bayasgalan G, Narandalai D, Freitas DL, Maia JA, Harden KP, Tucker-Drob EM, Kim B, Chong Y, Hong C, Shin HJ, Christensen K, Skytthe A, Kyvik KO, Derom CA, Vlietinck RF, Loos RJ, Cozen W, Hwang AE, Mack TM, He M, Ding X, Chang B, Silberg JL, Eaves LJ, Maes HH, Cutler TL, Hopper JL, Aujard K, Magnusson PK, Pedersen NL, Aslan AK, Song YM, Yang S, Lee K, Baker LA, Tuvblad C, Bjerregaard-Andersen M, Beck-Nielsen H, Sodemann M, Heikkilä K, Tan Q, Zhang D, Swan GE, Krasnow R, Jang KL, Knafo-Noam A, Mankuta D, Abramson L, Lichtenstein P, Krueger RF, McGue M, Pahlen S, Tynelius P, Duncan GE, Buchwald D, Corley RP, Huibregtse BM, Nelson TL, Whitfield KE, Franz CE, Kremen WS, Lyons MJ, Ooki S, Brandt I, Nilsen TS, Inui F, Watanabe M, Bartels M, van Beijsterveldt TC, Wardle J, Llewellyn CH, Fisher A, Rebato E, Martin NG, Iwatani Y, Hayakawa K, Sung J, Harris JR, Willemsen G, Busjahn A, Goldberg JH, Rasmussen F, Hur YM, Boomsma DI, S⊘rensen TI, Kaprio J. A cohort description of COllaborative project of Development of Anthropometrical measures in Twins (CODATwins) to study macro-environmental variation in genetic and environmental effects on anthropometric traits. Twin Res Hum Genet. 2015;27:1–13. doi: 10.1017/thg.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silventoinen K, Pietilainen KH, Tynelius P, S⊘rensen TI, Kaprio J, Rasmussen F. Genetic and environmental factors in relative weight from birth to age 18: The Swedish young male twins study. International Journal of Obesity. 2007b;31:615–621. doi: 10.1038/sj.ijo.0803577. [DOI] [PubMed] [Google Scholar]
- Sheay W, Ananth CV, Kinzler WL. Perinatal mortality in first- and second-born twins in the United States. Obstet Gynecol. 2004;103(1):63–70. doi: 10.1097/01.AOG.0000101291.14773.F0. [DOI] [PubMed] [Google Scholar]
- Smith GC, Pell JP, Dobbie R. Birth order, gestational age, and risk of delivery related perinatal death in twins: retrospective cohort study. BMJ. 2002;325:1004. doi: 10.1136/bmj.325.7371.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GC, Shah I, White IR, Pell JP, Dobbie R. Mode of delivery and the risk of delivery-related perinatal death among twins at term: a retrospective cohort study of 8073 births. BJOG. 2005;112:1139–44. doi: 10.1111/j.1471-0528.2005.00631.x. [DOI] [PubMed] [Google Scholar]
- Smith GC, Fleming KM. White IR. Birth order of twins and risk of perinatal death related to delivery in England, Northern Ireland, and Wales, 1994–2003: retrospective cohort study. BMJ. 2007;334:576. doi: 10.1136/bmj.39118.483819.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinwell ES, Blickstein I, Lusky A, Reichman B. Effect of birth order on neonatal morbidity and mortality, among very low birthweight twins: a population based study. Arch Dis Child Fetal Neonatal Ed. 2004;89:F145–F148. doi: 10.1136/adc.2002.021584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucie JE, Yang Q, Wen SW, Fung KF, Walker M. Neonatal mortality and morbidity rates in term twins with advancing gestational age. Am J Obstet Gynecol. 2006;195:172–177. doi: 10.1016/j.ajog.2006.01.018. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CEM, Overbeek LIH, Rozendaal L, McMaster MTB, Glasner TJ, Bartels M, Vink JM, Martin NG, Dolan CV, Boomsma DI. Chorionicity and heritability estimates from twin studies: The prenatal environment of twins and their resemblance across a large number of traits. Behav Genet. 2015 doi: 10.1007/s10519-015-9745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield JB, Treloar SA, Zhu G, Martin NG. Genetic and non-genetic factors affecting birth-weight and adult body mass index. Twin Research. 2001;4(5):365–370. doi: 10.1375/1369052012533. [DOI] [PubMed] [Google Scholar]