Abstract
Background:
Acute stroke is a time-sensitive condition in which rapid diagnosis must be made in order for thrombolytic treatment to be administered. A certain proportion of patients who receive thrombolysis will be found on further evaluation to have a diagnosis other than stroke, so-called “stroke mimics.” Little is known about the role of language discordance in the emergency department diagnosis of acute ischemic stroke.
Methods:
This is a retrospective analysis of all acute ischemic stroke patients who received IV tissue plasminogen activator (tPA) in our emergency department between July 2011 and December 2015. Baseline characteristics, patient language, and final diagnosis were compared between encounters in which the treating neurologist and patient spoke the same language (concordant cases) and encounters in which they did not (discordant cases).
Results:
A total of 350 patients received IV tPA during the study period. English was the primary language for 52.6%, Spanish for 44.9%, and other languages for 2.6%; 60.3% of cases were classified as language concordant and 39.7% as discordant. We found no significant difference in the proportion of stroke mimics in the language concordant compared to discordant groups (16.6% vs 9.4%, p = 0.06). Similarly, the proportion of stroke mimics did not differ between English- and Spanish-speaking patients (15.8% vs 11.5%, p = 0.27).
Conclusions:
Language discordance was not associated with acute stroke misdiagnosis among patients treated with IV tPA. Prospective evaluation of communication during acute stroke encounters is needed to gain clarity on the role of language discordance in acute stroke misdiagnosis.
Language discordance occurs when a patient and treating physician do not have proficiency in the same language and has been associated with decreased quality of acute care.1–3 However, little is known about the role of language discordance in the emergency department (ED) diagnosis of acute ischemic stroke (AIS).
The ED evaluation of patients with AIS is necessarily rapid because IV tissue plasminogen activator (tPA) must be given within 4.5 hours of the last-known-normal time. To safely administer IV tPA, a patient must be examined, last-known-normal time confirmed, exclusion criteria assessed, and a head CT obtained to exclude intracerebral hemorrhage. A proportion of patients who present with stroke symptoms and are treated with IV tPA are later found to have a diagnosis other than AIS upon further workup.4 These patients are considered to have stroke mimics (SMs). Although SM treatment is considered safe with low rates of intracerebral hemorrhage, the complication rate is not zero.5 In addition, there is a considerable cost associated with treatment of SMs.6 Other serious conditions may also be missed when an incorrect diagnosis of AIS is made.
We therefore sought to evaluate the role of language discordance on thrombolysis of SMs at a high-volume stroke center serving a large Spanish-speaking population. We hypothesized that language discordance between patients and neurologists would result in a greater proportion of SMs as language barriers may lead to inaccurate information gathering and misdiagnosis of AIS. The recent Institute of Medicine report on the harm and cost of diagnostic error makes this exploration particularly timely.7
METHODS
This was a retrospective, single-center, cross-sectional study. The electronic medical record was reviewed for all patients who received IV tPA in the ED at Columbia University Medical Center from July 1, 2011, through December 31, 2015. Demographic, clinical, final diagnosis, and patient language information was abstracted by manual chart review. SMs were defined as a final diagnosis other than AIS, aborted stroke, imaging-negative stroke, or TIA as assigned by the treating vascular neurology–boarded attending neurologist at the time of hospital discharge. Imaging-negative strokes were defined as a vascular diagnosis with lack of infarction on brain imaging, as documented by the treating vascular neurology–boarded attending neurologist at the time of hospital discharge. Final diagnoses of TIA, aborted or averted stroke, and diffusion-weighted imaging–negative stroke were considered imaging-negative strokes.
Patients' primary language was determined by self-report, as indicated at the time of ED triage. Fluency in languages other than English of treating neurologists was self-reported via standardized questionnaire administered to all neurology residents, who are the primary neurologists at and leaders of all acute stroke codes. The decision for thrombolysis is made in discussion with a vascular neurology fellow. Cases in which the treating neurology resident was fluent in the patient's primary language were categorized as concordant and those in which the treating neurologist was not fluent as discordant. In our ED, in-person Spanish interpreters are available 24 hours a day; telephone translation services are available for other languages. The official hospital policy is to provide interpreter services to all limited-English proficiency patients and only use family members or other unofficial interpreters when hospital-provided translation services are refused. Because the use of an interpreter is not reliably captured by our electronic medical record, we conducted a survey of current neurology residents on their impression of interpreter availability at acute stroke codes.
We compared baseline characteristics, relevant time intervals, and the proportion of SMs between language concordant and discordant groups. This was repeated for English- and Spanish-speaking patients regardless of treating physician language. Means (SDs) or medians (interquartile ranges) were reported for continuous variables. Fisher exact or χ2 tests were used for categorical variables; Mann–Whitney U test was used for all continuous variables aside from age, which was compared using a t test. A multivariable model adjusting for demographics (age entered continuously, sex) and pretreatment factors with significant differences on univariable analyses was also conducted. To determine the proportion of IV tPA–eligible patients who did not receive treatment in comparison to their primary language, we queried our prospective stroke registry over a period of 13 months for all patients with a final diagnosis of stroke who arrived in the ED within 4.5 hours of symptom onset. We considered p ≤ 0.05 to be statistically significant. Calculations were done using SPSS version 23.0 (IBM Corp., Armonk, NY).
Standard protocol approvals, registrations, and patient consents
The Columbia University institutional review board approved this study. A waiver of informed consent was granted because of the retrospective nature of this study. All study data were deidentified.
RESULTS
A total of 350 patients received IV tPA in the ED during the study period. Most patients were either English-speaking (52.6%) or Spanish-speaking (44.9%), with other languages accounting for a small portion (2.6%).
Sixty-three neurology residents treated patients during the study period, of which 15 (23.8%) were fluent in Spanish. There were 211 cases (60.3%) classified as concordant and 139 (39.7%) as discordant. All concordant cases were either English- or Spanish-speaking pairs, with the exception of one Russian-speaking pair. A survey of residents on interpreter services at stroke codes overall did not reveal major barriers to timely access or presence of these services during acute stroke evaluations.
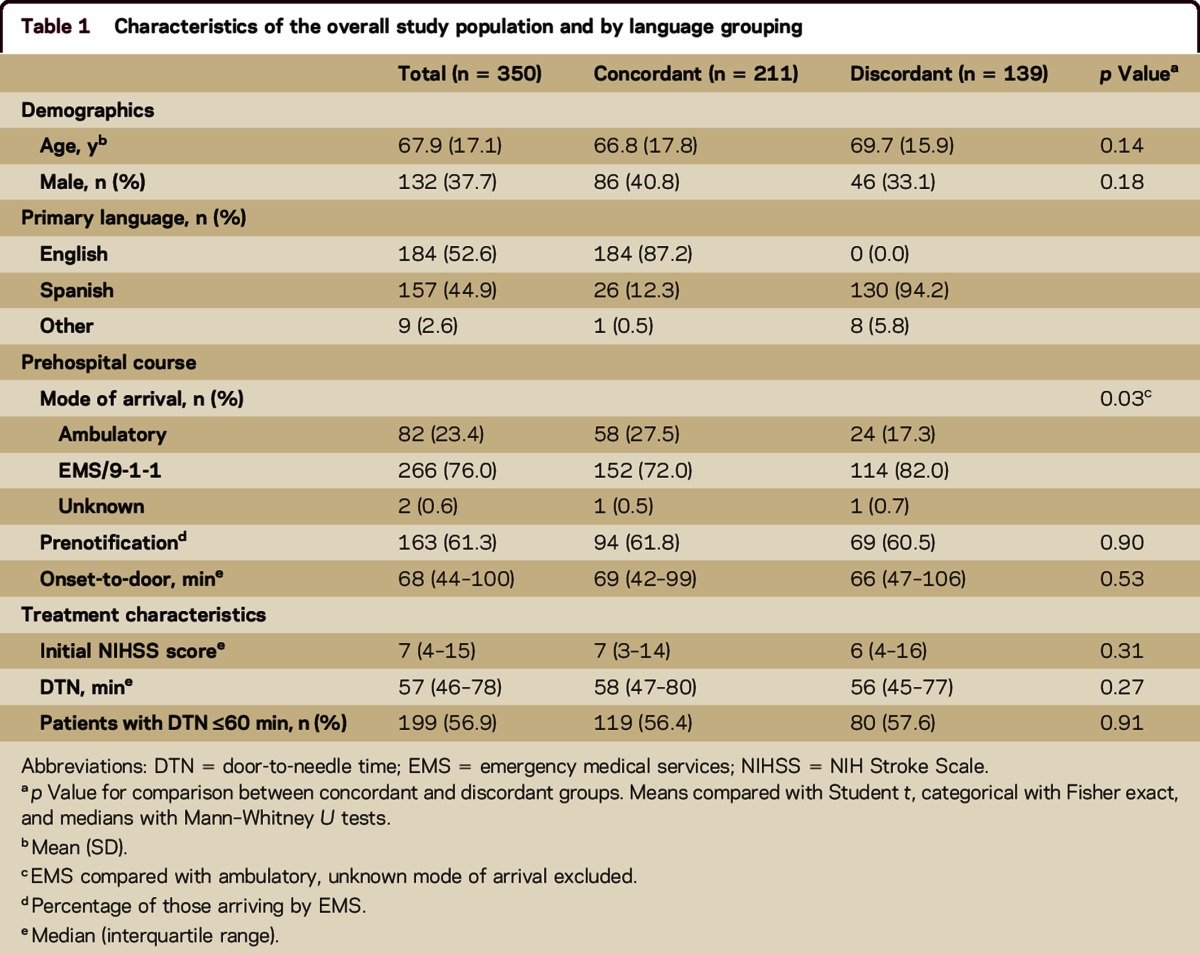
Overall mean age was 68 years, median NIH Stroke Scale (NIHSS) score was 7, and 266 (76.0%) arrived by emergency medical services (EMS). There were no differences in age, sex, and initial NIHSS score between concordant and discordant groups (table 1). Similarly, there were no differences in age, sex, and initial NIHSS score between English and Spanish speakers regardless of treating physician fluency. The proportion of patients arriving by EMS was higher in the discordant group (72.0% vs 82.0%, p = 0.03). There was no difference in EMS use among English speakers compared with Spanish speakers (72.1% vs 80.8%, p = 0.07). Among those arriving by EMS, rates of prenotification did not differ by either language concordance or primary language.
Table 1.
Characteristics of the overall study population and by language grouping
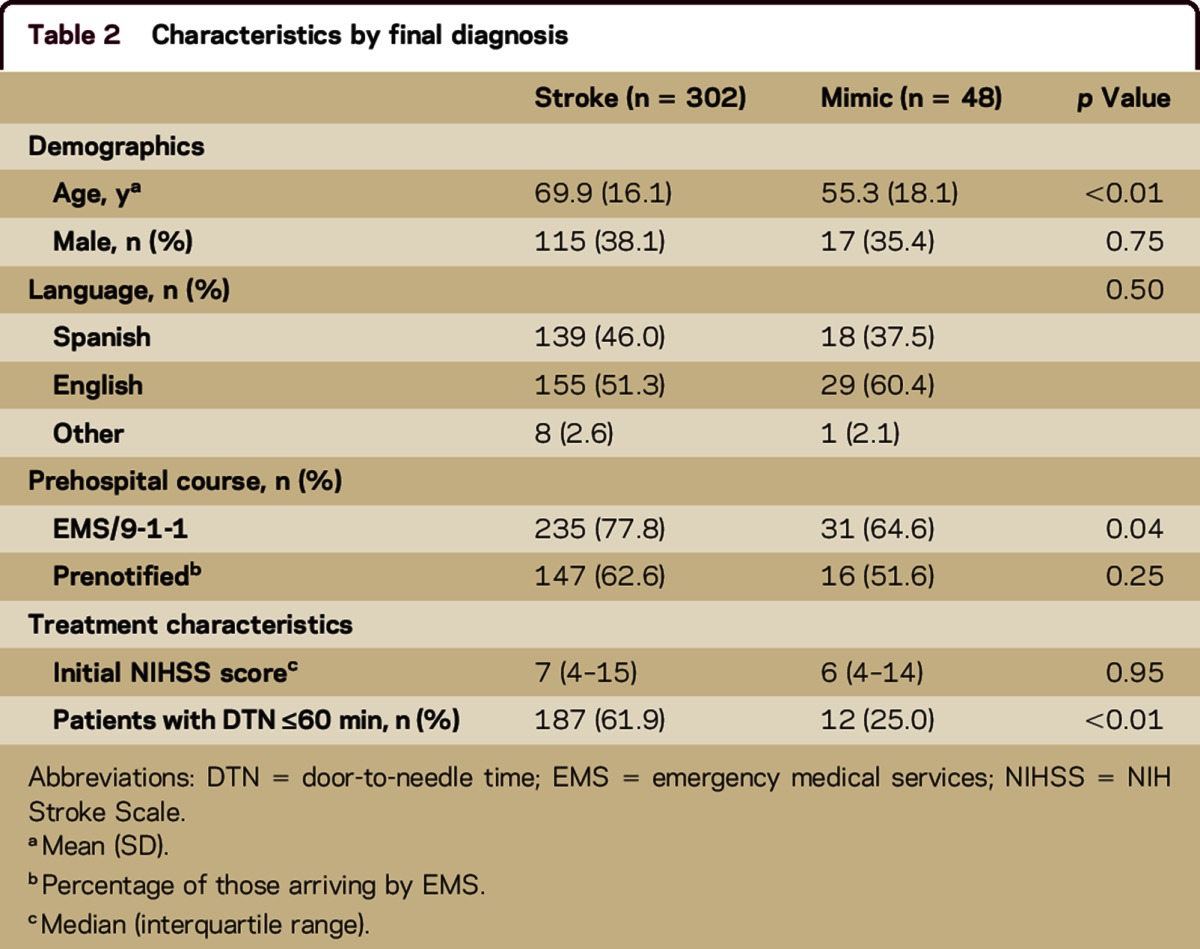
There were 48 (13.7%) SMs in total. Sex (proportion male 35.4% vs 38.1%, p = 0.75) and median NIHSS score (6 vs 7, p = 0.95) did not differ between those with SMs and those with confirmed strokes (table 2). However, those with SMs were, on average, younger (55 vs 70 years, p < 0.01) and less likely to use EMS (arrival by EMS 64.6% vs 77.8%, p = 0.04). Seizure (25%) was the most common final diagnosis in mimics followed by conversion disorder (19%) and migraine (17%).
Table 2.
Characteristics by final diagnosis
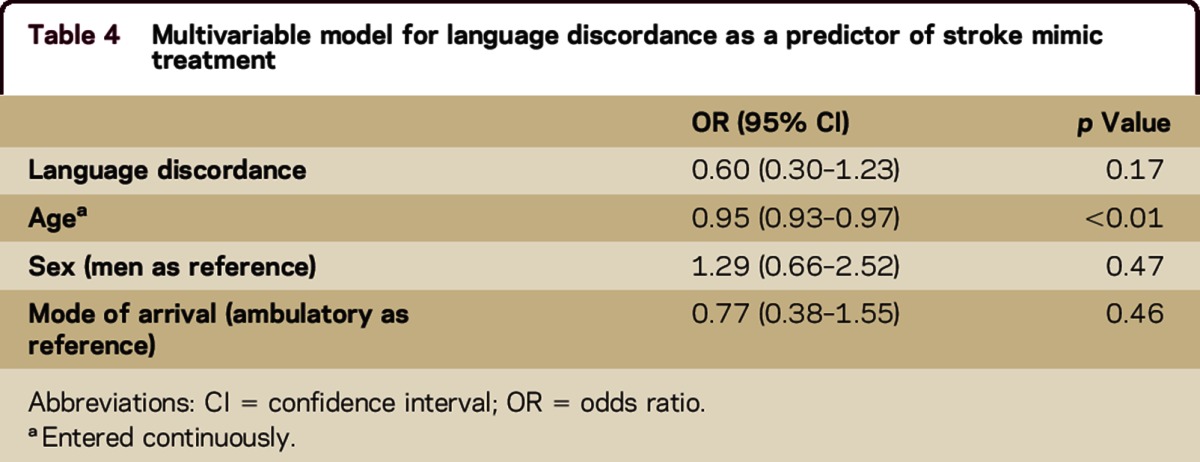
The proportion of SMs did not significantly differ in the concordant group compared to the discordant group (16.6% vs 9.4%, p = 0.06) (table 3). There was no difference in the proportion of SMs among English speakers compared with Spanish speakers (15.8% vs 11.5%, p = 0.27). In a multivariable model adjusting for demographics and mode of arrival (the only pretreatment factor with significance on univariable analyses; tables 1 and 2), there was no association between language discordance and SM treatment (odds ratio for language discordance 0.60, 95% confidence interval 0.30–1.23, p = 0.17) (table 4).
Table 3.
Final diagnosis by language grouping
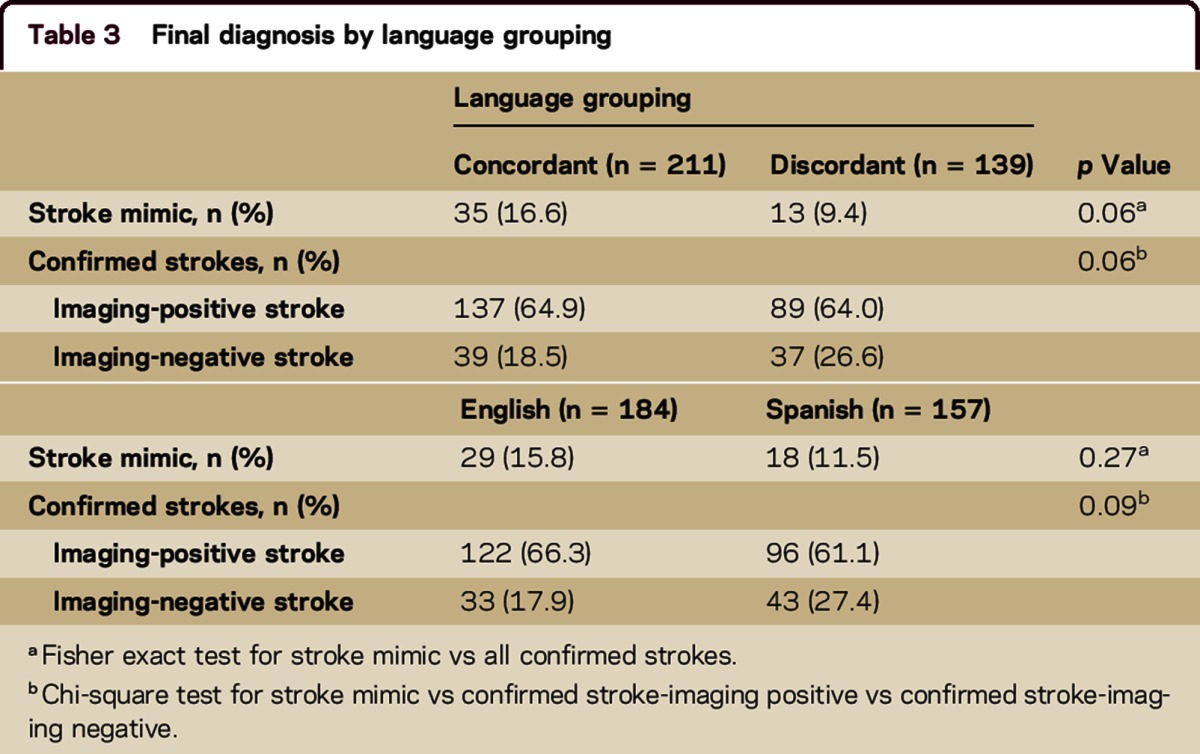
Table 4.
Multivariable model for language discordance as a predictor of stroke mimic treatment
A sensitivity analysis examining the effect of treatment times on our findings revealed a greater proportion of confirmed strokes with door-to-needle time ≤60 minutes compared to SMs (61.9% vs 25.0%, p < 0.01, table 2). However, the proportion of SMs with door-to-needle time ≤60 minutes did not differ between language concordant and discordant groups (8.4% [10] vs 2.5% [2], p = 0.13, 2-tailed Fisher exact test).
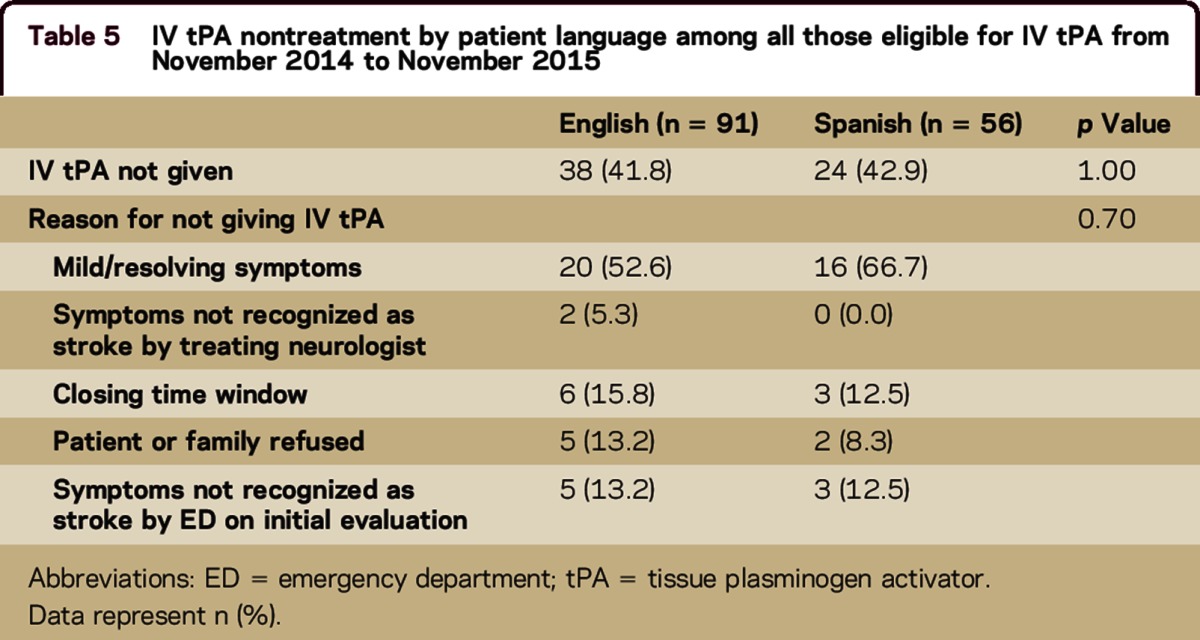
Data from our prospective stroke registry were available from November 2014 to November 2015. During this period, 147 patients without contraindications to IV tPA arrived in the ED within 4.5 hours of symptom onset. Of these, 62 (42.2%) were not treated with IV tPA. There was no difference in the proportion of English speakers compared with Spanish speakers who were not treated (41.8% vs 42.1%, p = 1.0). The most common reason for withholding thrombolysis was mild and resolving symptoms (58.1%). The reasons for nontreatment did not differ by patient language (table 5).
Table 5.
IV tPA nontreatment by patient language among all those eligible for IV tPA from November 2014 to November 2015
DISCUSSION
At our center, we did not observe an association between language discordance and the proportion of SMs among patients treated with IV tPA. Similarly, the proportion of SMs did not differ between English- and Spanish-speaking patients. We hypothesized that language barriers would lead to less accurate clinical history-taking and result in a greater proportion of SMs because of misdiagnosis from miscommunication; however, this was not the case. In fact, we found a trend toward a higher proportion of SMs in the language concordant group.
There are several possible explanations for our findings: (1) the decision to thrombolyse relies heavily on a focused, routine neurologic examination, which may not be affected by a language barrier; (2) language barriers alter the patterns of communication and may foster more parsimonious and targeted questioning from physicians; (3) a greater proportion of patients with SM who had limited English proficiency may not have been treated with tPA because of misinterpretation or delay in translation and thus were not captured in our study.
The first explanation may partially account for our findings of no difference in treatment of mimics by language grouping. However, the thrombolysis decision is more straightforward in severe strokes in which the neurologic deficit is obvious compared to minor strokes in which examination and history-taking may be more nuanced. The fact that we did not see a difference in the distribution of stroke severity between SMs and confirmed strokes argues against this point.
Regarding the second explanation, because of our retrospective design, we cannot determine with certainty how communication occurred in all discordant encounters. However, it is possible that questioning is more direct and focused in discordant interactions, which yields more accurate information. This may lead to increased detection of SMs in the presence of language barriers, and could account for the trend we observed toward a lower proportion of treated SMs in the discordant group.
Our retrospective design limits our ability to fully comment on the third explanation. Although door-to-needle times were longer in SMs compared with confirmed strokes, among SMs door-to-needle times did not differ by language grouping. In addition, overall door-to-needle times did not differ between language concordant and discordant groups. This suggests that language barriers do not contribute to prolonged treatment times in SMs. Data from our prospective stroke registry also provide some reassurance that selection bias is not a major factor in our findings; the frequency of not receiving thrombolysis did not vary by patient language.
There is limited prior work on the role of language discordance in acute stroke treatment. Results to date have been equivocal. The Brain Attack Surveillance in Corpus Christie project showed that in a population with a large proportion of Mexican Americans, primary language was not associated with either EMS use or time to ED presentation.8 Studies from Canada have demonstrated that among hospitalized stroke patients, language barriers are associated with increased lengths of stay and decreased inpatient mortality.1,9 Work from California using administrative claims data found lower risk-adjusted inpatient mortality for Spanish-speaking stroke patients.10 These studies did not capture language discordance or comment on the use of interpreters. We previously showed that language discordance did not affect time to thrombolysis.11
The proportion of SMs among tPA-treated patients we observed of 13.7% is consistent with that reported by other single-center studies and within the range reported elsewhere.5,12,13 Our SM population was demographically similar to that of prior studies in that they were younger than those with confirmed strokes. However, we found no sex differences between SMs and confirmed strokes, and our patients with SMs did not have decreased stroke severity compared to those with confirmed strokes; this differs from prior work.4,5 Regarding SM predictors, other studies have found lower rates of atrial fibrillation and hypertension in SMs compared with confirmed strokes. Without a detailed medical history, we cannot fully compare how our patients with SMs differed medically from patients with confirmed strokes.
There are limitations of this study. Our single-center, retrospective design has inherent selection bias because we only evaluated those treated with IV tPA; we cannot say how language discordance affected those who presented within the time window for IV tPA but were not treated. However, the limited data available from our prospective stroke registry are reassuring in that the frequency of not receiving IV tPA did not differ by patient language. The retrospective design also limits our ability to definitively determine important aspects of communication in language discordant interactions; specifically, we do not know how often interpretation was ad hoc, either by family, other staff members including ED physicians, or between patients and residents with limited proficiency. However, the results from our survey of residents are encouraging in that interpreters are generally thought to be readily present, and our hospital policy supports interpreter use with all patients who have limited English proficiency. Our study may have limited generalizability to centers that do not have around-the-clock access to professional interpreters. Lastly, we cannot exclude the possibility of misclassification of SMs. While our proportion of SMs is in line with other studies, our proportion of neuroimaging-negative strokes is somewhat higher than other series of patients treated with IV tPA.12,14
The strengths of our study include a fairly large sample of language discordant encounters. In addition, our mostly linguistically homogeneous non–English-speaking population allows exploration of the role of language discordance in acute stroke treatment among Spanish speakers. Although prospective confirmation is required, our results may support consideration of 24-hour access to professional interpreters at high-volume stroke centers with large non–English-speaking populations. Lastly, tracking SMs among thombolysed patients provides opportunities to study unique predictors of misdiagnosis, which may ultimately lead to interventions to improve diagnostic accuracy.
CONCLUSIONS
Language discordance was not associated with acute stroke misdiagnosis among patients treated with IV tPA at a single center with a large Spanish-speaking patient population. Prospective evaluation of communication during acute stroke encounters is needed to gain clarity on the role of language discordance in acute stroke misdiagnosis and the effect of in-person interpreters on these findings.
AUTHOR CONTRIBUTIONS
Sara K. Rostanski: study concept and design, acquisition of data, analysis and interpretation of data, draft of manuscript. Olajide Williams: revision of manuscript for intellectual content. Joshua I. Stillman: revision of manuscript for intellectual content. Randolph S. Marshall: revision of manuscript for intellectual content. Joshua Z. Willey: interpretation of data, revision of manuscript for intellectual content.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
S.K. Rostanski receives research support from NIH/National Institute of Neurological Disorders and Stroke StrokeNet Grant number 1U10 NS086728. O. Williams receives research support from NIH/National Institute of Neurological Disorders and Stroke and NYC Department of Health. J.I. Stillman participates in NIH-funded clinical trials research through NETT. R.S. Marshall serves on the editorial boards of JAMA Neurology, Stroke, Neurology®, and Annals of Neurology; receives publishing royalties from OnCall Neurology (Elsevier, 2003–present); and receives research support from NIH/National Institute of Neurological Disorders and Stroke. J.Z. Willey serves on Clinical Endpoint Committees for Reliant Heart and Cardiovascular Research Foundation; serves as a consultant for HeartWare Incorporated; and receives research support from AstraZeneca, Genentech, and NIH/National Institute of Neurological Disorders and Stroke. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
REFERENCES
- 1.John-Baptiste A, Naglie G, Tomlinson G, et al. . The effect of English language proficiency on length of stay and in-hospital mortality. J Gen Intern Med 2004;19:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hampers LC, Cha S, Gutglass DJ, Binns HJ, Krug SE. Language barriers and resource utilization in a pediatric emergency department. Pediatrics 1999;103:1253–1256. [DOI] [PubMed] [Google Scholar]
- 3.Meischke HW, Calhoun RE, Yip MP, Tu SP, Painter IS. The effect of language barriers on dispatching EMS response. Prehosp Emerg Care 2013;17:475–480. [DOI] [PubMed] [Google Scholar]
- 4.Zinkstok SM, Engelter ST, Gensicke H, et al. . Safety of thrombolysis in stroke mimics: results from a multicenter cohort study. Stroke 2013;44:1080–1084. [DOI] [PubMed] [Google Scholar]
- 5.Tsivgoulis G, Zand R, Katsanos AH, et al. . Safety of intravenous thrombolysis in stroke mimics: prospective 5-year study and comprehensive meta-analysis. Stroke 2015;46:1281–1287. [DOI] [PubMed] [Google Scholar]
- 6.Goyal N, Male S, Al Wafai A, Bellamkonda S, Zand R. Cost burden of stroke mimics and transient ischemic attack after intravenous tissue plasminogen activator treatment. J Stroke Cerebrovasc Dis 2015;24:828–833. [DOI] [PubMed] [Google Scholar]
- 7.National Academies of Sciences, Engineering, and Medicine. Improving Diagnosis in Health Care. Washington, DC: The National Academies Press; 2015. [Google Scholar]
- 8.Smith MA, Lisabeth LD, Bonikowski F, Morgenstern LB. The role of ethnicity, sex, and language on delay to hospital arrival for acute ischemic stroke. Stroke 2010;41:905–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah BR, Khan NA, O'Donnell MJ, Kapral MK. Impact of language barriers on stroke care and outcomes. Stroke 2015;46:813–818. [DOI] [PubMed] [Google Scholar]
- 10.Hines AL, Andrews RM, Moy E, Barrett ML, Coffey RM. Disparities in rates of inpatient mortality and adverse events: race/ethnicity and language as independent contributors. Int J Environ Res Public Health 2014;11:13017–13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rostanski SK, Stillman J, Williams O, Marshall RS, Yaghi S, Willey JZ. The influence of language discordance between patient and physician on time-to-thrombolysis in acute ischemic stroke. Neurohospitalist 2016;6:107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chernyshev OY, Martin-Schild S, Albright KC, et al. . Safety of tPA in stroke mimics and neuroimaging-negative cerebral ischemia. Neurology 2010;74:1340–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsivgoulis G, Alexandrov AV, Chang J, et al. . Safety and outcomes of intravenous thrombolysis in stroke mimics: a 6-year, single-care center study and a pooled analysis of reported series. Stroke 2011;42:1771–1774. [DOI] [PubMed] [Google Scholar]
- 14.Uchino K, Massaro L, Hammer MD. Transient ischemic attack after tissue plasminogen activator: aborted stroke or unnecessary stroke therapy? Cerebrovasc Dis 2010;29:57–61. [DOI] [PubMed] [Google Scholar]


